User login
Consideration of herbal products in pregnancy and lactation
In recent decades, natural products have had increased consumer attention in industrialized nations. One of the challenges is that “natural” can be more of a perception than a standard. “Herbal products” is a more frequently used and perhaps a more apt term. Herbal products come in many forms, including herbs used in food preparation, teas, infusions, caplets, dried extracts, essential oils, and tinctures.
Multiple prescription medications have pharmacologically active compounds that originated from herbal products, both historically and currently. Examples include the cardiac stimulant digoxin (foxglove plant), the antimalarial quinine (Cinchona bark), and antihypertensives (Rauwolfia serpentina). Indeed, the first pharmacologically active compound, morphine, was extracted from the seed pods of opium poppies approximately 200 years ago. This demonstrated that medications could be purified from plants and that a precise dose could be determined for administration. However, herbal products are grown and harvested in varying seasonal conditions and soil types, which, over time and geography, may contribute to variability in the levels of active compound in the final products.
The importance of active compound purification and consistent precise dosage in herbal products brings up the topic of regulation. Herbal products are considered dietary supplements and as such are Food and Drug Administration regulated as a food under the 1994 Dietary Supplement Health Education Act. Regulation as a food product does not involve the same level of scrutiny as a medication. There is no requirement that manufacturers check for purity and consistency of their product’s active compound(s). Manufacturers must ensure that the claims they make about herbal products are not false or misleading. They must also support their claims with evidence. However, there is no requirement for the manufacturers to submit this evidence to the FDA. This can translate into a discrepancy between the claim on the product label and scientific evidence that the product does what it claims to do. In other words, the product may not be effective.
With uncertain efficacy, the safety of herbal products comes into focus. Very few herbal products (or their specific active compounds) have been scientifically studied for safety in pregnancy and lactation. Further, herbal products may contain contaminants. Metals such as lead and mercury occur naturally. Yet, because of human activities, both may have collected in areas where herbal products are grown. From a safety perspective, both can be concerning in pregnancy or lactation. Lead and mercury are two examples of metal contaminants. Other contaminants may include pesticides, chemicals, and bacteria or other microorganisms. Some liquid herbal products such as tinctures contain alcohol, which should be avoided in pregnancy. An additional consideration would be the potential for herbal products, including any of their known or unknown product contents, to interact with prescribed medications or anesthesia.
Select examples of herbal products
Astragalus is the root of an herb and it is used for reasons of boosting immunity, energy, and other functions. These and its purported promotion of breast milk flow (galactagogue) are unsupported. Safety concerns include irregular heartbeat and dizziness, rendering it unsafe for use in pregnancy and of unknown efficacy and safety in lactation.
Kombucha is an herbal product made from leaves (tea), sugar, a culture, and other varying products. Like many herbal products, it is both manufactured and home brewed. It is used for probiotic and antioxidant reasons. As a fermented product, kombucha may contain 0.2%-0.5% alcohol. There is no known safe level of alcohol and no known safe type of alcohol for use in pregnancy. Alcohol exposure in pregnancy can result in fetal alcohol spectrum disorders, involving a range of birth defects and life-long intellectual, learning and behavioral disorders. Alcohol found in breast milk approximates the level of alcohol found in the maternal bloodstream. Alcohol-containing products should be avoided in pregnancy and lactation.
Nux vomica is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. It comes from the raw seeds (toxic) of an evergreen tree. It has serious safety concerns and yet it is still in use. It contains strychnine, which can harm both the pregnant individual and the developing fetus. It is not recommended in lactation.
Red raspberry leaf is a leaf, brewed and ingested as a tea. It is used for reasons of preventing miscarriage, relieving nausea and stomach discomfort, toning the uterus, reducing labor pain, increasing breast milk production, and other functions. In low doses, it appears to be safe. In high doses, it can induce smooth muscle relaxation. Efficacy has not been demonstrated with labor and delivery or in increasing breast milk production.
Tabacum is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. Its full name is Nicotiana tabacum (tobacco) and it contains 2%-8% nicotine, which should be avoided in pregnancy. Nicotine is a health danger for the pregnant individual and can damage a developing fetus’ brain and lungs.
Unless otherwise scientifically demonstrated, herbal products should be considered medications with pharmacologic activity, potential adverse effects, and potential toxicity in pregnancy and lactation. It’s easy for a patient to forget about reporting any nonprescription medications during a patient-provider visit. As a provider, purposefully asking about all over-the-counter and herbal products during each visit can prompt the patient to provide this important information. Further, it may facilitate discussion about the continuation/discontinuation of products of unknown safety and unknown benefit, culminating in the serious reflection: “Is it really worth the risk?”
For further information about the safety of herbal products, consult local Poison Control Centers, MothertoBaby, MothertoBaby affiliates, and the National Institutes of Health Drugs and Lactation Database, LactMed.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology, and represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy has worked with multiple pharmaceutical manufacturers regarding studies of medication safety in pregnancy, most recently Biohaven Pharmaceuticals, New Haven, CT.
.
In recent decades, natural products have had increased consumer attention in industrialized nations. One of the challenges is that “natural” can be more of a perception than a standard. “Herbal products” is a more frequently used and perhaps a more apt term. Herbal products come in many forms, including herbs used in food preparation, teas, infusions, caplets, dried extracts, essential oils, and tinctures.
Multiple prescription medications have pharmacologically active compounds that originated from herbal products, both historically and currently. Examples include the cardiac stimulant digoxin (foxglove plant), the antimalarial quinine (Cinchona bark), and antihypertensives (Rauwolfia serpentina). Indeed, the first pharmacologically active compound, morphine, was extracted from the seed pods of opium poppies approximately 200 years ago. This demonstrated that medications could be purified from plants and that a precise dose could be determined for administration. However, herbal products are grown and harvested in varying seasonal conditions and soil types, which, over time and geography, may contribute to variability in the levels of active compound in the final products.
The importance of active compound purification and consistent precise dosage in herbal products brings up the topic of regulation. Herbal products are considered dietary supplements and as such are Food and Drug Administration regulated as a food under the 1994 Dietary Supplement Health Education Act. Regulation as a food product does not involve the same level of scrutiny as a medication. There is no requirement that manufacturers check for purity and consistency of their product’s active compound(s). Manufacturers must ensure that the claims they make about herbal products are not false or misleading. They must also support their claims with evidence. However, there is no requirement for the manufacturers to submit this evidence to the FDA. This can translate into a discrepancy between the claim on the product label and scientific evidence that the product does what it claims to do. In other words, the product may not be effective.
With uncertain efficacy, the safety of herbal products comes into focus. Very few herbal products (or their specific active compounds) have been scientifically studied for safety in pregnancy and lactation. Further, herbal products may contain contaminants. Metals such as lead and mercury occur naturally. Yet, because of human activities, both may have collected in areas where herbal products are grown. From a safety perspective, both can be concerning in pregnancy or lactation. Lead and mercury are two examples of metal contaminants. Other contaminants may include pesticides, chemicals, and bacteria or other microorganisms. Some liquid herbal products such as tinctures contain alcohol, which should be avoided in pregnancy. An additional consideration would be the potential for herbal products, including any of their known or unknown product contents, to interact with prescribed medications or anesthesia.
Select examples of herbal products
Astragalus is the root of an herb and it is used for reasons of boosting immunity, energy, and other functions. These and its purported promotion of breast milk flow (galactagogue) are unsupported. Safety concerns include irregular heartbeat and dizziness, rendering it unsafe for use in pregnancy and of unknown efficacy and safety in lactation.
Kombucha is an herbal product made from leaves (tea), sugar, a culture, and other varying products. Like many herbal products, it is both manufactured and home brewed. It is used for probiotic and antioxidant reasons. As a fermented product, kombucha may contain 0.2%-0.5% alcohol. There is no known safe level of alcohol and no known safe type of alcohol for use in pregnancy. Alcohol exposure in pregnancy can result in fetal alcohol spectrum disorders, involving a range of birth defects and life-long intellectual, learning and behavioral disorders. Alcohol found in breast milk approximates the level of alcohol found in the maternal bloodstream. Alcohol-containing products should be avoided in pregnancy and lactation.
Nux vomica is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. It comes from the raw seeds (toxic) of an evergreen tree. It has serious safety concerns and yet it is still in use. It contains strychnine, which can harm both the pregnant individual and the developing fetus. It is not recommended in lactation.
Red raspberry leaf is a leaf, brewed and ingested as a tea. It is used for reasons of preventing miscarriage, relieving nausea and stomach discomfort, toning the uterus, reducing labor pain, increasing breast milk production, and other functions. In low doses, it appears to be safe. In high doses, it can induce smooth muscle relaxation. Efficacy has not been demonstrated with labor and delivery or in increasing breast milk production.
Tabacum is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. Its full name is Nicotiana tabacum (tobacco) and it contains 2%-8% nicotine, which should be avoided in pregnancy. Nicotine is a health danger for the pregnant individual and can damage a developing fetus’ brain and lungs.
