User login
ID Blog: Wuhan coronavirus – just a stop on the zoonotic highway
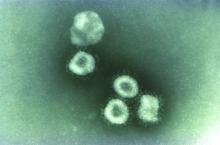
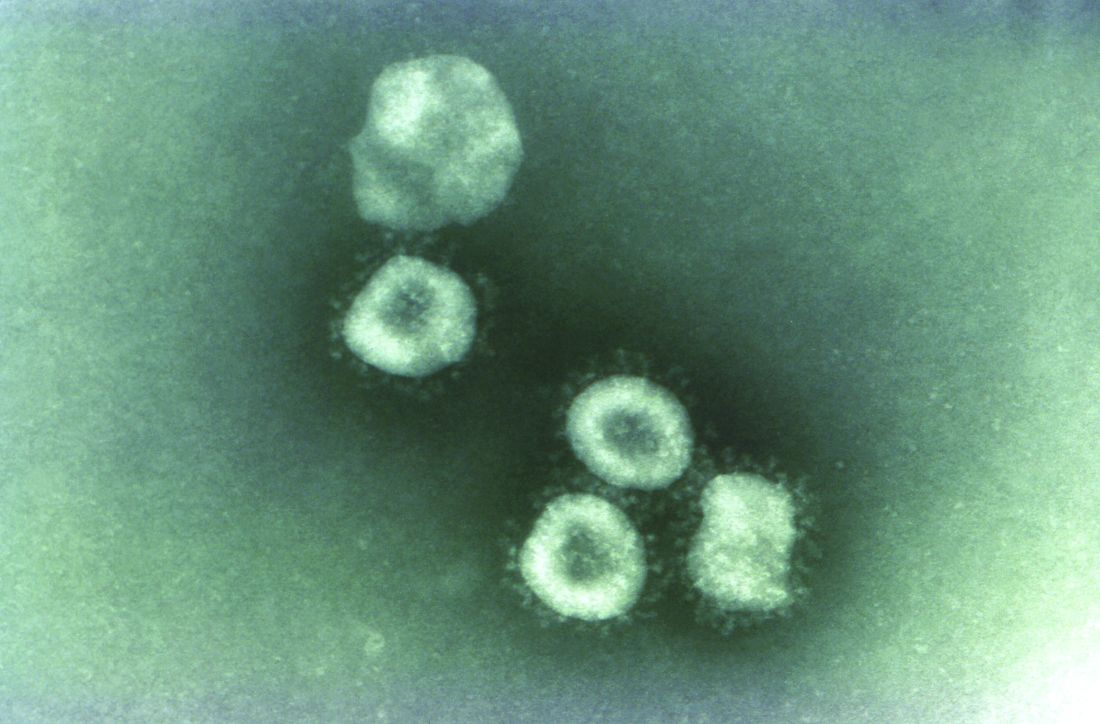
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
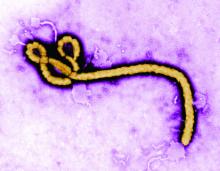
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Wuhan coronavirus cluster suggests human-to-human spread
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Echoes of SARS mark 2019 novel coronavirus outbreak
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
FROM THE LANCET
CDC: Five confirmed 2019-nCoV cases in the U.S.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Wuhan virus: What clinicians need to know
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
Mystery pneumonia in China has health officials on alert
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Without action, every child will be affected by climate change
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
A sepsis death linked to fecal microbiota transplantation
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two cases of bacteremia – one fatal – have been linked to a fecal microbiota transplant.
Major finding: Two patients developed bacteremia after receiving a fecal microbiota transplant from the same donor.
Study details: Case studies.
Disclosures: The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two authors were attached to a diagnostics company involved in the study.
Source: DeFillip Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.