User login
Transitioning patients with developmental disabilities to adult care
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
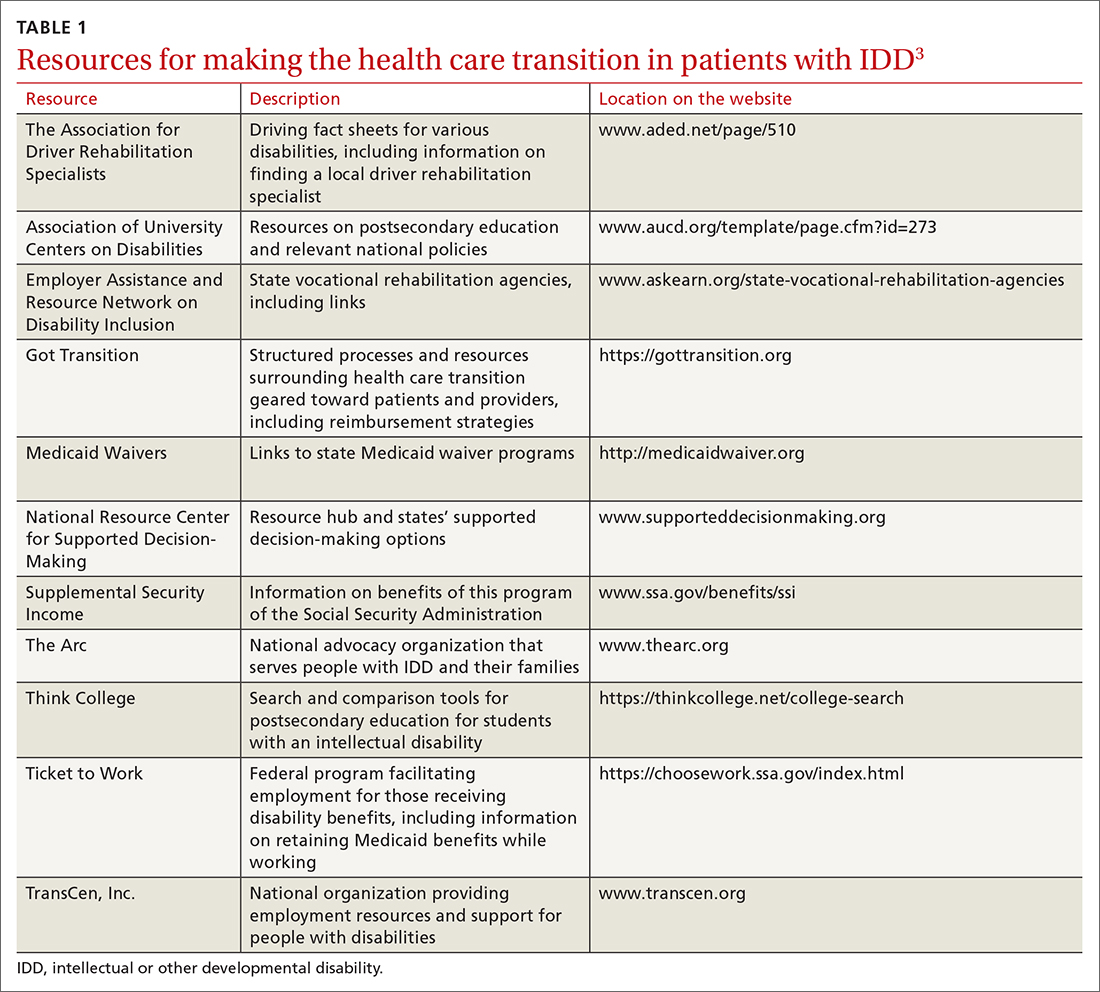
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
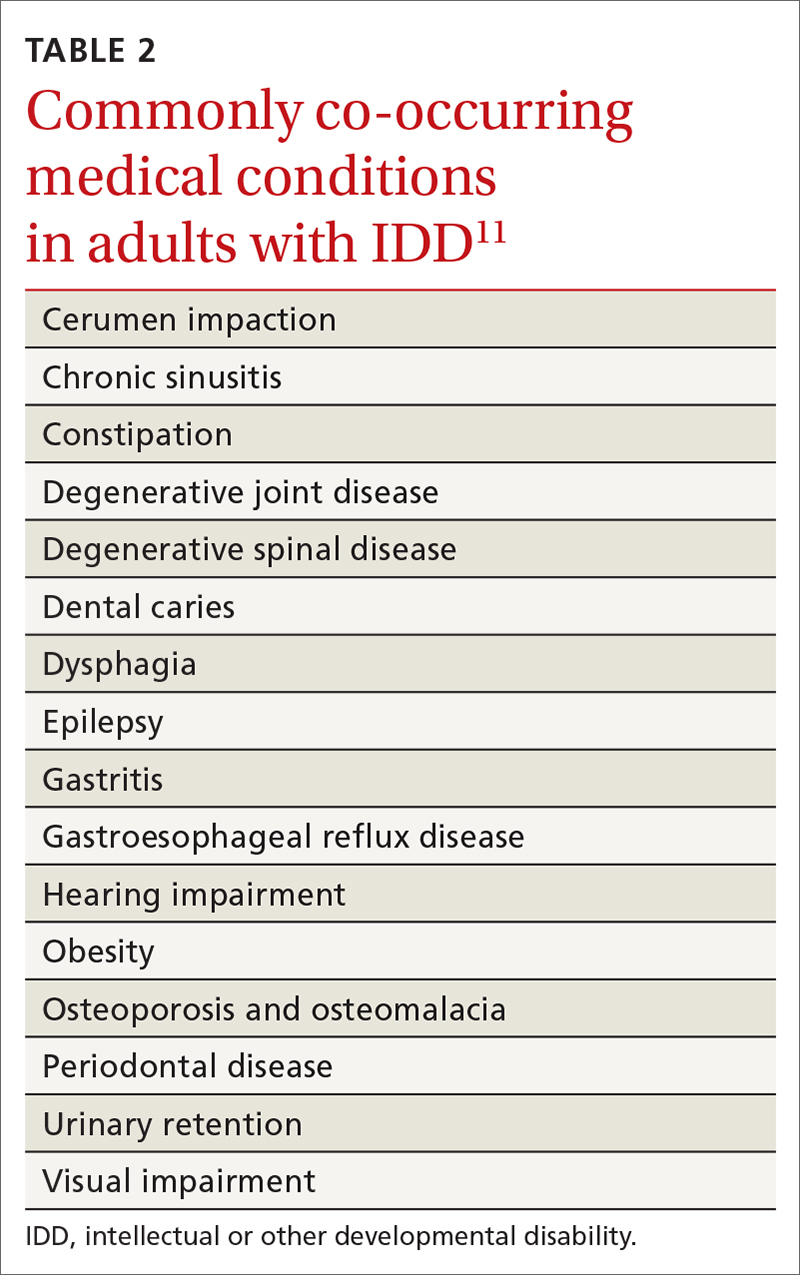
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
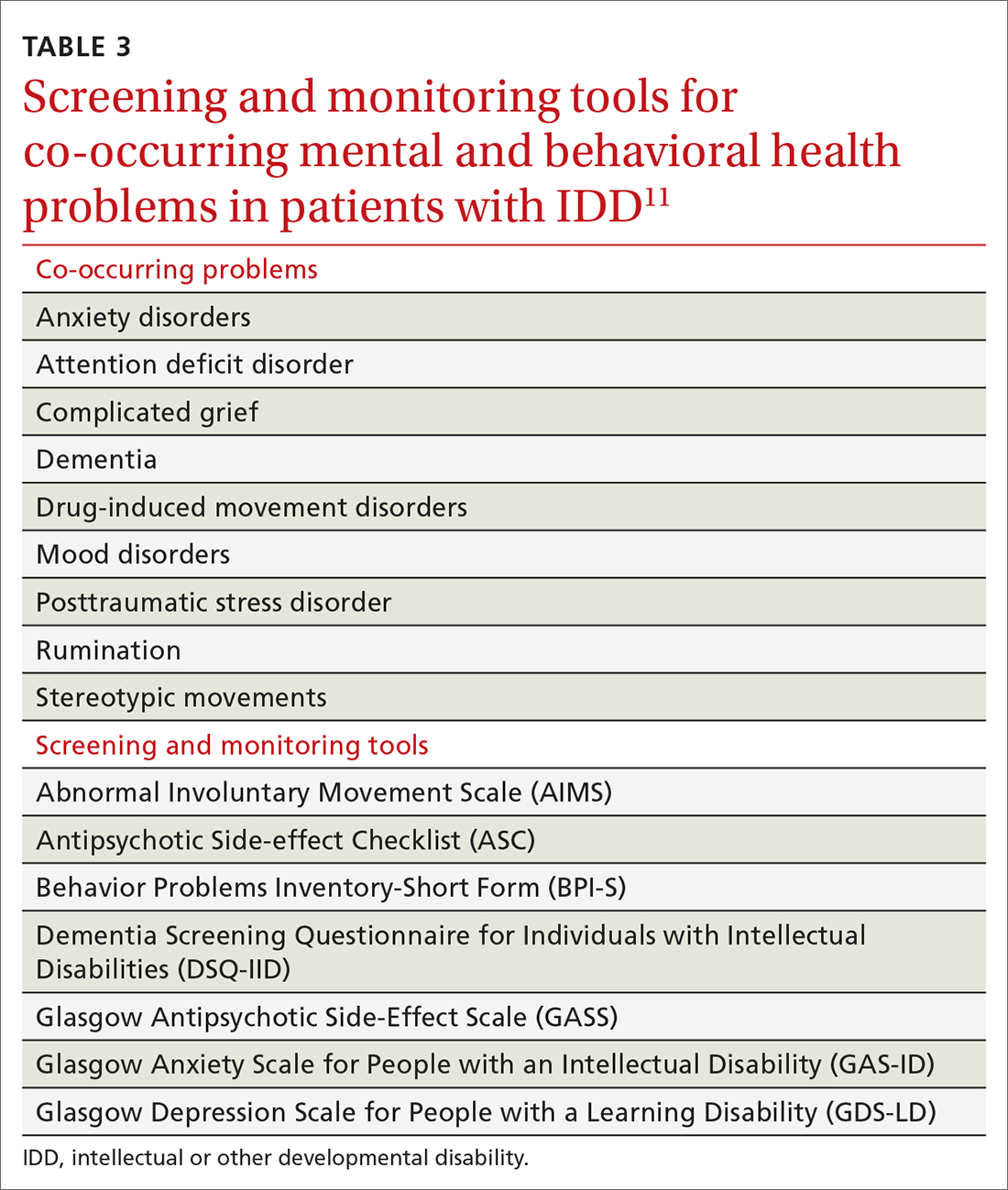
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
PRACTICE RECOMMENDATIONS
› Provide young people who have an intellectual or other developmental disability (IDD) with a defined, explicit process for making the transition into the adult health care system. A
› Conduct an annual comprehensive, systematic health assessment for patients who have IDD to improve detection of serious conditions and sensory impairments. A
› Encourage young people and adults with IDD to participate in regular physical activity to reduce psychosocial stressors and counteract metabolic syndromes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sen. Schumer backs federal decriminalization of marijuana
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
Tennessee fires top vaccine official as COVID cases increase
Tennessee officials have fired the state’s top vaccination manager, who faced recent criticism from Republican lawmakers about her efforts to vaccinate teens against COVID-19.