Unless otherwise scientifically demonstrated, herbal products should be considered medications with pharmacologic activity, potential adverse effects, and potential toxicity in pregnancy and lactation. It’s easy for a patient to forget about reporting any nonprescription medications during a patient-provider visit. As a provider, purposefully asking about all over-the-counter and herbal products during each visit can prompt the patient to provide this important information. Further, it may facilitate discussion about the continuation/discontinuation of products of unknown safety and unknown benefit, culminating in the serious reflection: “Is it really worth the risk?”
For further information about the safety of herbal products, consult local Poison Control Centers, MothertoBaby, MothertoBaby affiliates, and the National Institutes of Health Drugs and Lactation Database, LactMed.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology, and represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy has worked with multiple pharmaceutical manufacturers regarding studies of medication safety in pregnancy, most recently Biohaven Pharmaceuticals, New Haven, CT.
.
In recent decades, natural products have had increased consumer attention in industrialized nations. One of the challenges is that “natural” can be more of a perception than a standard. “Herbal products” is a more frequently used and perhaps a more apt term. Herbal products come in many forms, including herbs used in food preparation, teas, infusions, caplets, dried extracts, essential oils, and tinctures.
Multiple prescription medications have pharmacologically active compounds that originated from herbal products, both historically and currently. Examples include the cardiac stimulant digoxin (foxglove plant), the antimalarial quinine (Cinchona bark), and antihypertensives (Rauwolfia serpentina). Indeed, the first pharmacologically active compound, morphine, was extracted from the seed pods of opium poppies approximately 200 years ago. This demonstrated that medications could be purified from plants and that a precise dose could be determined for administration. However, herbal products are grown and harvested in varying seasonal conditions and soil types, which, over time and geography, may contribute to variability in the levels of active compound in the final products.
The importance of active compound purification and consistent precise dosage in herbal products brings up the topic of regulation. Herbal products are considered dietary supplements and as such are Food and Drug Administration regulated as a food under the 1994 Dietary Supplement Health Education Act. Regulation as a food product does not involve the same level of scrutiny as a medication. There is no requirement that manufacturers check for purity and consistency of their product’s active compound(s). Manufacturers must ensure that the claims they make about herbal products are not false or misleading. They must also support their claims with evidence. However, there is no requirement for the manufacturers to submit this evidence to the FDA. This can translate into a discrepancy between the claim on the product label and scientific evidence that the product does what it claims to do. In other words, the product may not be effective.
With uncertain efficacy, the safety of herbal products comes into focus. Very few herbal products (or their specific active compounds) have been scientifically studied for safety in pregnancy and lactation. Further, herbal products may contain contaminants. Metals such as lead and mercury occur naturally. Yet, because of human activities, both may have collected in areas where herbal products are grown. From a safety perspective, both can be concerning in pregnancy or lactation. Lead and mercury are two examples of metal contaminants. Other contaminants may include pesticides, chemicals, and bacteria or other microorganisms. Some liquid herbal products such as tinctures contain alcohol, which should be avoided in pregnancy. An additional consideration would be the potential for herbal products, including any of their known or unknown product contents, to interact with prescribed medications or anesthesia.
Select examples of herbal products
Astragalus is the root of an herb and it is used for reasons of boosting immunity, energy, and other functions. These and its purported promotion of breast milk flow (galactagogue) are unsupported. Safety concerns include irregular heartbeat and dizziness, rendering it unsafe for use in pregnancy and of unknown efficacy and safety in lactation.
Kombucha is an herbal product made from leaves (tea), sugar, a culture, and other varying products. Like many herbal products, it is both manufactured and home brewed. It is used for probiotic and antioxidant reasons. As a fermented product, kombucha may contain 0.2%-0.5% alcohol. There is no known safe level of alcohol and no known safe type of alcohol for use in pregnancy. Alcohol exposure in pregnancy can result in fetal alcohol spectrum disorders, involving a range of birth defects and life-long intellectual, learning and behavioral disorders. Alcohol found in breast milk approximates the level of alcohol found in the maternal bloodstream. Alcohol-containing products should be avoided in pregnancy and lactation.
Nux vomica is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. It comes from the raw seeds (toxic) of an evergreen tree. It has serious safety concerns and yet it is still in use. It contains strychnine, which can harm both the pregnant individual and the developing fetus. It is not recommended in lactation.
Red raspberry leaf is a leaf, brewed and ingested as a tea. It is used for reasons of preventing miscarriage, relieving nausea and stomach discomfort, toning the uterus, reducing labor pain, increasing breast milk production, and other functions. In low doses, it appears to be safe. In high doses, it can induce smooth muscle relaxation. Efficacy has not been demonstrated with labor and delivery or in increasing breast milk production.
Tabacum is an herbal product and is used for reasons of reducing nausea or vomiting in pregnancy. Its full name is Nicotiana tabacum (tobacco) and it contains 2%-8% nicotine, which should be avoided in pregnancy. Nicotine is a health danger for the pregnant individual and can damage a developing fetus’ brain and lungs.
Unless otherwise scientifically demonstrated, herbal products should be considered medications with pharmacologic activity, potential adverse effects, and potential toxicity in pregnancy and lactation. It’s easy for a patient to forget about reporting any nonprescription medications during a patient-provider visit. As a provider, purposefully asking about all over-the-counter and herbal products during each visit can prompt the patient to provide this important information. Further, it may facilitate discussion about the continuation/discontinuation of products of unknown safety and unknown benefit, culminating in the serious reflection: “Is it really worth the risk?”
For further information about the safety of herbal products, consult local Poison Control Centers, MothertoBaby, MothertoBaby affiliates, and the National Institutes of Health Drugs and Lactation Database, LactMed.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology, and represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy has worked with multiple pharmaceutical manufacturers regarding studies of medication safety in pregnancy, most recently Biohaven Pharmaceuticals, New Haven, CT.
.
Which behavioral health screening tool should you use—and when?
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3
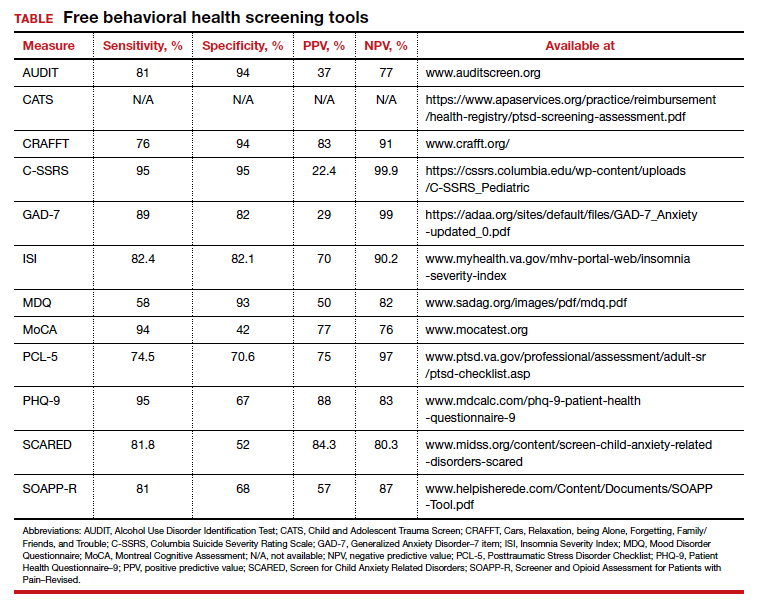
Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.
