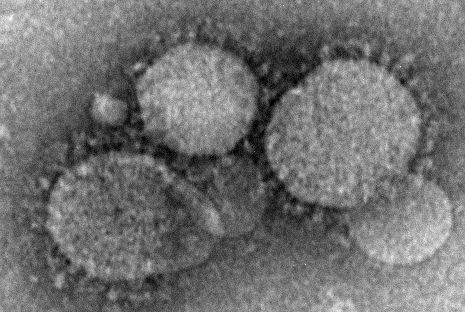User login
Anthony Fauci faces the ‘perpetual challenge’ of emerging infections
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
Chikungunya arthritis symptoms reduced with immunomodulatory drugs
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
The studies by Dr. Miner and his colleagues and Dr. Teo and his associates demonstrate the potential value of combination therapies for ameliorating heightened T-cell responses and their pathogenic role in joint inflammation. They explored how T-cell responses could be blunted during ongoing viral replication to control overt inflammation, an approach that also may be valuable for treating immune-mediated tissue damage associated with other infectious agents.
Selective T-cell immunomodulatory therapies that offset damaging immune responses offer an attractive option for future pharmacologic interventions for treating chikungunya virus–induced inflammatory disease. The small market size and the rapid sporadic nature of outbreaks could be major obstacles to the development and deployment of virus-specific interventions such as therapeutic antiviral neutralizing monoclonal antibodies or even vaccines. Targeted drug and immunotherapy treatments are likely to offer practical and beneficial options for most patients with chikungunya.
Preliminary reports in humans have suggested that methotrexate may be effective for treating chikungunya virus–induced arthritis. In Dr. Miner and colleagues’ study, a low dose of methotrexate (0.3 mg/kg) was ineffective at treating acute joint swelling in mice. It remains to be addressed whether a higher dose of methotrexate for a longer time period could be of benefit in the setting of chronic chikungunya virus–induced arthritis.
Philippe Gasque, MD, PhD, is with the Université de La Réunion, Saint-Denis, Réunion, and Marie Christine Jaffar-Bandjee, MD, PhD, is with the Centre Hospitalier Universitaire Félix Guyon, Saint-Denis, Réunion. They made these remarks in an editorial (Sci Transl Med. 2017;9:eaam6567).
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
Several different currently approved immunomodulatory therapies ameliorated arthritis symptoms in chikungunya-infected mice in two studies that separate teams of researchers published online Feb. 1 in Science Translational Medicine.
The first team, led by Jonathan J. Miner, MD, PhD, of Washington University in St. Louis tested six different approved oral and biologic antirheumatic agents (along with control agents) in chikungunya virus-infected mice with acute arthritis and foot swelling (Sci Transl Med. 2017;9:eaah3438).
When the researchers paired abatacept with an antiviral therapy (monoclonal anti-CHIKV human antibody) the combination “was highly effective at reducing joint inflammation, periarticular swelling, migration of inflammatory leukocytes, and infection, even when administered several days after virus inoculation,” Dr. Miner and his colleagues wrote.
The researchers concluded that a combination of anti-inflammatory and antibody-based antiviral therapy “may serve as a model for treating humans with arthritis caused by CHIKV or other related viruses.”
In the second study, researchers led by Teck-Hui Teo, PhD, of the Agency for Science, Technology and Research (A*STAR) in Singapore, further elucidated the mechanisms by which CHIKV proteins act on T cells (Sci Transl Med. 2017;9:eaal1333). They also found that CHIKV-infected mice treated with fingolimod (Gilenya), a drug that blocks T-cell migration from the lymph nodes to the joints and is approved for the treatment of multiple sclerosis, saw reduced arthritis symptoms even without reduction of viral replication.
Infection with the chikungunya virus can produce arthritis that mimics symptoms of rheumatoid arthritis and may in some cases lead to joint damage. Though the mechanisms driving chikungunya-related arthritis are not well understood, preliminary studies have suggested a T-cell–mediated adverse response.
The Singapore team received funding from its own agency, A*STAR, while the Washington University researchers received grants from the National Institutes of Health and the Rheumatology Research Foundation. Two coauthors on the U.S. study reported extensive commercial conflicts, including consulting and advisory relationships with pharmaceutical and vaccine manufacturers, and one patent.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: Abatacept, tofacitinib, and fingolimod all reduced arthritis symptoms, compared with controls.