Michelle Fiscus, MD, the medical director for vaccine-preventable diseases and immunization programs at the Tennessee Department of Health, was terminated on July 12. The termination letter doesn’t explain the reason for her dismissal, according to the newspaper, which received a copy of the letter.
“It was my job to provide evidence-based education and vaccine access so that Tennesseans could protect themselves against COVID-19,” Dr. Fiscus told the Tennessean. “I have now been terminated for doing exactly that.”
In May, Dr. Fiscus sent a memo to medical providers that described the state’s “Mature Minor Doctrine,” a legal mechanism established in 1987 that allows some minors between the ages if 14 and 17 years to receive medical care without parental consent. Tennessee is one of five states that allows health care providers to decide if a minor has the capacity to consent to care, according to CNN.
Dr. Fiscus said she sent the letter in response to providers’ questions and that it contained no new information. She also said the wording was approved by the health department’s attorney and the governor’s office, the newspaper reported.
At a June 16 hearing of the state’s Joint Government Operations Committee, however, Republican officials criticized the memo and Dr. Fiscus, saying that the state misinterpreted its legal authority. During the meeting, some lawmakers discussed dissolving the state health department to stop it from promoting vaccines to teens, the newspaper reported.
Since then, the health department has backed down from promoting vaccines to teens by deleting social media posts that recommended vaccines to anyone over age 12. Internal emails, which were obtained by the Tennessean, showed that department leaders ordered county-level employees to avoid holding vaccine events targeted toward adolescents.
Dr. Fiscus’s firing comes as vaccination efforts lag in the state. About 38% of residents have been fully vaccinated. At the current pace, Tennessee won’t pass the 50% mark until next March, according to an internal report obtained by the newspaper.
COVID-19 cases are beginning to climb again, particularly with the Delta variant circulating among unvaccinated residents. After months of a decline in cases, the average of daily cases has more than doubled since the end of June. The state’s test positivity rate has increased from 2% to 4.5% during that time as well.
In a long written statement, Dr. Fiscus said she was the 25th of 64 state and territorial immunization program directors to leave their positions during the pandemic, whether through resignation or termination. With a loss of institutional knowledge and leadership, COVID-19 vaccine efforts will fall behind.