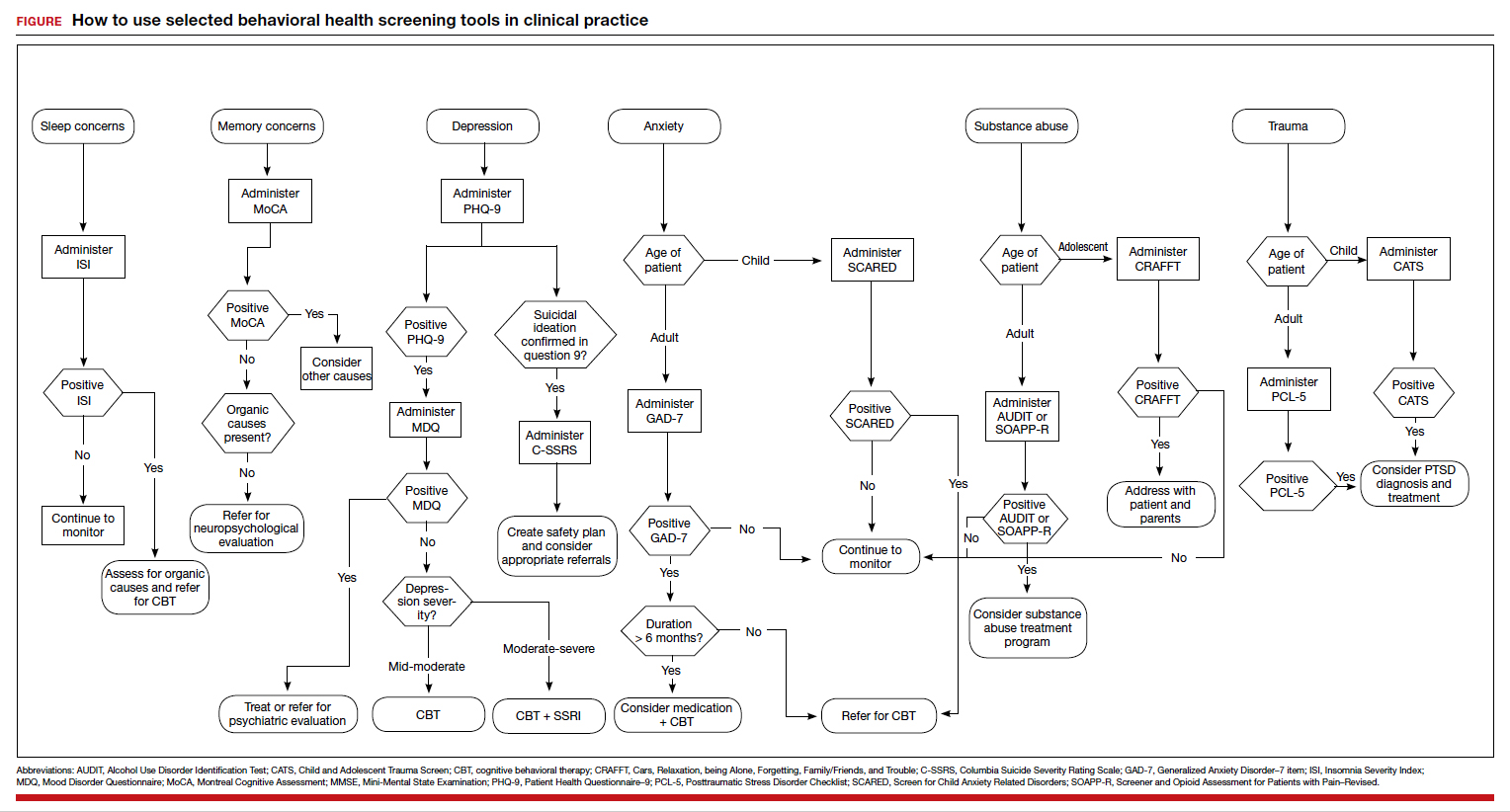
- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.

Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.

- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Women increasingly turn to CBD, with or without doc’s blessing
When 42-year-old Danielle Simone Brand started having hormonal migraines, she first turned to cannabidiol (CBD) oil, eventually adding an occasional pull on a prefilled tetrahydrocannabinol (THC) vape for nighttime use. She was careful to avoid THC during work hours. A parenting and cannabis writer, Ms. Brand had more than a cursory background in cannabinoid medicine and had spent time at her local California dispensary discussing various cannabinoid components that might help alleviate her pain.
A self-professed “do-it-yourselfer,” Ms. Brand continues to use cannabinoids for her monthly headaches, forgoing any other pain medication. “There are times for conventional medicine in partnership with your doctor, but when it comes to health and wellness, women should be empowered to make decisions and self-experiment,” she said in an interview.
Ms. Brand is not alone. Significant numbers of women are replacing or supplementing prescription medications with cannabinoids, often without consulting their primary care physician, ob.gyn., or other specialist. At times, women have tried to have these conversations, only to be met with silence or worse.
Take Linda Fuller, a 58-year-old yoga instructor from Long Island who says that she uses CBD and THC for chronic sacroiliac pain after a car accident and to alleviate stress-triggered eczema flares. “I’ve had doctors turn their backs on me; I’ve had nurse practitioners walk out on me in the middle of a sentence,” she said in an interview.
Ms. Fuller said her conversion to cannabinoid medicine is relatively new; she never used cannabis recreationally before her accident but now considers it a gift. She doesn’t keep aspirin in the house and refused pain medication immediately after she injured her back.
Diana Krach, a 34-year-old writer from Maryland, says she’s encountered roadblocks about her decision to use cannabinoids for endometriosis and for pain from Crohn’s disease. When she tried to discuss her CBD use with a gastroenterologist, he interrupted her: “Whatever pot you’re smoking isn’t going to work, you’re going on biologics.”
Ms. Krach had not been smoking anything but had turned to a CBD tincture for symptom relief after prescription pain medications failed to help.
Ms. Brand, Ms. Fuller, and Ms. Krach are the tip of the iceberg when it comes to women seeking symptom relief outside the medicine cabinet. A recent survey in the Journal of Women’s Health of almost 1,000 women show that 90% (most between the ages of 35 and 44) had used cannabis and would consider using it to treat gynecologic pain. Roughly 80% said they would consider using it for procedure-related pain or other conditions. Additionally, women have reported using cannabinoids for PTSD, sleep disturbances or insomnia, anxiety, and migraine headaches.
Observational survey data have likewise shown that 80% of women with advanced or recurrent gynecologic malignancies who were prescribed cannabis reported that it was equivalent or superior to other medications for relieving pain, neuropathy, nausea, insomnia, decreased appetite, and anxiety.
In another survey, almost half (45%) of women with gynecologic malignancies who used nonprescribed cannabis for the same symptoms reported that they had reduced their use of prescription narcotics after initiating use of cannabis.
The gray zone
There has been a surge in self-reported cannabis use among pregnant women in particular. The National Survey on Drug Use and Health findings for the periods 2002-2003 and 2016-2017 highlight increases in adjusted prevalence rates from 3.4% to 7% in past-month use among pregnant women overall and from 5.7% to 12.1% during the first trimester alone.
“The more that you talk to pregnant women, the more that you realize that a lot are using cannabinoids for something that is basically medicinal, for sleep, for anxiety, or for nausea,” Katrina Mark, MD, an ob.gyn. and associate professor of medicine at the University of Maryland, College Park, said in an interview. “I’m not saying it’s fine to use drugs in pregnancy, but it is a grayer conversation than a lot of colleagues want to believe. Telling women to quit seems foolish since the alternative is to be anxious, don’t sleep, don’t eat, or use a medication that also has risks to it.”
One observational study shows that pregnant women themselves are conflicted. Although the majority believe that cannabis is “natural” and “safe,” compared with prescription drugs, they aren’t entirely in the dark about potential risks. They often express frustration with practitioners’ responses when these topics are broached during office visits. An observational survey among women and practitioners published in 2020 highlights that only half of doctors openly discouraged perinatal cannabis use and that others opted out of the discussion entirely.
This is the experience of many of the women that this news organization spoke with. Ms. Krach pointed out that “there’s a big deficit in listening; the doctor is supposed to be working for our behalf, especially when it comes to reproductive health.”
Dr. Mark believed that a lot of the conversation has been clouded by the illegality of the substance but that cannabinoids deserve as much of a fair chance for discussion and consideration as other medicines, which also carry risks in pregnancy. “There’s literally no evidence that it will work in pregnancy [for these symptoms], but there’s no evidence that it doesn’t, either,” she said in an interview. “When I have this conversation with colleagues who do not share my views, I try to encourage them to look at the actual risks versus the benefits versus the alternatives.”
The ‘entourage effect’
Data supporting cannabinoids have been mostly laboratory based, case based, or observational. However, several well-designed (albeit small) trials have demonstrated efficacy for chronic pain conditions, including neuropathic and headache pain, as well as in Crohn’s disease. Most investigators have concluded that dosage is important and that there is a synergistic interaction between compounds (known as the “entourage effect”) that relates to cannabinoid efficacy or lack thereof, as well as possible adverse effects.
In addition to legality issues, the entourage effect is one of the most important factors related to the medical use of cannabinoids. “There are literally thousands of cultivars of cannabis, each with their own phytocannabinoid and terpenic profiles that may produce distinct therapeutic effects, [so] it is misguided to speak of cannabis in monolithic terms. It is like making broad claims about soup,” wrote coauthor Samoon Ahmad, MD, in Medical Marijuana: A Clinical Handbook.
Additionally, the role that reproductive hormones play is not entirely understood. Reproductive-aged women appear to be more susceptible to a “telescoping” (gender-related progression to dependence) effect in comparison with men. Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, said in an interview. She explained that research has shown that factors such as the degree of exposure, frequency of use, and menses confound this susceptibility.
It’s the data
Frustration over cannabinoid therapeutics abound, especially when it comes to data, legal issues, and lack of training. “The feedback that I hear from providers is that there isn’t enough information; we just don’t know enough about it,” Dr. Mark said, “but there is information that we do have, and ignoring it is not beneficial.”
Dr. Cooper concurred. Although she readily acknowledges that data from randomized, placebo-controlled trials are mostly lacking, she says, “There are signals in the literature providing evidence for the utility of cannabis and cannabinoids for pain and some other effects.”
Other practitioners said in an interview that some patients admit to using cannabinoids but that they lack the ample information to guide these patients. By and large, many women equate “natural” with “safe,” and some will experiment on their own to see what works.