Data source: Two studies testing multiple immunomodulatory or antirheumatic agents in chikungunya virus–infected mice.
Disclosures: The Agency for Science, Technology and Research (A*STAR) funded the Singapore researchers, while grants from the NIH and the Rheumatology Research Foundation funded the U.S. team. Two coauthors on the U.S. study disclosed extensive financial relationships with multiple pharmaceutical and vaccine manufacturers.
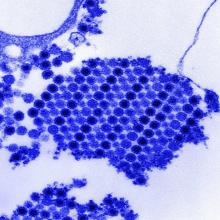
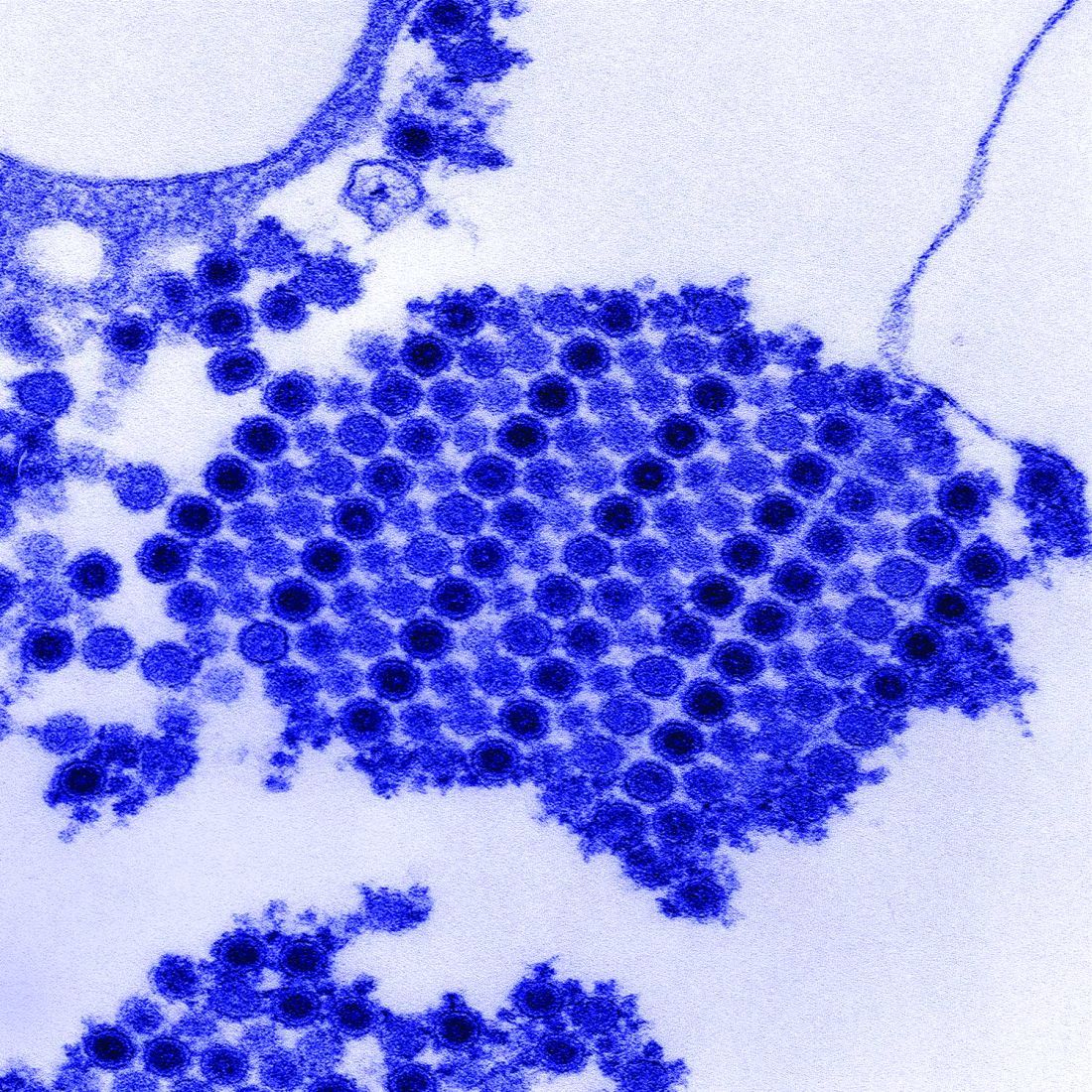
West Nile virus accounted for 95% of domestic arboviral disease in 2015
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
West Nile virus was the most common cause of domestically acquired arboviral disease in the United States in 2015, according to a report from the Centers for Disease Control and Prevention.