“Each of us should be waking up every morning with one question on our minds: ‘What can I do protect the people of Tennessee against COVID-19?’ ” she wrote. “Instead, our leaders are putting barriers in place to ensure the people of Tennessee remain at risk, even with the Delta variant bearing down upon us.”
A version of this article first appeared on WebMD.com.
Tennessee officials have fired the state’s top vaccination manager, who faced recent criticism from Republican lawmakers about her efforts to vaccinate teens against COVID-19.
Michelle Fiscus, MD, the medical director for vaccine-preventable diseases and immunization programs at the Tennessee Department of Health, was terminated on July 12. The termination letter doesn’t explain the reason for her dismissal, according to the newspaper, which received a copy of the letter.
“It was my job to provide evidence-based education and vaccine access so that Tennesseans could protect themselves against COVID-19,” Dr. Fiscus told the Tennessean. “I have now been terminated for doing exactly that.”
In May, Dr. Fiscus sent a memo to medical providers that described the state’s “Mature Minor Doctrine,” a legal mechanism established in 1987 that allows some minors between the ages if 14 and 17 years to receive medical care without parental consent. Tennessee is one of five states that allows health care providers to decide if a minor has the capacity to consent to care, according to CNN.
Dr. Fiscus said she sent the letter in response to providers’ questions and that it contained no new information. She also said the wording was approved by the health department’s attorney and the governor’s office, the newspaper reported.
At a June 16 hearing of the state’s Joint Government Operations Committee, however, Republican officials criticized the memo and Dr. Fiscus, saying that the state misinterpreted its legal authority. During the meeting, some lawmakers discussed dissolving the state health department to stop it from promoting vaccines to teens, the newspaper reported.
Since then, the health department has backed down from promoting vaccines to teens by deleting social media posts that recommended vaccines to anyone over age 12. Internal emails, which were obtained by the Tennessean, showed that department leaders ordered county-level employees to avoid holding vaccine events targeted toward adolescents.
Dr. Fiscus’s firing comes as vaccination efforts lag in the state. About 38% of residents have been fully vaccinated. At the current pace, Tennessee won’t pass the 50% mark until next March, according to an internal report obtained by the newspaper.
COVID-19 cases are beginning to climb again, particularly with the Delta variant circulating among unvaccinated residents. After months of a decline in cases, the average of daily cases has more than doubled since the end of June. The state’s test positivity rate has increased from 2% to 4.5% during that time as well.
In a long written statement, Dr. Fiscus said she was the 25th of 64 state and territorial immunization program directors to leave their positions during the pandemic, whether through resignation or termination. With a loss of institutional knowledge and leadership, COVID-19 vaccine efforts will fall behind.
“Each of us should be waking up every morning with one question on our minds: ‘What can I do protect the people of Tennessee against COVID-19?’ ” she wrote. “Instead, our leaders are putting barriers in place to ensure the people of Tennessee remain at risk, even with the Delta variant bearing down upon us.”
A version of this article first appeared on WebMD.com.
Tennessee officials have fired the state’s top vaccination manager, who faced recent criticism from Republican lawmakers about her efforts to vaccinate teens against COVID-19.
Michelle Fiscus, MD, the medical director for vaccine-preventable diseases and immunization programs at the Tennessee Department of Health, was terminated on July 12. The termination letter doesn’t explain the reason for her dismissal, according to the newspaper, which received a copy of the letter.
“It was my job to provide evidence-based education and vaccine access so that Tennesseans could protect themselves against COVID-19,” Dr. Fiscus told the Tennessean. “I have now been terminated for doing exactly that.”
In May, Dr. Fiscus sent a memo to medical providers that described the state’s “Mature Minor Doctrine,” a legal mechanism established in 1987 that allows some minors between the ages if 14 and 17 years to receive medical care without parental consent. Tennessee is one of five states that allows health care providers to decide if a minor has the capacity to consent to care, according to CNN.
Dr. Fiscus said she sent the letter in response to providers’ questions and that it contained no new information. She also said the wording was approved by the health department’s attorney and the governor’s office, the newspaper reported.
At a June 16 hearing of the state’s Joint Government Operations Committee, however, Republican officials criticized the memo and Dr. Fiscus, saying that the state misinterpreted its legal authority. During the meeting, some lawmakers discussed dissolving the state health department to stop it from promoting vaccines to teens, the newspaper reported.
