User login
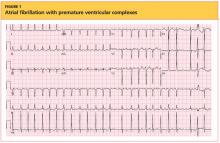
Elderly Woman with Shoulder Pain
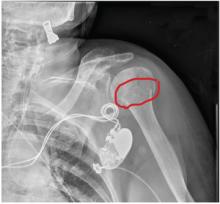
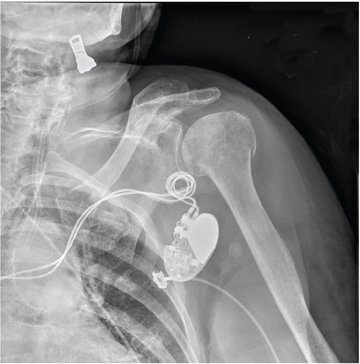
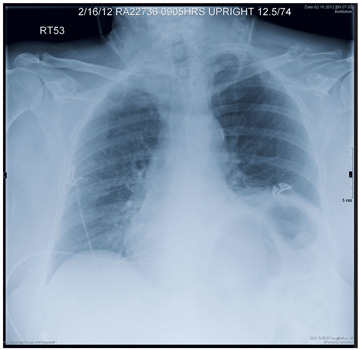
A 90-year-old woman is transferred to your facility from an outside hospital for evaluation of an intracranial hemorrhage secondary to a fall. The patient normally resides in a nursing home and has dementia. She was reportedly ambulating with her walker when she tripped and fell forward. In addition to dementia, her medical history is significant for sick sinus syndrome, for which she has a pacemaker. She also has hypertension and degenerative joint disease. Examination reveals an elderly female who is alert but very confused. Her vital signs are normal. She has moderate swelling and bruising on the left side of her forehead and left orbit. Her pupils react well. As you examine her, you note her unwillingness to use or move her left arm. When you inquire, she states, “It hurts.” Close examination of the left upper extremity shows no obvious deformity or swelling. She does have some tenderness over the left shoulder. You order a radiograph of the left shoulder (shown). What is your impression?
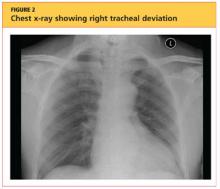
Elderly Man Failed To Mention Hip Pain After Fall
ANSWER
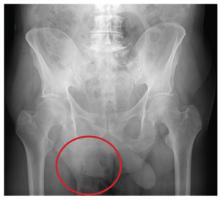
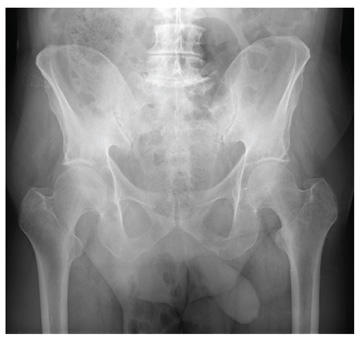
The radiograph demonstrates diffuse bony demineralization, as well as generalized degenerative changes. No obvious fracture or dislocation of the hip joint is seen.
Of note, though, are air lucencies within the area of the scrotum. This most likely represents a bowel finding and is strongly suggestive of an inguinal hernia. No acute intervention is warranted for this incidental finding; outpatient follow-up with general surgery was arranged.
ANSWER
The radiograph demonstrates diffuse bony demineralization, as well as generalized degenerative changes. No obvious fracture or dislocation of the hip joint is seen.
Of note, though, are air lucencies within the area of the scrotum. This most likely represents a bowel finding and is strongly suggestive of an inguinal hernia. No acute intervention is warranted for this incidental finding; outpatient follow-up with general surgery was arranged.
ANSWER
The radiograph demonstrates diffuse bony demineralization, as well as generalized degenerative changes. No obvious fracture or dislocation of the hip joint is seen.
Of note, though, are air lucencies within the area of the scrotum. This most likely represents a bowel finding and is strongly suggestive of an inguinal hernia. No acute intervention is warranted for this incidental finding; outpatient follow-up with general surgery was arranged.

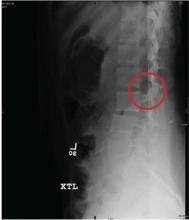
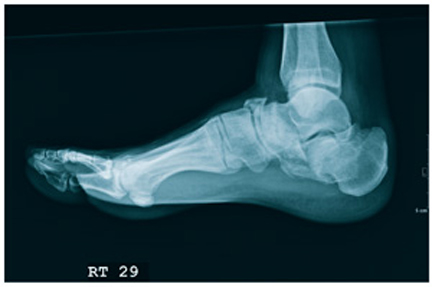
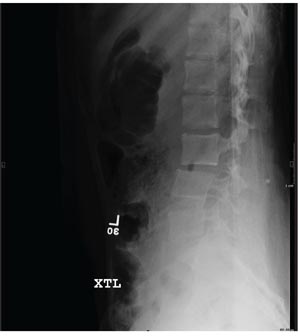
An 87-year-old man is admitted to the hospital with a traumatic subarachnoid hemorrhage secondary to a fall down some steps. He is taking warfarin for atrial fibrillation; because his INR is elevated, he is placed in the ICU for close observation and warfarin reversal. His medical history is otherwise unremarkable, except for mild hypertension. During rounds, he complains of severe hip pain, possibly related to the fall. He admits that he “probably didn’t mention this” while he was in the emergency department. Physical examination demonstrates moderate tenderness and bruising in the left hip. No obvious leg shortening is noted. There is pain in the left hip with abduction and adduction, as well as with internal and external rotation. Good distal pulses are noted, as well as good color and sensation. His vital signs are normal. You order a portable pelvis radiograph (shown). What is your impression?
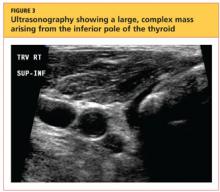
Severe Pain Following Car Crash
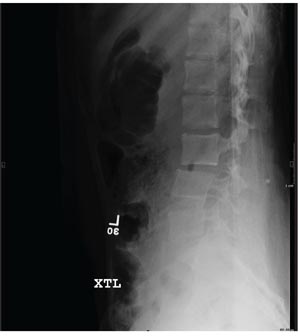
ANSWER
The radiograph shows malalignment at the L3-L4 level. There is a slight retrolisthesis, as well as widening of the interspinous disc space and the facets at this level. No compression fracture is seen.
Such findings are suggestive of a Chance fracture as a result of a hyperflexion injury. CT confirmed these findings. Such injuries are considered unstable and generally require surgical stabilization.
ANSWER
The radiograph shows malalignment at the L3-L4 level. There is a slight retrolisthesis, as well as widening of the interspinous disc space and the facets at this level. No compression fracture is seen.
Such findings are suggestive of a Chance fracture as a result of a hyperflexion injury. CT confirmed these findings. Such injuries are considered unstable and generally require surgical stabilization.
ANSWER
The radiograph shows malalignment at the L3-L4 level. There is a slight retrolisthesis, as well as widening of the interspinous disc space and the facets at this level. No compression fracture is seen.
Such findings are suggestive of a Chance fracture as a result of a hyperflexion injury. CT confirmed these findings. Such injuries are considered unstable and generally require surgical stabilization.
A 15-year-old girl is brought to your facility following a motor vehicle crash. She was a restrained back seat passenger in a vehicle that was struck from behind at a moderate rate of speed. Extensive damage to the vehicle was noted. The patient is complaining of severe abdominal and back pain. Medical history is unremarkable and vital signs are normal. Physical exam shows a female teen who is anxious but in no obvious distress. Her abdomen is firm, with diffuse tenderness and mild guarding. Palpation of her back reveals moderate tenderness in the lower lumbar spine. She is able to move all extremities well, with no other neurologic deficits noted. She is being sent for CT of the chest, abdomen, and pelvis; but first, a lateral radiograph of the lumbar spine is obtained (shown). What is your impression?
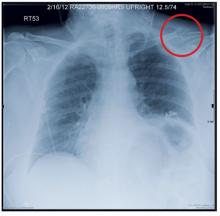
Grand Rounds: Man, 65, With Heart Failure Symptoms
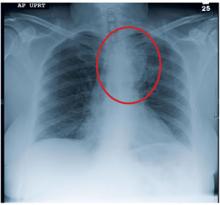
A black man, age 65, with no known history of cardiopulmonary disease presented with acute-onset exertional dyspnea and lower extremity edema. He also reported an episode of syncope, as well as occasional dizziness and abdominal bloating. He said he experienced exertional dyspnea while doing a routine step aerobic exercise. His exercise regimen included distance walking, yoga, and aerobics four to five days per week.
The patient’s medical history was remarkable for a single episode of a bleeding ulcer in previous years, low back pain, shoulder pain, and a septic arthritic hip. His social history was negative for use of tobacco, alcohol, or illegal drugs. He was married and had two biological daughters with fairly unremarkable medical histories. The patient had earned a master’s degree, worked full-time in the insurance business, and was an avid worldwide traveler. He reported diminished quality of life as a result of his acute-onset heart failure symptoms, which reduced his ability to exercise routinely, work full-time, or travel.
The patient’s sudden experience of exertional dyspnea prompted him to visit his primary care provider, who ordered an ECG that demonstrated low voltage patterns and a first-degree atrioventricular (AV) block. Subsequent stress echocardiography showed generalized thickening of the left ventricular myocardium. Posterior wall thickness measured 1.7 cm (normal range, 0.6 to 1.1 cm), septal thickness measured 1.9 cm (normal, 0.6 to 1.1 cm), and ejection fraction was 65%. The stress echocardiogram also showed a speckling pattern (brightly scattered spots) on the myocardium.
Although stress echocardiography results were negative for ischemic disease, the patient did experience dyspnea during the exam. He underwent cardiac catheterization, which indicated normal coronary arteries.
Additional diagnostic studies included cardiac MRI with and without contrast, which showed nulling of the heart muscle and delayed patchy hyperenhancement; this suggested myocardial tissue abnormality as result of amyloid fibril deposition.1 Both pulmonary and tricuspid aortic valves were normal, with no evidence of stenosis. No regional wall motion abnormalities were noted.
Laboratory findings during the work-up were lipid panel, unremarkable; complete blood count (CBC), mild anemia and leukopenia; and urinalysis, positive for proteinuria. Brain natriuretic peptide (BNP) was measured at 686 pg/mL (normal, 0.0 to 100 pg/mL), indicating moderate heart failure. A peripheral blood smear was negative for monoclonal plasma cells.
The patient’s physical exam was unremarkable except for 2+ pedal edema bilaterally. In consideration of normal coronary arteries on cardiac catheterization, the patient’s heart failure symptoms, and stress echocardiography abnormalities, a heart biopsy was ordered. An endomyocardial biopsy with Congo Red stain demonstrated an apple-green birefringent pattern viewed under high-definition polarized light microscope, which was consistent with amyloid deposition.2
The patient was given a diagnosis of primary amyloidosis by his local cardiologist despite negative findings on the peripheral blood smear for monoclonal plasma cells (which are typically found in primary amyloidosis).3 He presented to an institution well-known for its expertise in amyloidosis, for a second opinion. There, the diagnosis was negated, based on reevaluation of the patient’s previous heart specimen through immunohistochemical studies. These studies were positive for serum amyloid P, which is suggestive of transthyretin (TTR) or familial amyloidosis.4 Genetic testing revealed a familial amyloidosis DNA sequence analysis with the Val122Ile variant (ie, isoleucine for valine at position 1225). With the correct diagnosis confirmed, the patient was referred to another highly regarded institution to begin a work-up for cardiac transplantation. Meanwhile, he was cautiously treated with the loop diuretic furosemide to manage his shortness of breath and peripheral edema.
Fifteen months later (13 weeks after being listed for transplant), the patient underwent successful cardiac transplantation.
On pathologic review of the patient’s extricated heart, the myocardium was found to be grossly thickened (see figure, above) and weighed 540 g; the average adult heart weighs 300 to 350 g, depending on the patient’s size.6 Congo Red staining showed extensive amyloid deposits with infiltration throughout the myocardium.
Ninety percent of the amyloid deposits were interstitial, 5% were in the vessels, and 5% were noted in a nodular pattern. The left ventricular cavity showed dilated and thickened walls. Intramural and extramural blood vessels were infiltrated with amyloid as well.
Six months after transplantation, the patient underwent diagnostic testing to assess the function and structure of his new heart. Cardiac catheterization was negative for coronary artery disease. Thirteen months posttransplantation, endomyocardial biopsy with Congo Red stain was negative for amyloid deposition or organ rejection.
About 24 months posttransplantation, the patient was taking tacrolimus, pravastatin, pantoprazole, dapsone, propanolol, colchicine, and donepezil. Stress echocardiography demonstrated normal right and left ventricular systolic function; no wall-motion abnormalities or left ventricular hypertrophy were detected, and the right atrium was of normal size. There was abnormal structural enlargement of the left atrium at the site of anastamosis—a common finding in cardiac transplant patients. The aortic, tricuspid, and mitral valves were all normal.
At that time, it was decided not to repeat endomyocardial biopsy because of normal results on molecular expression testing (a noninvasive technique called AlloMap®7-9), which is performed to assess for heart transplant rejection. The patient’s lipid panel remained within normal limits. CBC indicated persistent anemia and leukopenia. Urine protein and BNP test results were not available.
Since undergoing cardiac transplantation, the patient has resumed his normal routine activities, including some type of exercise five days per week. He said his diet is maintained in moderation. He denied shortness of breath, chest pain, dizziness, or edema. He has returned to full-time employment and has vacationed in Croatia, Italy, and Central America.
DISCUSSION
Familial amyloidosis is an autosomal dominant disease characterized by the production of mutated proteins, most commonly ATTR. Presence of the ATTR Val 122Ile allele has been reported in 3.9% of all black Americans, and in one study, 23% of black Americans diagnosed with cardiac amyloidosis at autopsy were heterozygous for this variant allele.10-12 ATTR Val122Ile usually manifests in the fifth
or sixth decade of life with its characteristic presentation of infiltrative/restrictive cardiomyopathy,13 resulting in heart failure and sometimes peripheral neuropathy.10,11,14
Pathophysiology
In patients with ATTR Val122Ile, cardiomyopathy results from the deposition of mutant protein fibrils in the cardiac muscle, leading to restricted heart wall motion10,15 and stiffening of the cardiac ventricles, with subsequent disruption of the diastolic filling properties of the cardiac muscle.16 Fluid overload and heart failure follow.3,10,17 The atrium of the heart dilates, and the walls of the ventricles become thickened and fibrous.18 Liepnieks and Benson6 reported that the cadaver heart of one Val122Ile patient infiltrated with amyloid protein fibrils weighed 725 g—more than double the weight of an average adult heart.
In patients with cardiac amyloidosis, ECG can detect arrhythmia, and echocardiography shows cardiac enlargement; however, as in the case patient, cardiac catheterization shows normal coronary arteries.15,16,19,20 Thus, previously healthy patients who present with heart failure and negative results on cardiac catheterization should undergo further work-up for cardiac amyloidosis.19
Amyloidosis affects all populations globally.10 In systemic amyloidosis, amyloid releases into the plasma, infiltrating and impairing multiple organs. Poor survival has been reported in patients with heart failure symptoms resulting from amyloid deposition.20,21
Types of Amyloidosis
Primary amyloidosis, the most common of the three amyloidosis types, can be systemic or localized.22 It occurs when protein fibrils, developed from immunoglobin light chains or monoclonal plasma cells and measuring 7 to 10 nm in diameter, adhere to the heart, kidneys, peripheral nerves, eyes, and other organs.5,11,20,23,24 Known for its relation to multiple myeloma,19 primary amyloidosis is associated with a poor prognosis.3,10
Secondary amyloidosis results from a chronic inflammatory disorder, such as rheumatoid arthritis or ankylosing spondylitis—conditions that trigger the production of amyloid proteins.3,10 This type has also been associated with substance abuse and AIDS.23
Familial or hereditary amyloidosis, according to Benson,10 is a group of diseases, each resulting from mutation in a specific protein. In the United States, the most common type of familial amyloidosis is ATTR.11 More than 100 mutant types of ATTR proteins have been identified, each involving a specific nationality or group of nationalities.10,11,23
Mutant ATTR amyloid, when deposited in specific organs, leads to their dysfunction and ultimate failure.6 ATTR may affect the cardiac, gastric, renal, ophthalmic, or nervous system. Depending on the ATTR variant, the resulting clinical features are age- and time-dependent, with onset most common between the third and fifth decade of life.10
Prevalence
The prevalence of ATTR Val 122Ile amyloidosis is reportedly high in West Africa, and in the US African-American population (3.9%).4,11,14,25,26 In a study conducted at a county hospital in Indianapolis, 3% of black newborns were found positive for ATTR Val122Ile through DNA sampling of umbilical cord blood.25 These statistics are of concern, as ATTR amyloidosis could be a significant health concern in a patient population that is already medically underserved.
Yamashita et al25 estimate that 1.35 million Americans of African-American descent may be affected by ATTR Val122Ile and vulnerable to restrictive cardiomyopathy–related heart failure and death. At the very least, this disorder can impair quality of life, especially in the presence of other comorbid conditions.