A total of 2,282 cases of arboviral disease were reported to the CDC in 2015. Of those, 2,175 cases were caused by the West Nile virus. Of the patients with WNV, 1,616 were hospitalized because of the disease, and 146 died. Neuroinvasive WNV, which occurred in 1,455 cases, accounted for 1,382 of 1,616 WNV hospitalizations and 142 of 146 deaths.
Of the 107 non-WNV arbovirus cases reported to the CDC, 55 were La Crosse virus, 23 were St. Louis encephalitis, 11 were Jamestown Canyon virus, 7 were Powassan virus, and 6 were eastern equine encephalitis. In addition to La Crosse and Jamestown Canyon, 4 cases of additional California serogroup viruses were reported, as was 1 case of Cache Valley virus.
“Health care providers should consider arboviral infections in the differential diagnosis of cases of aseptic meningitis and encephalitis, obtain appropriate specimens for laboratory testing, and promptly report cases to public health authorities. Because human vaccines against domestic arboviruses are not available, prevention depends on community and household efforts to reduce vector populations, personal protective measures to decrease exposure to mosquitoes and ticks, and screening of blood donors,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6602a3).
FROM MORBIDITY AND MORTALITY REPORT
Replicative Zika RNA found in brain and placental tissue
U.S.-based researchers have isolated replicative Zika virus RNA from the brain tissue of infants with microcephaly, and the placenta and fetal tissues from women suspected of being infected with Zika virus during pregnancy.
In a paper published online in Emerging Infectious Diseases, Julu Bhatnagar, PhD, of the Infectious Diseases Pathology Branch at the Center for Emerging and Zoonotic Infectious Diseases in Atlanta, and her coauthors reported a case series in 52 patients – 8 infants with microcephaly who died and 44 women thought to have been infected with Zika virus during pregnancy – using Zika virus reverse transcription PCR and in situ hybridization assay to detect the virus.
“Nevertheless, localization of replicating Zika virus RNA directly in the tissues of patients with congenital and pregnancy-associated infections is critical for identifying cellular targets of Zika virus infection and virus persistence in various tissues and for further investigating the mechanism of Zika virus intrauterine transmission,” Dr. Bhatnagar said.
Using RT-PCR, the researchers were able to isolate Zika virus RNA from 32 (62%) of the case-patients – all 8 infants with microcephaly who died, and 24 women.
There were no major clinical differences between the women who tested positive for Zika virus with RT-PCR and those who tested negative; the most common symptoms in both groups were rash, fever, arthralgia, headache and conjunctivitis.
Among women who had an adverse pregnancy or birth outcome, 24 (75%) tested positive for Zika virus via RT-PCR, compared to 8 (36%) women with live-born healthy infants (P = .0082).
Symptom onset during the first trimester was associated with a significantly higher risk of adverse pregnancy and birth outcomes. Of the 24 women with positive RT-PCR results and adverse pregnancy outcomes, 23 had symptom onset during the first trimester, while all 8 patients with positive RT-PCR results but healthy infants had symptom onset in the third trimester (P less than .0001).
There were eight cases of infants with microcephaly who died within a few minutes to 2 months after birth, and five women who delivered live infants with microcephaly who survived.
All but one of these tested positive for Zika virus by RT-PCR, either in brain tissue or placental/fetal tissue, and all the women experienced symptom onset during the first trimester. Zika virus was not detected in other tissues from the infants.
Researchers also found that the levels of Zika virus RNA in the brain tissues of the infants who had microcephaly and died were around 1,200-fold higher than the levels observed in second or third trimester or full-term placentas.
Using in situ hybridization assays, researchers found Zika virus RNA in half of the tissues of the 32 case-patients who tested positive with RT-PCR.
“Zika virus replicative RNA, detected by using sense probe, was observed in the neural cells, neurons, and degenerating glial cells within the cerebral cortex of the brain,” the authors wrote.
