User login
Pregnant Woman, 39, With Hypertension and New-Onset Proteinuria
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
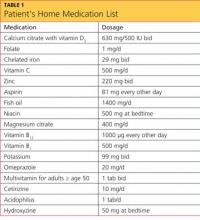
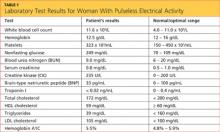
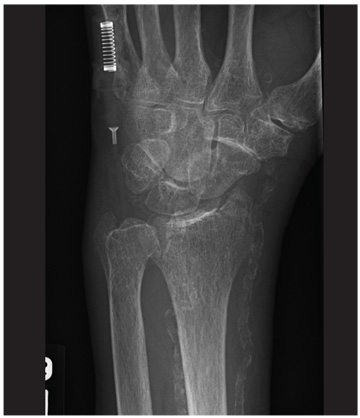
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
Chest Wall and Knee Pain Following Motor Vehicle Collision
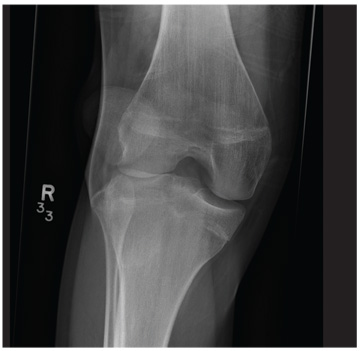
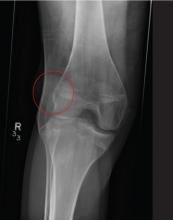
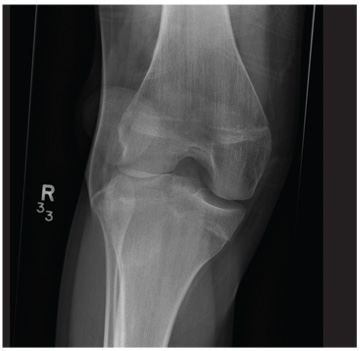
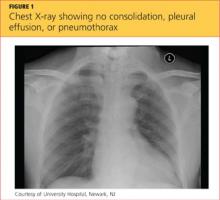
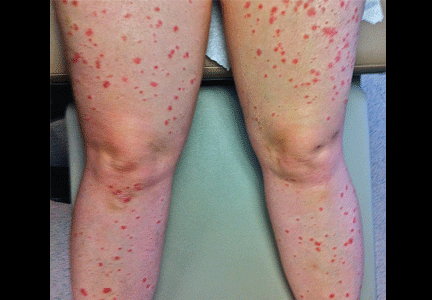
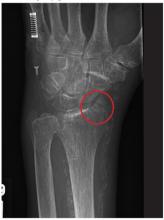
A 20-year-old man presents following a motor vehicle collision in which the car he was driving was broadsided by another vehicle. His air bag deployed, and the patient is now complaining of right-sided chest wall pain and right knee pain. His medical history is unremarkable. In a primary survey, the patient appears stable, with normal vital signs. Inspection of his right knee shows some deformity of the joint, with mild swelling and moderate tenderness. The patient is unable to perform flexion with his right knee. Good distal pulses are present, and sensation is intact. Radiograph of the right knee is obtained. What is your impression?
A short story of the short QT syndrome
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
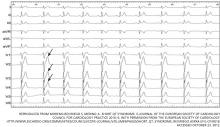
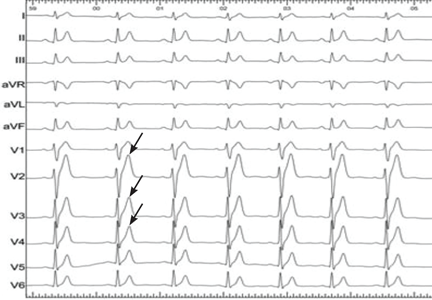
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
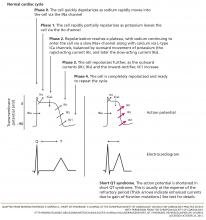
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
KEY POINTS
- Short QT syndrome is a genetic disease described initially in young patients who had atrial fibrillation or who died suddenly with no apparent structural heart disease.
- The diagnosis is established by the finding of a short QT interval. However, other factors including personal and family history are also important in establishing the diagnosis.
