User login
Cardiogenic shock teams again tied to lower mortality
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
New AHA guidance targets obesity-related hypertension
Whereas previous scientific statements from the American Heart Association have addressed how diet, physical activity, and weight control can help prevent and manage hypertension, a new AHA statement focuses on obesity-related hypertension.
The document, which was published online Sept. 20, 2021, in Hypertension, also identifies knowledge gaps and suggests future research directions.
“Given [that] obesity is a major risk factor for hypertension, and hypertension is one of the greatest (if not the greatest) attributable risk factors for most cardiovascular diseases, we thought it was important to focus on weight loss strategies and update what we know about the treatment options that are available to treat obesity hypertension,” writing group chair Michael E. Hall, MD, told this news organization.
“Medical and surgical strategies may help with long-term weight and blood pressure improvement, in addition to a heart-healthy diet and physical activity,” he noted in a press release from the AHA. “We often don’t consider medications or metabolic surgery until after there has been target organ damage, such as heart injury or having a stroke.”
However, by acting earlier, “we may be able to prevent these complications,” added Dr. Hall, associate division director for cardiovascular diseases at the University of Mississippi Medical Center in Jackson.
“This is not a call for greater use of one specific therapy,” he clarified. “However, we do know that more aggressive treatments including antiobesity medications or metabolic surgery are underutilized.”
According to Dr. Hall, “we treat the secondary problem [i.e., the hypertension or diabetes], but we are not treating the root cause [obesity] as aggressively.”
“Hopefully this statement will increase awareness that there are several [treatment] options [and] bring attention to this major health issue,” he said.
He added that the most important question, in his mind, is how best to tackle obesity among children and adolescents to lower their risk of hypertension and other associated complications.
The statement is aimed at both primary care providers and specialists.
Diet, physical activity help, but weight regain common
Losing 5%-10% of body weight can lead to a more than 5–mm Hg reduction in systolic blood pressure and a 4–mm Hg reduction in diastolic blood pressure, the statement notes. Losing 10 kg may lower systolic blood pressure by 5-20 mm Hg.
To manage weight, control hypertension, and reduce the risk of cardiovascular disease, guidelines recommend the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet, which both emphasize fruits, vegetables, legumes, nuts, and seeds, with moderate intake of fish, seafood, poultry, and dairy, and low intake of red and processed meats and sweets. The Mediterranean diet also includes olive oil and moderate consumption of (mainly red) wine.
The effect of intermittent fasting on blood pressure control is not clear, the statement noted.
It added that typically 150-225 minutes and 225-420 minutes of physical activity per week can produce weight loss of 2-3 kg or 5-7.5 kg respectively, and 200-300 minutes of physical activity per week is needed to maintain this weight loss.
“Successful weight-loss maintenance over years therefore typically requires high levels of [physical activity] and limited sedentary time, frequent weight monitoring, and high levels of dietary restraint,” and weight regain is common, the authors summarize.
Other options to address obesity, hypertension
Weight-loss pharmacotherapies and metabolic surgery are other options to treat obesity and lower hypertension.
The statement reports that four drugs are approved by the Food and Drug Administration for long-term weight loss: Orlistat (Xenical, Alli), phentermine/topiramate extended release (Qsymia), naltrexone/bupropion (Contrave), and liraglutide 3.0 mg (Saxenda). On June 4, the FDA approved a fifth drug, semaglutide (Wegovy).
The long-term effects of antiobesity medications on blood pressure are mixed.
However, “prescription rates for these drugs remain low, likely because of limited insurance coverage and low levels of clinical proficiency with treating obesity,” Dr. Hall and colleagues write.
Metabolic surgery could be a weight loss option for certain patients, and it is associated with blood pressure lowering.
In the 100-patient Gastric Bypass to Treat Obese Patients With Steady Hypertension (GATEWAY) trial, published in Circulation in 2018, more patients in the Roux-en-Y gastric-bypass group than the control group (84% vs. 13%) met the primary outcome of a 30% or greater reduction in the number of blood pressure-lowering medications at 12 months while maintaining an office blood pressure less than 140/90 mm Hg.
Unanswered questions, future research directions
In 2015-2016, an estimated 18.5% of U.S. children and adolescents aged 2-19 years had obesity, the statement notes. Children with obesity have a twofold increased risk of incident hypertension, and those with severe obesity have an over fourfold increased risk of this outcome, compared with children who have a healthy weight.