Clinical Presentation
The presence of exertional syncope at presentation is ominous, as it may be a marker of severe restrictive cardiomyopathy, postural hypotension due to excessive diuresis or autonomic neuropathy, ventricular arrhythmias from localized hypoperfusion, and rarely from cardiac tamponade due to pericardial involvement.27 Despite widespread involvement of the conduction system in specimens at autopsy, high-grade IV block is unusual.3
Diagnostic Studies
ECG. Both ECG and Holter monitoring can detect the arrhythmias and conduction disturbances (eg, first-degree AV block, low voltage patterns) associated with cardiac amyloidosis. Patients often experience syncopal and near-syncopal episodes as a result of conduction disturbances.28 Patients with conditions such as cardiac amyloidosis who present with severe heart failure are at high risk for sudden death secondary to conduction disturbances. Many have benefited from implanted defibrillators.29
Echocardiography. In patients with ATTR Val122Ile cardiac amyloidosis, echocardiography reveals thickened ventricular walls (ie, measuring ≥ 15 mm; normal, ≤ 11 mm).19 Amyloid-restrictive cardiomyopathy is associated with a marked dissociation between short- and long-axis systolic function, in cases in which left ventricular ejection fraction is normal.30
Echocardiography may demonstrate the characteristic specular or granular sparkling appearance that signifies advanced disease.15,21 Only a minority have this pattern in the myocardium, however, and changes in echocardiographic technology have made this finding less noticeable.30
MRI. Among more recently used diagnostic studies, cardiac magnetic resonance (CMR) has been reported to demonstrate late gadolinium enhancement (LGE) in perhaps 80% of patients with familial amyloidosis and cardiac involvement (as determined through biopsy and Congo Red stain). LGE-CMR shows darkening of the cardiac tissue, a common occurrence in amyloidosis.31
LGE is associated with increased thickness of both left and right ventricles, lower ECG voltage patterns, elevated BNP, and elevated troponin T.31,32 Globally, LGE is associated with the worst prognosis in patients with cardiac amyloidosis. Use of LGE-CMR testing can help facilitate early detection of cardiac amyloidosis in patients who may be vulnerable to cardiac damage.31
Cardiac catheterization. In patients with cardiac amyloidosis, cardiac catheterization usually shows normal coronary arteries.3
Diagnosis
Early diagnosis of ATTR cardiac amyloidosis is crucial to the patient’s survival; it should be ruled out in any African-American patient with unexplained heart failure and echocardiography showing increased wall thickness with a nondilated left ventricular cavity. Additional clues include significant proteinuria, hepatomegaly disproportionate to the degree of heart failure, or corresponding neuropathy. Known family history of the disease, along with variant type, allows for a prompt and correct diagnosis.10
It has been reported that most clinicians who encounter heart failure, particularly in black patients, do not consider amyloidosis in the differential diagnosis, because of the high prevalence of hypertension and congestive heart failure in this population.10,15,19 As a result, amyloidosis often goes undiagnosed.19
Findings of enlarged and thickened cardiac walls on echocardiography but normal coronary arteries on cardiac catheterization should alert the treating clinician to further work-up for cardiac amyloidosis.19 In such a patient, according to Kristen et al,21 endomyocardial biopsy with Congo Red staining is the gold standard for diagnosis of amyloidosis.
In ATTR Val122Ile familial amyloidosis, it is unclear whether patients who are homozygous for the disease present with symptoms at earlier onset with more progressive illness or die sooner than those who are heterozygous.33 Nevertheless, once the diagnosis is confirmed, it is important to determine the patient’s specific variant type by DNA testing so that appropriate treatment can be initiated and the patient’s prognosis evaluated.5,10,13
Treatment
For familial amyloidosis in general, some researchers advocate liver transplantation to remove the source of mutant amyloid protein and stop all deposition of amyloid fibrils; this procedure can be followed later by transplantation of other affected organs (including the heart).5,23 Maurer et al34 have reported improved one-year survival rates among patients with ATTR amyloidosis who underwent both cardiac and liver transplantation: 75%, versus 23% in patients who did not receive transplanted organs.
Management of cardiac amyloidosis usually requires a twofold approach: treating associated congestive heart failure, and preventing further deposition of amyloid.24 In the case patient (as in most patients with ATTR amyloidosis), heart transplantation was deemed the only life-sustaining treatment option.11,19,33
Pharmacotherapeutic options are limited for patients with ATTR Val122Ile familial amyloidosis. Conventional heart failure agents (eg, ACE inhibitors, angiotensin receptor blockers, digoxin, β-blockers, calcium channel blockers) can exacerbate heart failure symptoms, leading to a potentially life-threatening arrhythmia.3,11,19,24,35 Amyloid fibrils bind to digitalis, increasing susceptibility to digitalis toxicity; and to nifedipine, causing hemodynamic deterioration. Verapamil should be avoided, as it may induce severe left ventricular dysfunction. ACE inhibitors often provoke profound hypotension in primary amyloidosis.24,35
Diuretics, too (eg, furosemide, as was prescribed for the case patient), must be used with caution.3 These agents have been used to treat fluid overload and the resulting peripheral edema and shortness of breath found in ATTR Val122Ile patients who experience heart failure.36 According to Dubrey et al,5 cautious use of diuretics is necessary for management of heart failure symptoms in these patients.
Because the risk for intracardiac thrombus is high, anticoagulation (using agents other than β-blockers or calcium channel blockers) should be implemented unless compelling risks are involved.11,24 Amiodarone is relatively well tolerated for ventricular tachydysrhythmias and in atrial fibrillation if the goal is maintaining sinus rhythm.37
Regarding heart transplantation in patients with familial amyloidosis, Jacob et al33 hypothesize that since mutant amyloid protein is synthesized by the liver, it would take approximately 50 years for a transplanted heart to become affected by amyloid deposition. In a 59-year-old Afro-Caribbean man with familial amyloidosis who underwent cardiac transplantation, Hamour et al11 reported that the donor heart remained amyloid-free three years posttransplantation, as demonstrated by serial cardiac biopsy.
On the Horizon
Clinical trials are now under way to examine pharmacotherapeutic options for patients with ATTR amyloidosis. Now being examined in clinical trials, for example, is Fx-1006A, a drug that stabilizes ATTR and prevents the misfolding of the amyloid protein fibril, in turn preventing it from binding to the target organ.38 Similarly, ALN-TTR, a drug believed to prevent disease manifestation and possibly facilitate disease regression, is being investigated in early human trials.39
Additionally, the use of genetic testing is recommended in at-risk individuals to identify the TTR gene. Affected patients may benefit from prophylactic medical management, which would halt amyloidogenesis of TTR—and possibly treat the condition as well.35 Pharmacotherapeutic agents like diflunisal, an NSAID, antagonize the aggregation of TTR protein and hinder formation of the amyloid fibrils.40
CONCLUSION
ATTR Val122Ile familial amyloidosis is a rare disorder that causes abnormal synthesis of amyloid protein in the liver, which then infiltrates the cardiac structure, leading to restrictive cardiomyopathy and progressive heart failure. Patients who present with symptoms of heart failure, cardiac enlargement on echocardiography, and a finding of granular speckling patterns, though not specific on echocardiography, should prompt the health care provider to refer the patient to a cardiologist familiar with cardiac amyloidosis for further work-up.
Diagnosed patients must undergo genetic testing to determine the specific variant type so that prompt treatment can be initiated. In patients with ATTR Val122Ile familial amyloidosis, the treatment of choice is cardiac transplantation. Although the mutant amyloid protein continues to be synthesized in the liver, the donor heart is unlikely to become affected by this substance for many years. Appropriately treated patients can maintain good quality of life, free of heart failure.
REFERENCES
1. Lim RP, Srichai MB, Lee VS. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. AJR Am J Roentgenol. 2007;188 (6):1675-1681.
2. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17(3-4):101-104.
3. Kendall H. Cardiac amyloidosis. Crit Care Nurse. 2010;30(2):16-23.
4. Eriksson M, Büttener J, Todorov T, et al. Prevalence of germline mutations in the TTR gene in a consecutive series of surgical pathology specimens with AATR amyloid. Am J Surg Pathol. 2009;33(1):58-65.
5. Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97(1):75-84.
6. Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14(4):277-282.
7. XDx Expression Diagnostics. Allomap®: molecular expression testing (2004). www.allomap.com. Accessed May 14, 2012.
8. Mandras SA, Crespo J, Patel HM. Innovative application of immunologic principles in heart transplantation. Ochsner J. 2010;10(4):231-235.
9. Yamani MH, Taylor DO, Rodriguez R, et al. Transplant vasculopathy is associated with increased AlloMap gene expression score. J Heart Lung Transplant. 2007;26(4):403-406.
10. Benson MD. The hereditary amyloidoses. Best Pract Res Clin Rheumatol. 2003;17(6):909-927.
11. Hamour IM, Lachmann HJ, Goodman HJ, et al. Heart transplantation for homozygous familial transthyretin (TTR) V122I cardiac amyloidosis. Am J Transplant. 2008;8(5):1056-1059.
12. Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466-473.
13. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-361.
14. Askanas V, Engel WK, McFerrin J, Vattemi G. Transthyretin Val122Ile, accumulated Abeta, and inclusion-body myositis aspects in cultured muscle. Neurology. 2003;61(2):257-260.
15. Hamidi Asl K, Nakamura M, Yamashita T, Benson MD. Cardiac amyloidoses associated with the transthyretin lle122 mutation in a Caucasian family. Amyloid. 2001;8(4):263-269.
16. Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol. 2009;24(3): 214-220.
17. Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009;10 (8):iii23-iii33.
18. Bruce J. Getting to the heart of cardiomyopathies. Nursing. 2005;35(8):44-47.
19. Falk RH. The neglected entity of familial cardiac amyloidosis in African Americans. Ethn Dis. 2002;12(1):141-143.
20. Piper C, Butz T, Farr M, et al. How to diagnose cardiac amyloidosis early: impact of ECG, tissue Doppler echocardiography, and myocardial biopsy. Amyloid. 2010;17(1):1-9.
21. Kristen AV, Meyer FJ, Perz JB, et al. Risk stratification in cardiac amyloidosis: novel approaches. Transplantation. 2005;80(1 suppl):S151-S155.
22. Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179-183.
23. Picken MM. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens. 2007; 16(3):196-203.
24. Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011;124(9):1079-1085.
25. Yamashita T, Asl KH, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile 122 allele frequency in an African-American population. Amyloid. 2005;12(2):127-130.
26. Benson MD, Yazaki M, Magy N. Laboratory assessment of transthyretin amyloidosis. Clin Chem Lab Med. 2002;40(12):1262-1265.
27. Chamarthi B, Dubrey SW, Cha K, et al. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80(9):1242-1245.
28. Correia MJ, Coutinho CA, Conceiçao I, et al. Role of heart rate variability in the assessment of autonomic dysfunction in type I familial amyloidotic polyneuropathy. Folia Cardiol. 2005;12(suppl C):459-462.
29. Kadish A, Mehra M. Heart failure devices: Implantable cardioverter-defibrillators and biventricular pacing therapy. Circulation. 2005; 111(24):3327-3335.
30. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410-415.
31. Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155-164.
32. Fealey ME, Edwards WD, Buadi FK, et al. Echocardiographic features of cardiac amyloidosis presenting as endomyocardial disease in a 54-year-old male. J Cardiol. 2009;54(1):162-166.
33. Jacob EK, Edwards WD, Zucker M, et al. Homozygous transthyretin mutation in an African American male. J Mol Diagn. 2007; 9(1):127-131.
34. Maurer MS, Raina A, Hesdorffer C, et al. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplantation. 2007;83(5):539-545.
35. Buxbaum J, Alexander A, Koziol J, et al. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African-Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159(5):864-870.
36. Rose BD, Colucci WS. Use of diuretics in heart failure (2010). www.uptodate.com/con tents/use-of-diuretics-in-patients-with-heart-failure. Accessed May 14, 2012.
37. Trappe H-J. Treating critical supraventricular and ventricular arrhythmias. J Emerg Trauma Shock. 2010;3(2):143-152.
38. Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14(30):3219-3230.
39. Alnylam Pharmaceuticals. TTR Amyloidosis: ALN-TTR (2011). www.alnylam.com/Programs-and-Pipeline/Programs/index.php. Accessed May 14, 2012.
40. Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin: potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355-374.
A black man, age 65, with no known history of cardiopulmonary disease presented with acute-onset exertional dyspnea and lower extremity edema. He also reported an episode of syncope, as well as occasional dizziness and abdominal bloating. He said he experienced exertional dyspnea while doing a routine step aerobic exercise. His exercise regimen included distance walking, yoga, and aerobics four to five days per week.
The patient’s medical history was remarkable for a single episode of a bleeding ulcer in previous years, low back pain, shoulder pain, and a septic arthritic hip. His social history was negative for use of tobacco, alcohol, or illegal drugs. He was married and had two biological daughters with fairly unremarkable medical histories. The patient had earned a master’s degree, worked full-time in the insurance business, and was an avid worldwide traveler. He reported diminished quality of life as a result of his acute-onset heart failure symptoms, which reduced his ability to exercise routinely, work full-time, or travel.
The patient’s sudden experience of exertional dyspnea prompted him to visit his primary care provider, who ordered an ECG that demonstrated low voltage patterns and a first-degree atrioventricular (AV) block. Subsequent stress echocardiography showed generalized thickening of the left ventricular myocardium. Posterior wall thickness measured 1.7 cm (normal range, 0.6 to 1.1 cm), septal thickness measured 1.9 cm (normal, 0.6 to 1.1 cm), and ejection fraction was 65%. The stress echocardiogram also showed a speckling pattern (brightly scattered spots) on the myocardium.
Although stress echocardiography results were negative for ischemic disease, the patient did experience dyspnea during the exam. He underwent cardiac catheterization, which indicated normal coronary arteries.
Additional diagnostic studies included cardiac MRI with and without contrast, which showed nulling of the heart muscle and delayed patchy hyperenhancement; this suggested myocardial tissue abnormality as result of amyloid fibril deposition.1 Both pulmonary and tricuspid aortic valves were normal, with no evidence of stenosis. No regional wall motion abnormalities were noted.
Laboratory findings during the work-up were lipid panel, unremarkable; complete blood count (CBC), mild anemia and leukopenia; and urinalysis, positive for proteinuria. Brain natriuretic peptide (BNP) was measured at 686 pg/mL (normal, 0.0 to 100 pg/mL), indicating moderate heart failure. A peripheral blood smear was negative for monoclonal plasma cells.
The patient’s physical exam was unremarkable except for 2+ pedal edema bilaterally. In consideration of normal coronary arteries on cardiac catheterization, the patient’s heart failure symptoms, and stress echocardiography abnormalities, a heart biopsy was ordered. An endomyocardial biopsy with Congo Red stain demonstrated an apple-green birefringent pattern viewed under high-definition polarized light microscope, which was consistent with amyloid deposition.2
The patient was given a diagnosis of primary amyloidosis by his local cardiologist despite negative findings on the peripheral blood smear for monoclonal plasma cells (which are typically found in primary amyloidosis).3 He presented to an institution well-known for its expertise in amyloidosis, for a second opinion. There, the diagnosis was negated, based on reevaluation of the patient’s previous heart specimen through immunohistochemical studies. These studies were positive for serum amyloid P, which is suggestive of transthyretin (TTR) or familial amyloidosis.4 Genetic testing revealed a familial amyloidosis DNA sequence analysis with the Val122Ile variant (ie, isoleucine for valine at position 1225). With the correct diagnosis confirmed, the patient was referred to another highly regarded institution to begin a work-up for cardiac transplantation. Meanwhile, he was cautiously treated with the loop diuretic furosemide to manage his shortness of breath and peripheral edema.
Fifteen months later (13 weeks after being listed for transplant), the patient underwent successful cardiac transplantation.
On pathologic review of the patient’s extricated heart, the myocardium was found to be grossly thickened (see figure, above) and weighed 540 g; the average adult heart weighs 300 to 350 g, depending on the patient’s size.6 Congo Red staining showed extensive amyloid deposits with infiltration throughout the myocardium.
Ninety percent of the amyloid deposits were interstitial, 5% were in the vessels, and 5% were noted in a nodular pattern. The left ventricular cavity showed dilated and thickened walls. Intramural and extramural blood vessels were infiltrated with amyloid as well.
Six months after transplantation, the patient underwent diagnostic testing to assess the function and structure of his new heart. Cardiac catheterization was negative for coronary artery disease. Thirteen months posttransplantation, endomyocardial biopsy with Congo Red stain was negative for amyloid deposition or organ rejection.
About 24 months posttransplantation, the patient was taking tacrolimus, pravastatin, pantoprazole, dapsone, propanolol, colchicine, and donepezil. Stress echocardiography demonstrated normal right and left ventricular systolic function; no wall-motion abnormalities or left ventricular hypertrophy were detected, and the right atrium was of normal size. There was abnormal structural enlargement of the left atrium at the site of anastamosis—a common finding in cardiac transplant patients. The aortic, tricuspid, and mitral valves were all normal.
At that time, it was decided not to repeat endomyocardial biopsy because of normal results on molecular expression testing (a noninvasive technique called AlloMap®7-9), which is performed to assess for heart transplant rejection. The patient’s lipid panel remained within normal limits. CBC indicated persistent anemia and leukopenia. Urine protein and BNP test results were not available.