- The current recommendations for managing patients with short QT syndrome are not evidence-based. We encourage consultation with centers that have special interest in QT-interval-related disorders.
- Placement of an implantable cardioverter-defibrillator is considered the standard of care, especially in survivors of sudden cardiac death, ventricular fibrillation, or ventricular tachycardia. Unfortunately, a higher incidence of inappropriate shocks adds to the challenges of managing this potentially deadly disease.
Man, 56, With Wrist Pain After a Fall
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.
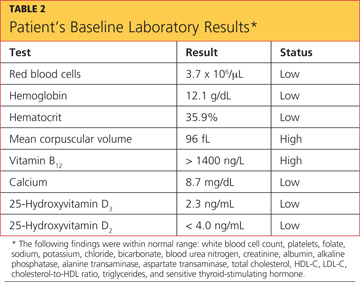
X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.
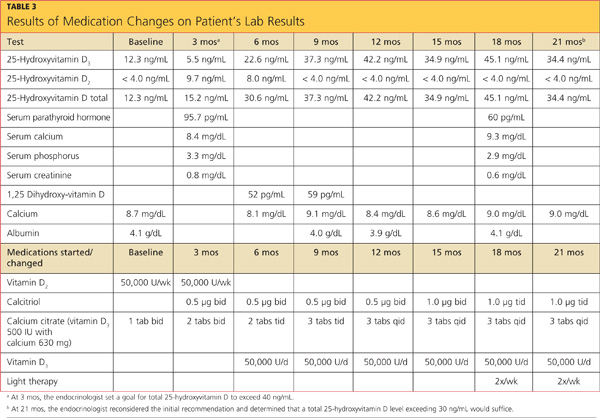
The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.

X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.

The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
A white man, age 56, presented to his primary care clinician with wrist pain and swelling. Two days earlier, he had fallen from a step stool and landed on his right wrist. He treated the pain by resting, elevating his arm, applying ice, and taking ibuprofen 800 mg tid. He said he had lost strength in his hand and arm and was experiencing numbness and tingling in his right hand and fingers.
The patient’s medical history included hypertension, type 2 diabetes mellitus, morbid obesity, obstructive sleep apnea, asthma, carpel tunnel syndrome, and peripheral neuropathy. His surgical history was significant for duodenal switch gastric bypass surgery, performed eight years earlier, and his weight at the time of presentation was 200 lb; before his gastric bypass, he weighed 385 lb. Since the surgery, his hypertension, diabetes, asthma, and sleep apnea had all resolved. Table 1 shows a list of medications he was taking at the time of presentation.
The patient, a registered nurse, had been married for 30 years and had one child. He had quit smoking 15 years earlier, with a 43–pack-year smoking history. He reported social drinking but denied any recreational drug use. He was unaware of having any allergies to food or medication.
His vital signs on presentation were blood pressure, 110/75 mm Hg; heart rate, 53 beats/min; respiration, 18 breaths/min; O2 saturation, 97% on room air; and temperature, 97.5°F.
Physical exam revealed that the patient’s right wrist was ecchymotic and swollen with +1 pitting edema. The skin was warm and dry to the touch. Decreased range of motion was noted in the right wrist, compared with the left. Pain with point tenderness was noted at the right lateral wrist. Pulses were +3 with capillary refill of less than 3 seconds. The rest of the exam was unremarkable.
The differential diagnosis included fracture secondary to the fall, osteoporosis, osteopenia, osteomalacia, Paget’s disease, tumor, infection, and sprain or strain of the wrist. A wrist x-ray was ordered, as were the following baseline labs: complete blood count with differential (CBC), vitamin B12 and folate levels, blood chemistry, lipid profile, liver profile, total vitamin D, and sensitive thyroid-stimulating hormone. Test results are shown in Table 2.

X-ray of the wrist showed fracture only, making it possible to rule out Paget’s disease (ie, no patchy white areas noted in the bone) and tumor (no masses seen) as the immediate cause of fracture. Normal body temperature and normal white blood cell count eliminated the possibility of infection.
Because the patient was only 56 and had a history of bariatric surgery, further testing was pursued to investigate a cause for the weakened bone. Bone mineral density (BMD) testing revealed the following results:
• The lumbar spine in frontal projection measured 0.968 g/cm2 with a T-score of –2.2 and a Z-score of –2.2.