Those experiments are not without risk, which is why “it’s just as important for physicians to talk to their patients about cannabis use as it is for patients to be forthcoming about that use,” said Dr. Cooper. “It could have implications on their overall health as well as interactions with other drugs that they’re using.”
That balance from a clinical perspective on cannabis is crucial, wrote coauthor Kenneth Hill, MD, in Medical Marijuana: A Clinical Handbook. “Without it,” he wrote, “the window of opportunity for a patient to accept treatment that she needs may not be open very long.”
A version of this article first appeared on Medscape.com.
When 42-year-old Danielle Simone Brand started having hormonal migraines, she first turned to cannabidiol (CBD) oil, eventually adding an occasional pull on a prefilled tetrahydrocannabinol (THC) vape for nighttime use. She was careful to avoid THC during work hours. A parenting and cannabis writer, Ms. Brand had more than a cursory background in cannabinoid medicine and had spent time at her local California dispensary discussing various cannabinoid components that might help alleviate her pain.
A self-professed “do-it-yourselfer,” Ms. Brand continues to use cannabinoids for her monthly headaches, forgoing any other pain medication. “There are times for conventional medicine in partnership with your doctor, but when it comes to health and wellness, women should be empowered to make decisions and self-experiment,” she said in an interview.
Ms. Brand is not alone. Significant numbers of women are replacing or supplementing prescription medications with cannabinoids, often without consulting their primary care physician, ob.gyn., or other specialist. At times, women have tried to have these conversations, only to be met with silence or worse.
Take Linda Fuller, a 58-year-old yoga instructor from Long Island who says that she uses CBD and THC for chronic sacroiliac pain after a car accident and to alleviate stress-triggered eczema flares. “I’ve had doctors turn their backs on me; I’ve had nurse practitioners walk out on me in the middle of a sentence,” she said in an interview.
Ms. Fuller said her conversion to cannabinoid medicine is relatively new; she never used cannabis recreationally before her accident but now considers it a gift. She doesn’t keep aspirin in the house and refused pain medication immediately after she injured her back.
Diana Krach, a 34-year-old writer from Maryland, says she’s encountered roadblocks about her decision to use cannabinoids for endometriosis and for pain from Crohn’s disease. When she tried to discuss her CBD use with a gastroenterologist, he interrupted her: “Whatever pot you’re smoking isn’t going to work, you’re going on biologics.”
Ms. Krach had not been smoking anything but had turned to a CBD tincture for symptom relief after prescription pain medications failed to help.
Ms. Brand, Ms. Fuller, and Ms. Krach are the tip of the iceberg when it comes to women seeking symptom relief outside the medicine cabinet. A recent survey in the Journal of Women’s Health of almost 1,000 women show that 90% (most between the ages of 35 and 44) had used cannabis and would consider using it to treat gynecologic pain. Roughly 80% said they would consider using it for procedure-related pain or other conditions. Additionally, women have reported using cannabinoids for PTSD, sleep disturbances or insomnia, anxiety, and migraine headaches.
Observational survey data have likewise shown that 80% of women with advanced or recurrent gynecologic malignancies who were prescribed cannabis reported that it was equivalent or superior to other medications for relieving pain, neuropathy, nausea, insomnia, decreased appetite, and anxiety.
In another survey, almost half (45%) of women with gynecologic malignancies who used nonprescribed cannabis for the same symptoms reported that they had reduced their use of prescription narcotics after initiating use of cannabis.
The gray zone
There has been a surge in self-reported cannabis use among pregnant women in particular. The National Survey on Drug Use and Health findings for the periods 2002-2003 and 2016-2017 highlight increases in adjusted prevalence rates from 3.4% to 7% in past-month use among pregnant women overall and from 5.7% to 12.1% during the first trimester alone.
“The more that you talk to pregnant women, the more that you realize that a lot are using cannabinoids for something that is basically medicinal, for sleep, for anxiety, or for nausea,” Katrina Mark, MD, an ob.gyn. and associate professor of medicine at the University of Maryland, College Park, said in an interview. “I’m not saying it’s fine to use drugs in pregnancy, but it is a grayer conversation than a lot of colleagues want to believe. Telling women to quit seems foolish since the alternative is to be anxious, don’t sleep, don’t eat, or use a medication that also has risks to it.”
One observational study shows that pregnant women themselves are conflicted. Although the majority believe that cannabis is “natural” and “safe,” compared with prescription drugs, they aren’t entirely in the dark about potential risks. They often express frustration with practitioners’ responses when these topics are broached during office visits. An observational survey among women and practitioners published in 2020 highlights that only half of doctors openly discouraged perinatal cannabis use and that others opted out of the discussion entirely.
This is the experience of many of the women that this news organization spoke with. Ms. Krach pointed out that “there’s a big deficit in listening; the doctor is supposed to be working for our behalf, especially when it comes to reproductive health.”
Dr. Mark believed that a lot of the conversation has been clouded by the illegality of the substance but that cannabinoids deserve as much of a fair chance for discussion and consideration as other medicines, which also carry risks in pregnancy. “There’s literally no evidence that it will work in pregnancy [for these symptoms], but there’s no evidence that it doesn’t, either,” she said in an interview. “When I have this conversation with colleagues who do not share my views, I try to encourage them to look at the actual risks versus the benefits versus the alternatives.”
The ‘entourage effect’
Data supporting cannabinoids have been mostly laboratory based, case based, or observational. However, several well-designed (albeit small) trials have demonstrated efficacy for chronic pain conditions, including neuropathic and headache pain, as well as in Crohn’s disease. Most investigators have concluded that dosage is important and that there is a synergistic interaction between compounds (known as the “entourage effect”) that relates to cannabinoid efficacy or lack thereof, as well as possible adverse effects.
In addition to legality issues, the entourage effect is one of the most important factors related to the medical use of cannabinoids. “There are literally thousands of cultivars of cannabis, each with their own phytocannabinoid and terpenic profiles that may produce distinct therapeutic effects, [so] it is misguided to speak of cannabis in monolithic terms. It is like making broad claims about soup,” wrote coauthor Samoon Ahmad, MD, in Medical Marijuana: A Clinical Handbook.
Additionally, the role that reproductive hormones play is not entirely understood. Reproductive-aged women appear to be more susceptible to a “telescoping” (gender-related progression to dependence) effect in comparison with men. Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, said in an interview. She explained that research has shown that factors such as the degree of exposure, frequency of use, and menses confound this susceptibility.
It’s the data
Frustration over cannabinoid therapeutics abound, especially when it comes to data, legal issues, and lack of training. “The feedback that I hear from providers is that there isn’t enough information; we just don’t know enough about it,” Dr. Mark said, “but there is information that we do have, and ignoring it is not beneficial.”
Dr. Cooper concurred. Although she readily acknowledges that data from randomized, placebo-controlled trials are mostly lacking, she says, “There are signals in the literature providing evidence for the utility of cannabis and cannabinoids for pain and some other effects.”
Other practitioners said in an interview that some patients admit to using cannabinoids but that they lack the ample information to guide these patients. By and large, many women equate “natural” with “safe,” and some will experiment on their own to see what works.
Those experiments are not without risk, which is why “it’s just as important for physicians to talk to their patients about cannabis use as it is for patients to be forthcoming about that use,” said Dr. Cooper. “It could have implications on their overall health as well as interactions with other drugs that they’re using.”
That balance from a clinical perspective on cannabis is crucial, wrote coauthor Kenneth Hill, MD, in Medical Marijuana: A Clinical Handbook. “Without it,” he wrote, “the window of opportunity for a patient to accept treatment that she needs may not be open very long.”
A version of this article first appeared on Medscape.com.
When 42-year-old Danielle Simone Brand started having hormonal migraines, she first turned to cannabidiol (CBD) oil, eventually adding an occasional pull on a prefilled tetrahydrocannabinol (THC) vape for nighttime use. She was careful to avoid THC during work hours. A parenting and cannabis writer, Ms. Brand had more than a cursory background in cannabinoid medicine and had spent time at her local California dispensary discussing various cannabinoid components that might help alleviate her pain.
A self-professed “do-it-yourselfer,” Ms. Brand continues to use cannabinoids for her monthly headaches, forgoing any other pain medication. “There are times for conventional medicine in partnership with your doctor, but when it comes to health and wellness, women should be empowered to make decisions and self-experiment,” she said in an interview.
Ms. Brand is not alone. Significant numbers of women are replacing or supplementing prescription medications with cannabinoids, often without consulting their primary care physician, ob.gyn., or other specialist. At times, women have tried to have these conversations, only to be met with silence or worse.
Take Linda Fuller, a 58-year-old yoga instructor from Long Island who says that she uses CBD and THC for chronic sacroiliac pain after a car accident and to alleviate stress-triggered eczema flares. “I’ve had doctors turn their backs on me; I’ve had nurse practitioners walk out on me in the middle of a sentence,” she said in an interview.
