User login
Commentary: Functional assessment developed for older adults with sickle cell disease
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
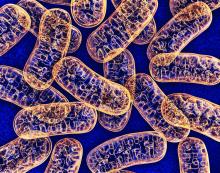
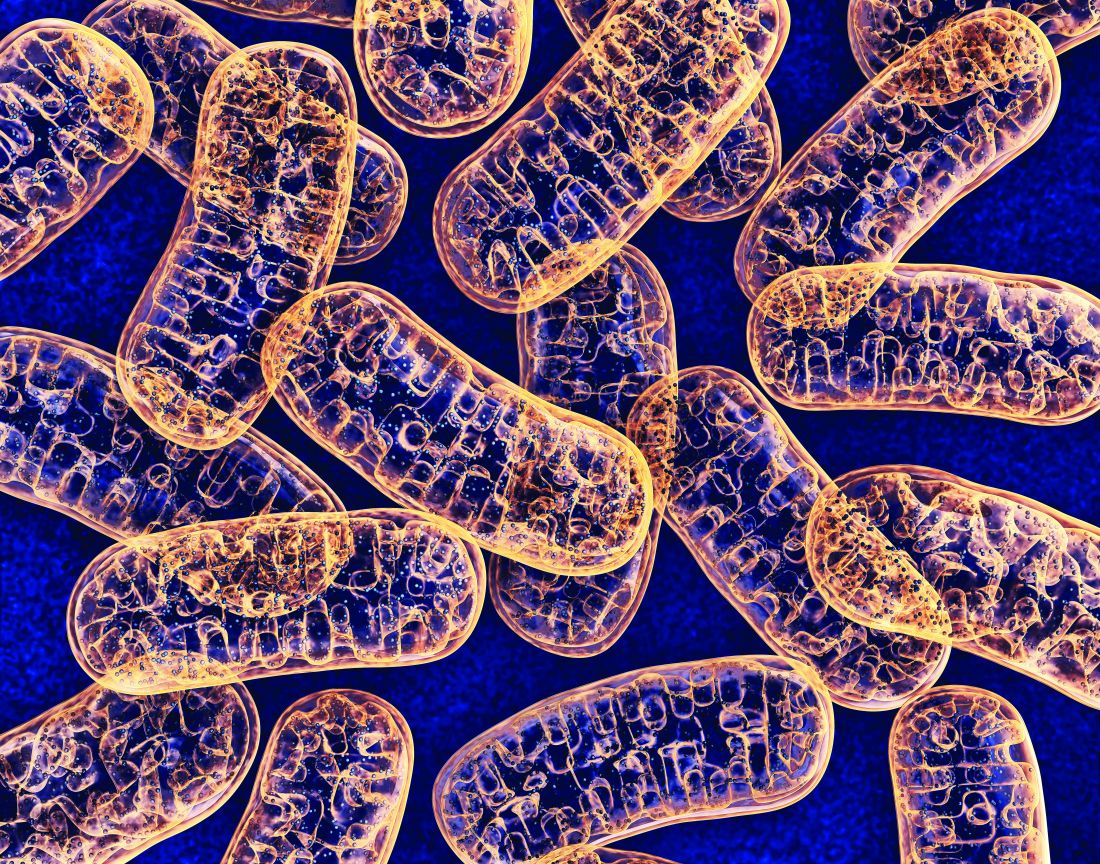
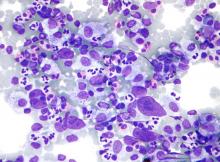
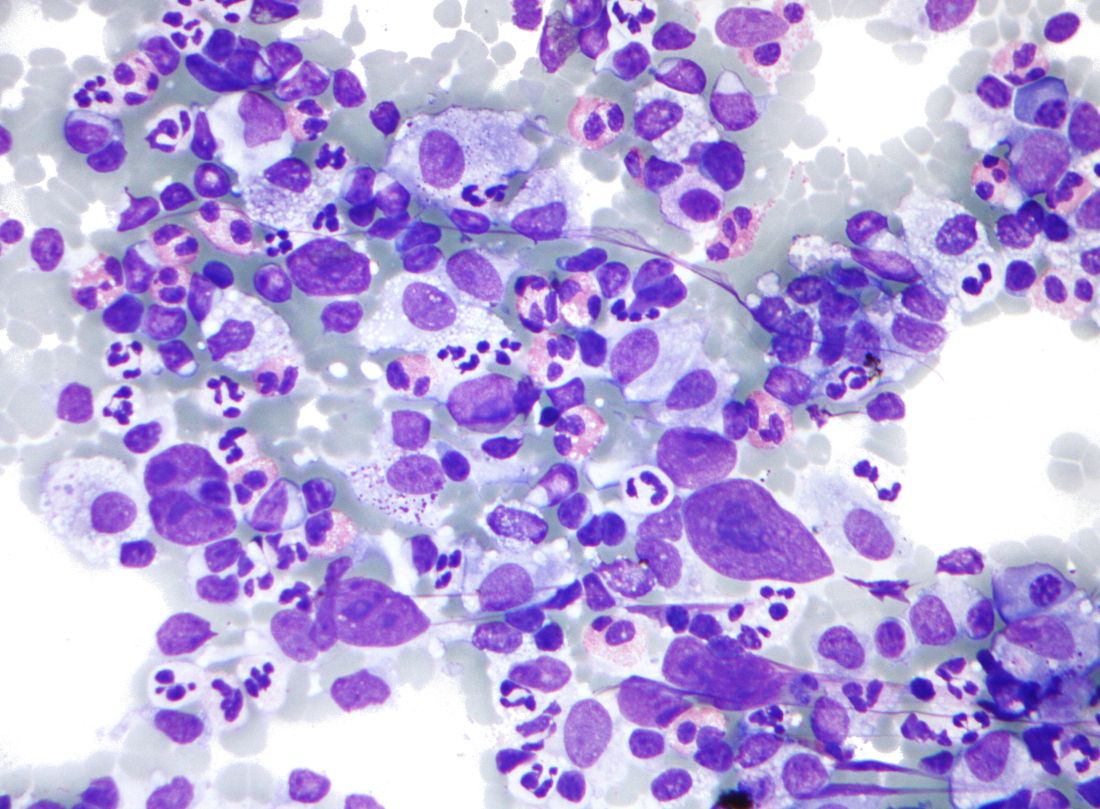
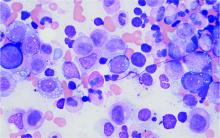
Mitochondrial DNA predicts survival in pediatric acute myeloid leukemia
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
FROM MITOCHONDRION
Predicting outcomes in therapy-related AML
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
Frontline brentuximab vedotin shows promise in high-risk pediatric Hodgkin lymphoma
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
A frontline treatment regimen including brentuximab vedotin (Bv) was well tolerated, was highly effective, and significantly reduced radiation exposure in pediatric patients with high-risk Hodgkin lymphoma, according to the results of an open-label, phase 2 trial.
Of 77 patients enrolled in the investigator-initiated, single-arm, multicenter trial, 27 (35%) achieved complete remission (CR) without radiation at the early response assessment (ERA) after two cycles of therapy, reported Monika L. Metzger, MD, of St. Jude Children’s Research Hospital, Memphis, Tenn. and colleagues. The report was published online in the Journal of Clinical Oncology.
The addition of Bv also resulted in superior event-free survival (97.4%) and overall survival (98.7%) at median follow-up of 3.4 years, compared with previously published pediatric trials, such as the HOD99 trial (EFS and OS of 80.8% and 96.5%, respectively), the authors noted.
Bv chemotherapy
Bv, a targeted anti-CD30 antibody-drug conjugate, received expanded Food and Drug Administration approval in March 2018 for frontline use in combination with chemotherapy in adults with stage III or IV classical Hodgkin lymphoma (HL). The current study is the first to include Bv as part of a chemotherapy regimen in the frontline setting for pediatric classical HL, the authors noted, adding that their primary aim was to reduce prescribed radiation thereby limiting late toxicities associated with radiation in this population.
Patients enrolled were children and adolescents aged 18 years and under with stage IIB, IIIB, or IV classical HL. Bv was used in place of vincristine in the standard OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) frontline regimen for pediatric HL.
The Bv-based chemotherapy regimen was well tolerated and mostly limited to low-grade nausea, vomiting, and constipation, and the most common adverse events were hematologic events occurring mainly during the first two cycles of chemotherapy.
“Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation,” they wrote, explaining that neuropathy is more common with vincristine.