Since undergoing cardiac transplantation, the patient has resumed his normal routine activities, including some type of exercise five days per week. He said his diet is maintained in moderation. He denied shortness of breath, chest pain, dizziness, or edema. He has returned to full-time employment and has vacationed in Croatia, Italy, and Central America.
DISCUSSION
Familial amyloidosis is an autosomal dominant disease characterized by the production of mutated proteins, most commonly ATTR. Presence of the ATTR Val 122Ile allele has been reported in 3.9% of all black Americans, and in one study, 23% of black Americans diagnosed with cardiac amyloidosis at autopsy were heterozygous for this variant allele.10-12 ATTR Val122Ile usually manifests in the fifth
or sixth decade of life with its characteristic presentation of infiltrative/restrictive cardiomyopathy,13 resulting in heart failure and sometimes peripheral neuropathy.10,11,14
Pathophysiology
In patients with ATTR Val122Ile, cardiomyopathy results from the deposition of mutant protein fibrils in the cardiac muscle, leading to restricted heart wall motion10,15 and stiffening of the cardiac ventricles, with subsequent disruption of the diastolic filling properties of the cardiac muscle.16 Fluid overload and heart failure follow.3,10,17 The atrium of the heart dilates, and the walls of the ventricles become thickened and fibrous.18 Liepnieks and Benson6 reported that the cadaver heart of one Val122Ile patient infiltrated with amyloid protein fibrils weighed 725 g—more than double the weight of an average adult heart.
In patients with cardiac amyloidosis, ECG can detect arrhythmia, and echocardiography shows cardiac enlargement; however, as in the case patient, cardiac catheterization shows normal coronary arteries.15,16,19,20 Thus, previously healthy patients who present with heart failure and negative results on cardiac catheterization should undergo further work-up for cardiac amyloidosis.19
Amyloidosis affects all populations globally.10 In systemic amyloidosis, amyloid releases into the plasma, infiltrating and impairing multiple organs. Poor survival has been reported in patients with heart failure symptoms resulting from amyloid deposition.20,21
Types of Amyloidosis
Primary amyloidosis, the most common of the three amyloidosis types, can be systemic or localized.22 It occurs when protein fibrils, developed from immunoglobin light chains or monoclonal plasma cells and measuring 7 to 10 nm in diameter, adhere to the heart, kidneys, peripheral nerves, eyes, and other organs.5,11,20,23,24 Known for its relation to multiple myeloma,19 primary amyloidosis is associated with a poor prognosis.3,10
Secondary amyloidosis results from a chronic inflammatory disorder, such as rheumatoid arthritis or ankylosing spondylitis—conditions that trigger the production of amyloid proteins.3,10 This type has also been associated with substance abuse and AIDS.23
Familial or hereditary amyloidosis, according to Benson,10 is a group of diseases, each resulting from mutation in a specific protein. In the United States, the most common type of familial amyloidosis is ATTR.11 More than 100 mutant types of ATTR proteins have been identified, each involving a specific nationality or group of nationalities.10,11,23
Mutant ATTR amyloid, when deposited in specific organs, leads to their dysfunction and ultimate failure.6 ATTR may affect the cardiac, gastric, renal, ophthalmic, or nervous system. Depending on the ATTR variant, the resulting clinical features are age- and time-dependent, with onset most common between the third and fifth decade of life.10
Prevalence
The prevalence of ATTR Val 122Ile amyloidosis is reportedly high in West Africa, and in the US African-American population (3.9%).4,11,14,25,26 In a study conducted at a county hospital in Indianapolis, 3% of black newborns were found positive for ATTR Val122Ile through DNA sampling of umbilical cord blood.25 These statistics are of concern, as ATTR amyloidosis could be a significant health concern in a patient population that is already medically underserved.
Yamashita et al25 estimate that 1.35 million Americans of African-American descent may be affected by ATTR Val122Ile and vulnerable to restrictive cardiomyopathy–related heart failure and death. At the very least, this disorder can impair quality of life, especially in the presence of other comorbid conditions.
Clinical Presentation
The presence of exertional syncope at presentation is ominous, as it may be a marker of severe restrictive cardiomyopathy, postural hypotension due to excessive diuresis or autonomic neuropathy, ventricular arrhythmias from localized hypoperfusion, and rarely from cardiac tamponade due to pericardial involvement.27 Despite widespread involvement of the conduction system in specimens at autopsy, high-grade IV block is unusual.3
Diagnostic Studies
ECG. Both ECG and Holter monitoring can detect the arrhythmias and conduction disturbances (eg, first-degree AV block, low voltage patterns) associated with cardiac amyloidosis. Patients often experience syncopal and near-syncopal episodes as a result of conduction disturbances.28 Patients with conditions such as cardiac amyloidosis who present with severe heart failure are at high risk for sudden death secondary to conduction disturbances. Many have benefited from implanted defibrillators.29
Echocardiography. In patients with ATTR Val122Ile cardiac amyloidosis, echocardiography reveals thickened ventricular walls (ie, measuring ≥ 15 mm; normal, ≤ 11 mm).19 Amyloid-restrictive cardiomyopathy is associated with a marked dissociation between short- and long-axis systolic function, in cases in which left ventricular ejection fraction is normal.30
Echocardiography may demonstrate the characteristic specular or granular sparkling appearance that signifies advanced disease.15,21 Only a minority have this pattern in the myocardium, however, and changes in echocardiographic technology have made this finding less noticeable.30
MRI. Among more recently used diagnostic studies, cardiac magnetic resonance (CMR) has been reported to demonstrate late gadolinium enhancement (LGE) in perhaps 80% of patients with familial amyloidosis and cardiac involvement (as determined through biopsy and Congo Red stain). LGE-CMR shows darkening of the cardiac tissue, a common occurrence in amyloidosis.31
LGE is associated with increased thickness of both left and right ventricles, lower ECG voltage patterns, elevated BNP, and elevated troponin T.31,32 Globally, LGE is associated with the worst prognosis in patients with cardiac amyloidosis. Use of LGE-CMR testing can help facilitate early detection of cardiac amyloidosis in patients who may be vulnerable to cardiac damage.31
Cardiac catheterization. In patients with cardiac amyloidosis, cardiac catheterization usually shows normal coronary arteries.3
Diagnosis
Early diagnosis of ATTR cardiac amyloidosis is crucial to the patient’s survival; it should be ruled out in any African-American patient with unexplained heart failure and echocardiography showing increased wall thickness with a nondilated left ventricular cavity. Additional clues include significant proteinuria, hepatomegaly disproportionate to the degree of heart failure, or corresponding neuropathy. Known family history of the disease, along with variant type, allows for a prompt and correct diagnosis.10
It has been reported that most clinicians who encounter heart failure, particularly in black patients, do not consider amyloidosis in the differential diagnosis, because of the high prevalence of hypertension and congestive heart failure in this population.10,15,19 As a result, amyloidosis often goes undiagnosed.19
Findings of enlarged and thickened cardiac walls on echocardiography but normal coronary arteries on cardiac catheterization should alert the treating clinician to further work-up for cardiac amyloidosis.19 In such a patient, according to Kristen et al,21 endomyocardial biopsy with Congo Red staining is the gold standard for diagnosis of amyloidosis.
In ATTR Val122Ile familial amyloidosis, it is unclear whether patients who are homozygous for the disease present with symptoms at earlier onset with more progressive illness or die sooner than those who are heterozygous.33 Nevertheless, once the diagnosis is confirmed, it is important to determine the patient’s specific variant type by DNA testing so that appropriate treatment can be initiated and the patient’s prognosis evaluated.5,10,13
Treatment
For familial amyloidosis in general, some researchers advocate liver transplantation to remove the source of mutant amyloid protein and stop all deposition of amyloid fibrils; this procedure can be followed later by transplantation of other affected organs (including the heart).5,23 Maurer et al34 have reported improved one-year survival rates among patients with ATTR amyloidosis who underwent both cardiac and liver transplantation: 75%, versus 23% in patients who did not receive transplanted organs.
Management of cardiac amyloidosis usually requires a twofold approach: treating associated congestive heart failure, and preventing further deposition of amyloid.24 In the case patient (as in most patients with ATTR amyloidosis), heart transplantation was deemed the only life-sustaining treatment option.11,19,33
Pharmacotherapeutic options are limited for patients with ATTR Val122Ile familial amyloidosis. Conventional heart failure agents (eg, ACE inhibitors, angiotensin receptor blockers, digoxin, β-blockers, calcium channel blockers) can exacerbate heart failure symptoms, leading to a potentially life-threatening arrhythmia.3,11,19,24,35 Amyloid fibrils bind to digitalis, increasing susceptibility to digitalis toxicity; and to nifedipine, causing hemodynamic deterioration. Verapamil should be avoided, as it may induce severe left ventricular dysfunction. ACE inhibitors often provoke profound hypotension in primary amyloidosis.24,35
Diuretics, too (eg, furosemide, as was prescribed for the case patient), must be used with caution.3 These agents have been used to treat fluid overload and the resulting peripheral edema and shortness of breath found in ATTR Val122Ile patients who experience heart failure.36 According to Dubrey et al,5 cautious use of diuretics is necessary for management of heart failure symptoms in these patients.
Because the risk for intracardiac thrombus is high, anticoagulation (using agents other than β-blockers or calcium channel blockers) should be implemented unless compelling risks are involved.11,24 Amiodarone is relatively well tolerated for ventricular tachydysrhythmias and in atrial fibrillation if the goal is maintaining sinus rhythm.37
Regarding heart transplantation in patients with familial amyloidosis, Jacob et al33 hypothesize that since mutant amyloid protein is synthesized by the liver, it would take approximately 50 years for a transplanted heart to become affected by amyloid deposition. In a 59-year-old Afro-Caribbean man with familial amyloidosis who underwent cardiac transplantation, Hamour et al11 reported that the donor heart remained amyloid-free three years posttransplantation, as demonstrated by serial cardiac biopsy.
On the Horizon
Clinical trials are now under way to examine pharmacotherapeutic options for patients with ATTR amyloidosis. Now being examined in clinical trials, for example, is Fx-1006A, a drug that stabilizes ATTR and prevents the misfolding of the amyloid protein fibril, in turn preventing it from binding to the target organ.38 Similarly, ALN-TTR, a drug believed to prevent disease manifestation and possibly facilitate disease regression, is being investigated in early human trials.39
Additionally, the use of genetic testing is recommended in at-risk individuals to identify the TTR gene. Affected patients may benefit from prophylactic medical management, which would halt amyloidogenesis of TTR—and possibly treat the condition as well.35 Pharmacotherapeutic agents like diflunisal, an NSAID, antagonize the aggregation of TTR protein and hinder formation of the amyloid fibrils.40
CONCLUSION
ATTR Val122Ile familial amyloidosis is a rare disorder that causes abnormal synthesis of amyloid protein in the liver, which then infiltrates the cardiac structure, leading to restrictive cardiomyopathy and progressive heart failure. Patients who present with symptoms of heart failure, cardiac enlargement on echocardiography, and a finding of granular speckling patterns, though not specific on echocardiography, should prompt the health care provider to refer the patient to a cardiologist familiar with cardiac amyloidosis for further work-up.
Diagnosed patients must undergo genetic testing to determine the specific variant type so that prompt treatment can be initiated. In patients with ATTR Val122Ile familial amyloidosis, the treatment of choice is cardiac transplantation. Although the mutant amyloid protein continues to be synthesized in the liver, the donor heart is unlikely to become affected by this substance for many years. Appropriately treated patients can maintain good quality of life, free of heart failure.
REFERENCES
1. Lim RP, Srichai MB, Lee VS. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. AJR Am J Roentgenol. 2007;188 (6):1675-1681.
2. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17(3-4):101-104.
3. Kendall H. Cardiac amyloidosis. Crit Care Nurse. 2010;30(2):16-23.
4. Eriksson M, Büttener J, Todorov T, et al. Prevalence of germline mutations in the TTR gene in a consecutive series of surgical pathology specimens with AATR amyloid. Am J Surg Pathol. 2009;33(1):58-65.
5. Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97(1):75-84.
6. Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14(4):277-282.
7. XDx Expression Diagnostics. Allomap®: molecular expression testing (2004). www.allomap.com. Accessed May 14, 2012.
8. Mandras SA, Crespo J, Patel HM. Innovative application of immunologic principles in heart transplantation. Ochsner J. 2010;10(4):231-235.
9. Yamani MH, Taylor DO, Rodriguez R, et al. Transplant vasculopathy is associated with increased AlloMap gene expression score. J Heart Lung Transplant. 2007;26(4):403-406.
10. Benson MD. The hereditary amyloidoses. Best Pract Res Clin Rheumatol. 2003;17(6):909-927.
11. Hamour IM, Lachmann HJ, Goodman HJ, et al. Heart transplantation for homozygous familial transthyretin (TTR) V122I cardiac amyloidosis. Am J Transplant. 2008;8(5):1056-1059.
12. Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466-473.
13. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-361.
14. Askanas V, Engel WK, McFerrin J, Vattemi G. Transthyretin Val122Ile, accumulated Abeta, and inclusion-body myositis aspects in cultured muscle. Neurology. 2003;61(2):257-260.
15. Hamidi Asl K, Nakamura M, Yamashita T, Benson MD. Cardiac amyloidoses associated with the transthyretin lle122 mutation in a Caucasian family. Amyloid. 2001;8(4):263-269.
16. Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol. 2009;24(3): 214-220.
17. Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009;10 (8):iii23-iii33.
18. Bruce J. Getting to the heart of cardiomyopathies. Nursing. 2005;35(8):44-47.
19. Falk RH. The neglected entity of familial cardiac amyloidosis in African Americans. Ethn Dis. 2002;12(1):141-143.
20. Piper C, Butz T, Farr M, et al. How to diagnose cardiac amyloidosis early: impact of ECG, tissue Doppler echocardiography, and myocardial biopsy. Amyloid. 2010;17(1):1-9.
21. Kristen AV, Meyer FJ, Perz JB, et al. Risk stratification in cardiac amyloidosis: novel approaches. Transplantation. 2005;80(1 suppl):S151-S155.
22. Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179-183.
23. Picken MM. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens. 2007; 16(3):196-203.
24. Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011;124(9):1079-1085.
25. Yamashita T, Asl KH, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile 122 allele frequency in an African-American population. Amyloid. 2005;12(2):127-130.
26. Benson MD, Yazaki M, Magy N. Laboratory assessment of transthyretin amyloidosis. Clin Chem Lab Med. 2002;40(12):1262-1265.
27. Chamarthi B, Dubrey SW, Cha K, et al. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80(9):1242-1245.
28. Correia MJ, Coutinho CA, Conceiçao I, et al. Role of heart rate variability in the assessment of autonomic dysfunction in type I familial amyloidotic polyneuropathy. Folia Cardiol. 2005;12(suppl C):459-462.
29. Kadish A, Mehra M. Heart failure devices: Implantable cardioverter-defibrillators and biventricular pacing therapy. Circulation. 2005; 111(24):3327-3335.
30. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410-415.
31. Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155-164.
32. Fealey ME, Edwards WD, Buadi FK, et al. Echocardiographic features of cardiac amyloidosis presenting as endomyocardial disease in a 54-year-old male. J Cardiol. 2009;54(1):162-166.
33. Jacob EK, Edwards WD, Zucker M, et al. Homozygous transthyretin mutation in an African American male. J Mol Diagn. 2007; 9(1):127-131.
34. Maurer MS, Raina A, Hesdorffer C, et al. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplantation. 2007;83(5):539-545.
35. Buxbaum J, Alexander A, Koziol J, et al. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African-Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159(5):864-870.
36. Rose BD, Colucci WS. Use of diuretics in heart failure (2010). www.uptodate.com/con tents/use-of-diuretics-in-patients-with-heart-failure. Accessed May 14, 2012.
37. Trappe H-J. Treating critical supraventricular and ventricular arrhythmias. J Emerg Trauma Shock. 2010;3(2):143-152.
38. Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14(30):3219-3230.
39. Alnylam Pharmaceuticals. TTR Amyloidosis: ALN-TTR (2011). www.alnylam.com/Programs-and-Pipeline/Programs/index.php. Accessed May 14, 2012.
40. Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin: potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355-374.
A black man, age 65, with no known history of cardiopulmonary disease presented with acute-onset exertional dyspnea and lower extremity edema. He also reported an episode of syncope, as well as occasional dizziness and abdominal bloating. He said he experienced exertional dyspnea while doing a routine step aerobic exercise. His exercise regimen included distance walking, yoga, and aerobics four to five days per week.
The patient’s medical history was remarkable for a single episode of a bleeding ulcer in previous years, low back pain, shoulder pain, and a septic arthritic hip. His social history was negative for use of tobacco, alcohol, or illegal drugs. He was married and had two biological daughters with fairly unremarkable medical histories. The patient had earned a master’s degree, worked full-time in the insurance business, and was an avid worldwide traveler. He reported diminished quality of life as a result of his acute-onset heart failure symptoms, which reduced his ability to exercise routinely, work full-time, or travel.