Dr. Hall and colleagues emphasized that, “as the prevalence of obesity continues to increase, hypertension and associated cardiorenal diseases will also increase unless more effective strategies to prevent and treat obesity are developed.”
They identified 17 unanswered questions (knowledge gaps) that can guide the direction of future research. These include:
- What new strategies and science-based guidelines are needed to curb the growing evidence of childhood obesity?
- Does intentional weight loss with pharmacotherapy or metabolic surgery in childhood and early adulthood prevent hypertension and subsequent target organ damage in later life?
- What is the optimal amount of time that clinicians should allow before recommending more aggressive weight management strategies (that is, antiobesity medications or metabolic surgery) or hypertension strategies beyond lifestyle changes?
“To me,” Dr. Hall said, “addressing childhood obesity hypertension and determining optimal timing of antiobesity therapies are the most important [issues]. Certainly, these therapies (i.e., diets, medications, surgeries) have some risks, but we don’t have a clear understanding if their benefits outweigh these risks in younger obese people or whether initiating these therapies before the onset of target organ damage such as heart failure” outweigh the risks.
Dr. Hall has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
Whereas previous scientific statements from the American Heart Association have addressed how diet, physical activity, and weight control can help prevent and manage hypertension, a new AHA statement focuses on obesity-related hypertension.
The document, which was published online Sept. 20, 2021, in Hypertension, also identifies knowledge gaps and suggests future research directions.
“Given [that] obesity is a major risk factor for hypertension, and hypertension is one of the greatest (if not the greatest) attributable risk factors for most cardiovascular diseases, we thought it was important to focus on weight loss strategies and update what we know about the treatment options that are available to treat obesity hypertension,” writing group chair Michael E. Hall, MD, told this news organization.
“Medical and surgical strategies may help with long-term weight and blood pressure improvement, in addition to a heart-healthy diet and physical activity,” he noted in a press release from the AHA. “We often don’t consider medications or metabolic surgery until after there has been target organ damage, such as heart injury or having a stroke.”
However, by acting earlier, “we may be able to prevent these complications,” added Dr. Hall, associate division director for cardiovascular diseases at the University of Mississippi Medical Center in Jackson.
“This is not a call for greater use of one specific therapy,” he clarified. “However, we do know that more aggressive treatments including antiobesity medications or metabolic surgery are underutilized.”
According to Dr. Hall, “we treat the secondary problem [i.e., the hypertension or diabetes], but we are not treating the root cause [obesity] as aggressively.”
“Hopefully this statement will increase awareness that there are several [treatment] options [and] bring attention to this major health issue,” he said.
He added that the most important question, in his mind, is how best to tackle obesity among children and adolescents to lower their risk of hypertension and other associated complications.
The statement is aimed at both primary care providers and specialists.
Diet, physical activity help, but weight regain common
Losing 5%-10% of body weight can lead to a more than 5–mm Hg reduction in systolic blood pressure and a 4–mm Hg reduction in diastolic blood pressure, the statement notes. Losing 10 kg may lower systolic blood pressure by 5-20 mm Hg.
To manage weight, control hypertension, and reduce the risk of cardiovascular disease, guidelines recommend the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet, which both emphasize fruits, vegetables, legumes, nuts, and seeds, with moderate intake of fish, seafood, poultry, and dairy, and low intake of red and processed meats and sweets. The Mediterranean diet also includes olive oil and moderate consumption of (mainly red) wine.
The effect of intermittent fasting on blood pressure control is not clear, the statement noted.
It added that typically 150-225 minutes and 225-420 minutes of physical activity per week can produce weight loss of 2-3 kg or 5-7.5 kg respectively, and 200-300 minutes of physical activity per week is needed to maintain this weight loss.
“Successful weight-loss maintenance over years therefore typically requires high levels of [physical activity] and limited sedentary time, frequent weight monitoring, and high levels of dietary restraint,” and weight regain is common, the authors summarize.
Other options to address obesity, hypertension
Weight-loss pharmacotherapies and metabolic surgery are other options to treat obesity and lower hypertension.
The statement reports that four drugs are approved by the Food and Drug Administration for long-term weight loss: Orlistat (Xenical, Alli), phentermine/topiramate extended release (Qsymia), naltrexone/bupropion (Contrave), and liraglutide 3.0 mg (Saxenda). On June 4, the FDA approved a fifth drug, semaglutide (Wegovy).
The long-term effects of antiobesity medications on blood pressure are mixed.