Ms. Fuller said her conversion to cannabinoid medicine is relatively new; she never used cannabis recreationally before her accident but now considers it a gift. She doesn’t keep aspirin in the house and refused pain medication immediately after she injured her back.
Diana Krach, a 34-year-old writer from Maryland, says she’s encountered roadblocks about her decision to use cannabinoids for endometriosis and for pain from Crohn’s disease. When she tried to discuss her CBD use with a gastroenterologist, he interrupted her: “Whatever pot you’re smoking isn’t going to work, you’re going on biologics.”
Ms. Krach had not been smoking anything but had turned to a CBD tincture for symptom relief after prescription pain medications failed to help.
Ms. Brand, Ms. Fuller, and Ms. Krach are the tip of the iceberg when it comes to women seeking symptom relief outside the medicine cabinet. A recent survey in the Journal of Women’s Health of almost 1,000 women show that 90% (most between the ages of 35 and 44) had used cannabis and would consider using it to treat gynecologic pain. Roughly 80% said they would consider using it for procedure-related pain or other conditions. Additionally, women have reported using cannabinoids for PTSD, sleep disturbances or insomnia, anxiety, and migraine headaches.
Observational survey data have likewise shown that 80% of women with advanced or recurrent gynecologic malignancies who were prescribed cannabis reported that it was equivalent or superior to other medications for relieving pain, neuropathy, nausea, insomnia, decreased appetite, and anxiety.
In another survey, almost half (45%) of women with gynecologic malignancies who used nonprescribed cannabis for the same symptoms reported that they had reduced their use of prescription narcotics after initiating use of cannabis.
The gray zone
There has been a surge in self-reported cannabis use among pregnant women in particular. The National Survey on Drug Use and Health findings for the periods 2002-2003 and 2016-2017 highlight increases in adjusted prevalence rates from 3.4% to 7% in past-month use among pregnant women overall and from 5.7% to 12.1% during the first trimester alone.
“The more that you talk to pregnant women, the more that you realize that a lot are using cannabinoids for something that is basically medicinal, for sleep, for anxiety, or for nausea,” Katrina Mark, MD, an ob.gyn. and associate professor of medicine at the University of Maryland, College Park, said in an interview. “I’m not saying it’s fine to use drugs in pregnancy, but it is a grayer conversation than a lot of colleagues want to believe. Telling women to quit seems foolish since the alternative is to be anxious, don’t sleep, don’t eat, or use a medication that also has risks to it.”
One observational study shows that pregnant women themselves are conflicted. Although the majority believe that cannabis is “natural” and “safe,” compared with prescription drugs, they aren’t entirely in the dark about potential risks. They often express frustration with practitioners’ responses when these topics are broached during office visits. An observational survey among women and practitioners published in 2020 highlights that only half of doctors openly discouraged perinatal cannabis use and that others opted out of the discussion entirely.
This is the experience of many of the women that this news organization spoke with. Ms. Krach pointed out that “there’s a big deficit in listening; the doctor is supposed to be working for our behalf, especially when it comes to reproductive health.”
Dr. Mark believed that a lot of the conversation has been clouded by the illegality of the substance but that cannabinoids deserve as much of a fair chance for discussion and consideration as other medicines, which also carry risks in pregnancy. “There’s literally no evidence that it will work in pregnancy [for these symptoms], but there’s no evidence that it doesn’t, either,” she said in an interview. “When I have this conversation with colleagues who do not share my views, I try to encourage them to look at the actual risks versus the benefits versus the alternatives.”
The ‘entourage effect’
Data supporting cannabinoids have been mostly laboratory based, case based, or observational. However, several well-designed (albeit small) trials have demonstrated efficacy for chronic pain conditions, including neuropathic and headache pain, as well as in Crohn’s disease. Most investigators have concluded that dosage is important and that there is a synergistic interaction between compounds (known as the “entourage effect”) that relates to cannabinoid efficacy or lack thereof, as well as possible adverse effects.
In addition to legality issues, the entourage effect is one of the most important factors related to the medical use of cannabinoids. “There are literally thousands of cultivars of cannabis, each with their own phytocannabinoid and terpenic profiles that may produce distinct therapeutic effects, [so] it is misguided to speak of cannabis in monolithic terms. It is like making broad claims about soup,” wrote coauthor Samoon Ahmad, MD, in Medical Marijuana: A Clinical Handbook.
Additionally, the role that reproductive hormones play is not entirely understood. Reproductive-aged women appear to be more susceptible to a “telescoping” (gender-related progression to dependence) effect in comparison with men. Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, said in an interview. She explained that research has shown that factors such as the degree of exposure, frequency of use, and menses confound this susceptibility.
It’s the data
Frustration over cannabinoid therapeutics abound, especially when it comes to data, legal issues, and lack of training. “The feedback that I hear from providers is that there isn’t enough information; we just don’t know enough about it,” Dr. Mark said, “but there is information that we do have, and ignoring it is not beneficial.”
Dr. Cooper concurred. Although she readily acknowledges that data from randomized, placebo-controlled trials are mostly lacking, she says, “There are signals in the literature providing evidence for the utility of cannabis and cannabinoids for pain and some other effects.”
Other practitioners said in an interview that some patients admit to using cannabinoids but that they lack the ample information to guide these patients. By and large, many women equate “natural” with “safe,” and some will experiment on their own to see what works.
Those experiments are not without risk, which is why “it’s just as important for physicians to talk to their patients about cannabis use as it is for patients to be forthcoming about that use,” said Dr. Cooper. “It could have implications on their overall health as well as interactions with other drugs that they’re using.”
That balance from a clinical perspective on cannabis is crucial, wrote coauthor Kenneth Hill, MD, in Medical Marijuana: A Clinical Handbook. “Without it,” he wrote, “the window of opportunity for a patient to accept treatment that she needs may not be open very long.”
A version of this article first appeared on Medscape.com.
Cesarean myomectomy: Safe operation or surgical folly?
Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.
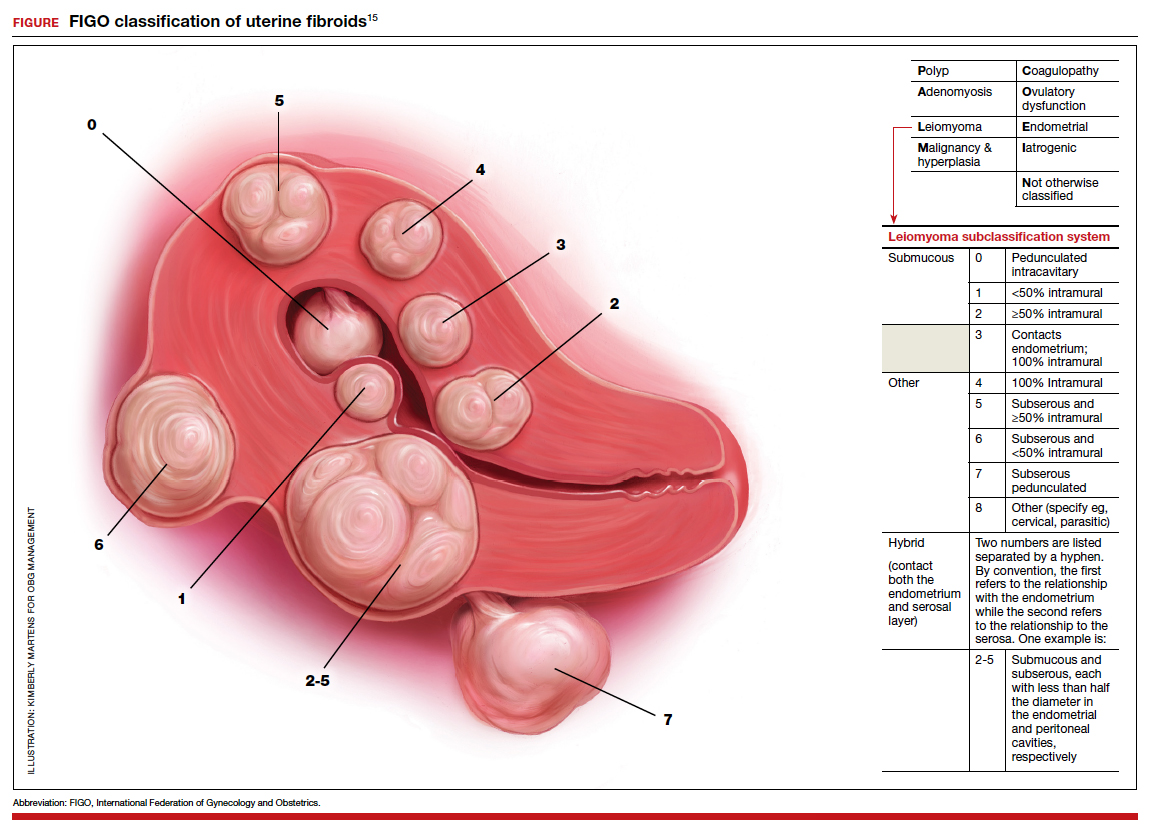
The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
A case of BV during pregnancy: Best management approach
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.