The patient’s sudden experience of exertional dyspnea prompted him to visit his primary care provider, who ordered an ECG that demonstrated low voltage patterns and a first-degree atrioventricular (AV) block. Subsequent stress echocardiography showed generalized thickening of the left ventricular myocardium. Posterior wall thickness measured 1.7 cm (normal range, 0.6 to 1.1 cm), septal thickness measured 1.9 cm (normal, 0.6 to 1.1 cm), and ejection fraction was 65%. The stress echocardiogram also showed a speckling pattern (brightly scattered spots) on the myocardium.
Although stress echocardiography results were negative for ischemic disease, the patient did experience dyspnea during the exam. He underwent cardiac catheterization, which indicated normal coronary arteries.
Additional diagnostic studies included cardiac MRI with and without contrast, which showed nulling of the heart muscle and delayed patchy hyperenhancement; this suggested myocardial tissue abnormality as result of amyloid fibril deposition.1 Both pulmonary and tricuspid aortic valves were normal, with no evidence of stenosis. No regional wall motion abnormalities were noted.
Laboratory findings during the work-up were lipid panel, unremarkable; complete blood count (CBC), mild anemia and leukopenia; and urinalysis, positive for proteinuria. Brain natriuretic peptide (BNP) was measured at 686 pg/mL (normal, 0.0 to 100 pg/mL), indicating moderate heart failure. A peripheral blood smear was negative for monoclonal plasma cells.
The patient’s physical exam was unremarkable except for 2+ pedal edema bilaterally. In consideration of normal coronary arteries on cardiac catheterization, the patient’s heart failure symptoms, and stress echocardiography abnormalities, a heart biopsy was ordered. An endomyocardial biopsy with Congo Red stain demonstrated an apple-green birefringent pattern viewed under high-definition polarized light microscope, which was consistent with amyloid deposition.2
The patient was given a diagnosis of primary amyloidosis by his local cardiologist despite negative findings on the peripheral blood smear for monoclonal plasma cells (which are typically found in primary amyloidosis).3 He presented to an institution well-known for its expertise in amyloidosis, for a second opinion. There, the diagnosis was negated, based on reevaluation of the patient’s previous heart specimen through immunohistochemical studies. These studies were positive for serum amyloid P, which is suggestive of transthyretin (TTR) or familial amyloidosis.4 Genetic testing revealed a familial amyloidosis DNA sequence analysis with the Val122Ile variant (ie, isoleucine for valine at position 1225). With the correct diagnosis confirmed, the patient was referred to another highly regarded institution to begin a work-up for cardiac transplantation. Meanwhile, he was cautiously treated with the loop diuretic furosemide to manage his shortness of breath and peripheral edema.
Fifteen months later (13 weeks after being listed for transplant), the patient underwent successful cardiac transplantation.
On pathologic review of the patient’s extricated heart, the myocardium was found to be grossly thickened (see figure, above) and weighed 540 g; the average adult heart weighs 300 to 350 g, depending on the patient’s size.6 Congo Red staining showed extensive amyloid deposits with infiltration throughout the myocardium.
Ninety percent of the amyloid deposits were interstitial, 5% were in the vessels, and 5% were noted in a nodular pattern. The left ventricular cavity showed dilated and thickened walls. Intramural and extramural blood vessels were infiltrated with amyloid as well.
Six months after transplantation, the patient underwent diagnostic testing to assess the function and structure of his new heart. Cardiac catheterization was negative for coronary artery disease. Thirteen months posttransplantation, endomyocardial biopsy with Congo Red stain was negative for amyloid deposition or organ rejection.
About 24 months posttransplantation, the patient was taking tacrolimus, pravastatin, pantoprazole, dapsone, propanolol, colchicine, and donepezil. Stress echocardiography demonstrated normal right and left ventricular systolic function; no wall-motion abnormalities or left ventricular hypertrophy were detected, and the right atrium was of normal size. There was abnormal structural enlargement of the left atrium at the site of anastamosis—a common finding in cardiac transplant patients. The aortic, tricuspid, and mitral valves were all normal.
At that time, it was decided not to repeat endomyocardial biopsy because of normal results on molecular expression testing (a noninvasive technique called AlloMap®7-9), which is performed to assess for heart transplant rejection. The patient’s lipid panel remained within normal limits. CBC indicated persistent anemia and leukopenia. Urine protein and BNP test results were not available.
Since undergoing cardiac transplantation, the patient has resumed his normal routine activities, including some type of exercise five days per week. He said his diet is maintained in moderation. He denied shortness of breath, chest pain, dizziness, or edema. He has returned to full-time employment and has vacationed in Croatia, Italy, and Central America.
DISCUSSION
Familial amyloidosis is an autosomal dominant disease characterized by the production of mutated proteins, most commonly ATTR. Presence of the ATTR Val 122Ile allele has been reported in 3.9% of all black Americans, and in one study, 23% of black Americans diagnosed with cardiac amyloidosis at autopsy were heterozygous for this variant allele.10-12 ATTR Val122Ile usually manifests in the fifth
or sixth decade of life with its characteristic presentation of infiltrative/restrictive cardiomyopathy,13 resulting in heart failure and sometimes peripheral neuropathy.10,11,14
Pathophysiology
In patients with ATTR Val122Ile, cardiomyopathy results from the deposition of mutant protein fibrils in the cardiac muscle, leading to restricted heart wall motion10,15 and stiffening of the cardiac ventricles, with subsequent disruption of the diastolic filling properties of the cardiac muscle.16 Fluid overload and heart failure follow.3,10,17 The atrium of the heart dilates, and the walls of the ventricles become thickened and fibrous.18 Liepnieks and Benson6 reported that the cadaver heart of one Val122Ile patient infiltrated with amyloid protein fibrils weighed 725 g—more than double the weight of an average adult heart.
In patients with cardiac amyloidosis, ECG can detect arrhythmia, and echocardiography shows cardiac enlargement; however, as in the case patient, cardiac catheterization shows normal coronary arteries.15,16,19,20 Thus, previously healthy patients who present with heart failure and negative results on cardiac catheterization should undergo further work-up for cardiac amyloidosis.19
Amyloidosis affects all populations globally.10 In systemic amyloidosis, amyloid releases into the plasma, infiltrating and impairing multiple organs. Poor survival has been reported in patients with heart failure symptoms resulting from amyloid deposition.20,21
Types of Amyloidosis
Primary amyloidosis, the most common of the three amyloidosis types, can be systemic or localized.22 It occurs when protein fibrils, developed from immunoglobin light chains or monoclonal plasma cells and measuring 7 to 10 nm in diameter, adhere to the heart, kidneys, peripheral nerves, eyes, and other organs.5,11,20,23,24 Known for its relation to multiple myeloma,19 primary amyloidosis is associated with a poor prognosis.3,10
Secondary amyloidosis results from a chronic inflammatory disorder, such as rheumatoid arthritis or ankylosing spondylitis—conditions that trigger the production of amyloid proteins.3,10 This type has also been associated with substance abuse and AIDS.23
Familial or hereditary amyloidosis, according to Benson,10 is a group of diseases, each resulting from mutation in a specific protein. In the United States, the most common type of familial amyloidosis is ATTR.11 More than 100 mutant types of ATTR proteins have been identified, each involving a specific nationality or group of nationalities.10,11,23
Mutant ATTR amyloid, when deposited in specific organs, leads to their dysfunction and ultimate failure.6 ATTR may affect the cardiac, gastric, renal, ophthalmic, or nervous system. Depending on the ATTR variant, the resulting clinical features are age- and time-dependent, with onset most common between the third and fifth decade of life.10
Prevalence
The prevalence of ATTR Val 122Ile amyloidosis is reportedly high in West Africa, and in the US African-American population (3.9%).4,11,14,25,26 In a study conducted at a county hospital in Indianapolis, 3% of black newborns were found positive for ATTR Val122Ile through DNA sampling of umbilical cord blood.25 These statistics are of concern, as ATTR amyloidosis could be a significant health concern in a patient population that is already medically underserved.
Yamashita et al25 estimate that 1.35 million Americans of African-American descent may be affected by ATTR Val122Ile and vulnerable to restrictive cardiomyopathy–related heart failure and death. At the very least, this disorder can impair quality of life, especially in the presence of other comorbid conditions.
Clinical Presentation
The presence of exertional syncope at presentation is ominous, as it may be a marker of severe restrictive cardiomyopathy, postural hypotension due to excessive diuresis or autonomic neuropathy, ventricular arrhythmias from localized hypoperfusion, and rarely from cardiac tamponade due to pericardial involvement.27 Despite widespread involvement of the conduction system in specimens at autopsy, high-grade IV block is unusual.3
Diagnostic Studies
ECG. Both ECG and Holter monitoring can detect the arrhythmias and conduction disturbances (eg, first-degree AV block, low voltage patterns) associated with cardiac amyloidosis. Patients often experience syncopal and near-syncopal episodes as a result of conduction disturbances.28 Patients with conditions such as cardiac amyloidosis who present with severe heart failure are at high risk for sudden death secondary to conduction disturbances. Many have benefited from implanted defibrillators.29
Echocardiography. In patients with ATTR Val122Ile cardiac amyloidosis, echocardiography reveals thickened ventricular walls (ie, measuring ≥ 15 mm; normal, ≤ 11 mm).19 Amyloid-restrictive cardiomyopathy is associated with a marked dissociation between short- and long-axis systolic function, in cases in which left ventricular ejection fraction is normal.30
Echocardiography may demonstrate the characteristic specular or granular sparkling appearance that signifies advanced disease.15,21 Only a minority have this pattern in the myocardium, however, and changes in echocardiographic technology have made this finding less noticeable.30
MRI. Among more recently used diagnostic studies, cardiac magnetic resonance (CMR) has been reported to demonstrate late gadolinium enhancement (LGE) in perhaps 80% of patients with familial amyloidosis and cardiac involvement (as determined through biopsy and Congo Red stain). LGE-CMR shows darkening of the cardiac tissue, a common occurrence in amyloidosis.31
LGE is associated with increased thickness of both left and right ventricles, lower ECG voltage patterns, elevated BNP, and elevated troponin T.31,32 Globally, LGE is associated with the worst prognosis in patients with cardiac amyloidosis. Use of LGE-CMR testing can help facilitate early detection of cardiac amyloidosis in patients who may be vulnerable to cardiac damage.31
Cardiac catheterization. In patients with cardiac amyloidosis, cardiac catheterization usually shows normal coronary arteries.3
Diagnosis
Early diagnosis of ATTR cardiac amyloidosis is crucial to the patient’s survival; it should be ruled out in any African-American patient with unexplained heart failure and echocardiography showing increased wall thickness with a nondilated left ventricular cavity. Additional clues include significant proteinuria, hepatomegaly disproportionate to the degree of heart failure, or corresponding neuropathy. Known family history of the disease, along with variant type, allows for a prompt and correct diagnosis.10
It has been reported that most clinicians who encounter heart failure, particularly in black patients, do not consider amyloidosis in the differential diagnosis, because of the high prevalence of hypertension and congestive heart failure in this population.10,15,19 As a result, amyloidosis often goes undiagnosed.19
Findings of enlarged and thickened cardiac walls on echocardiography but normal coronary arteries on cardiac catheterization should alert the treating clinician to further work-up for cardiac amyloidosis.19 In such a patient, according to Kristen et al,21 endomyocardial biopsy with Congo Red staining is the gold standard for diagnosis of amyloidosis.
In ATTR Val122Ile familial amyloidosis, it is unclear whether patients who are homozygous for the disease present with symptoms at earlier onset with more progressive illness or die sooner than those who are heterozygous.33 Nevertheless, once the diagnosis is confirmed, it is important to determine the patient’s specific variant type by DNA testing so that appropriate treatment can be initiated and the patient’s prognosis evaluated.5,10,13
Treatment
For familial amyloidosis in general, some researchers advocate liver transplantation to remove the source of mutant amyloid protein and stop all deposition of amyloid fibrils; this procedure can be followed later by transplantation of other affected organs (including the heart).5,23 Maurer et al34 have reported improved one-year survival rates among patients with ATTR amyloidosis who underwent both cardiac and liver transplantation: 75%, versus 23% in patients who did not receive transplanted organs.
Management of cardiac amyloidosis usually requires a twofold approach: treating associated congestive heart failure, and preventing further deposition of amyloid.24 In the case patient (as in most patients with ATTR amyloidosis), heart transplantation was deemed the only life-sustaining treatment option.11,19,33
Pharmacotherapeutic options are limited for patients with ATTR Val122Ile familial amyloidosis. Conventional heart failure agents (eg, ACE inhibitors, angiotensin receptor blockers, digoxin, β-blockers, calcium channel blockers) can exacerbate heart failure symptoms, leading to a potentially life-threatening arrhythmia.3,11,19,24,35 Amyloid fibrils bind to digitalis, increasing susceptibility to digitalis toxicity; and to nifedipine, causing hemodynamic deterioration. Verapamil should be avoided, as it may induce severe left ventricular dysfunction. ACE inhibitors often provoke profound hypotension in primary amyloidosis.24,35
Diuretics, too (eg, furosemide, as was prescribed for the case patient), must be used with caution.3 These agents have been used to treat fluid overload and the resulting peripheral edema and shortness of breath found in ATTR Val122Ile patients who experience heart failure.36 According to Dubrey et al,5 cautious use of diuretics is necessary for management of heart failure symptoms in these patients.
Because the risk for intracardiac thrombus is high, anticoagulation (using agents other than β-blockers or calcium channel blockers) should be implemented unless compelling risks are involved.11,24 Amiodarone is relatively well tolerated for ventricular tachydysrhythmias and in atrial fibrillation if the goal is maintaining sinus rhythm.37
Regarding heart transplantation in patients with familial amyloidosis, Jacob et al33 hypothesize that since mutant amyloid protein is synthesized by the liver, it would take approximately 50 years for a transplanted heart to become affected by amyloid deposition. In a 59-year-old Afro-Caribbean man with familial amyloidosis who underwent cardiac transplantation, Hamour et al11 reported that the donor heart remained amyloid-free three years posttransplantation, as demonstrated by serial cardiac biopsy.
On the Horizon
Clinical trials are now under way to examine pharmacotherapeutic options for patients with ATTR amyloidosis. Now being examined in clinical trials, for example, is Fx-1006A, a drug that stabilizes ATTR and prevents the misfolding of the amyloid protein fibril, in turn preventing it from binding to the target organ.38 Similarly, ALN-TTR, a drug believed to prevent disease manifestation and possibly facilitate disease regression, is being investigated in early human trials.39
Additionally, the use of genetic testing is recommended in at-risk individuals to identify the TTR gene. Affected patients may benefit from prophylactic medical management, which would halt amyloidogenesis of TTR—and possibly treat the condition as well.35 Pharmacotherapeutic agents like diflunisal, an NSAID, antagonize the aggregation of TTR protein and hinder formation of the amyloid fibrils.40
CONCLUSION
ATTR Val122Ile familial amyloidosis is a rare disorder that causes abnormal synthesis of amyloid protein in the liver, which then infiltrates the cardiac structure, leading to restrictive cardiomyopathy and progressive heart failure. Patients who present with symptoms of heart failure, cardiac enlargement on echocardiography, and a finding of granular speckling patterns, though not specific on echocardiography, should prompt the health care provider to refer the patient to a cardiologist familiar with cardiac amyloidosis for further work-up.
Diagnosed patients must undergo genetic testing to determine the specific variant type so that prompt treatment can be initiated. In patients with ATTR Val122Ile familial amyloidosis, the treatment of choice is cardiac transplantation. Although the mutant amyloid protein continues to be synthesized in the liver, the donor heart is unlikely to become affected by this substance for many years. Appropriately treated patients can maintain good quality of life, free of heart failure.
REFERENCES
1. Lim RP, Srichai MB, Lee VS. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. AJR Am J Roentgenol. 2007;188 (6):1675-1681.
2. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17(3-4):101-104.
3. Kendall H. Cardiac amyloidosis. Crit Care Nurse. 2010;30(2):16-23.
4. Eriksson M, Büttener J, Todorov T, et al. Prevalence of germline mutations in the TTR gene in a consecutive series of surgical pathology specimens with AATR amyloid. Am J Surg Pathol. 2009;33(1):58-65.
5. Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97(1):75-84.
6. Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14(4):277-282.
7. XDx Expression Diagnostics. Allomap®: molecular expression testing (2004). www.allomap.com. Accessed May 14, 2012.
8. Mandras SA, Crespo J, Patel HM. Innovative application of immunologic principles in heart transplantation. Ochsner J. 2010;10(4):231-235.
9. Yamani MH, Taylor DO, Rodriguez R, et al. Transplant vasculopathy is associated with increased AlloMap gene expression score. J Heart Lung Transplant. 2007;26(4):403-406.
10. Benson MD. The hereditary amyloidoses. Best Pract Res Clin Rheumatol. 2003;17(6):909-927.
11. Hamour IM, Lachmann HJ, Goodman HJ, et al. Heart transplantation for homozygous familial transthyretin (TTR) V122I cardiac amyloidosis. Am J Transplant. 2008;8(5):1056-1059.
12. Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466-473.
13. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-361.
14. Askanas V, Engel WK, McFerrin J, Vattemi G. Transthyretin Val122Ile, accumulated Abeta, and inclusion-body myositis aspects in cultured muscle. Neurology. 2003;61(2):257-260.