However, “prescription rates for these drugs remain low, likely because of limited insurance coverage and low levels of clinical proficiency with treating obesity,” Dr. Hall and colleagues write.
Metabolic surgery could be a weight loss option for certain patients, and it is associated with blood pressure lowering.
In the 100-patient Gastric Bypass to Treat Obese Patients With Steady Hypertension (GATEWAY) trial, published in Circulation in 2018, more patients in the Roux-en-Y gastric-bypass group than the control group (84% vs. 13%) met the primary outcome of a 30% or greater reduction in the number of blood pressure-lowering medications at 12 months while maintaining an office blood pressure less than 140/90 mm Hg.
Unanswered questions, future research directions
In 2015-2016, an estimated 18.5% of U.S. children and adolescents aged 2-19 years had obesity, the statement notes. Children with obesity have a twofold increased risk of incident hypertension, and those with severe obesity have an over fourfold increased risk of this outcome, compared with children who have a healthy weight.
Dr. Hall and colleagues emphasized that, “as the prevalence of obesity continues to increase, hypertension and associated cardiorenal diseases will also increase unless more effective strategies to prevent and treat obesity are developed.”
They identified 17 unanswered questions (knowledge gaps) that can guide the direction of future research. These include:
- What new strategies and science-based guidelines are needed to curb the growing evidence of childhood obesity?
- Does intentional weight loss with pharmacotherapy or metabolic surgery in childhood and early adulthood prevent hypertension and subsequent target organ damage in later life?
- What is the optimal amount of time that clinicians should allow before recommending more aggressive weight management strategies (that is, antiobesity medications or metabolic surgery) or hypertension strategies beyond lifestyle changes?
“To me,” Dr. Hall said, “addressing childhood obesity hypertension and determining optimal timing of antiobesity therapies are the most important [issues]. Certainly, these therapies (i.e., diets, medications, surgeries) have some risks, but we don’t have a clear understanding if their benefits outweigh these risks in younger obese people or whether initiating these therapies before the onset of target organ damage such as heart failure” outweigh the risks.
Dr. Hall has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
Whereas previous scientific statements from the American Heart Association have addressed how diet, physical activity, and weight control can help prevent and manage hypertension, a new AHA statement focuses on obesity-related hypertension.
The document, which was published online Sept. 20, 2021, in Hypertension, also identifies knowledge gaps and suggests future research directions.
“Given [that] obesity is a major risk factor for hypertension, and hypertension is one of the greatest (if not the greatest) attributable risk factors for most cardiovascular diseases, we thought it was important to focus on weight loss strategies and update what we know about the treatment options that are available to treat obesity hypertension,” writing group chair Michael E. Hall, MD, told this news organization.
“Medical and surgical strategies may help with long-term weight and blood pressure improvement, in addition to a heart-healthy diet and physical activity,” he noted in a press release from the AHA. “We often don’t consider medications or metabolic surgery until after there has been target organ damage, such as heart injury or having a stroke.”
However, by acting earlier, “we may be able to prevent these complications,” added Dr. Hall, associate division director for cardiovascular diseases at the University of Mississippi Medical Center in Jackson.
“This is not a call for greater use of one specific therapy,” he clarified. “However, we do know that more aggressive treatments including antiobesity medications or metabolic surgery are underutilized.”
According to Dr. Hall, “we treat the secondary problem [i.e., the hypertension or diabetes], but we are not treating the root cause [obesity] as aggressively.”
“Hopefully this statement will increase awareness that there are several [treatment] options [and] bring attention to this major health issue,” he said.
He added that the most important question, in his mind, is how best to tackle obesity among children and adolescents to lower their risk of hypertension and other associated complications.
The statement is aimed at both primary care providers and specialists.
Diet, physical activity help, but weight regain common
Losing 5%-10% of body weight can lead to a more than 5–mm Hg reduction in systolic blood pressure and a 4–mm Hg reduction in diastolic blood pressure, the statement notes. Losing 10 kg may lower systolic blood pressure by 5-20 mm Hg.
To manage weight, control hypertension, and reduce the risk of cardiovascular disease, guidelines recommend the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet, which both emphasize fruits, vegetables, legumes, nuts, and seeds, with moderate intake of fish, seafood, poultry, and dairy, and low intake of red and processed meats and sweets. The Mediterranean diet also includes olive oil and moderate consumption of (mainly red) wine.
The effect of intermittent fasting on blood pressure control is not clear, the statement noted.