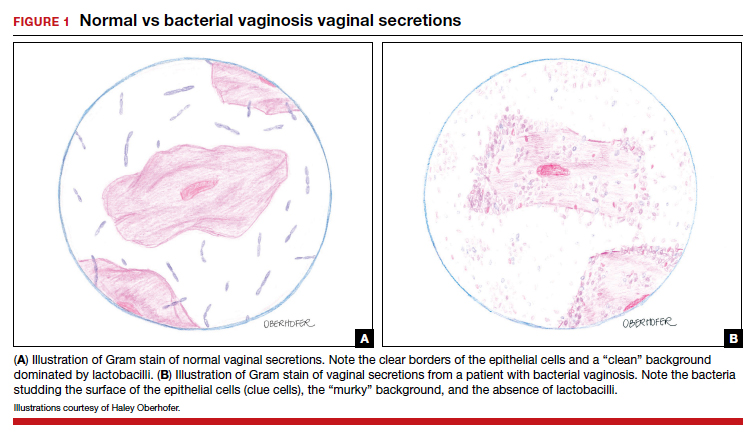
BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.
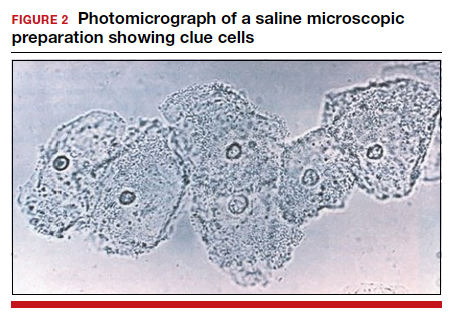
For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.
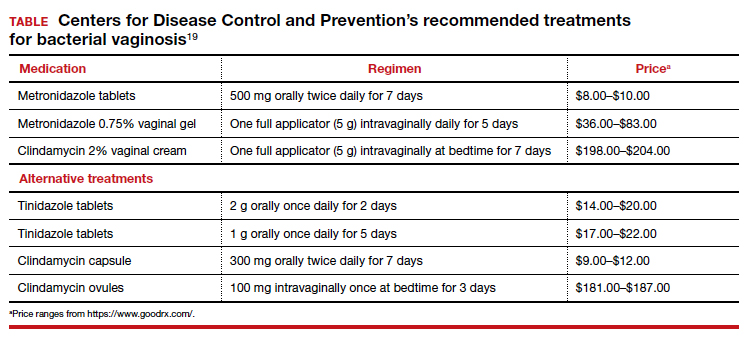
Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
Racism and gynecologic surgery: A time to act
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).
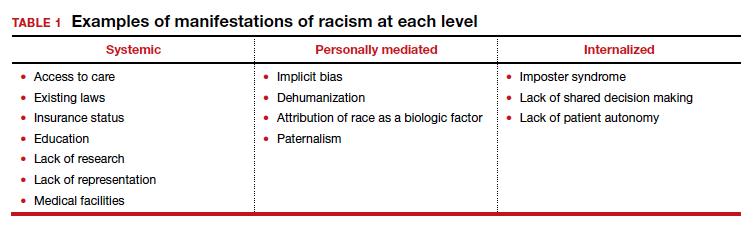
Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Product update: Breast biopsy system, tamponade mini-sponge, ovulation prediction device and app
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
The current and future state of uterus transplantation
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.
Since the first baby was born after a uterus transplantation in Sweden in 2014, uterus transplantation has been rapidly transitioning toward clinical reality.1 Several teams in the United States and multiple teams worldwide have performed the procedure, with the total number of worldwide surgeries performed nearing 100.
Uterus transplantation is the first and only true treatment for women with absolute uterine factor infertility – estimated to affect 1 in 500 women – and is filling an unmet need for this population of women. Women who have sought participation in uterus transplantation research have had complex and meaningful reasons and motivations for doing so.2 Combined with an accumulation of successful pregnancies, this makes continued research and technical improvement a worthy endeavor.
Most of the births thus far have occurred through the living-donor model; the initial Swedish trial involved nine women, seven of whom completed the procedure with viable transplants from living donors, and gave birth to eight healthy children. (Two required hysterectomy prior to attempted embryo transfer.3)
The Cleveland Clinic opted to build its first – and still ongoing – trial focusing on deceased-donor uterus transplants on the premise that such an approach obviates any risk to the donor and presents the fewest ethical challenges at the current time. Of eight uterus transplants performed thus far at the Cleveland Clinic, there have been three live births and two graft failures. As of early 2021, there was one ongoing pregnancy and two patients in preparation for embryo transfer.
Thus far, neither the living- nor deceased-donor model of uterus transplantation has been demonstrated to be superior. However, as data accrues from deceased donor studies, we will be able to more directly compare outcomes.
In the meantime, alongside a rapid ascent of clinical landmarks – the first live birth in the United States from living-donor uterus transplantation in 2017 at Baylor University Medical Center in Houston,4 for instance, and the first live birth in the United States from deceased-donor uterus transplantation in 2019 at the Cleveland Clinic – there have been significant improvements in surgical retrieval of the uterus and in the optimization of graft performance.5
Most notably, the utero-ovarian vein has been used successfully in living donors to achieve venous drainage of the graft. This has lessened the risks of deep pelvic dissection in the living donor and made the transition to laparoscopic and robotic approaches in the living donor much easier.
Donor procurement, venous drainage
Adequate circulatory inflow and outflow for the transplanted uterus are essential both for the prevention of ischemia and thrombosis, which have been major causes of graft failure, and for meeting the increased demands of blood flow during pregnancy. Of the two, the outflow is the more challenging component.
Venous drainage traditionally has been accomplished through the use of the uterine veins, which drain into the internal iliac veins; often the vascular graft will include a portion of the internal iliac vessel which can be connected via anastomoses to the external iliac vein classically in deceased donors. Typically, the gynecologic surgeon on the team performs the vaginal anastomosis and suspension of the uterus, while the transplant surgeons perform the venous and arterial anastomoses.
In the living-donor model, procurement and dissection of these often unpredictable and tortuous complexes in the deep pelvis – particularly the branching uterine veins that lie in close proximity to the ureter, bladder, other blood vessels, and rectum – can be risky. The anatomic variants in the uterine vein are numerous, and even in one patient, a comprehensive dissection on one side cannot be expected to be mirrored on the contralateral side.
In addition to the risk of injury to the donor, the anastomosis may be unsuccessful as the veins are thinly walled and challenging to suture. As such, multiple modifications have been developed, often adapted to the donor’s anatomy and the caliber and accessibility of vessels. Preoperative vascular imaging with CT and/or MRI may help to identify suitable candidates and also may facilitate presurgical planning of which vessels may be selected for use.
Recently, surgeons performing living-donor transplantations have successfully used the more accessible and less risky ovarian and/or utero-ovarian veins for venous anastomosis. In 2019, for instance, a team in Pune, India, reported laparoscopically dissecting the donor ovarian veins and a portion of the internal iliac artery, and completing anastomosis with bilateral donor internal iliac arteries to recipient internal iliac arteries, and bilateral donor ovarian veins to recipient external iliac veins.6 It is significant that these smaller-caliber vessels were found to able to support the uterus through pregnancy.
We must be cautious, however, to avoid removing donors’ ovaries. Oophorectomy for women in their 40s can result in significant long-term medical sequelae. Surgeons at Baylor have achieved at least one live birth after harvesting the donor’s utero-ovarian veins while conserving the ovaries – a significant advancement for the living-donor model.4
There is tremendous interest in developing minimally invasive approaches to further reduce living-donor risk. The Swedish team has completed a series of eight robotic hysterectomies in living-donor uterus transplantations as part of a second trial. Addressing the reality of a learning curve, their study was designed around a step-wise approach, mastering initial steps first – e.g., dissections of the uterovaginal fossa, arteries, and ureters – and ultimately converting to laparotomy.7 In the United States, Baylor University has now completed at least five completely robotic living-donor hysterectomies with complete vaginal extraction.
Published data on robotic surgery suggests that surgical access and perioperative visualization of the vessels may be improved. And as minimally invasive approaches are adopted and improved, the length of donor surgery – 10-13 hours of operating room time in the original Swedish series – should diminish, as should the morbidity associated with laparotomy.
Surgical acquisition of a uterine graft from a deceased donor diminishes concerns for injury to nearby structures. Therefore, although it is a technically similar procedure, a deceased-donor model allows more flexibility with the length, caliber, and number of vessels that can be used for anastomosis. The internal iliac vessels and even portions of the external iliac vessels and ovarian vessels can be used to allow maximum flexibility.8
Surgical technique for uterus recipients
For the recipient surgery, entry is achieved via a midline, vertical laparotomy. The external iliac vessels are exposed, and the sites of vascular anastomoses are identified. The peritoneal reflection of the bladder is identified and dissected away to expose the anterior vagina, and the vagina is opened to a diameter that matches the donor, typically using a monopolar electrosurgical cutting instrument.