15. Hamidi Asl K, Nakamura M, Yamashita T, Benson MD. Cardiac amyloidoses associated with the transthyretin lle122 mutation in a Caucasian family. Amyloid. 2001;8(4):263-269.
16. Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol. 2009;24(3): 214-220.
17. Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009;10 (8):iii23-iii33.
18. Bruce J. Getting to the heart of cardiomyopathies. Nursing. 2005;35(8):44-47.
19. Falk RH. The neglected entity of familial cardiac amyloidosis in African Americans. Ethn Dis. 2002;12(1):141-143.
20. Piper C, Butz T, Farr M, et al. How to diagnose cardiac amyloidosis early: impact of ECG, tissue Doppler echocardiography, and myocardial biopsy. Amyloid. 2010;17(1):1-9.
21. Kristen AV, Meyer FJ, Perz JB, et al. Risk stratification in cardiac amyloidosis: novel approaches. Transplantation. 2005;80(1 suppl):S151-S155.
22. Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179-183.
23. Picken MM. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens. 2007; 16(3):196-203.
24. Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011;124(9):1079-1085.
25. Yamashita T, Asl KH, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile 122 allele frequency in an African-American population. Amyloid. 2005;12(2):127-130.
26. Benson MD, Yazaki M, Magy N. Laboratory assessment of transthyretin amyloidosis. Clin Chem Lab Med. 2002;40(12):1262-1265.
27. Chamarthi B, Dubrey SW, Cha K, et al. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80(9):1242-1245.
28. Correia MJ, Coutinho CA, Conceiçao I, et al. Role of heart rate variability in the assessment of autonomic dysfunction in type I familial amyloidotic polyneuropathy. Folia Cardiol. 2005;12(suppl C):459-462.
29. Kadish A, Mehra M. Heart failure devices: Implantable cardioverter-defibrillators and biventricular pacing therapy. Circulation. 2005; 111(24):3327-3335.
30. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410-415.
31. Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155-164.
32. Fealey ME, Edwards WD, Buadi FK, et al. Echocardiographic features of cardiac amyloidosis presenting as endomyocardial disease in a 54-year-old male. J Cardiol. 2009;54(1):162-166.
33. Jacob EK, Edwards WD, Zucker M, et al. Homozygous transthyretin mutation in an African American male. J Mol Diagn. 2007; 9(1):127-131.
34. Maurer MS, Raina A, Hesdorffer C, et al. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplantation. 2007;83(5):539-545.
35. Buxbaum J, Alexander A, Koziol J, et al. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African-Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159(5):864-870.
36. Rose BD, Colucci WS. Use of diuretics in heart failure (2010). www.uptodate.com/con tents/use-of-diuretics-in-patients-with-heart-failure. Accessed May 14, 2012.
37. Trappe H-J. Treating critical supraventricular and ventricular arrhythmias. J Emerg Trauma Shock. 2010;3(2):143-152.
38. Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14(30):3219-3230.
39. Alnylam Pharmaceuticals. TTR Amyloidosis: ALN-TTR (2011). www.alnylam.com/Programs-and-Pipeline/Programs/index.php. Accessed May 14, 2012.
40. Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin: potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355-374.
Woman With Back Pain Unable To Walk
ANSWER
The radiograph demonstrates a fairly large mass on the medial aspect of the left upper lobe. Such findings are usually associated with bronchogenic carcinomas.
Among the documentation from the transferring facility was a copy of a CT scan of the patient’s chest. On that study, the mass is seen; it appears to extend to the posterior chest wall, with extensive involvement and destruction of the ribs and posterior elements of T4 and T5. There is also evidence of some spinal cord compression, which would explain the patient’s presenting complaint.
Subsequent CT-guided biopsy demonstrated the lesion to be a non–small cell carcinoma. Due to the already extensive involvement, surgery was not an option, and the patient was referred for palliative radiation therapy.
ANSWER
The radiograph demonstrates a fairly large mass on the medial aspect of the left upper lobe. Such findings are usually associated with bronchogenic carcinomas.
Among the documentation from the transferring facility was a copy of a CT scan of the patient’s chest. On that study, the mass is seen; it appears to extend to the posterior chest wall, with extensive involvement and destruction of the ribs and posterior elements of T4 and T5. There is also evidence of some spinal cord compression, which would explain the patient’s presenting complaint.
Subsequent CT-guided biopsy demonstrated the lesion to be a non–small cell carcinoma. Due to the already extensive involvement, surgery was not an option, and the patient was referred for palliative radiation therapy.
ANSWER
The radiograph demonstrates a fairly large mass on the medial aspect of the left upper lobe. Such findings are usually associated with bronchogenic carcinomas.
Among the documentation from the transferring facility was a copy of a CT scan of the patient’s chest. On that study, the mass is seen; it appears to extend to the posterior chest wall, with extensive involvement and destruction of the ribs and posterior elements of T4 and T5. There is also evidence of some spinal cord compression, which would explain the patient’s presenting complaint.
Subsequent CT-guided biopsy demonstrated the lesion to be a non–small cell carcinoma. Due to the already extensive involvement, surgery was not an option, and the patient was referred for palliative radiation therapy.
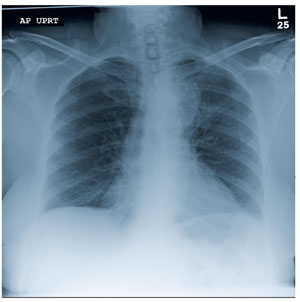
A 63-year-old woman is transferred to your facility for evaluation of acute lower extremity paralysis. She has had ongoing back pain for the past several months. Yesterday, she noticed her legs were dragging; last night, she was unable to walk, which prompted her trip to the local emergency department. She says she had “some sort of scan” there and was told that she had a tumor on her spine. She denies any injury or trauma. Her medical history is significant for reflux, hypertension, and extensive tobacco use. She is in no obvious distress, and her vital signs are normal. Physical exam demonstrates extreme weakness in both lower extremities, from the hips down and worse on the left side. She has decreased sensation and reflexes as well. As you review the rest of her transfer paperwork and try to find any imaging studies or reports, the radiology technician collects the patient for a chest radiograph (the result of which is shown). What is your impression?
Grand Rounds: Man, 62, With New-Onset Atrial Fibrillation
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
A 62-year-old black nursing home resident was transported to the hospital emergency department with fever of 102°F, new-onset atrial fibrillation (A-fib), and dementia. His medical history was significant for hypertension and multiple strokes.
His inpatient work-up for A-fib and dementia revealed a thyroid-stimulating hormone (TSH) level below 0.005 µIU/mL (normal range, 0.3 to 3.0 µIU/mL). Results of thyroid function testing (TFT) revealed a triiodothyronine (T3) level within normal range but a free thyroxine (T4) level of 2.9 ng/dL (normal range, 0.7 to 1.5 ng/dL) and a total T4 of 17.8 µg/dL (normal, 4.5 to 12.0 µg/dL). The abnormal TSH and T4 levels were considered suggestive of a thyrotoxic state, warranting an endocrinology consult. Cardiology was consulted regarding new-onset A-fib.
During history taking, the patient denied any shortness of breath, cough, palpitations, heat intolerance, anxiety, tremors, insomnia, dysphagia, diarrhea, dysuria, weight loss, or recent ingestion of iodine-containing medications or supplements.
On examination, the patient was febrile, with a blood pressure of 106/71 mm Hg; pulse, 74 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 98% to 99% on room air. ECG showed a normal sinus rhythm and a ventricular rate of 64 beats/min.
The patient's weight was 58.9 kg, and his height, 63" (BMI, 22.8). The patient had no skin changes, and his mucous membranes were slightly moist. The patient's head was atraumatic and normocephalic. His extraocular movements were intact, and his pupils were equal, round, and reactive to light, with nonicteric sclera. There was no proptosis or ophthalmoplegia. The patient's neck was supple, with no jugular venous distension, tracheal deviation, or thyromegaly.
The cardiovascular exam revealed an irregular heartbeat, and repeat ECG showed A-fib with a ventricular rate of 151 beats/min (see Figure 1). The patient's chest was clear, with no wheezing or rhonchi. The abdomen was soft and slightly obese, and bowel sounds were present. The neurologic examination revealed no hyperreflexia. The patient's mental status was altered at times and he was alert, awake, and oriented to others. His speech was slightly slow, and some left-sided weakness was noted.
As recommended during the endocrinology consult, the patient underwent an I-123 sodium iodide thyroid scan, which showed faint uptake at the base of the neck, slightly to the left of midline; and a 24-hour radioactive iodide uptake (RAIU), which measured 2.8% (normal range, 8% to 35%).
The patient's chest X-ray showed a right tracheal deviation not previously noted on physical examination (see Figure 2); the possible cause of a thyroid mass was considered. Subsequent ultrasonography of the thyroid revealed generally normal dimensions and parenchymal echogenicity. However, a large complex mass was detected, arising from the inferior pole of the thyroid and displacing the trachea toward the right (see Figure 3). According to the radiologist's notes, the mass contained both solid and cystic elements, scattered calcifications, and foci of flow on color Doppler. It measured about 6 cm in the largest (transverse) dimension. A 2.0-mm nodule was noted in the isthmus, slightly to the right of midline, consistent with multinodular goiter.
Following the cardiology consult, a diltiazem drip was initiated, but the patient was later optimized on flecainide for rhythm control and metoprolol for rate control. He was also initially anticoagulated using a heparin drip and bridged to warfarin, with target international normalized ratio (INR) between 2.0 and 3.0. Echocardiography revealed normal systolic function with ejection fraction of 55%, left ventricular hypertrophy, pulmonary artery systolic pressure of 35 mm Hg, and no pericardial effusions or valvular disease.
Regarding the patient's unexplained fever, results of chest imaging were negative for signs of pneumonia or atelectasis, which might have suggested a pulmonary cause. Urinalysis results were normal. Complete blood count showed no leukocytosis. The patient's fever subsided within 48 hours.
The differential diagnosis included Graves' disease, toxic multinodular goiter, Jod-Basedow syndrome, and subacute thyroiditis.
Graves' disease, an autoimmune disease with an unknown trigger, is the most common cause of hyperthyroidism. In affected patients, the thyroid gland overproduces thyroid hormones, leading to thyrotoxicosis. Thyrotoxicosis can result in multiple clinical signs and symptoms, including Graves' ophthalmopathy, pretibial myxedema, and goiter; TFT results typically include elevated T3 and T4 and low TSH.1-5 In the case patient (who had no history of thyroid disease, nor clinical signs or symptoms of Graves' disease), low uptake of iodine on thyroid scan precluded this diagnosis.
Toxic multinodular goiter, the second most common cause of hyperthyroidism, can be responsible for A-fib, tachycardia, and congestive heart failure.6,7 Iodine deficiency causes enlargement of the thyroid gland, where numerous nodules can develop, as seen in the case patient. These nodules can function independently, sometimes producing excess thyroid hormone; this leads to hyperplasia of the thyroid gland, resulting in a nontoxic multinodular goiter. From this goiter, a toxic multinodular goiter can emerge insidiously. However, in this condition, RAIU typically exceeds 30%; in the case patient, low 24-hour RAIU (2.8%) and the absence of functioning nodules on scanning made it possible to rule out this diagnosis.
Jod-Basedow syndrome refers to hyperthyroidism that develops as a result of administration of iodide, either as a dietary supplement or as IV contrast medium, or as an adverse effect of the antiarrhythmic drug amiodarone. This phenomenon is usually seen in a patient with endemic goiter.8-11 The relatively limited nature of the case patient's goiter and absence of a precipitating exposure to iodine made this diagnosis highly unlikely.
Subacute thyroiditis is a condition to which the patient's abnormal TFT results could reasonably be attributed. The patient had a substernal multinodular goiter that could not be palpated on physical examination, but it was visualized in the extended lower neck during thyroid scintigraphy.3 RAIU was minimal—a typical finding in this disorder,6 as TSH is suppressed by leakage of the excessive amounts of thyroid hormone. A tentative diagnosis of subacute thyroiditis was made.
As subacute thyroiditis is a self-limiting disorder, the patient was not started on any medications for hyperthyroidism but was advised to follow up with his primary care provider or an endocrinologist for repeat TFT and for fine-needle aspiration biopsy of the large thyroid nodule (a complex mass, containing cystic elements and calcifications, with a potential for malignancy) to rule out thyroid cancer.
Repeat ECG before discharge showed normal sinus rhythm with a ventricular rate of 74 beats/min. The patient was alert, awake, and oriented at discharge. He was continued on flecainide, metoprolol, and warfarin and advised to follow up with his primary care provider regarding his target INR.
DISCUSSION
The incidence of subacute thyroiditis, according to findings reported in 2003 from the Rochester Epidemiology Project in Olmsted County, Minnesota,12 is 12.1 cases per 100,000/year, with a higher incidence in women than men. It is most common in young adults and decreases with advancing age. Coxsackie virus, adenovirus, mumps, echovirus, influenza, and Epstein-Barr virus have been implicated in the disorder.12,13
Subacute thyroiditis is associated with a triphasic clinical course of hyperthyroidism, then hypothyroidism, then a return to normal thyroid function—as was seen in the case patient. Onset of subacute thyroiditis has been associated with recent viral infection, which may serve as a precipitant. The cause of this patient's high fever was never identified; thus, the etiology may have been viral.
The initial high thyroid hormone levels result from inflammation of thyroid tissue and release of preformed thyroid hormone into the circulation.6 At this point, TSH is suppressed and patients have very low RAIU, as was true in the case patient.
The condition is self-limiting and does not require treatment in the majority of patients, as TFT results return to normal levels within about two months.6 Patients can appear extremely ill due to thyrotoxicosis from subacute thyroiditis, but this usually lasts no longer than six to eight weeks.3 Subacute thyroiditis can be associated with atrial arrhythmia or heart failure.14,15
PATIENT OUTCOME
New-onset A-fib was attributed to the patient's thyrotoxicosis, which in turn was caused by subacute thyroiditis. He had a multinodular goiter, although he had not received any iodine supplements or IV contrast. As in most cases of subacute thyroiditis, no precipitating event was identified. However, given this patient's residence in a nursing facility and presentation with a high fever with no identifiable cause, a viral etiology for his subacute thyroiditis is possible.6
The patient's dementia may have been secondary to acute thyrotoxicosis, as his mental state improved during the hospital stay. His vitamin B12, folate, and A1C levels were within normal range. CT of the head showed multiple chronic infarcts and cerebral atrophy, and MRI of the brain indicated microvascular ischemic disease.
The patient was readmitted one month later for an episode of near-syncope (which, it was concluded, was a vasovagal episode). At that time, his TSH was found normal at 1.350 µIU/mL. Flecainide and metoprolol were discontinued; he was started on diltiazem for continued rate and rhythm control (as recommended by cardiology) and continued on warfarin.
CONCLUSION
In this case, subacute thyroiditis was most likely caused by a viral infection that led to destruction of the normal thyroid follicles and release of their preformed thyroid hormone into the circulation; this in turn led to sudden-onset A-fib. The diagnosis of subacute thyroiditis was suggested based on the abnormalities seen in this patient's TFT results, coupled with the suppressed RAIU—a typical finding in this disease.
Because subacute thyroiditis is a self-limiting condition, there is no role for antithyroid medication. Instead, treatment should be focused on relieving the patient's symptoms, such as ß-blockade or calcium channel blockers for tachycardia and corticosteroids or NSAIDs for neck pain.
REFERENCES
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-1248.
2. Delgado Hurtado JJ, Pineda M. Images in medicine: Graves' disease. N Engl J Med. 2011; 364(20):1955.
3. Al-Sharif AA, Abujbara MA, Chiacchio S, et al. Contribution of radioiodine uptake measurement and thyroid scintigraphy to the differential diagnosis of thyrotoxicosis. Hell J Nucl Med. 2010;13(2):132-137.
4. Buccelletti F, Carroccia A, Marsiliani D, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med. 2011;29(9):1158-1162.
5. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364(6):542-550.
6. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
7. Erickson D, Gharib H, Li H, van Heerden JA. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8(4):277-282.
8. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118(7):706-714.
9. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95(6):2529-2535.
10. El-Shirbiny AM, Stavrou SS, Dnistrian A, et al. Jod-Basedow syndrome following oral iodine and radioiodinated-antibody administration. J Nucl Med. 1997;38(11):1816-1817.
11. Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
12. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100-2105.
13. Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
14. Volpé R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253-255.
15. Lee SL. Subacute thyroiditis (2009). http://emedicine.medscape.com/article/125648-overview. Accessed April 17, 2012.
Woman with Malaise, Cough, and Shoulder Pain
ANSWER
The radiograph demonstrates a slightly elevated left hemidiaphragm, which is nonspecific, as well as normal to slightly increased lung markings. There is no definite infiltrate or consolidation noted.
Of note, there is a displaced fracture of the mid-distal left clavicle, which may be partially healed. There appears to be a focal lytic lesion within that area. The surrounding bone is extremely osteopenic as well.
These findings, especially in the absence of injury or trauma, raise the question of a pathologic fracture, and further workup is warranted. Subsequent workup on this patient demonstrated a large renal mass, which was felt to be, most likely, a metastatic lesion.