Since then, the health department has backed down from promoting vaccines to teens by deleting social media posts that recommended vaccines to anyone over age 12. Internal emails, which were obtained by the Tennessean, showed that department leaders ordered county-level employees to avoid holding vaccine events targeted toward adolescents.
Dr. Fiscus’s firing comes as vaccination efforts lag in the state. About 38% of residents have been fully vaccinated. At the current pace, Tennessee won’t pass the 50% mark until next March, according to an internal report obtained by the newspaper.
COVID-19 cases are beginning to climb again, particularly with the Delta variant circulating among unvaccinated residents. After months of a decline in cases, the average of daily cases has more than doubled since the end of June. The state’s test positivity rate has increased from 2% to 4.5% during that time as well.
In a long written statement, Dr. Fiscus said she was the 25th of 64 state and territorial immunization program directors to leave their positions during the pandemic, whether through resignation or termination. With a loss of institutional knowledge and leadership, COVID-19 vaccine efforts will fall behind.
“Each of us should be waking up every morning with one question on our minds: ‘What can I do protect the people of Tennessee against COVID-19?’ ” she wrote. “Instead, our leaders are putting barriers in place to ensure the people of Tennessee remain at risk, even with the Delta variant bearing down upon us.”
A version of this article first appeared on WebMD.com.
Talking about guns: Website helps physicians follow through on pledge
The group has developed a national resource for clinicians who wish to address the problem of gun violence deaths in the United States, which continue to mount by the day.
Signatures came quickly in 2018 after the Annals of Internal Medicine asked physicians to sign a formal pledge in which they commit to talking with their patients about firearms. To date, the list has grown to more than 3,600, and it remains open for additional signatories.
The effort built on data showing that before people commit violence with firearms, they often have notable risk factors that prompt them to see a physician.
At the time the pledge campaign was launched, frustration and despair had hit new highs after the school shooting of Feb. 14, 2018, in Parkland, Florida, in which 17 people were killed. That occurred just 4 months after the mass shooting in Las Vegas, Nevada, on Oct. 1, 2017, in which 58 people were gunned down.
An editorial by Garen J. Wintemute, MD, MPH, helped kick off the drive.
More deaths than WWII combat fatalities
Dr. Wintemute cited some grim statistics, writing that “nationwide in 2016, there was an average of 97 deaths from firearm violence per day: 35,476 altogether. In the 10 years ending with 2016, deaths of U.S. civilians from firearm violence exceeded American combat fatalities in World War II.”
Amy Barnhorst, MD, vice chair of psychiatry at UC Davis, who was one of the early signers of the pledge, told this news organization that data analyst Rocco Pallin, MPH, with the UC Davis Violence Prevention Research Program (VPRP), quickly started managing commitments to the pledge and developed a “What You Can Do” intervention for physicians looking for help on how to prevent firearm injury and death.
Those efforts snowballed, and a need arose for a centralized public resource. In 2019, the state of California gave $3.8 million to the VPRP, which helped launch the BulletPoints Project, which Dr. Barnhorst now directs.
The website provides clinicians with evidence-based direction on how to have the conversations with patients. It walks them through various scenarios and details what can be done if what they learn during a patient interview requires action.
Dr. Barnhorst said the team is working on formalized online educational courses for mental health professionals and medical clinicians that will be hosted through various national organizations.
Christine Laine, MD, editor-in-chief of the Annals of Internal Medicine, said in an interview that although almost 4,000 persons have made the pledge, that number should be higher. She notes that the American College of Physicians has about 165,000 members, and even that is only a fraction of all physicians and clinicians.
“Signing the pledge helps raise awareness that this is a public health issue and, within the realm of health care providers, that they should be counseling patients about reducing risk, the same way we counsel people to wear bike helmets and use seat belts,” she said.
Dr. Barnhorst says those who don’t want to sign the pledge usually cite time considerations and that they already talk with patients about a list of public health issues. They also say they don’t know how to have the conversations or what they should do if what they hear in the interviews requires action.
“We can’t do anything about the time, but we can do something about the resources,” Dr. Barnhorst said.
Some clinicians, she said, worry that patients will get angry if physicians ask about guns, or they believe it’s illegal to ask.
“But there’s no law preventing physicians from asking these questions,” she said.
Dr. Wintemute told this news organization that he is not discouraged that only about 4,000 have signed the pledge. Rather, he was encouraged that the signatures came so quickly. He also notes that the number of persons who are interested far exceeds the number who have made the pledge.
Boosting the pledge numbers will likely take a new push in the form of published articles, he added, and those are in the works.
Among the next steps is conducting pre- and post-tests to see whether BulletPoints is effectively conveying the information for users, he said.