The vault of the donor vagina will be attached to the recipient’s existing vagina or vaginal pouch. It is important to identify recipient vaginal mucosa and incorporate it into the vaginal anastomosis to reduce the risk of vaginal stricture. We recommend that the vaginal mucosa be tagged with PDS II sutures or grasped with allis clamps to prevent retraction.
Surgical teams have taken multiple approaches to vaginal anastomosis. The Cleveland Clinic has used both a running suture as well as a horizontal mattress stitch for closure. For the latter, a 30-inch double-armed 2.0 Vicryl allows for complete suturing of the recipient vagina – with eight stitches placed circumferentially – before the uterus is placed. Both ends of the suture are passed intra-abdominal to intravaginal in the recipient.9
Once the donor uterus is suspended, attention focuses on vascular anastomosis, with bilateral end-to-side anastomosis between the donor anterior division of the internal iliac arteries and the external iliac vessels of the recipient, and with venous drainage commonly achieved through the uterine veins draining into the internal or external iliac vein of the recipient. As mentioned, recent cases involving living donors have also demonstrated success with the use of ovarian and/or utero-ovarian veins. Care should be taken to avoid having tension or twisting across the anastomosis.
After adequate graft perfusion is confirmed, with the uterus turning from a dusky color to a pink and well-perfused organ, the vaginal anastomosis is completed, with the arms of the double-armed suture passed through the donor vagina, from intravaginal to intra-abdominal. Tension should be evenly spread along the recipient and donor vagina in order to reduce the formation of granulation tissue and the severity of future vaginal stricturing.
For uterine fixation, polypropylene sutures are placed between the graft uterosacral ligaments and recipient uterine rudiments, and between the graft round ligaments and the recipient pelvic side wall at the level of the deep inguinal ring.
Current uterus transplantation protocols require removal of the uterus after one or two live births are achieved, so that recipients will not be exposed to long-term immunosuppression.
Complications and controversies
Postoperative vaginal strictures can make embryo transfer difficult and are a common complication in both living- and deceased-donor models. The Cleveland Clinic team has applied techniques from vaginal reconstructive surgery to try to reduce the occurrence of postoperative strictures – mainly increasing attention paid to anastomosis tissue–site preparation and closure of the anastomosis using a tension-free interrupted suture technique, as described above.9 The jury is out on whether such changes are sufficient, and a more complete understanding of the causes of vaginal stricture is needed.
Other perioperative complications include infection and graft thrombosis, both of which typically result in urgent graft hysterectomy. During pregnancy, one of our patients experienced abnormal placentation, though this was not thought to be related to uterus transplantation.5
The U.S. Uterus Transplant Consortium (USUTC) is a group of active programs that are sharing ideas and outcomes and advocating for continued research in this rapidly developing field. Uterine transplants require collaboration with transplant surgery, transplant medicine, infectious disease, gynecologic surgery, high-risk obstetrics, and other specialties. While significant progress has been made in a short period of time, uterine transplantation is still in its early stages, and transplants should be done in institutions that have the capacity for mentorship, bioethical oversight, and long-term follow-up of donors, recipients, and offspring.
The USUTC has recently proposed guidelines for nomenclature related to operative technique, vascular anatomy, and uterine transplantation outcomes.10 It proposes standardizing the names for the four veins originating from the uterus (to eliminate current inconsistency), which will be important as optimal strategies for vascular anastomoses are discussed and determined.
In addition, the consortium is creating a registry for the rigorous collection of data on procedures and outcomes (from menstruation and pregnancy through delivery, graft removal, and long-term follow-up). A registry has also been proposed by the International Society for Uterine Transplantation.
A major question remains in our field: Is the living-donor or deceased-donor uterus transplant the best approach? Knowledge of the quality of the uterus is greater preoperatively within a living-donor model, but no matter how minimally invasive the technique, the donor still assumes some risk of prolonged surgery and extensive pelvic dissection for a transplant that is not lifesaving.
On the other hand, deceased-donor transplants require additional layers of organization and coordination, and the availability of suitable deceased-donor uteri will likely not be sufficient to meet the current demand. Many of us in the field believe that the future of uterine transplantation will involve some combination of living- and deceased-donor transplants – similar to other solid organ transplant programs.
Dr. Flyckt and Dr. Richards reported that they have no relevant financial disclosures.
Correction, 2/2/21: An earlier version of this article misstated Dr. Richards' name in the photo caption.
References
1. Lancet. 2015;14:385:607-16.
2. AJOB Empir Bioeth. 2019;10(1):23-5.
3. Transplantation. 2020;104(7):1312-5.
4. Am J Transplant. 2018;18(5):1270-4.
5. Am J Obstet Gynecol. 2020;223(2):143-51.
6. J Minimally Invasive Gynecol. 2019;26:628-35.
7. Acta Obstet Gynecol Scand. 2020;99(9):1222-9.
8. Fertil Steril. 2018;110(1):183.
9. Fertil Steril. 2020 Jul 16. doi: 10.1016/j.fertnstert.2020.05.017
10 Am J Transplant. 2020;20(12):3319-25.
Uterus transplantation for absolute uterine factor infertility
Until the advent of uterus transplantation, there was no restorative procedure available to a woman presenting with an absent uterus or nonfunctioning uterus; that is, absolute uterine factor infertility (AUFI). It is estimated that 1 in 500 women of childbearing age are affected by AUFI.1,2 An absent uterus may be secondary to uterine agenesis or Mayer-Rokitansky-Küster-Hauser syndrome (MRKH), which occurs in 1 in 4,500 women.3,4 (Because women with MRKH have a normal karyotype, their children can be normal, without urogenital malformations.5)
Given the fact that roughly 240,000 hysterectomies are performed in the United States each year for women aged under 44 years, hysterectomy is the most common cause of acquired AUFI.6AUFI may also be secondary to a uterus that will not support a viable pregnancy; that is, a nonfunctional uterus. In this case, medical or surgical treatment is impossible to enable normal physiological uterine function to produce a successful pregnancy. Causal factors include Müllerian anomalies, severe intrauterine adhesions/Asherman syndrome, uterine fibroids not amendable to surgical therapy, and radiation injury not responsive to medical therapy.
Prior to uterus transplantation, parenthood could only be achieved via adoption, foster parenting, or gestational carrier. While utilizing a gestational carrier is legal in most U.S. states, most countries of western Europe as well as Brazil and Japan, to name a few, do not allow the use of gestational carriers. For some women, moreover, the desire is not only to have a baby, but to carry a child as well.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rebecca Flyckt, MD, division chief of reproductive endocrinology and infertility at University Hospitals Cleveland Medical Center and associate professor at Case Western Reserve University, Cleveland, and Elliott G. Richards, MD, director of reproductive endocrinology and infertility research at the Cleveland Clinic, to discuss the current and future state of uterus transplantation.
Dr. Flyckt and Dr. Richards have both contributed to the uterus transplantation team at the Cleveland Clinic and are founding members of the U.S. Uterus Transplant Consortium. They are well published in the field of minimally invasive gynecology and reproductive endocrinology and infertility. It is truly a pleasure to welcome them both to this edition of the Master Class in Gynecologic Surgery.
References
1. Fertil Steril. 2014 May;101(5):1228-36.
2. Acta Biomater. 2014 Dec;10(12):5034-42.
3. Hum Reprod Update. Mar-Apr 2001;7(2):161-74.
4. Obstet Gynecol Surv. 2000 Oct;55(10):644-9.
5. Fertil Steril. 1997 Feb;67(2):387-9
6. Am J Public Health. 2003 Feb;93(2):307-12.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
Until the advent of uterus transplantation, there was no restorative procedure available to a woman presenting with an absent uterus or nonfunctioning uterus; that is, absolute uterine factor infertility (AUFI). It is estimated that 1 in 500 women of childbearing age are affected by AUFI.1,2 An absent uterus may be secondary to uterine agenesis or Mayer-Rokitansky-Küster-Hauser syndrome (MRKH), which occurs in 1 in 4,500 women.3,4 (Because women with MRKH have a normal karyotype, their children can be normal, without urogenital malformations.5)
Given the fact that roughly 240,000 hysterectomies are performed in the United States each year for women aged under 44 years, hysterectomy is the most common cause of acquired AUFI.6AUFI may also be secondary to a uterus that will not support a viable pregnancy; that is, a nonfunctional uterus. In this case, medical or surgical treatment is impossible to enable normal physiological uterine function to produce a successful pregnancy. Causal factors include Müllerian anomalies, severe intrauterine adhesions/Asherman syndrome, uterine fibroids not amendable to surgical therapy, and radiation injury not responsive to medical therapy.