It added that typically 150-225 minutes and 225-420 minutes of physical activity per week can produce weight loss of 2-3 kg or 5-7.5 kg respectively, and 200-300 minutes of physical activity per week is needed to maintain this weight loss.
“Successful weight-loss maintenance over years therefore typically requires high levels of [physical activity] and limited sedentary time, frequent weight monitoring, and high levels of dietary restraint,” and weight regain is common, the authors summarize.
Other options to address obesity, hypertension
Weight-loss pharmacotherapies and metabolic surgery are other options to treat obesity and lower hypertension.
The statement reports that four drugs are approved by the Food and Drug Administration for long-term weight loss: Orlistat (Xenical, Alli), phentermine/topiramate extended release (Qsymia), naltrexone/bupropion (Contrave), and liraglutide 3.0 mg (Saxenda). On June 4, the FDA approved a fifth drug, semaglutide (Wegovy).
The long-term effects of antiobesity medications on blood pressure are mixed.
However, “prescription rates for these drugs remain low, likely because of limited insurance coverage and low levels of clinical proficiency with treating obesity,” Dr. Hall and colleagues write.
Metabolic surgery could be a weight loss option for certain patients, and it is associated with blood pressure lowering.
In the 100-patient Gastric Bypass to Treat Obese Patients With Steady Hypertension (GATEWAY) trial, published in Circulation in 2018, more patients in the Roux-en-Y gastric-bypass group than the control group (84% vs. 13%) met the primary outcome of a 30% or greater reduction in the number of blood pressure-lowering medications at 12 months while maintaining an office blood pressure less than 140/90 mm Hg.
Unanswered questions, future research directions
In 2015-2016, an estimated 18.5% of U.S. children and adolescents aged 2-19 years had obesity, the statement notes. Children with obesity have a twofold increased risk of incident hypertension, and those with severe obesity have an over fourfold increased risk of this outcome, compared with children who have a healthy weight.
Dr. Hall and colleagues emphasized that, “as the prevalence of obesity continues to increase, hypertension and associated cardiorenal diseases will also increase unless more effective strategies to prevent and treat obesity are developed.”
They identified 17 unanswered questions (knowledge gaps) that can guide the direction of future research. These include:
- What new strategies and science-based guidelines are needed to curb the growing evidence of childhood obesity?
- Does intentional weight loss with pharmacotherapy or metabolic surgery in childhood and early adulthood prevent hypertension and subsequent target organ damage in later life?
- What is the optimal amount of time that clinicians should allow before recommending more aggressive weight management strategies (that is, antiobesity medications or metabolic surgery) or hypertension strategies beyond lifestyle changes?
“To me,” Dr. Hall said, “addressing childhood obesity hypertension and determining optimal timing of antiobesity therapies are the most important [issues]. Certainly, these therapies (i.e., diets, medications, surgeries) have some risks, but we don’t have a clear understanding if their benefits outweigh these risks in younger obese people or whether initiating these therapies before the onset of target organ damage such as heart failure” outweigh the risks.
Dr. Hall has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
FDA approves Abbott’s Portico valve for TAVR
The Food and Drug Administration has approved the Portico with FlexNav (Abbott) transcatheter aortic valve replacement (TAVR) system for patients with “symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery,” the company has announced.
The approval indication is in line with the entry criteria of PORTICO IDE, the investigational device exemption trial from which the FDA largely made its decision.
With the self-expanding Portico valve, Abbott joins two other companies with TAVR valves on the U.S. market: Medtronic with the self-expanding Corevalve Evolut (Medtronic) line, and Edwards Lifesciences with its Sapien (Edwards Lifesciences) valves, both of which can be used in patients at low surgical risk.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the Portico with FlexNav (Abbott) transcatheter aortic valve replacement (TAVR) system for patients with “symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery,” the company has announced.
The approval indication is in line with the entry criteria of PORTICO IDE, the investigational device exemption trial from which the FDA largely made its decision.
With the self-expanding Portico valve, Abbott joins two other companies with TAVR valves on the U.S. market: Medtronic with the self-expanding Corevalve Evolut (Medtronic) line, and Edwards Lifesciences with its Sapien (Edwards Lifesciences) valves, both of which can be used in patients at low surgical risk.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the Portico with FlexNav (Abbott) transcatheter aortic valve replacement (TAVR) system for patients with “symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery,” the company has announced.
The approval indication is in line with the entry criteria of PORTICO IDE, the investigational device exemption trial from which the FDA largely made its decision.