ANSWER
The radiograph demonstrates a slightly elevated left hemidiaphragm, which is nonspecific, as well as normal to slightly increased lung markings. There is no definite infiltrate or consolidation noted.
Of note, there is a displaced fracture of the mid-distal left clavicle, which may be partially healed. There appears to be a focal lytic lesion within that area. The surrounding bone is extremely osteopenic as well.
These findings, especially in the absence of injury or trauma, raise the question of a pathologic fracture, and further workup is warranted. Subsequent workup on this patient demonstrated a large renal mass, which was felt to be, most likely, a metastatic lesion.
ANSWER
The radiograph demonstrates a slightly elevated left hemidiaphragm, which is nonspecific, as well as normal to slightly increased lung markings. There is no definite infiltrate or consolidation noted.
Of note, there is a displaced fracture of the mid-distal left clavicle, which may be partially healed. There appears to be a focal lytic lesion within that area. The surrounding bone is extremely osteopenic as well.
These findings, especially in the absence of injury or trauma, raise the question of a pathologic fracture, and further workup is warranted. Subsequent workup on this patient demonstrated a large renal mass, which was felt to be, most likely, a metastatic lesion.
A 63-year-old woman presents to your clinic with complaints of general malaise, weakness, and occasional cough—symptoms that started a couple of days ago. Also, her left shoulder has been hurting her “more than usual.” She denies any fever, chills, nausea, or vomiting. She admits to smoking two packs of cigarettes per day and having hypertension. Otherwise, her medical history is unremarkable. During the physical exam, you observe that the patient is an older female in no obvious distress. She is afebrile, and the rest of her vital signs, including pulse oximetry, are normal. Her breath sounds demonstrate scattered rhonchi but overall are clear. She does have localized tenderness over her left shoulder, with decreased range of motion in that arm secondary to the pain and stiffness in it. You send a blood sample to the lab to check her complete blood count and obtain a chest radiograph, which is shown. What is your impression?
Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon
Over the past year, it has been hard to avoid news reports involving people getting high on “bath salts” and “incense” (also known as “Spice” or “K2”). Addiction treatment professionals have been overwhelmed by questions regarding why one would want to “snort bath salts” or “smoke incense.”
These substances are not what they appear to be. They are sold as bath salts and incense and are labeled “not for human consumption” simply to avoid regulation by the US Food and Drug Administration (FDA). In reality, they are powerful psychoactive drugs, with effects that mimic those of more commonly abused drugs such as amphetamines and marijuana. Until recently, they were legally available over the counter at quick-marts, head shops, and on the Internet. Because they are relatively new, they may not be detectable on routine urine drug screens, and users may be unaware of the specific chemicals contained in them.
These drugs, which we have collectively termed synthetic legal intoxicating drugs (SLIDs), are increasing dramatically in use.1–3 A survey of youths at a rave party indicated that 21% had used one of them on at least one occasion.4 The general impression held by the drug-using public is that SLIDs are relatively cheap, are not detected on standard urine drug screens, can produce a powerful high, and, until recently, were readily available through legitimate sources.
Physicians need to be aware of SLIDs in order to recognize and manage the intoxication syndromes associated with these substances when encountered in clinical practice, and in order to educate patients about their potential dangers.
SYNTHETIC CANNABINOIDS MARKETED AS INCENSE
Herbal incense products that could be smoked as an alternative to marijuana started appearing on the Internet in Europe in 2004. By 2008, when such products first appeared in the United States, their use in Europe was already widespread.
Initially, consumers were led to believe that such herbal smoking blends were safe, legal alternatives to marijuana, and that it was the proprietary blend of herbs that was responsible for the “natural” high. Spice, a specific brand name, was originally trademarked in England as incense and also as an herbal smoking product.5
Legal authorities, however, suspected that these herbal blends were adulterated with synthetic substances. In December 2008, the first such substance was found when Austrian authorities isolated a synthetic cannabinoid, JWH-018, from an herbal incense product.6 By the end of 2009, five other synthetic cannabinoids—CP-47,497, HU-210, JWH-073, JWH-250, and JWH-398—had been isolated from various herbal incense samples around the world.7
The synthetic cannabinoids in herbal incense products are not derived from the hemp plant (Cannabis sativa), but are synthesized in laboratories and are formulated to interact with the endogenous cannabinoid receptors in the brain to produce psychoactive effects.
Synthetic cannabinoids are full agonists; natural THC is only a partial agonist
Two types of cannabinoid receptors have been discovered in humans: CB1 and CB2. Both types are found in the central nervous system, and CB2 is also found extensively in the periphery. CB1 is the receptor responsible for the psychoactive effects of cannabinoids, including altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time.6 The physiologic role of CB2 remains uncertain.
The major psychoactive cannabinoid in naturally occurring marijuana is delta-9-tetrahydrocannabinol (THC). The so-called classic cannabinoids, such as HU-210, are analogues of THC and are based on its chemical structure. The rest of the synthetic cannabinoids commonly found in incense products differ in chemical structure from naturally occurring cannabinoids such as THC, but have activity at the CB1 receptor and are thus psychoactive.
Of clinical relevance is that THC is only a partial agonist at the CB1 receptor, while all synthetic cannabinoids commonly found in incense products are full agonists at CB1.7 This difference is important because partial agonists bind to receptors but stimulate them only partially and therefore exhibit a plateau effect in terms of dose vs clinical response. In contrast, full agonists have no ceiling on the dose-response relationship and therefore have a greater potential for overdose and severe toxic effects.
Despite uncertainties, use is widespread
Most of the synthetic cannabinoids in herbal incense products were developed for research purposes, and there are almost no reliable scientific data on their effects in humans. Of additional concern is that no research has been conducted on their pyrolytic effects, ie, how these chemicals are transformed when they are burned, such as when consumers smoke them. Furthermore, herbal incense products often vary in their active substances and concentrations, so consumers really do not know what they are getting.
Despite the many uncertainties, the use of these products is widespread. Data submitted to the US Drug Enforcement Administration (DEA) from a major toxicology laboratory indicated that from July through November of 2010, 3,700 samples tested positive for either JWH-018 or JWH-073. This report also indicated that 30% to 35% of specimens submitted by juvenile probation departments were positive for synthetic cannabinoids.8
MEDICAL CONCERNS OVER SYNTHETIC CANNABINOIDS
Amid the mysteries surrounding synthetic cannabinoids, one thing is clear: users are increasingly seeking medical attention. In 2010, there were 2,906 calls to poison control centers across the United States pertaining to “synthetic marijuana”; in 2011 there were 6,959 calls, and in January 2012, 639 such calls had been placed.9
The duration of the intoxicating effects of synthetic cannabinoids is generally longer than that of THC, but this seems to be variable. JWH-018, for instance, seems to have a shorter duration of action, at around 1 to 2 hours, while a longer, 5- to 6-hour intoxicating effect has been observed with CP-47,497.7,12
Serious adverse effects
Although the prevalence of serious adverse effects associated with the use of synthetic cannabinoids is not known, a number of serious complications have been recognized.
Seizures. One case of seizure has been reported in association with the use of synthetic cannabinoids, specifically JWH-018.12 This case involved a previously healthy 48-year-old man who had ingested a powder that was subsequently confirmed to be JWH-018, which he mixed with alcohol. Of further concern in this case is that this individual developed a refractory supraventricular tachycardia that required cardioversion on the first hospital day.
The authors speculated that the seizure may have been due to a dose-response mechanism that resulted in either the release of presynaptic excitatory neurotransmitters or the decreased release of inhibitory neurotransmitters. They further postulated that the supraventricular tachycardia could have been caused by one of two mechanisms previously reported in association with CB1 agonists: an increase in circulating catecholamines or heightened oxidative demands on the myocardium.12
Psychosis. The occurrence of psychotic symptoms such as hallucinations and paranoid delusions in association with synthetic cannabinoids is not surprising, given the well-documented link between marijuana use and psychosis.13,14
A case report of a 25-year-old patient with a 7-year history of recurrent psychosis that was initially triggered by cannabis use indicated that the use of 3 g of herbal incense on three occasions was associated with worsening of previous psychotic symptoms and the emergence of command and paranoid types of auditory hallucination.10
Semistructured interviews of 15 patients in a forensic rehabilitative service, all of whom had a history of psychotic illness, showed that 69% experienced symptoms consistent with psychotic relapse after smoking an herbal incense product containing JWH-018.15
It is possible that psychotic symptoms may be more prominent with synthetic cannabinoids than with natural marijuana because not only are synthetic cannabinoids more potent and work as full agonists, but, unlike marijuana, they do not contain cannabidiol, which is thought to have antipsychotic efficacy.10,16 However, the risk of psychotic symptoms in association with synthetic cannabinoid usage in otherwise healthy people is unknown.
Regulation lags behind
Growing concern over the perceived dangers posed by synthetic cannabinoids has led to a ban on some of the more common ones contained in herbal incense preparations. On March 1, 2011, the US DEA temporarily placed five synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) under schedule I (banned substances).
Such a ban, however, may be futile because there are an estimated 100 synthetic cannabinoids that have yet to enter the market, and when one is banned, a new one is likely to be introduced immediately as a replacement.8
SYNTHETIC STIMULANTS MARKETED AS BATH SALTS
Like the herbal incense products, “bath salts” may likewise not be what they appear to be. They too may be labeled “not for human consumption” in an effort to bypass laws governing mind-altering substances.
Several pharmacologically active substances have been marketed as bath salts. Two of the more common ingredients are 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylcathinone (mephedrone).
MDPV is a dopamine and norepineph-rine reuptake inhibitor that acts as a powerful stimulant. It has no FDA-approved medical use, but it is an analogue of the stimulant pyrovalerone, which was once used to treat chronic fatigue.17
MDPV seems to be the most common substance found in bath salt products in the United States. A sample of this substance was first seized on the streets by German authorities in 2007. A study in Finland conducted from August 2009 to September 2010 estimated that 5.7% of all arrests for driving under the influence (DUI) unrelated to alcohol consumption involved MDPV intoxication.17 In 2009, the National Forensic Laboratory Information System of the US DEA had seized only two samples of MDPV, but by 2010 that had increased to 161.18
Mephedrone is derived from phenethylamine and is closely related to cathinone, the active ingredient in the African khat plant (Catha edulis).19 Khat has a history of abuse, and the chemical structure of cathinone and its derivatives is similar to that of amphetamine.
Mephedrone, a powerful stimulant, is suspected of working as a monoamine reuptake inhibitor, and it may also directly induce the presynaptic release of monoamines.20 The net effect is an increase in serotonin, norepineph-rine, and dopamine levels at neuronal synapses.
Mephedrone was first described in 1929 by chemist Saem de Burnaga Sanchez, and it remained an obscure research chemical for many years.21 It was formally recognized as a drug of abuse in Europe in 2007, and by 2009 it was the sixth most frequently used such drug in Europe.8,22
Although MDPV and mephedrone are the most common psychoactive ingredients in bath salts, many other synthetic drugs have been found on the market.
A temporary ban
On September 7, 2011, the US government made it illegal to possess or sell any substance containing MDPV, mephedrone, or methy-lone. This temporary restriction was to remain in effect for 1 year to give the DEA time to collect data to support a move to permanently control these substances.3
Like synthetic cannabinoids, however, synthetic stimulants are very difficult to regulate because they are a large group of substances. As soon as one substance is outlawed, another synthetic stimulant will likely take its place.
MEDICAL CONCERNS REGARDING SYNTHETIC STIMULANTS
The medical and psychiatric sequelae that are associated with the use of bath salts have sent an increasing number of people to emergency rooms. The number of bath-salt-related calls to US poison control centers increased dramatically from 303 in 2010 to 4,720 by August 31, 2011. Most of these calls were related to tachycardia, agitation, hallucinations, extreme paranoia, delusions, and elevations in blood pressure.3
A report of 35 cases of people who had used bath salts and who had reported to Michigan emergency rooms between November 13, 2010, and March 31, 2011, indicated that agitation was present in 66%, tachycardia in 63%, delusions and hallucinations in 40%, seizure or tremor in 29%, hypertension in 23%, drowsiness in 23%, paranoia in 20%, and mydriasis in 20%; one patient was dead on arrival. Of the 34 patients who were alive on arrival, 17 (50%) were hospitalized, 15 were released, and 2 left against medical advice. In the patients in this study, 63% had injected the drug, 26% snorted it, and 11% ingested it orally.2 Toxicology results obtained during an autopsy on the one person who died revealed a high level of MDPV, and the coroner ruled that MDPV toxicity was the primary cause of death.2
Though the pharmacokinetic properties of mephedrone are unknown, James et al24 noted that an interesting feature is that its clinical effects seem to persist for more than 24 hours after the last exposure to the drug, which would not be expected based on the rapid elimination of other similar cathinones.
Sympathomimetic toxicity. Many of the symptoms listed in Table 2 are consistent with a sympathomimetic syndrome. In a case series reported by Regan et al,26 most of the 57 patients exhibited cardiovascular findings consistent with sympathomimetic toxicity.
In the study by James et al,24 one of the patients with chest pain had electrocardiographic changes consistent with acute myocardial infarction. Though it is not possible to conclude from a single case that mephedrone poses a risk of myocardial infarction, such a risk has been reported with khat.28 More research is needed to determine whether mephedrone poses a risk of cardiac events when used by people with or without an underlying cardiac condition.
Seizure also seems to be a relatively common feature associated with mephedrone use in case series of emergency room presentations. The US Centers for Disease Control and Prevention l2 reported that of 35 patients who had used bath salts, 40% experienced seizures or “tremors.” A recent case series27 of 15 patients presenting to an emergency department after mephedrone use reported that 20% had experienced seizures. In the study by James et al,24 four patients (3% of the total group) experienced seizures after using mephedrone. It should be noted that, aside from people presenting to emergency rooms, seizures are rarely reported in the wider population of mephedrone users.
Psychotic symptoms are also quite common in users of synthetic stimulants who present to emergency rooms, occurring, as previously stated, in 14% to 40% of cases.2,24
In a small case series, Penders and Gestring29 pointed out some common features in three patients who had used MDPV and had presented with psychosis: sleep problems, inattention, vivid hallucinations of intruders, fearfulness, and inability to remember many of the events surrounding their drug use. The authors concluded that the psychotic syndrome present in their three patients was indicative of a short-term delirium rather than a substance-induced psychosis based on the presence of attention deficits and memory problems. The patients in this series responded well to brief hospitalization and antipsychotic medications.
As with seizure, extreme presentations such as psychosis are infrequently mentioned except in people requiring treatment at a hospital. There are simply no data regarding the prevalence of psychotic symptoms in the larger group of all synthetic stimulant users.
SUSPECT SLID INTOXICATION IN ‘PSYCHIATRIC’ PATIENTS
Despite the temporary ban on the more common substances found in Spice and bath salts, it is premature for the medical community to breath a sigh of relief. Producers of these products are already likely bringing to market new ones containing similar but as yet nonbanned substances. Furthermore, such bans will do little to affect Internet commerce; rather than go to a head shop, consumers will order the products online.
Doctors in urgent care centers, emergency rooms, and on general medical floors should pay close attention to any patient without a known psychiatric history who is acting in a bizarre fashion. Most SLID-intoxicated patients will present with anxiety, agitation, and psychosis. Rather than assume that they are psychiatric patients, one should consider the possibility of SLID intoxication and pay close attention to the possible medical sequelae associated with SLID use, such as elevated blood pressure, tachycardia, and seizure.
Benzodiazepines, especially lorazepam (Ativan), have been the agents most commonly used to treat both agitation and seizures associated with SLID intoxication.
Antipsychotics should be used judiciously because of their propensity to lower the seizure threshold, and patients with synthetic stimulant toxicity are already at increased risk of seizure.
A psychiatric consult should be considered in the event of any suspected toxicity or for any patient whose behavior is difficult to manage.
Restraints may be needed in some circumstances when agitation cannot be controlled with benzodiazepines alone, to ensure safety for the patient as well as that of others in the emergency department.
Routine laboratory tests should be part of the workup of patients suspected of being under the influence of SLIDs. These include a complete blood cell count, complete metabolic panel, and urine toxicology (Table 3).23,25 A routine urine toxicology study will likely be negative, but either the patient or collateral information may give you a general idea of what the patient used, in which case the sample could be sent out for special tests for the more common substances found in herbal incense or bath salt products.
Electroencephalography may be indicated if there is any question as to whether the patient may have suffered a seizure. There should be a low threshold to order electrocardiography, especially in the case of synthetic stimulant intoxication.
Serial cardiac enzymes may be warranted if a patient with synthetic-stimulant intoxicated has chest pain.
Education, addiction treatment. Much is unknown about the risk of SLIDs, but given the adverse events reported in the literature, it seems likely that those with underlying cardiac or psychiatric issues may be at higher risk for the most serious drug-related consequences. With regard to synthetic stimulants, Winstock et al20 recommend a harm-reduction approach involving educating patients about avoiding the development of tolerance, not engaging in polydrug use, not injecting, and paying special attention to remaining cool and well hydrated.
Experience shows that once SLID patients get through their acute crisis and are no longer psychotic, they tend to be forthright in divulging what they used to get high. At that point, consideration should be given to consulting an addiction treatment specialist for further evaluation of the patient’s drug use history and for formulation of a treatment plan to help ensure that the patient doesn’t return to using these drugs.