Zika virus genomic and replicative RNA also was found in the placental chorionic villi – predominantly in the Hofbauer cells – in 9 (75%) of the 12 women with positive RT-PCR results who had experienced an adverse pregnancy outcome during the first or second trimester. The authors said this indicated the possibility that the Hofbauer cells may play a role in disseminating or transferring the virus to the fetal brain.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” the authors wrote. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
No conflicts of interest were declared.
[email protected]
On Twitter @idpractitioner
U.S.-based researchers have isolated replicative Zika virus RNA from the brain tissue of infants with microcephaly, and the placenta and fetal tissues from women suspected of being infected with Zika virus during pregnancy.
In a paper published online in Emerging Infectious Diseases, Julu Bhatnagar, PhD, of the Infectious Diseases Pathology Branch at the Center for Emerging and Zoonotic Infectious Diseases in Atlanta, and her coauthors reported a case series in 52 patients – 8 infants with microcephaly who died and 44 women thought to have been infected with Zika virus during pregnancy – using Zika virus reverse transcription PCR and in situ hybridization assay to detect the virus.
“Nevertheless, localization of replicating Zika virus RNA directly in the tissues of patients with congenital and pregnancy-associated infections is critical for identifying cellular targets of Zika virus infection and virus persistence in various tissues and for further investigating the mechanism of Zika virus intrauterine transmission,” Dr. Bhatnagar said.
Using RT-PCR, the researchers were able to isolate Zika virus RNA from 32 (62%) of the case-patients – all 8 infants with microcephaly who died, and 24 women.
There were no major clinical differences between the women who tested positive for Zika virus with RT-PCR and those who tested negative; the most common symptoms in both groups were rash, fever, arthralgia, headache and conjunctivitis.
Among women who had an adverse pregnancy or birth outcome, 24 (75%) tested positive for Zika virus via RT-PCR, compared to 8 (36%) women with live-born healthy infants (P = .0082).
Symptom onset during the first trimester was associated with a significantly higher risk of adverse pregnancy and birth outcomes. Of the 24 women with positive RT-PCR results and adverse pregnancy outcomes, 23 had symptom onset during the first trimester, while all 8 patients with positive RT-PCR results but healthy infants had symptom onset in the third trimester (P less than .0001).
There were eight cases of infants with microcephaly who died within a few minutes to 2 months after birth, and five women who delivered live infants with microcephaly who survived.
All but one of these tested positive for Zika virus by RT-PCR, either in brain tissue or placental/fetal tissue, and all the women experienced symptom onset during the first trimester. Zika virus was not detected in other tissues from the infants.
Researchers also found that the levels of Zika virus RNA in the brain tissues of the infants who had microcephaly and died were around 1,200-fold higher than the levels observed in second or third trimester or full-term placentas.
Using in situ hybridization assays, researchers found Zika virus RNA in half of the tissues of the 32 case-patients who tested positive with RT-PCR.
“Zika virus replicative RNA, detected by using sense probe, was observed in the neural cells, neurons, and degenerating glial cells within the cerebral cortex of the brain,” the authors wrote.
Zika virus genomic and replicative RNA also was found in the placental chorionic villi – predominantly in the Hofbauer cells – in 9 (75%) of the 12 women with positive RT-PCR results who had experienced an adverse pregnancy outcome during the first or second trimester. The authors said this indicated the possibility that the Hofbauer cells may play a role in disseminating or transferring the virus to the fetal brain.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” the authors wrote. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
No conflicts of interest were declared.
[email protected]
On Twitter @idpractitioner
U.S.-based researchers have isolated replicative Zika virus RNA from the brain tissue of infants with microcephaly, and the placenta and fetal tissues from women suspected of being infected with Zika virus during pregnancy.
In a paper published online in Emerging Infectious Diseases, Julu Bhatnagar, PhD, of the Infectious Diseases Pathology Branch at the Center for Emerging and Zoonotic Infectious Diseases in Atlanta, and her coauthors reported a case series in 52 patients – 8 infants with microcephaly who died and 44 women thought to have been infected with Zika virus during pregnancy – using Zika virus reverse transcription PCR and in situ hybridization assay to detect the virus.
“Nevertheless, localization of replicating Zika virus RNA directly in the tissues of patients with congenital and pregnancy-associated infections is critical for identifying cellular targets of Zika virus infection and virus persistence in various tissues and for further investigating the mechanism of Zika virus intrauterine transmission,” Dr. Bhatnagar said.