Radiation exposure
Residual node radiotherapy (RNRT) was delivered at a prescribed dose of 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy only to nodal sites that did not achieve a CR at the early response assessment (ERA) after two cycles of therapy.
“Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with [involved-field radiation therapy] (78.1 J vs. 249.6 J),” the authors wrote. “Doses to specific organs were also compared ... [t]he mean heart dose was reduced to 5.29 Gy from 16.9 Gy, and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy.”
Women also had significantly less breast radiation exposure (mean of 3.21 Gy vs. 6.85 Gy in HOD99).
One irradiated patient experienced disease progression at the end of therapy, but remained disease free more than 6 years following salvage therapy, and one unexpected death occurred, the authors said.
“We have already reduced the use of radiation for low-risk Hodgkin lymphoma patients. In this study we’ve shown that it is also possible to either omit or reduce the extent of radiation for high-risk patients, using highly focal methods such as proton beam radiation or intensity modulated radiation,” co–senior author Matthew Krasin, MD, of St. Jude’s department of radiation oncology, stated in a press release.
Next steps
Co–senior author Melissa Hudson, MD, the St. Jude cancer survivorship division director, added that “[b]eing able to offer Hodgkin lymphoma patients a targeted therapy in the frontline setting is an exciting development.
“The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial,” the authors concluded, noting that “[l]onger follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.”
The study was sponsored by Seattle Genetics. The research at St. Jude was funded in part by grants from the National Cancer Institute and ALSAC (American Lebanese Syrian Associated Charities), St. Jude’s fundraising and awareness organization. Dr. Metzger reported research funding from Seattle Genetics. Dr. Krasin reported a consulting or advisory role for Debiopharm Group. Dr. Hudson reported a consulting or advisory role for Oncology Research Information Exchange Network, Princess Máxima Center.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
GENUINE improvements: Ublituximab plus ibrutinib for CLL
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine failure in patients with blood cancers
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel antiplatelet drug: Hope for efficacy without bleeding?
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
Palliative care helpful but underutilized for blood cancer patients
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
FROM THE JOURNAL OF PAIN AND SYMPTOM MANAGEMENT
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”