• Total BMD of the left hip was 0.863 g/cm2 with a T-score of –1.7 and a Z-score of –1.4.
• Total BMD of the left femoral neck was 0.863 g/cm2 with a T-score of 1.7 and a Z-score of –1.1.
These findings suggested osteopenia1,2 (not osteoporosis) in all sites, with a 12% decrease of BMD in the spine (suggesting increased risk for spinal fracture) and a 16.3% decrease of BMD in the hip since the patient’s most recent bone scan five years earlier (radiologist’s report). Other abnormal findings were elevated parathyroid hormone (PTH) serum, 95.7 pg/mL (reference range, 10 to 65 pg/mL); low total calcium serum, 8.7 mg/dL (reference range, 8.9 to 10.2 mg/dL), and low 25-hydroxyvitamin D total, 12.3 ng/mL (reference range, 25 to 80 ng/mL).
A 2010 clinical practice guideline from the Endocrine Society3 specifies that after malabsorptive surgery, vitamin D and calcium supplementation should be adjusted by a qualified medical professional, based on serum markers and measures of bone density. An endocrinologist who was consulted at the patient’s initial visit prescribed the following medications: vitamin D2, 50,000 U/wk PO; combined calcium citrate (vitamin D3) 500 IU with calcium 630 mg, 1 tab bid; and calcitriol 0.5 μg bid.
The patient’s final diagnosis was osteomalacia secondary to gastric bypass surgery. (See “Making the Diagnosis of Osteomalacia.”4-6)

DISCUSSION
According to 2008 data from the World Health Organization (WHO),7 1.4 billion persons older than 20 worldwide were overweight, and 200 million men and 300 million women were considered obese—meaning that one in every 10 adults worldwide is overweight or obese. In 2010, the WHO reports, 40 million children younger than 5 worldwide were considered overweight.7 Health care providers need to be prepared to care for the increasing number of patients who will undergo bariatric surgeries to treat obesity and its related comorbidities.8
Postoperative follow-up for the malabsorption deficiencies related to bariatric procedures should be performed every six months, including obtaining levels of alkaline phosphatase and others previously discussed. In addition, the Endocrine Society guideline3 recommends measuring levels of vitamin B12, albumin, pre-albumin, iron, and ferritin, and obtaining a CBC, a liver profile, glucose reading, creatinine measurement, and a metabolic profile at one month and two months after surgery, then every six months until two years after surgery, then annually if findings are stable.
Furthermore, the Endocrine Society3 recommends obtaining zinc levels every six months for the first year, then annually. An annual vitamin A level is optional.9 Yearly bone density testing is recommended until the patient’s BMD is deemed stable.3
Additionally, Koch and Finelli10 recommend performing the following labs postoperatively: hemoglobin A1C every three months; copper, magnesium, whole blood thiamine, vitamin B12, and a 24-hour urinary calcium every six months for the first three years, then once a year if findings remain stable.
Use of alcohol should be discouraged among patients who have undergone bariatric surgery, as its use alters micronutrient requirements and metabolism. Alcohol consumption may also contribute to dumping syndrome (ie, rapid gastric emptying).11
Any patient with a history of malabsorptive bypass surgery who complains of neurologic, visual, or skin disorders, anemia, or edema may require a further workup to rule out other absorptive deficiencies. These include vitamins A, E, and B12, zinc, folate, thiamine, niacin, selenium, and ferritin.10
Osteomalacia
Metabolic bone diseases can result from genetics, dietary factors, medication use, surgery, or hormonal irregularities. They alter the normal biochemical reactions in bone structure.