Using RT-PCR, the researchers were able to isolate Zika virus RNA from 32 (62%) of the case-patients – all 8 infants with microcephaly who died, and 24 women.
There were no major clinical differences between the women who tested positive for Zika virus with RT-PCR and those who tested negative; the most common symptoms in both groups were rash, fever, arthralgia, headache and conjunctivitis.
Among women who had an adverse pregnancy or birth outcome, 24 (75%) tested positive for Zika virus via RT-PCR, compared to 8 (36%) women with live-born healthy infants (P = .0082).
Symptom onset during the first trimester was associated with a significantly higher risk of adverse pregnancy and birth outcomes. Of the 24 women with positive RT-PCR results and adverse pregnancy outcomes, 23 had symptom onset during the first trimester, while all 8 patients with positive RT-PCR results but healthy infants had symptom onset in the third trimester (P less than .0001).
There were eight cases of infants with microcephaly who died within a few minutes to 2 months after birth, and five women who delivered live infants with microcephaly who survived.
All but one of these tested positive for Zika virus by RT-PCR, either in brain tissue or placental/fetal tissue, and all the women experienced symptom onset during the first trimester. Zika virus was not detected in other tissues from the infants.
Researchers also found that the levels of Zika virus RNA in the brain tissues of the infants who had microcephaly and died were around 1,200-fold higher than the levels observed in second or third trimester or full-term placentas.
Using in situ hybridization assays, researchers found Zika virus RNA in half of the tissues of the 32 case-patients who tested positive with RT-PCR.
“Zika virus replicative RNA, detected by using sense probe, was observed in the neural cells, neurons, and degenerating glial cells within the cerebral cortex of the brain,” the authors wrote.
Zika virus genomic and replicative RNA also was found in the placental chorionic villi – predominantly in the Hofbauer cells – in 9 (75%) of the 12 women with positive RT-PCR results who had experienced an adverse pregnancy outcome during the first or second trimester. The authors said this indicated the possibility that the Hofbauer cells may play a role in disseminating or transferring the virus to the fetal brain.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” the authors wrote. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
No conflicts of interest were declared.
[email protected]
On Twitter @idpractitioner
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Researchers isolated replicative Zika virus RNA from the brain tissue of infants with microcephaly, and the placental tissues of women suspected of being infected with Zika virus during pregnancy.
Major finding: Among women who had an adverse pregnancy or birth outcome, 75% tested positive for Zika virus via RT-PCR, compared to 36% of women with live-born healthy infants.
Data source: Case series of 8 infants with microcephaly who died and 44 women thought to have been infected with Zika virus during pregnancy.
Disclosures: No conflicts of interest were declared.
Big data ready to revolutionize infectious diseases research
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
An all-encompassing definition of “big data” in health care remains elusive, but most researchers and public health experts believe big data may be poised to revolutionize the field of infectious diseases research.
Big data could provide the means to finally achieve effective and timely surveillance systems, which form a “pillar of infectious disease control,” according to Shweta Bansal, PhD, of the department of biology, Georgetown University, Washington, and her associates (J Infect Dis. 2016 Nov;214[S4]:S375-9. doi: 10.1093/infdis/jiw400).
“Further, full situational awareness requires availability of multiple surveillance data streams that capture mild and severe clinical outcomes (death certificates, hospital admissions, and emergency department and outpatient visits), as well as laboratory-based information (confirmed cases, genetic sequences, and serologic findings),” Dr. Simonsen added.
But unlike marketing or meteorology, two fields that have “perfected the art of real-time acquisition and analysis of highly resolved digital data,” the field of infectious diseases research has suffered from slow and incomplete surveillance of emerging and reemerging pathogens and pandemics, Dr. Bansal said.
What has changed in recent years is that physicians and researchers now have better access to patient information. Today, electronic health records and nontraditional patient data sources such as social media and remote sensing technology provide multiple surveillance data streams, and millions of people around the world can participate as the Internet, cell phones, and computers pervade even low income countries.
Several private and federal public health agencies have already launched successful initiatives “to use electronic data and patient records in a more timely fashion to track important events,” Dr. Simonsen said. For example, the Food and Drug Administration’s Sentinel Initiative aims to augment traditional surveillance (which relies on passive case reporting by physicians) with private sector electronic health data to identify severe adverse drug events.