SLIDs POSE A REAL CHALLENGE
SLIDs present a real challenge to law enforcement, governments, the public, and the addiction treatment community. There is currently no way to routinely test for these substances. Furthermore, any tests that are developed or laws that are enacted will be easily evaded, as there are many more synthetic substances waiting in the wings to be released.
Don’t be lulled into thinking that SLIDs are gone with the recent bans against some of the more common substances. More SLIDs are coming, and more morbidity should be expected in medical settings.
Doctors in emergency departments and other settings need to be prepared for the agitated and often psychotic presentation of SLID-intoxicated patients and should be ready with benzodiazepines, restraints, and a calm and reassuring manner. And for patients who present with psychotic symptoms, medical staff should also be ready to consider involuntary short-term commitment to an inpatient psychiatric unit.
Once they recover, patients need to be educated about the dangers of substances such as SLIDs that, because of their novelty, may be perceived as less dangerous alternatives to traditional illicit drugs.
- Wehrman J. Fake marijuana spurs more than 4,500 calls to US poison centers. American Association of Poison Control Centers (AAPCC), May 12, 2011. http://www.aapcc.org/dnn/Portals/0/prrel/updatedk2-may112011.pdf. Accessed February 20, 2012.
- Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan, November 13, 2010–March 31, 2011. MMWR Morb Mortal Wkly Rep 2011; 60( 19):624–627.
- Canton L. Poison control centers applaud DEA’s ban of bath salts. American Association of Poison Control Centers (AAPCC). September 8, 2011. http://www.mc.vanderbilt.edu/root/vumc.php?site=poisoncenter&doc=36028. Accessed February 20, 2012.
- Banta-Green C. “Club drug” use patterns and related behaviors in Seattle, King County. Survey data collected for STEPS (Stemming the Tide of Ecstasy through Prevention Strategies). Report to public health-Seattle, King County, Feb. 9, 2004.
- Erowid EF, Erowid F. Spice & spin-offs: prohibition’s high-tech cannabis substitutes. June 2009. http://www.erowid.org/chemicals/spice_product/spice_product_article1.shtml. Accessed February 20, 2012.
- Cary P. Spice, K2 and the problem of synthetic cannabinoids. Drug Court Practitioner Fact Sheet 2010; 6:2–3.
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA 2009 thematic paper—understanding the ‘Spice’ phenomenon. Luxembourg: Office for Official Publications of the European Communities, 2009.
- Rannazzi T. The dangers of synthetic cannabinoids and stimulants. Testimony before the Senate Caucus on International Narcotics Control, United States Senate. April 6, 2011. http://www.justice.gov/dea/speeches/110412_testimony.pdf. Accessed February 20, 2012.
- American Association of Poison Control Centers. Poison centers report calls about synthetic marijuana. www.AAPCC.org. Accessed February 22, 2012.
- Müller H, Sperling W, Körhrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 2010; 118:309–310.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011: 49;760–764.
- Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 2010; 197:157–162.
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. BMJ 2006; 332:172–175.
- Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328.
- Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend 2011; 117:152–157.
- Huffman JW, Thompson AL, Wilety JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem 2008; 16:322–335.
- Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int 2011; 210:195–200.
- Drug Enforcement Administration. 3,4-Methylenedioxypyrovalerone (MDPV). (Street names: “bath salts,” Ivory Wave,” “plant fertilizer,” “Vanilla Sky,” “Energy-1”). October 2011. www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf. Accessed February 20, 2012.
- Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol 1992; 70:77–86.
- Winstock AR, Marsen J, Mitcheson L. What should be done about mephedrone? BMJ 2010; 340:c1605.
- Saem de Burnaga Sanchez J. Sur un homologue de l’ éphédrine. Bulletin de la Societé Chimique de France 1929; 45:284–286.
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction 2011; 106:1991–1996.
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction 2011; 106:154–161.
- James D, Adams RD, Spears R, et al; National Poisons Information Service. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J 2011; 28:686–689.
- Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of 4-methylmethcathinone (4-MMC) with associated sympathomimetic toxicity. J Med Toxicol 2010; 6:327–330.
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J 2011; 28:1055–1058.
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J 2011; 28:280–282.
- Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol 2005; 59:574–581.
- Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: “bath salts.” Gen Hosp Psychiatry 2011; 33:525–526.
Over the past year, it has been hard to avoid news reports involving people getting high on “bath salts” and “incense” (also known as “Spice” or “K2”). Addiction treatment professionals have been overwhelmed by questions regarding why one would want to “snort bath salts” or “smoke incense.”
These substances are not what they appear to be. They are sold as bath salts and incense and are labeled “not for human consumption” simply to avoid regulation by the US Food and Drug Administration (FDA). In reality, they are powerful psychoactive drugs, with effects that mimic those of more commonly abused drugs such as amphetamines and marijuana. Until recently, they were legally available over the counter at quick-marts, head shops, and on the Internet. Because they are relatively new, they may not be detectable on routine urine drug screens, and users may be unaware of the specific chemicals contained in them.
These drugs, which we have collectively termed synthetic legal intoxicating drugs (SLIDs), are increasing dramatically in use.1–3 A survey of youths at a rave party indicated that 21% had used one of them on at least one occasion.4 The general impression held by the drug-using public is that SLIDs are relatively cheap, are not detected on standard urine drug screens, can produce a powerful high, and, until recently, were readily available through legitimate sources.
Physicians need to be aware of SLIDs in order to recognize and manage the intoxication syndromes associated with these substances when encountered in clinical practice, and in order to educate patients about their potential dangers.
SYNTHETIC CANNABINOIDS MARKETED AS INCENSE
Herbal incense products that could be smoked as an alternative to marijuana started appearing on the Internet in Europe in 2004. By 2008, when such products first appeared in the United States, their use in Europe was already widespread.
Initially, consumers were led to believe that such herbal smoking blends were safe, legal alternatives to marijuana, and that it was the proprietary blend of herbs that was responsible for the “natural” high. Spice, a specific brand name, was originally trademarked in England as incense and also as an herbal smoking product.5
Legal authorities, however, suspected that these herbal blends were adulterated with synthetic substances. In December 2008, the first such substance was found when Austrian authorities isolated a synthetic cannabinoid, JWH-018, from an herbal incense product.6 By the end of 2009, five other synthetic cannabinoids—CP-47,497, HU-210, JWH-073, JWH-250, and JWH-398—had been isolated from various herbal incense samples around the world.7
The synthetic cannabinoids in herbal incense products are not derived from the hemp plant (Cannabis sativa), but are synthesized in laboratories and are formulated to interact with the endogenous cannabinoid receptors in the brain to produce psychoactive effects.
Synthetic cannabinoids are full agonists; natural THC is only a partial agonist
Two types of cannabinoid receptors have been discovered in humans: CB1 and CB2. Both types are found in the central nervous system, and CB2 is also found extensively in the periphery. CB1 is the receptor responsible for the psychoactive effects of cannabinoids, including altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time.6 The physiologic role of CB2 remains uncertain.
The major psychoactive cannabinoid in naturally occurring marijuana is delta-9-tetrahydrocannabinol (THC). The so-called classic cannabinoids, such as HU-210, are analogues of THC and are based on its chemical structure. The rest of the synthetic cannabinoids commonly found in incense products differ in chemical structure from naturally occurring cannabinoids such as THC, but have activity at the CB1 receptor and are thus psychoactive.
Of clinical relevance is that THC is only a partial agonist at the CB1 receptor, while all synthetic cannabinoids commonly found in incense products are full agonists at CB1.7 This difference is important because partial agonists bind to receptors but stimulate them only partially and therefore exhibit a plateau effect in terms of dose vs clinical response. In contrast, full agonists have no ceiling on the dose-response relationship and therefore have a greater potential for overdose and severe toxic effects.
Despite uncertainties, use is widespread
Most of the synthetic cannabinoids in herbal incense products were developed for research purposes, and there are almost no reliable scientific data on their effects in humans. Of additional concern is that no research has been conducted on their pyrolytic effects, ie, how these chemicals are transformed when they are burned, such as when consumers smoke them. Furthermore, herbal incense products often vary in their active substances and concentrations, so consumers really do not know what they are getting.
Despite the many uncertainties, the use of these products is widespread. Data submitted to the US Drug Enforcement Administration (DEA) from a major toxicology laboratory indicated that from July through November of 2010, 3,700 samples tested positive for either JWH-018 or JWH-073. This report also indicated that 30% to 35% of specimens submitted by juvenile probation departments were positive for synthetic cannabinoids.8
MEDICAL CONCERNS OVER SYNTHETIC CANNABINOIDS
Amid the mysteries surrounding synthetic cannabinoids, one thing is clear: users are increasingly seeking medical attention. In 2010, there were 2,906 calls to poison control centers across the United States pertaining to “synthetic marijuana”; in 2011 there were 6,959 calls, and in January 2012, 639 such calls had been placed.9
The duration of the intoxicating effects of synthetic cannabinoids is generally longer than that of THC, but this seems to be variable. JWH-018, for instance, seems to have a shorter duration of action, at around 1 to 2 hours, while a longer, 5- to 6-hour intoxicating effect has been observed with CP-47,497.7,12
Serious adverse effects
Although the prevalence of serious adverse effects associated with the use of synthetic cannabinoids is not known, a number of serious complications have been recognized.
Seizures. One case of seizure has been reported in association with the use of synthetic cannabinoids, specifically JWH-018.12 This case involved a previously healthy 48-year-old man who had ingested a powder that was subsequently confirmed to be JWH-018, which he mixed with alcohol. Of further concern in this case is that this individual developed a refractory supraventricular tachycardia that required cardioversion on the first hospital day.
The authors speculated that the seizure may have been due to a dose-response mechanism that resulted in either the release of presynaptic excitatory neurotransmitters or the decreased release of inhibitory neurotransmitters. They further postulated that the supraventricular tachycardia could have been caused by one of two mechanisms previously reported in association with CB1 agonists: an increase in circulating catecholamines or heightened oxidative demands on the myocardium.12
Psychosis. The occurrence of psychotic symptoms such as hallucinations and paranoid delusions in association with synthetic cannabinoids is not surprising, given the well-documented link between marijuana use and psychosis.13,14
A case report of a 25-year-old patient with a 7-year history of recurrent psychosis that was initially triggered by cannabis use indicated that the use of 3 g of herbal incense on three occasions was associated with worsening of previous psychotic symptoms and the emergence of command and paranoid types of auditory hallucination.10
Semistructured interviews of 15 patients in a forensic rehabilitative service, all of whom had a history of psychotic illness, showed that 69% experienced symptoms consistent with psychotic relapse after smoking an herbal incense product containing JWH-018.15
It is possible that psychotic symptoms may be more prominent with synthetic cannabinoids than with natural marijuana because not only are synthetic cannabinoids more potent and work as full agonists, but, unlike marijuana, they do not contain cannabidiol, which is thought to have antipsychotic efficacy.10,16 However, the risk of psychotic symptoms in association with synthetic cannabinoid usage in otherwise healthy people is unknown.
Regulation lags behind
Growing concern over the perceived dangers posed by synthetic cannabinoids has led to a ban on some of the more common ones contained in herbal incense preparations. On March 1, 2011, the US DEA temporarily placed five synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) under schedule I (banned substances).
Such a ban, however, may be futile because there are an estimated 100 synthetic cannabinoids that have yet to enter the market, and when one is banned, a new one is likely to be introduced immediately as a replacement.8
SYNTHETIC STIMULANTS MARKETED AS BATH SALTS
Like the herbal incense products, “bath salts” may likewise not be what they appear to be. They too may be labeled “not for human consumption” in an effort to bypass laws governing mind-altering substances.
Several pharmacologically active substances have been marketed as bath salts. Two of the more common ingredients are 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylcathinone (mephedrone).
MDPV is a dopamine and norepineph-rine reuptake inhibitor that acts as a powerful stimulant. It has no FDA-approved medical use, but it is an analogue of the stimulant pyrovalerone, which was once used to treat chronic fatigue.17
MDPV seems to be the most common substance found in bath salt products in the United States. A sample of this substance was first seized on the streets by German authorities in 2007. A study in Finland conducted from August 2009 to September 2010 estimated that 5.7% of all arrests for driving under the influence (DUI) unrelated to alcohol consumption involved MDPV intoxication.17 In 2009, the National Forensic Laboratory Information System of the US DEA had seized only two samples of MDPV, but by 2010 that had increased to 161.18
Mephedrone is derived from phenethylamine and is closely related to cathinone, the active ingredient in the African khat plant (Catha edulis).19 Khat has a history of abuse, and the chemical structure of cathinone and its derivatives is similar to that of amphetamine.
Mephedrone, a powerful stimulant, is suspected of working as a monoamine reuptake inhibitor, and it may also directly induce the presynaptic release of monoamines.20 The net effect is an increase in serotonin, norepineph-rine, and dopamine levels at neuronal synapses.
Mephedrone was first described in 1929 by chemist Saem de Burnaga Sanchez, and it remained an obscure research chemical for many years.21 It was formally recognized as a drug of abuse in Europe in 2007, and by 2009 it was the sixth most frequently used such drug in Europe.8,22
Although MDPV and mephedrone are the most common psychoactive ingredients in bath salts, many other synthetic drugs have been found on the market.
A temporary ban
On September 7, 2011, the US government made it illegal to possess or sell any substance containing MDPV, mephedrone, or methy-lone. This temporary restriction was to remain in effect for 1 year to give the DEA time to collect data to support a move to permanently control these substances.3
Like synthetic cannabinoids, however, synthetic stimulants are very difficult to regulate because they are a large group of substances. As soon as one substance is outlawed, another synthetic stimulant will likely take its place.
MEDICAL CONCERNS REGARDING SYNTHETIC STIMULANTS
The medical and psychiatric sequelae that are associated with the use of bath salts have sent an increasing number of people to emergency rooms. The number of bath-salt-related calls to US poison control centers increased dramatically from 303 in 2010 to 4,720 by August 31, 2011. Most of these calls were related to tachycardia, agitation, hallucinations, extreme paranoia, delusions, and elevations in blood pressure.3
A report of 35 cases of people who had used bath salts and who had reported to Michigan emergency rooms between November 13, 2010, and March 31, 2011, indicated that agitation was present in 66%, tachycardia in 63%, delusions and hallucinations in 40%, seizure or tremor in 29%, hypertension in 23%, drowsiness in 23%, paranoia in 20%, and mydriasis in 20%; one patient was dead on arrival. Of the 34 patients who were alive on arrival, 17 (50%) were hospitalized, 15 were released, and 2 left against medical advice. In the patients in this study, 63% had injected the drug, 26% snorted it, and 11% ingested it orally.2 Toxicology results obtained during an autopsy on the one person who died revealed a high level of MDPV, and the coroner ruled that MDPV toxicity was the primary cause of death.2
Though the pharmacokinetic properties of mephedrone are unknown, James et al24 noted that an interesting feature is that its clinical effects seem to persist for more than 24 hours after the last exposure to the drug, which would not be expected based on the rapid elimination of other similar cathinones.
Sympathomimetic toxicity. Many of the symptoms listed in Table 2 are consistent with a sympathomimetic syndrome. In a case series reported by Regan et al,26 most of the 57 patients exhibited cardiovascular findings consistent with sympathomimetic toxicity.
In the study by James et al,24 one of the patients with chest pain had electrocardiographic changes consistent with acute myocardial infarction. Though it is not possible to conclude from a single case that mephedrone poses a risk of myocardial infarction, such a risk has been reported with khat.28 More research is needed to determine whether mephedrone poses a risk of cardiac events when used by people with or without an underlying cardiac condition.
Seizure also seems to be a relatively common feature associated with mephedrone use in case series of emergency room presentations. The US Centers for Disease Control and Prevention l2 reported that of 35 patients who had used bath salts, 40% experienced seizures or “tremors.” A recent case series27 of 15 patients presenting to an emergency department after mephedrone use reported that 20% had experienced seizures. In the study by James et al,24 four patients (3% of the total group) experienced seizures after using mephedrone. It should be noted that, aside from people presenting to emergency rooms, seizures are rarely reported in the wider population of mephedrone users.
Psychotic symptoms are also quite common in users of synthetic stimulants who present to emergency rooms, occurring, as previously stated, in 14% to 40% of cases.2,24
In a small case series, Penders and Gestring29 pointed out some common features in three patients who had used MDPV and had presented with psychosis: sleep problems, inattention, vivid hallucinations of intruders, fearfulness, and inability to remember many of the events surrounding their drug use. The authors concluded that the psychotic syndrome present in their three patients was indicative of a short-term delirium rather than a substance-induced psychosis based on the presence of attention deficits and memory problems. The patients in this series responded well to brief hospitalization and antipsychotic medications.
As with seizure, extreme presentations such as psychosis are infrequently mentioned except in people requiring treatment at a hospital. There are simply no data regarding the prevalence of psychotic symptoms in the larger group of all synthetic stimulant users.
SUSPECT SLID INTOXICATION IN ‘PSYCHIATRIC’ PATIENTS
Despite the temporary ban on the more common substances found in Spice and bath salts, it is premature for the medical community to breath a sigh of relief. Producers of these products are already likely bringing to market new ones containing similar but as yet nonbanned substances. Furthermore, such bans will do little to affect Internet commerce; rather than go to a head shop, consumers will order the products online.