Prior to uterus transplantation, parenthood could only be achieved via adoption, foster parenting, or gestational carrier. While utilizing a gestational carrier is legal in most U.S. states, most countries of western Europe as well as Brazil and Japan, to name a few, do not allow the use of gestational carriers. For some women, moreover, the desire is not only to have a baby, but to carry a child as well.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rebecca Flyckt, MD, division chief of reproductive endocrinology and infertility at University Hospitals Cleveland Medical Center and associate professor at Case Western Reserve University, Cleveland, and Elliott G. Richards, MD, director of reproductive endocrinology and infertility research at the Cleveland Clinic, to discuss the current and future state of uterus transplantation.
Dr. Flyckt and Dr. Richards have both contributed to the uterus transplantation team at the Cleveland Clinic and are founding members of the U.S. Uterus Transplant Consortium. They are well published in the field of minimally invasive gynecology and reproductive endocrinology and infertility. It is truly a pleasure to welcome them both to this edition of the Master Class in Gynecologic Surgery.
References
1. Fertil Steril. 2014 May;101(5):1228-36.
2. Acta Biomater. 2014 Dec;10(12):5034-42.
3. Hum Reprod Update. Mar-Apr 2001;7(2):161-74.
4. Obstet Gynecol Surv. 2000 Oct;55(10):644-9.
5. Fertil Steril. 1997 Feb;67(2):387-9
6. Am J Public Health. 2003 Feb;93(2):307-12.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
Until the advent of uterus transplantation, there was no restorative procedure available to a woman presenting with an absent uterus or nonfunctioning uterus; that is, absolute uterine factor infertility (AUFI). It is estimated that 1 in 500 women of childbearing age are affected by AUFI.1,2 An absent uterus may be secondary to uterine agenesis or Mayer-Rokitansky-Küster-Hauser syndrome (MRKH), which occurs in 1 in 4,500 women.3,4 (Because women with MRKH have a normal karyotype, their children can be normal, without urogenital malformations.5)
Given the fact that roughly 240,000 hysterectomies are performed in the United States each year for women aged under 44 years, hysterectomy is the most common cause of acquired AUFI.6AUFI may also be secondary to a uterus that will not support a viable pregnancy; that is, a nonfunctional uterus. In this case, medical or surgical treatment is impossible to enable normal physiological uterine function to produce a successful pregnancy. Causal factors include Müllerian anomalies, severe intrauterine adhesions/Asherman syndrome, uterine fibroids not amendable to surgical therapy, and radiation injury not responsive to medical therapy.
Prior to uterus transplantation, parenthood could only be achieved via adoption, foster parenting, or gestational carrier. While utilizing a gestational carrier is legal in most U.S. states, most countries of western Europe as well as Brazil and Japan, to name a few, do not allow the use of gestational carriers. For some women, moreover, the desire is not only to have a baby, but to carry a child as well.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rebecca Flyckt, MD, division chief of reproductive endocrinology and infertility at University Hospitals Cleveland Medical Center and associate professor at Case Western Reserve University, Cleveland, and Elliott G. Richards, MD, director of reproductive endocrinology and infertility research at the Cleveland Clinic, to discuss the current and future state of uterus transplantation.
Dr. Flyckt and Dr. Richards have both contributed to the uterus transplantation team at the Cleveland Clinic and are founding members of the U.S. Uterus Transplant Consortium. They are well published in the field of minimally invasive gynecology and reproductive endocrinology and infertility. It is truly a pleasure to welcome them both to this edition of the Master Class in Gynecologic Surgery.
References
1. Fertil Steril. 2014 May;101(5):1228-36.
2. Acta Biomater. 2014 Dec;10(12):5034-42.
3. Hum Reprod Update. Mar-Apr 2001;7(2):161-74.
4. Obstet Gynecol Surv. 2000 Oct;55(10):644-9.
5. Fertil Steril. 1997 Feb;67(2):387-9
6. Am J Public Health. 2003 Feb;93(2):307-12.
Dr. Miller is professor of obstetrics & gynecology in the department of clinical sciences, Rosalind Franklin University, North Chicago, and director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
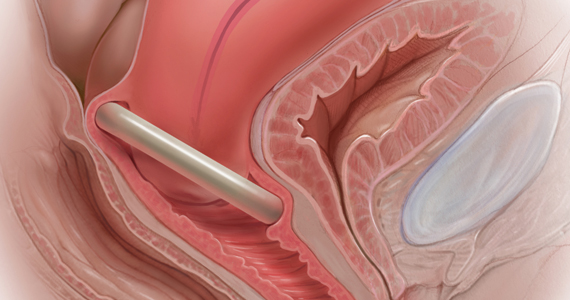
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13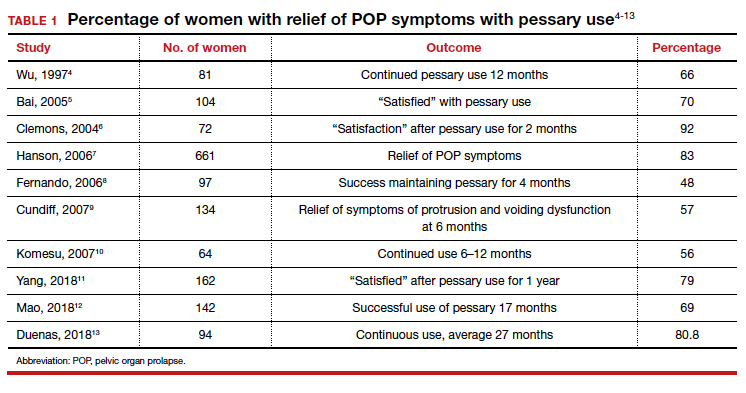
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17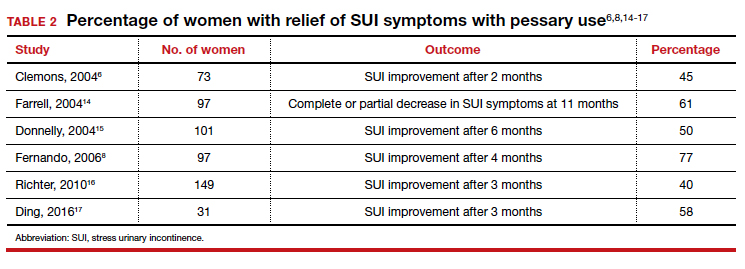
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
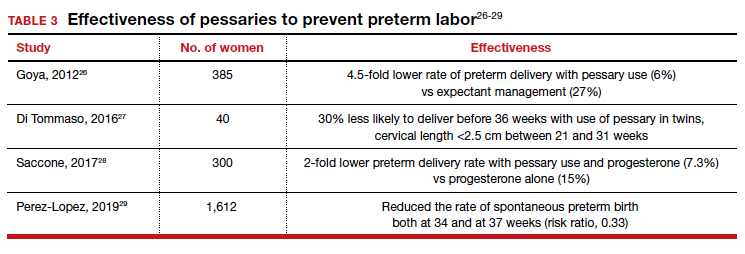
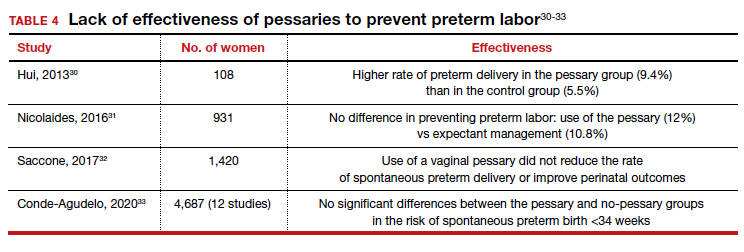
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
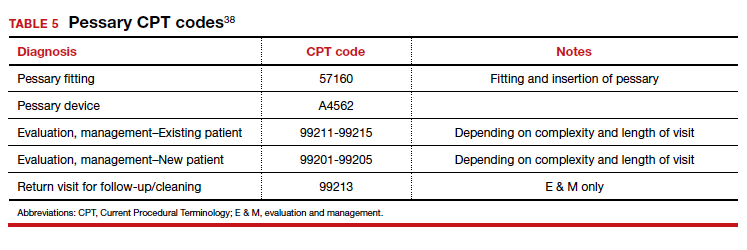
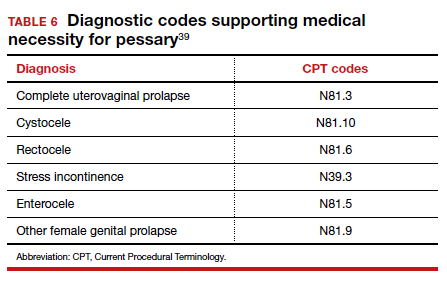
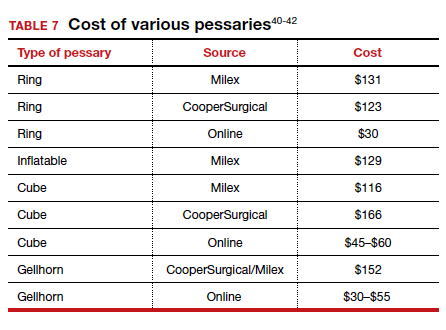
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.