The Centers for Disease Control and Prevention’s BioSense platform collects electronic health records to achieve “real-time awareness and tracking of pandemic influenza or any other novel health threat.” Google tracks influenza epidemics by analyzing Internet search query data. In Germany, researchers use medical claims data to track vaccination rates. In Canada, public health analysts compile multiple sources of disease outbreak information into online computational systems and then use this information to identify and track novel outbreaks and drug resistance.
The authors of these two papers warn that while big data is promising, it must be “balanced by caution.” Privacy concerns, barriers in access to e-health systems, and ill-fitting big data models must be addressed, and continued validation against traditional surveillance systems is imperative.
The authors of both papers reported no relevant conflicts of interest.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF INFECTIOUS DISEASES
Combine qSOFA and SIRS for best sepsis score
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Of the 36% of patients who met only one of the three qSOFA criteria, 6% died in the hospital. Of those patients, two-thirds were SIRS positive, meaning that they met two or more SIRS criteria.
Data source: Two studies of almost 7,000 septic patients.
Disclosures: There was no industry funding for the work, and the investigators had no relevant financial disclosures.
Bezlotoxumab reduces CDI recurrence across antibiotic subgroups
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
NEW ORLEANS – The monoclonal antibody bezlotoxumab significantly reduces the risk of Clostridium difficile infection (CDI) recurrence in adults receiving standard of care antibiotic treatment, regardless of whether that treatment is with metronidazole, vancomycin, or fidaxomicin, according to an analysis of data from the MODIFY I and II trials.
The global, randomized, double-blind, placebo-controlled trials demonstrated that bezlotoxumab was safe and effective for preventing CDI recurrence, and the current prespecified analysis further showed that choice of standard of care antibiotic therapy did not affect the outcomes, Erik R. Dubberke, MD, of Washington University in St. Louis, reported at IDWeek, an annual scientific meeting on infectious diseases.
Consistent with the overall study results, clinical cure rates were similar with bezlotoxumab and placebo, regardless of the standard of care antibiotic received (80% vs. 80.3%, respectively, overall; 81% vs. 81.3% in the metronidazole group; 78.5% vs. 79.6% in the vancomycin group; and 86.7% vs. 76.9% in the fidaxomicin group), he said.
However, CDI recurrence rates were lower among those who received bezlotoxumab, compared with those who received placebo in all three standard of care subgroups, with 10%, 15%, and 12% fewer bezlotoxumab vs. placebo patients experiencing recurrence in the metronidazole, vancomycin, and fidaxomicin groups, respectively, and 12% fewer experiencing recurrence overall.
The primary endpoint of CDI recurrence was defined in the studies as a new episode of diarrhea (at least three unformed stools in 24 hours) and a positive stool test for toxigenic C. difficile after clinical cure of the baseline CDI episode. Clinical cure was defined as standard of care antibiotics given for 14 days or less and no diarrhea for 2 consecutive days after completing standard of care treatment.
Of note, the patients in the vancomycin group were older and sicker, and had more risk factors for CDI, he said, noting, for example, that 57% of vancomycin patients were aged at least 65 years and 33% were aged at least 75 years, vs. 46% and 26% for metronidazole, respectively, and 46% and 18% for fidaxomicin; 72% of vancomycin patients were inpatients, compared with 65% and 50% of metronidazole and fidaxomicin patients.
Further, 19% of vancomycin patients, vs. 13% and 14% of metronidazole and fidaxomicin patients met criteria for severe CDI.
The findings of the current analysis are important, because the incidence of CDI recurrence is about 25%, and the risk increases with each subsequent recurrence, Dr. Dubberke said, concluding that this novel, nonantibiotic approach to prevention of CDI recurrence using bezlotoxumab, which recently received Food and Drug Administration approval for this indication, is of benefit for reducing that risk, regardless of the antibiotic used as part of standard of care therapy for CDI.
Dr. Dubberke reported serving as an investigator, adviser and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
AT IDWEEK 2016
Key clinical point:
Major finding: 12% fewer bezlotoxumab vs. placebo patients experienced CDI recurrence.
Data source: A prespecified analysis of data from 1,554 subjects from the MODIFY I and II trials.
Disclosures: Dr. Dubberke reported serving as an investigator, adviser, and/or consultant for Merck, Rebiotix, Sanofi Pasteur, and Summit, and receiving consultant fees and/or grant/research support from these companies.