Another is pushing for advances in petitioning for “extreme risk protection orders,” which would require a gun owner to temporarily relinquish any firearms and ammunition and not purchase additional firearms.
Dr. Wintemute said that currently, Maryland is the only state in which health care professionals can petition for extreme risk protection orders. In any state that has the law, a health care professional can contact law enforcement about “a person who is at very high risk for violence in the very near future” but who has not committed a crime and is not mentally ill and so cannot be legally detained.
For physicians to include gun counseling as a routine part of patient care will likely require hearing from peers who are finding the time to do this effectively and hearing that it matters, he said.
“It’s going to take that on-the-ground diffusion of information, just as it has with vaccine hesitancy,” he said.
He notes that data on how to stop firearm violence are sparse and approaches so far have extrapolated from information on how to stop other health threats, such as smoking and drinking.
But that is changing rapidly, he said: “There’s funding from the CDC for research into the kind of work we’re doing.”
Measuring the success of those efforts is difficult.
One sign of change in the past 3 years, Dr. Wintemute says, is that there’s recognition among health care professionals and the public that this fits into clinicians’ “lane.”
Mass shootings not the largest source of gun violence
Mass shootings continue to dominate news about fatal shootings, but Dr. Barnhorst notes that such shootings represent a very small part – reportedly 1% to 2% – of the firearm deaths in the United States. Almost two-thirds of the deaths are suicides. Domestic violence deaths make up another large sector.
But it’s the mass shootings that stick in the collective U.S. consciousness, and the rising and unrelenting numbers can lead to a sense of futility.
Dr. Barnhorst, Dr. Laine, and Dr. Wintemute acknowledge they don’t know to what degree physicians’ talking to patients about firearms can help. But they do not doubt it’s worthy of the effort.
Dr. Laine said that during the past year, COVID-19 overshadowed the focus on the pledge, but he notes the signup for the pledge remains open. Information on firearm injury is collected on the Annals website.
Dr. Barnhorst says there is no good answer to the question of how many lives need to be saved before talking with patients about firearms becomes worth the effort. “For me,” she said, “that number is very, very low.”
Dr. Laine puts the number at one.
“If a physician talking to their patients about firearms prevents one suicide, then the intervention is a success,” she said.
Dr. Laine, Dr. Barnhorst, and Dr. Wintemute report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The group has developed a national resource for clinicians who wish to address the problem of gun violence deaths in the United States, which continue to mount by the day.
Signatures came quickly in 2018 after the Annals of Internal Medicine asked physicians to sign a formal pledge in which they commit to talking with their patients about firearms. To date, the list has grown to more than 3,600, and it remains open for additional signatories.
The effort built on data showing that before people commit violence with firearms, they often have notable risk factors that prompt them to see a physician.
At the time the pledge campaign was launched, frustration and despair had hit new highs after the school shooting of Feb. 14, 2018, in Parkland, Florida, in which 17 people were killed. That occurred just 4 months after the mass shooting in Las Vegas, Nevada, on Oct. 1, 2017, in which 58 people were gunned down.
An editorial by Garen J. Wintemute, MD, MPH, helped kick off the drive.
More deaths than WWII combat fatalities
Dr. Wintemute cited some grim statistics, writing that “nationwide in 2016, there was an average of 97 deaths from firearm violence per day: 35,476 altogether. In the 10 years ending with 2016, deaths of U.S. civilians from firearm violence exceeded American combat fatalities in World War II.”
Amy Barnhorst, MD, vice chair of psychiatry at UC Davis, who was one of the early signers of the pledge, told this news organization that data analyst Rocco Pallin, MPH, with the UC Davis Violence Prevention Research Program (VPRP), quickly started managing commitments to the pledge and developed a “What You Can Do” intervention for physicians looking for help on how to prevent firearm injury and death.
Those efforts snowballed, and a need arose for a centralized public resource. In 2019, the state of California gave $3.8 million to the VPRP, which helped launch the BulletPoints Project, which Dr. Barnhorst now directs.
The website provides clinicians with evidence-based direction on how to have the conversations with patients. It walks them through various scenarios and details what can be done if what they learn during a patient interview requires action.
Dr. Barnhorst said the team is working on formalized online educational courses for mental health professionals and medical clinicians that will be hosted through various national organizations.
Christine Laine, MD, editor-in-chief of the Annals of Internal Medicine, said in an interview that although almost 4,000 persons have made the pledge, that number should be higher. She notes that the American College of Physicians has about 165,000 members, and even that is only a fraction of all physicians and clinicians.