The three most common forms of metabolic bone disease are osteoporosis, osteopenia, and osteomalacia. The WHO diagnostic classifications and associated T-scores for bone mineral density1,2 indicate a T-score above –1.0 as normal. A score between –1.0 and –2.5 is indicative of osteopenia, and a score below –2.5 indicates osteoporosis. A T-score below –2.5 in the patient with a history of fragility fracture indicates severe osteoporosis.1,2
In osteomalacia, bone volume remains unchanged, but mineralization of osteoid in the mature compact and spongy bone is either delayed or inadequate. The remolding cycle continues unchanged in the formation of osteoid, but mineral calcification and deposition do not occur.3-5
Osteomalacia is normally considered a rare disorder, but it may become more common as increasing numbers of patients undergo gastric bypass operations.12,13 Primary care practitioners should monitor for this condition in such patients before serious bone loss or other problems develop.9,13,14
Vitamin D deficiency (see “Vitamin D Metabolism,”4,15-19 below), whether or not the result of gastric bypass surgery, is a major risk factor for osteomalacia. Disorders of the small bowel, the hepatobiliary system, and the pancreas are all common causes of vitamin D deficiency. Liver disease interferes with the metabolism of vitamin D. Diseases of the pancreas may cause a deficiency of bile salts, which are vital for the intestinal absorption of vitamin D.17

Restriction and Malabsorption
The case patient had undergone a gastric bypass (duodenal switch), in which a large portion of the stomach is removed and a large part of the small bowel rerouted—with both parts of the procedure causing malabsorption.11 It is in the small bowel that absorption of vitamin D and calcium takes place.
The duodenal switch gastric bypass surgery causes both restriction and malabsorption. Though similar to a biliopancreatic diversion, the duodenal switch preserves the distal stomach and the pylorus20 by way of a sleeve gastrectomy that is performed to reduce the gastric reservoir; the common channel length after revision is 100 cm, not 50 cm (as in conventional biliopancreatic diversion).13 The sleeve gastrectomy involves removal of parietal cells, reducing production of hydrochloric acid (which is necessary to break down food), and hindering the absorption of certain nutrients, including the fat-soluble vitamins, vitamin B12, and iron.12 Patients who take H2-blockers or proton pump inhibitors experience an additional decrease in the production and availability of HCl and may have an increased risk for fracture.14,20,21
In addition to its biliopancreatic diversion component, the duodenal switch diverts a large portion of the small bowel, with food restricted from moving through it. Vitamin D and protein are normally absorbed at the jejunum and ileum, but only when bile salts are present; after a duodenal switch, bile and pancreatic enzymes are not introduced into the small intestines until 75 to 100 cm before they reach the large intestine. Thus, absorption of vitamin D, protein, calcium, and other nutrients is impaired.20,22
Since phosphorus and magnesium are also absorbed at the sites of the duodenum and jejunum, malabsorption of these nutrients may occur in a patient who has undergone a duodenal switch. Although vitamin B12 is absorbed at the site of the distal ileum, it also requires gastric acid to free it from the food. Zinc absorption, which normally occurs at the site of the jejunum, may be impaired after duodenal switch surgery, and calcium supplementation, though essential, may further reduce zinc absorption.9 Iron absorption requires HCl, facilitated by the presence of vitamin C. Use of H2-blockers and proton pump inhibitors may impair iron metabolism, resulting in anemia.20
In a randomized controlled trial, Aasheim et al23 compared the effects of Roux-en-Y gastric bypass with those of duodenal switch gastric bypass on patients’ vitamin metabolism. The researchers concluded that patients who undergo a duodenal switch are at greater risk for vitamin A and D deficiencies in the first year after surgery; and for thiamine deficiency in the months following surgery as a result of malabsorption, compared with patients who undergo Roux-en-Y gastric bypass.20,23
Patient Management
The case patient’s care necessitated consultations with endocrinology, dermatology, and gastroenterology (GI). Table 3 (below) shows the laboratory findings and the medication changes prompted by the patient’s physical exam and lab results. Table 4 lists the findings from other lab studies ordered throughout the patient’s course of treatment.

The endocrinologist was consulted at the first sign of osteopenia, and a workup was soon initiated, followed by treatment. GI was consulted six months after the beginning of treatment, when the patient began to complain of reflux while sleeping and frequent diarrhea throughout the day.
Results of esophagogastroduodenoscopy with biopsy ruled out celiac disease and mucosal ulceration, but a small hiatal hernia that was detected (< 3 cm) was determined to be an aggravating factor for the patient’s reflux. The patient was instructed in lifestyle modifications for hiatal hernia, including the need to remain upright one to two hours after eating before going to sleep to prevent aspiration. The patient was instructed to avoid taking iron and calcium within two hours of each other and to limit his alcohol intake. He was also educated in precautions against falls.