With the self-expanding Portico valve, Abbott joins two other companies with TAVR valves on the U.S. market: Medtronic with the self-expanding Corevalve Evolut (Medtronic) line, and Edwards Lifesciences with its Sapien (Edwards Lifesciences) valves, both of which can be used in patients at low surgical risk.
A version of this article first appeared on Medscape.com.
EMPEROR-Preserved: Empagliflozin’s HFpEF efficacy catalyzes a heart failure redefinition
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
FROM HFSA 2021
COVID vaccine preprint study prompts Twitter outrage
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
Texts boost activity, quality of life in patients with heart failure and diabetes
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
FROM HFSA 2021
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
PRESERVED-HF: Dapagliflozin improves physical limitations in patients with HFpEF
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
FROM HFSA 2021
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”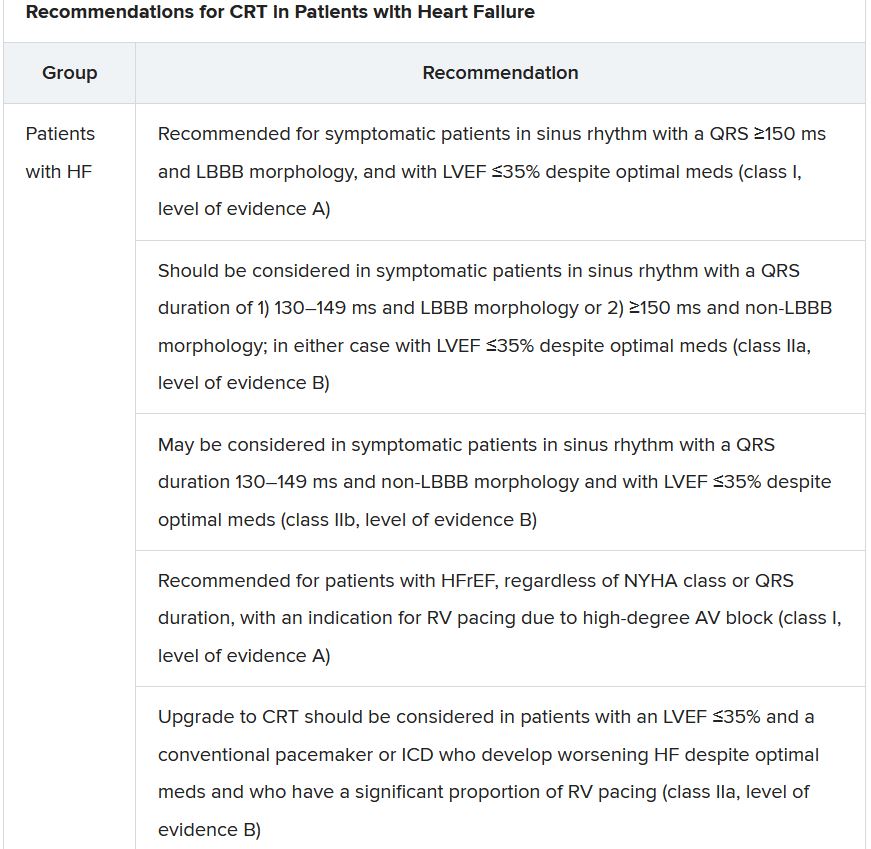
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.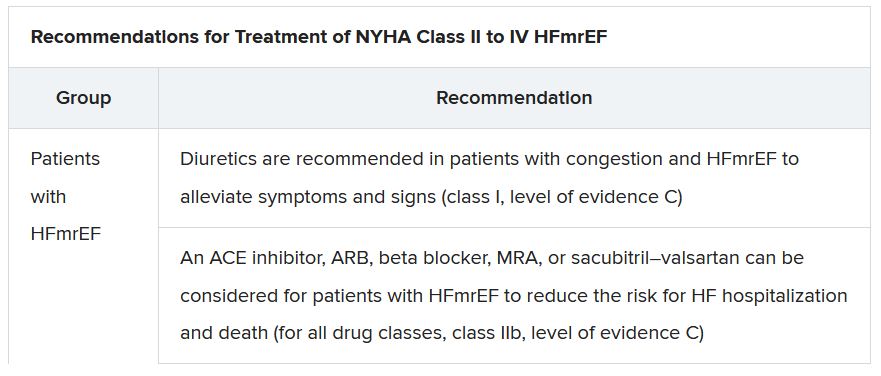
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
