Health care workers at risk for mild MERS-CoV infections
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Health care workers directly caring for patients with Middle East respiratory syndrome coronavirus (MERS-CoV) are more highly predisposed to contracting the virus, but in a milder form than that of their patients, thus making it difficult to diagnose and treat.
In a study published in Emerging Infectious Diseases, health care professionals (HCP) from the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, were examined to determine their likelihood for getting MERS-CoV based on their proximity to patients who already had it.
“Healthcare settings are important amplifiers of transmission,” explained the investigators, led by Basem M. Alraddadi, MD. “Current MERS-CoV infection control recommendations are based on experience with other viruses rather than on a complete understanding of the epidemiology of MERS-CoV transmission.”
Dr. Alraddadi and his coinvestigators identified 363 HCP, all of whom would be placed into one of three cohorts based on the department in which they worked most extensively: the Medical Intensive Care Unit (MICU), the emergency department (ED), and the neurology unit. A total of 292 HCP were ultimately enrolled in the study: 131 in MICU, 127 in ED, and 34 in neurology. After 9 subjects were excluded because of unavailability of serum specimens, 128 MICU, 122 ED, and 33 neurology unit workers remained.
While none of the neurology unit workers contracted the virus, 15 MICU workers (11.7%) and 5 ED workers (4.1%) did, for a total of 20 out of the 250 subjects in those two cohorts (8%). Radiology technicians were the most susceptible, as 5 of 17 (29.4%) got the virus, followed by 13 of 138 nurses (9.4%), 1 of 31 respiratory therapists (3.2%), and 1 of 41 physicians (2.4%).
“HCP who reported always covering their nose and mouth with either a medical mask or N95 respirator had lower risk for infection than did HCP reporting not always or never doing so, [while] those who reported always using N95 respirators for direct patient contact were less likely to be seropositive, a trend that approached statistical significance (P = .07),” the authors noted.
The most frequent symptoms reported by those surveyed were muscle pain, fevers, headaches, dry cough, and shortness of breath. In the 20-case HCP sample, however, 12 subjects (60%) only had mild illness while 3 (15%) were asymptomatic, making it very hard to diagnose and treat their infection. Three subjects (15%) had severe illness, while another two (10%) had moderate illness, meaning they were admitted to hospital but did not require any mechanical ventilation.
“Our study did not identify strong associations with underlying chronic illnesses, most likely because the prevalence of such conditions was low ([less than] 10%) in this population, [but] HCPs with a history of smoking had a risk for infection almost 3 times that of nonsmokers,” the authors wrote (Emerg Infect Dis. 2016 Nov. doi: 10.3201/eid2211.160920).
The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. Dr. Alraddadi and his coauthors did not report any disclosures.
Key clinical point:
Major finding: Among workers who actually treated MERS-CoV patients, 20 out of 250 (8%) contracted the virus, while none of the clerical staff or patient transporters did.
Data source: Retrospective, single-center study of 363 health care personnel during May-June 2014.
Disclosures: The Ministry of Health of Saudi Arabia and the Centers for Disease Control and Prevention funded the study. The authors reported no financial disclosures.
ZMapp shows promise for Ebola treatment, but misses efficacy goal
ZMapp, a potential monoclonal antibody treatment for Ebola virus disease, did not meet its efficacy goal in a clinical trial of patients in Liberia, Sierra Leone, and Guinea, according to a report in the New England Journal of Medicine.
ZMapp, developed by Mapp Biopharmaceutical, comprises three different laboratory-generated monoclonal antibodies. The drug targets the main surface protein of the Ebola virus. Earlier studies in nonhuman primates demonstrated that ZMapp had strong antiviral activity and prevented death when administered as late as 5 days after experimental infection with the Zaire Ebola virus strain.
The process of ZMapp infusion was generally safe, with only one serious adverse event – hypertension – attributed to the infusion itself. Of the 93 infusions attempted in the ZMapp group, 8 were stopped because of adverse events, and 9 were slowed down to accommodate for side effects.
While ZMapp did not meet its goal, the relative risk of death was 40% lower in the ZMapp group then in the control group. Treatment with ZMapp was delayed for a week after patients became symptomatic, exceeding the 5-day window where ZMapp has been shown to have at least 90% effectiveness. In addition, of the eight patients who died in the ZMapp group, seven died before the second of the three planned ZMapp infusions were delivered.