“Signing the pledge helps raise awareness that this is a public health issue and, within the realm of health care providers, that they should be counseling patients about reducing risk, the same way we counsel people to wear bike helmets and use seat belts,” she said.
Dr. Barnhorst says those who don’t want to sign the pledge usually cite time considerations and that they already talk with patients about a list of public health issues. They also say they don’t know how to have the conversations or what they should do if what they hear in the interviews requires action.
“We can’t do anything about the time, but we can do something about the resources,” Dr. Barnhorst said.
Some clinicians, she said, worry that patients will get angry if physicians ask about guns, or they believe it’s illegal to ask.
“But there’s no law preventing physicians from asking these questions,” she said.
Dr. Wintemute told this news organization that he is not discouraged that only about 4,000 have signed the pledge. Rather, he was encouraged that the signatures came so quickly. He also notes that the number of persons who are interested far exceeds the number who have made the pledge.
Boosting the pledge numbers will likely take a new push in the form of published articles, he added, and those are in the works.
Among the next steps is conducting pre- and post-tests to see whether BulletPoints is effectively conveying the information for users, he said.
Another is pushing for advances in petitioning for “extreme risk protection orders,” which would require a gun owner to temporarily relinquish any firearms and ammunition and not purchase additional firearms.
Dr. Wintemute said that currently, Maryland is the only state in which health care professionals can petition for extreme risk protection orders. In any state that has the law, a health care professional can contact law enforcement about “a person who is at very high risk for violence in the very near future” but who has not committed a crime and is not mentally ill and so cannot be legally detained.
For physicians to include gun counseling as a routine part of patient care will likely require hearing from peers who are finding the time to do this effectively and hearing that it matters, he said.
“It’s going to take that on-the-ground diffusion of information, just as it has with vaccine hesitancy,” he said.
He notes that data on how to stop firearm violence are sparse and approaches so far have extrapolated from information on how to stop other health threats, such as smoking and drinking.
But that is changing rapidly, he said: “There’s funding from the CDC for research into the kind of work we’re doing.”
Measuring the success of those efforts is difficult.
One sign of change in the past 3 years, Dr. Wintemute says, is that there’s recognition among health care professionals and the public that this fits into clinicians’ “lane.”
Mass shootings not the largest source of gun violence
Mass shootings continue to dominate news about fatal shootings, but Dr. Barnhorst notes that such shootings represent a very small part – reportedly 1% to 2% – of the firearm deaths in the United States. Almost two-thirds of the deaths are suicides. Domestic violence deaths make up another large sector.
But it’s the mass shootings that stick in the collective U.S. consciousness, and the rising and unrelenting numbers can lead to a sense of futility.
Dr. Barnhorst, Dr. Laine, and Dr. Wintemute acknowledge they don’t know to what degree physicians’ talking to patients about firearms can help. But they do not doubt it’s worthy of the effort.
Dr. Laine said that during the past year, COVID-19 overshadowed the focus on the pledge, but he notes the signup for the pledge remains open. Information on firearm injury is collected on the Annals website.
Dr. Barnhorst says there is no good answer to the question of how many lives need to be saved before talking with patients about firearms becomes worth the effort. “For me,” she said, “that number is very, very low.”
Dr. Laine puts the number at one.
“If a physician talking to their patients about firearms prevents one suicide, then the intervention is a success,” she said.
Dr. Laine, Dr. Barnhorst, and Dr. Wintemute report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The group has developed a national resource for clinicians who wish to address the problem of gun violence deaths in the United States, which continue to mount by the day.
Signatures came quickly in 2018 after the Annals of Internal Medicine asked physicians to sign a formal pledge in which they commit to talking with their patients about firearms. To date, the list has grown to more than 3,600, and it remains open for additional signatories.
The effort built on data showing that before people commit violence with firearms, they often have notable risk factors that prompt them to see a physician.
At the time the pledge campaign was launched, frustration and despair had hit new highs after the school shooting of Feb. 14, 2018, in Parkland, Florida, in which 17 people were killed. That occurred just 4 months after the mass shooting in Las Vegas, Nevada, on Oct. 1, 2017, in which 58 people were gunned down.
An editorial by Garen J. Wintemute, MD, MPH, helped kick off the drive.
More deaths than WWII combat fatalities
Dr. Wintemute cited some grim statistics, writing that “nationwide in 2016, there was an average of 97 deaths from firearm violence per day: 35,476 altogether. In the 10 years ending with 2016, deaths of U.S. civilians from firearm violence exceeded American combat fatalities in World War II.”