Dermatology was consulted nine months into treatment so that light therapy could be initiated, allowing the patient to take advantage of the body’s natural pathway to manufacture vitamin D3.
CONCLUSION
For post–bariatric surgery patients, primary care practitioners are in a position to coordinate care recommendations from multiple specialists, including those in nutrition, to determine the best course of action.
This case illustrates complications of bariatric surgery (malabsorption of key vitamins and minerals, wrist fracture, osteopenia, osteomalacia) that require diagnosis and treatment. The specialists and the primary care practitioner, along with the patient, had to weigh the risks and benefits of continued proton pump inhibitor use, as such medications can increase the risk for fracture. They also addressed the patient’s anemia and remained attentive to his preventive health care needs.
REFERENCES
1. Brusin JH. Update on bone densitometry. Radiol Technol. 2009;81(2):153BD-170BD.
2. Wilson CR. Essentials of bone densitometry for the medical physicist. Presented at: The American Association of Physicists in Medicine 2003 Annual Meeting; July 22-26, 2003; San Diego, CA.
3. Heber D, Greenway FL, Kaplan LM. et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(11):4825-4843.
4. Osteomalacia: step-by-step diagnostic approach (2011). http://bestpractice.bmj.com/best-practice/monograph/517/diagnosis/step-by-step.html. Accessed December 18, 2012.
5. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited : a report on 28 cases. Clin Rheumatol. 2011;30(5):639-645.
6. Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519-523.
7. World Health Organization. Obesity and overweight (May 2012). Fact Sheet No 311. www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2012.
8. Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg. 2009;75(2):103-112.
9. Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23(4):396-405.
10. Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39(1):109-124.
11. Manchester S, Roye GD. Bariatric surgery: an overview for dietetics professionals. Nutr Today. 2011;46(6):264-275.
12. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544-546.
13. Iannelli A, Schneck AS, Dahman M, et al. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23(10):2385-2389.
14. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085.
15. Holrick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98 (10):1024-1027.
16. Kalro BN. Vitamin D and the skeleton. Alt Ther Womens Health. 2009;2(4):25-32.
17. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1568-1617.
18. Huether SE. Structure and function of the renal and urologic systems. In: McCance KL, Huether SE, Brashers VL, Rote NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1344-1364.
19. Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321-331.
20. Decker GA, Swain JM, Crowell MD. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol. 2007;102(11):2571-2580.
21. Targownik LE, Lix LM, Metge C, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326.
22. Ybarra J, Sánchez-Hernández J, Pérez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am. 2007;42(1):19-27.
23. Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15-22.
Is Chest Pain Related to Prior Fracture?
ANSWER
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
ANSWER
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
ANSWER
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
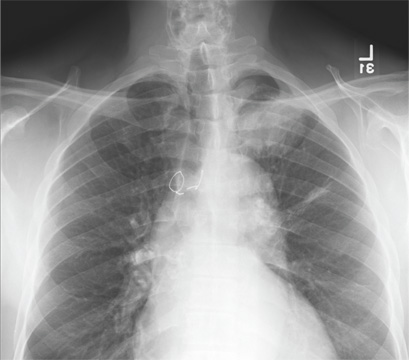
A 61-year-old man presents to your urgent care center for evaluation of “chest pain” he has been experiencing for almost four weeks. He denies any injury or trauma. He describes the pain as “sharp” and “stabbing” and says occasionally it is associated with breathing, localized primarily to the left side. There is no radiation of the pain. He denies fever, nausea, weight loss, night sweats, and hemoptysis. He has smoked a half-pack of cigarettes daily for more than 40 years. His medical history is otherwise unremarkable, except that he was told he had “high blood pressure” and he had his sternum repaired several years ago, following fracture in an accident. Vital signs are as follows: temperature, 36.4°C; blood pressure, 174/100 mm Hg; ventricular rate, 88 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 100% on room air. He appears to be in no obvious distress. Lung sounds are normal, as is the rest of the physical examination. You obtain a chest radiograph. What is your impression?
Grand Rounds: Woman, 38, With Pulseless Electrical Activity
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
Tattooing: Medical uses and problems
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
KEY POINTS
- Tattoos that state an advance directive for health care are not recognized as meeting the legal requirements for advance directives. They should only be considered as a guide to treatment decisions.