The abrupt end to the Ebola epidemic prevented the ZMapp trial from being adequately completed. A goal of 100 patients in both groups was desired but could not be reached.
“Despite the concerted efforts of many dedicated researchers domestically and internationally who participated in this and other trials, the outbreak appears to have ended with no incontrovertible evidence that any single treatment intervention, or combination of interventions, was unequivocally superior to the types of supportive medical care typically provided,” the investigators said.
Find the full study in the New England Journal of Medicine (doi: 10.1056/NEJMoa1604330).
ZMapp, a potential monoclonal antibody treatment for Ebola virus disease, did not meet its efficacy goal in a clinical trial of patients in Liberia, Sierra Leone, and Guinea, according to a report in the New England Journal of Medicine.
ZMapp, developed by Mapp Biopharmaceutical, comprises three different laboratory-generated monoclonal antibodies. The drug targets the main surface protein of the Ebola virus. Earlier studies in nonhuman primates demonstrated that ZMapp had strong antiviral activity and prevented death when administered as late as 5 days after experimental infection with the Zaire Ebola virus strain.
The process of ZMapp infusion was generally safe, with only one serious adverse event – hypertension – attributed to the infusion itself. Of the 93 infusions attempted in the ZMapp group, 8 were stopped because of adverse events, and 9 were slowed down to accommodate for side effects.
While ZMapp did not meet its goal, the relative risk of death was 40% lower in the ZMapp group then in the control group. Treatment with ZMapp was delayed for a week after patients became symptomatic, exceeding the 5-day window where ZMapp has been shown to have at least 90% effectiveness. In addition, of the eight patients who died in the ZMapp group, seven died before the second of the three planned ZMapp infusions were delivered.
The abrupt end to the Ebola epidemic prevented the ZMapp trial from being adequately completed. A goal of 100 patients in both groups was desired but could not be reached.
“Despite the concerted efforts of many dedicated researchers domestically and internationally who participated in this and other trials, the outbreak appears to have ended with no incontrovertible evidence that any single treatment intervention, or combination of interventions, was unequivocally superior to the types of supportive medical care typically provided,” the investigators said.
Find the full study in the New England Journal of Medicine (doi: 10.1056/NEJMoa1604330).
ZMapp, a potential monoclonal antibody treatment for Ebola virus disease, did not meet its efficacy goal in a clinical trial of patients in Liberia, Sierra Leone, and Guinea, according to a report in the New England Journal of Medicine.
ZMapp, developed by Mapp Biopharmaceutical, comprises three different laboratory-generated monoclonal antibodies. The drug targets the main surface protein of the Ebola virus. Earlier studies in nonhuman primates demonstrated that ZMapp had strong antiviral activity and prevented death when administered as late as 5 days after experimental infection with the Zaire Ebola virus strain.
The process of ZMapp infusion was generally safe, with only one serious adverse event – hypertension – attributed to the infusion itself. Of the 93 infusions attempted in the ZMapp group, 8 were stopped because of adverse events, and 9 were slowed down to accommodate for side effects.
While ZMapp did not meet its goal, the relative risk of death was 40% lower in the ZMapp group then in the control group. Treatment with ZMapp was delayed for a week after patients became symptomatic, exceeding the 5-day window where ZMapp has been shown to have at least 90% effectiveness. In addition, of the eight patients who died in the ZMapp group, seven died before the second of the three planned ZMapp infusions were delivered.
The abrupt end to the Ebola epidemic prevented the ZMapp trial from being adequately completed. A goal of 100 patients in both groups was desired but could not be reached.
“Despite the concerted efforts of many dedicated researchers domestically and internationally who participated in this and other trials, the outbreak appears to have ended with no incontrovertible evidence that any single treatment intervention, or combination of interventions, was unequivocally superior to the types of supportive medical care typically provided,” the investigators said.
Find the full study in the New England Journal of Medicine (doi: 10.1056/NEJMoa1604330).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Further evidence links Zika, Guillain-Barré syndrome
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Key clinical point:
Major finding: 97% of subjects had Zika-like symptoms prior to onset of GBS, and 40% of GBS patients who underwent RT-PCR testing were positive for Zika virus.
Data source: Study of 68 GBS patients from six hospitals in Colombia, with 42 patients undergoing RT-PCR analysis.
Disclosures: Funding provided by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.