Amy Barnhorst, MD, vice chair of psychiatry at UC Davis, who was one of the early signers of the pledge, told this news organization that data analyst Rocco Pallin, MPH, with the UC Davis Violence Prevention Research Program (VPRP), quickly started managing commitments to the pledge and developed a “What You Can Do” intervention for physicians looking for help on how to prevent firearm injury and death.
Those efforts snowballed, and a need arose for a centralized public resource. In 2019, the state of California gave $3.8 million to the VPRP, which helped launch the BulletPoints Project, which Dr. Barnhorst now directs.
The website provides clinicians with evidence-based direction on how to have the conversations with patients. It walks them through various scenarios and details what can be done if what they learn during a patient interview requires action.
Dr. Barnhorst said the team is working on formalized online educational courses for mental health professionals and medical clinicians that will be hosted through various national organizations.
Christine Laine, MD, editor-in-chief of the Annals of Internal Medicine, said in an interview that although almost 4,000 persons have made the pledge, that number should be higher. She notes that the American College of Physicians has about 165,000 members, and even that is only a fraction of all physicians and clinicians.
“Signing the pledge helps raise awareness that this is a public health issue and, within the realm of health care providers, that they should be counseling patients about reducing risk, the same way we counsel people to wear bike helmets and use seat belts,” she said.
Dr. Barnhorst says those who don’t want to sign the pledge usually cite time considerations and that they already talk with patients about a list of public health issues. They also say they don’t know how to have the conversations or what they should do if what they hear in the interviews requires action.
“We can’t do anything about the time, but we can do something about the resources,” Dr. Barnhorst said.
Some clinicians, she said, worry that patients will get angry if physicians ask about guns, or they believe it’s illegal to ask.
“But there’s no law preventing physicians from asking these questions,” she said.
Dr. Wintemute told this news organization that he is not discouraged that only about 4,000 have signed the pledge. Rather, he was encouraged that the signatures came so quickly. He also notes that the number of persons who are interested far exceeds the number who have made the pledge.
Boosting the pledge numbers will likely take a new push in the form of published articles, he added, and those are in the works.
Among the next steps is conducting pre- and post-tests to see whether BulletPoints is effectively conveying the information for users, he said.
Another is pushing for advances in petitioning for “extreme risk protection orders,” which would require a gun owner to temporarily relinquish any firearms and ammunition and not purchase additional firearms.
Dr. Wintemute said that currently, Maryland is the only state in which health care professionals can petition for extreme risk protection orders. In any state that has the law, a health care professional can contact law enforcement about “a person who is at very high risk for violence in the very near future” but who has not committed a crime and is not mentally ill and so cannot be legally detained.
For physicians to include gun counseling as a routine part of patient care will likely require hearing from peers who are finding the time to do this effectively and hearing that it matters, he said.
“It’s going to take that on-the-ground diffusion of information, just as it has with vaccine hesitancy,” he said.
He notes that data on how to stop firearm violence are sparse and approaches so far have extrapolated from information on how to stop other health threats, such as smoking and drinking.
But that is changing rapidly, he said: “There’s funding from the CDC for research into the kind of work we’re doing.”
Measuring the success of those efforts is difficult.
One sign of change in the past 3 years, Dr. Wintemute says, is that there’s recognition among health care professionals and the public that this fits into clinicians’ “lane.”
Mass shootings not the largest source of gun violence
Mass shootings continue to dominate news about fatal shootings, but Dr. Barnhorst notes that such shootings represent a very small part – reportedly 1% to 2% – of the firearm deaths in the United States. Almost two-thirds of the deaths are suicides. Domestic violence deaths make up another large sector.
But it’s the mass shootings that stick in the collective U.S. consciousness, and the rising and unrelenting numbers can lead to a sense of futility.
Dr. Barnhorst, Dr. Laine, and Dr. Wintemute acknowledge they don’t know to what degree physicians’ talking to patients about firearms can help. But they do not doubt it’s worthy of the effort.
Dr. Laine said that during the past year, COVID-19 overshadowed the focus on the pledge, but he notes the signup for the pledge remains open. Information on firearm injury is collected on the Annals website.
Dr. Barnhorst says there is no good answer to the question of how many lives need to be saved before talking with patients about firearms becomes worth the effort. “For me,” she said, “that number is very, very low.”
Dr. Laine puts the number at one.
“If a physician talking to their patients about firearms prevents one suicide, then the intervention is a success,” she said.
Dr. Laine, Dr. Barnhorst, and Dr. Wintemute report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Placental allograft, cytology processor, cell-free RNA testing, and male infertility
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
Mistrust and Mandates: COVID-19 Vaccination in the Military
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
CDC director cites rise in hospitalizations in urging teen vaccinations
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.