- Tattooing for medical-alert purposes is part of current culture. People with diabetes should avoid tattooing of feet or lower legs in view of impaired healing.
- Endoscopic tattooing is commonly used to aid visualization of diseased bowel segments during laparoscopic surgical procedures. Complications are rare but include mild chronic inflammation, abscesses, inflammatory pseudotumors, focal peritonitis, and peritoneal staining.
- Improper sterilization of tattooing needles can cause a wide range of infectious diseases and skin reactions.
A rash after streptococcal infection
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
Man with Consistent Headaches
ANSWER
The image shows obvious mass effect throughout the left hemisphere. On close examination, there is evidence of an isodense subdural collection within the left frontoparietal region. This is causing a left-to-right shift of almost 11 mm.
This finding is most likely a subacute subdural hematoma, probably seven to 14 days old. Further questioning reveals that the patient had fallen in the shower approximately two weeks prior and hit his head. The patient was admitted for observation and subsequently underwent a craniotomy for evacuation of the subdural hematoma.
ANSWER
The image shows obvious mass effect throughout the left hemisphere. On close examination, there is evidence of an isodense subdural collection within the left frontoparietal region. This is causing a left-to-right shift of almost 11 mm.
This finding is most likely a subacute subdural hematoma, probably seven to 14 days old. Further questioning reveals that the patient had fallen in the shower approximately two weeks prior and hit his head. The patient was admitted for observation and subsequently underwent a craniotomy for evacuation of the subdural hematoma.
ANSWER
The image shows obvious mass effect throughout the left hemisphere. On close examination, there is evidence of an isodense subdural collection within the left frontoparietal region. This is causing a left-to-right shift of almost 11 mm.
This finding is most likely a subacute subdural hematoma, probably seven to 14 days old. Further questioning reveals that the patient had fallen in the shower approximately two weeks prior and hit his head. The patient was admitted for observation and subsequently underwent a craniotomy for evacuation of the subdural hematoma.
You are called to the emergency department (ED) in reference to a patient who was sent there by radiology with a reported “brain mass” noted on imaging. During further investigation in the ED, the patient, who is in his 50s, stated that he has had headaches for the past several weeks; he consulted his primary care provider, who ordered outpatient MRI of the brain—the test that ultimately led to his arrival in the ED. Since the MRI results were not immediately available for review, the ED staff obtained noncontrast CT of the head. The ED provider notes that it “looks like there is something there causing significant mass effect and shift.” When you arrive to see the patient, you note that he is awake, alert, and oriented times three. His Glasgow Coma Scale score is 15, and his vitals signs are normal. He states he has a mild headache, rating it a 3 out of 10 on a pain scale. His only other complaint is mild right-sided weakness, which he has noticed in the past week or so. Clinically, the strength in his right upper and lower extremities is good. His medical history is significant for prostate cancer and hypertension. A single cut from the CT of his head is shown. What is your impression?
Wrist Pain After a Fall
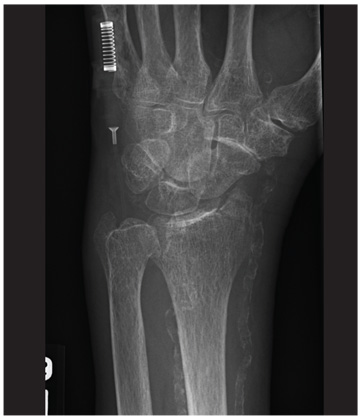
A 90-year-old man is brought to your facility for evaluation after a fall. The patient states he was out in his yard, near his garden, when he “just passed out.” He landed in an ant bed and was eventually found by a neighbor, who brought him for evaluation. The patient says he has felt weak for the past several days. He has no other constitutional complaints. He is also experiencing bilateral wrist pain, he presumes as a result of receiving multiple ant bites. His medical history is significant for diabetes. His vital signs are normal. Inspection of both wrists demonstrates mild to moderate circumferential swelling with several raised, reddened bumps. Both wrists are tender; range of motion does cause some tenderness. Sensation is intact, and good capillary refill time is noted. While waiting for lab results, you obtain a radiograph of the left wrist (shown). What is your impression?