Doctors in urgent care centers, emergency rooms, and on general medical floors should pay close attention to any patient without a known psychiatric history who is acting in a bizarre fashion. Most SLID-intoxicated patients will present with anxiety, agitation, and psychosis. Rather than assume that they are psychiatric patients, one should consider the possibility of SLID intoxication and pay close attention to the possible medical sequelae associated with SLID use, such as elevated blood pressure, tachycardia, and seizure.
Benzodiazepines, especially lorazepam (Ativan), have been the agents most commonly used to treat both agitation and seizures associated with SLID intoxication.
Antipsychotics should be used judiciously because of their propensity to lower the seizure threshold, and patients with synthetic stimulant toxicity are already at increased risk of seizure.
A psychiatric consult should be considered in the event of any suspected toxicity or for any patient whose behavior is difficult to manage.
Restraints may be needed in some circumstances when agitation cannot be controlled with benzodiazepines alone, to ensure safety for the patient as well as that of others in the emergency department.
Routine laboratory tests should be part of the workup of patients suspected of being under the influence of SLIDs. These include a complete blood cell count, complete metabolic panel, and urine toxicology (Table 3).23,25 A routine urine toxicology study will likely be negative, but either the patient or collateral information may give you a general idea of what the patient used, in which case the sample could be sent out for special tests for the more common substances found in herbal incense or bath salt products.
Electroencephalography may be indicated if there is any question as to whether the patient may have suffered a seizure. There should be a low threshold to order electrocardiography, especially in the case of synthetic stimulant intoxication.
Serial cardiac enzymes may be warranted if a patient with synthetic-stimulant intoxicated has chest pain.
Education, addiction treatment. Much is unknown about the risk of SLIDs, but given the adverse events reported in the literature, it seems likely that those with underlying cardiac or psychiatric issues may be at higher risk for the most serious drug-related consequences. With regard to synthetic stimulants, Winstock et al20 recommend a harm-reduction approach involving educating patients about avoiding the development of tolerance, not engaging in polydrug use, not injecting, and paying special attention to remaining cool and well hydrated.
Experience shows that once SLID patients get through their acute crisis and are no longer psychotic, they tend to be forthright in divulging what they used to get high. At that point, consideration should be given to consulting an addiction treatment specialist for further evaluation of the patient’s drug use history and for formulation of a treatment plan to help ensure that the patient doesn’t return to using these drugs.
SLIDs POSE A REAL CHALLENGE
SLIDs present a real challenge to law enforcement, governments, the public, and the addiction treatment community. There is currently no way to routinely test for these substances. Furthermore, any tests that are developed or laws that are enacted will be easily evaded, as there are many more synthetic substances waiting in the wings to be released.
Don’t be lulled into thinking that SLIDs are gone with the recent bans against some of the more common substances. More SLIDs are coming, and more morbidity should be expected in medical settings.
Doctors in emergency departments and other settings need to be prepared for the agitated and often psychotic presentation of SLID-intoxicated patients and should be ready with benzodiazepines, restraints, and a calm and reassuring manner. And for patients who present with psychotic symptoms, medical staff should also be ready to consider involuntary short-term commitment to an inpatient psychiatric unit.
Once they recover, patients need to be educated about the dangers of substances such as SLIDs that, because of their novelty, may be perceived as less dangerous alternatives to traditional illicit drugs.
Over the past year, it has been hard to avoid news reports involving people getting high on “bath salts” and “incense” (also known as “Spice” or “K2”). Addiction treatment professionals have been overwhelmed by questions regarding why one would want to “snort bath salts” or “smoke incense.”
These substances are not what they appear to be. They are sold as bath salts and incense and are labeled “not for human consumption” simply to avoid regulation by the US Food and Drug Administration (FDA). In reality, they are powerful psychoactive drugs, with effects that mimic those of more commonly abused drugs such as amphetamines and marijuana. Until recently, they were legally available over the counter at quick-marts, head shops, and on the Internet. Because they are relatively new, they may not be detectable on routine urine drug screens, and users may be unaware of the specific chemicals contained in them.
These drugs, which we have collectively termed synthetic legal intoxicating drugs (SLIDs), are increasing dramatically in use.1–3 A survey of youths at a rave party indicated that 21% had used one of them on at least one occasion.4 The general impression held by the drug-using public is that SLIDs are relatively cheap, are not detected on standard urine drug screens, can produce a powerful high, and, until recently, were readily available through legitimate sources.
Physicians need to be aware of SLIDs in order to recognize and manage the intoxication syndromes associated with these substances when encountered in clinical practice, and in order to educate patients about their potential dangers.
SYNTHETIC CANNABINOIDS MARKETED AS INCENSE
Herbal incense products that could be smoked as an alternative to marijuana started appearing on the Internet in Europe in 2004. By 2008, when such products first appeared in the United States, their use in Europe was already widespread.
Initially, consumers were led to believe that such herbal smoking blends were safe, legal alternatives to marijuana, and that it was the proprietary blend of herbs that was responsible for the “natural” high. Spice, a specific brand name, was originally trademarked in England as incense and also as an herbal smoking product.5
Legal authorities, however, suspected that these herbal blends were adulterated with synthetic substances. In December 2008, the first such substance was found when Austrian authorities isolated a synthetic cannabinoid, JWH-018, from an herbal incense product.6 By the end of 2009, five other synthetic cannabinoids—CP-47,497, HU-210, JWH-073, JWH-250, and JWH-398—had been isolated from various herbal incense samples around the world.7
The synthetic cannabinoids in herbal incense products are not derived from the hemp plant (Cannabis sativa), but are synthesized in laboratories and are formulated to interact with the endogenous cannabinoid receptors in the brain to produce psychoactive effects.
Synthetic cannabinoids are full agonists; natural THC is only a partial agonist
Two types of cannabinoid receptors have been discovered in humans: CB1 and CB2. Both types are found in the central nervous system, and CB2 is also found extensively in the periphery. CB1 is the receptor responsible for the psychoactive effects of cannabinoids, including altered consciousness, euphoria, relaxation, perceptual disturbances, intensified sensory experiences, cognitive impairment, and increased reaction time.6 The physiologic role of CB2 remains uncertain.
The major psychoactive cannabinoid in naturally occurring marijuana is delta-9-tetrahydrocannabinol (THC). The so-called classic cannabinoids, such as HU-210, are analogues of THC and are based on its chemical structure. The rest of the synthetic cannabinoids commonly found in incense products differ in chemical structure from naturally occurring cannabinoids such as THC, but have activity at the CB1 receptor and are thus psychoactive.
Of clinical relevance is that THC is only a partial agonist at the CB1 receptor, while all synthetic cannabinoids commonly found in incense products are full agonists at CB1.7 This difference is important because partial agonists bind to receptors but stimulate them only partially and therefore exhibit a plateau effect in terms of dose vs clinical response. In contrast, full agonists have no ceiling on the dose-response relationship and therefore have a greater potential for overdose and severe toxic effects.
Despite uncertainties, use is widespread
Most of the synthetic cannabinoids in herbal incense products were developed for research purposes, and there are almost no reliable scientific data on their effects in humans. Of additional concern is that no research has been conducted on their pyrolytic effects, ie, how these chemicals are transformed when they are burned, such as when consumers smoke them. Furthermore, herbal incense products often vary in their active substances and concentrations, so consumers really do not know what they are getting.
Despite the many uncertainties, the use of these products is widespread. Data submitted to the US Drug Enforcement Administration (DEA) from a major toxicology laboratory indicated that from July through November of 2010, 3,700 samples tested positive for either JWH-018 or JWH-073. This report also indicated that 30% to 35% of specimens submitted by juvenile probation departments were positive for synthetic cannabinoids.8
MEDICAL CONCERNS OVER SYNTHETIC CANNABINOIDS
Amid the mysteries surrounding synthetic cannabinoids, one thing is clear: users are increasingly seeking medical attention. In 2010, there were 2,906 calls to poison control centers across the United States pertaining to “synthetic marijuana”; in 2011 there were 6,959 calls, and in January 2012, 639 such calls had been placed.9
The duration of the intoxicating effects of synthetic cannabinoids is generally longer than that of THC, but this seems to be variable. JWH-018, for instance, seems to have a shorter duration of action, at around 1 to 2 hours, while a longer, 5- to 6-hour intoxicating effect has been observed with CP-47,497.7,12
Serious adverse effects
Although the prevalence of serious adverse effects associated with the use of synthetic cannabinoids is not known, a number of serious complications have been recognized.
Seizures. One case of seizure has been reported in association with the use of synthetic cannabinoids, specifically JWH-018.12 This case involved a previously healthy 48-year-old man who had ingested a powder that was subsequently confirmed to be JWH-018, which he mixed with alcohol. Of further concern in this case is that this individual developed a refractory supraventricular tachycardia that required cardioversion on the first hospital day.
The authors speculated that the seizure may have been due to a dose-response mechanism that resulted in either the release of presynaptic excitatory neurotransmitters or the decreased release of inhibitory neurotransmitters. They further postulated that the supraventricular tachycardia could have been caused by one of two mechanisms previously reported in association with CB1 agonists: an increase in circulating catecholamines or heightened oxidative demands on the myocardium.12
Psychosis. The occurrence of psychotic symptoms such as hallucinations and paranoid delusions in association with synthetic cannabinoids is not surprising, given the well-documented link between marijuana use and psychosis.13,14
A case report of a 25-year-old patient with a 7-year history of recurrent psychosis that was initially triggered by cannabis use indicated that the use of 3 g of herbal incense on three occasions was associated with worsening of previous psychotic symptoms and the emergence of command and paranoid types of auditory hallucination.10
Semistructured interviews of 15 patients in a forensic rehabilitative service, all of whom had a history of psychotic illness, showed that 69% experienced symptoms consistent with psychotic relapse after smoking an herbal incense product containing JWH-018.15
It is possible that psychotic symptoms may be more prominent with synthetic cannabinoids than with natural marijuana because not only are synthetic cannabinoids more potent and work as full agonists, but, unlike marijuana, they do not contain cannabidiol, which is thought to have antipsychotic efficacy.10,16 However, the risk of psychotic symptoms in association with synthetic cannabinoid usage in otherwise healthy people is unknown.
Regulation lags behind
Growing concern over the perceived dangers posed by synthetic cannabinoids has led to a ban on some of the more common ones contained in herbal incense preparations. On March 1, 2011, the US DEA temporarily placed five synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) under schedule I (banned substances).
Such a ban, however, may be futile because there are an estimated 100 synthetic cannabinoids that have yet to enter the market, and when one is banned, a new one is likely to be introduced immediately as a replacement.8
SYNTHETIC STIMULANTS MARKETED AS BATH SALTS
Like the herbal incense products, “bath salts” may likewise not be what they appear to be. They too may be labeled “not for human consumption” in an effort to bypass laws governing mind-altering substances.
Several pharmacologically active substances have been marketed as bath salts. Two of the more common ingredients are 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylcathinone (mephedrone).
MDPV is a dopamine and norepineph-rine reuptake inhibitor that acts as a powerful stimulant. It has no FDA-approved medical use, but it is an analogue of the stimulant pyrovalerone, which was once used to treat chronic fatigue.17
MDPV seems to be the most common substance found in bath salt products in the United States. A sample of this substance was first seized on the streets by German authorities in 2007. A study in Finland conducted from August 2009 to September 2010 estimated that 5.7% of all arrests for driving under the influence (DUI) unrelated to alcohol consumption involved MDPV intoxication.17 In 2009, the National Forensic Laboratory Information System of the US DEA had seized only two samples of MDPV, but by 2010 that had increased to 161.18
Mephedrone is derived from phenethylamine and is closely related to cathinone, the active ingredient in the African khat plant (Catha edulis).19 Khat has a history of abuse, and the chemical structure of cathinone and its derivatives is similar to that of amphetamine.
Mephedrone, a powerful stimulant, is suspected of working as a monoamine reuptake inhibitor, and it may also directly induce the presynaptic release of monoamines.20 The net effect is an increase in serotonin, norepineph-rine, and dopamine levels at neuronal synapses.
Mephedrone was first described in 1929 by chemist Saem de Burnaga Sanchez, and it remained an obscure research chemical for many years.21 It was formally recognized as a drug of abuse in Europe in 2007, and by 2009 it was the sixth most frequently used such drug in Europe.8,22
Although MDPV and mephedrone are the most common psychoactive ingredients in bath salts, many other synthetic drugs have been found on the market.
A temporary ban
On September 7, 2011, the US government made it illegal to possess or sell any substance containing MDPV, mephedrone, or methy-lone. This temporary restriction was to remain in effect for 1 year to give the DEA time to collect data to support a move to permanently control these substances.3
Like synthetic cannabinoids, however, synthetic stimulants are very difficult to regulate because they are a large group of substances. As soon as one substance is outlawed, another synthetic stimulant will likely take its place.
MEDICAL CONCERNS REGARDING SYNTHETIC STIMULANTS
The medical and psychiatric sequelae that are associated with the use of bath salts have sent an increasing number of people to emergency rooms. The number of bath-salt-related calls to US poison control centers increased dramatically from 303 in 2010 to 4,720 by August 31, 2011. Most of these calls were related to tachycardia, agitation, hallucinations, extreme paranoia, delusions, and elevations in blood pressure.3
A report of 35 cases of people who had used bath salts and who had reported to Michigan emergency rooms between November 13, 2010, and March 31, 2011, indicated that agitation was present in 66%, tachycardia in 63%, delusions and hallucinations in 40%, seizure or tremor in 29%, hypertension in 23%, drowsiness in 23%, paranoia in 20%, and mydriasis in 20%; one patient was dead on arrival. Of the 34 patients who were alive on arrival, 17 (50%) were hospitalized, 15 were released, and 2 left against medical advice. In the patients in this study, 63% had injected the drug, 26% snorted it, and 11% ingested it orally.2 Toxicology results obtained during an autopsy on the one person who died revealed a high level of MDPV, and the coroner ruled that MDPV toxicity was the primary cause of death.2
Though the pharmacokinetic properties of mephedrone are unknown, James et al24 noted that an interesting feature is that its clinical effects seem to persist for more than 24 hours after the last exposure to the drug, which would not be expected based on the rapid elimination of other similar cathinones.
Sympathomimetic toxicity. Many of the symptoms listed in Table 2 are consistent with a sympathomimetic syndrome. In a case series reported by Regan et al,26 most of the 57 patients exhibited cardiovascular findings consistent with sympathomimetic toxicity.
In the study by James et al,24 one of the patients with chest pain had electrocardiographic changes consistent with acute myocardial infarction. Though it is not possible to conclude from a single case that mephedrone poses a risk of myocardial infarction, such a risk has been reported with khat.28 More research is needed to determine whether mephedrone poses a risk of cardiac events when used by people with or without an underlying cardiac condition.
Seizure also seems to be a relatively common feature associated with mephedrone use in case series of emergency room presentations. The US Centers for Disease Control and Prevention l2 reported that of 35 patients who had used bath salts, 40% experienced seizures or “tremors.” A recent case series27 of 15 patients presenting to an emergency department after mephedrone use reported that 20% had experienced seizures. In the study by James et al,24 four patients (3% of the total group) experienced seizures after using mephedrone. It should be noted that, aside from people presenting to emergency rooms, seizures are rarely reported in the wider population of mephedrone users.
Psychotic symptoms are also quite common in users of synthetic stimulants who present to emergency rooms, occurring, as previously stated, in 14% to 40% of cases.2,24
In a small case series, Penders and Gestring29 pointed out some common features in three patients who had used MDPV and had presented with psychosis: sleep problems, inattention, vivid hallucinations of intruders, fearfulness, and inability to remember many of the events surrounding their drug use. The authors concluded that the psychotic syndrome present in their three patients was indicative of a short-term delirium rather than a substance-induced psychosis based on the presence of attention deficits and memory problems. The patients in this series responded well to brief hospitalization and antipsychotic medications.
As with seizure, extreme presentations such as psychosis are infrequently mentioned except in people requiring treatment at a hospital. There are simply no data regarding the prevalence of psychotic symptoms in the larger group of all synthetic stimulant users.
SUSPECT SLID INTOXICATION IN ‘PSYCHIATRIC’ PATIENTS
Despite the temporary ban on the more common substances found in Spice and bath salts, it is premature for the medical community to breath a sigh of relief. Producers of these products are already likely bringing to market new ones containing similar but as yet nonbanned substances. Furthermore, such bans will do little to affect Internet commerce; rather than go to a head shop, consumers will order the products online.
Doctors in urgent care centers, emergency rooms, and on general medical floors should pay close attention to any patient without a known psychiatric history who is acting in a bizarre fashion. Most SLID-intoxicated patients will present with anxiety, agitation, and psychosis. Rather than assume that they are psychiatric patients, one should consider the possibility of SLID intoxication and pay close attention to the possible medical sequelae associated with SLID use, such as elevated blood pressure, tachycardia, and seizure.
Benzodiazepines, especially lorazepam (Ativan), have been the agents most commonly used to treat both agitation and seizures associated with SLID intoxication.
Antipsychotics should be used judiciously because of their propensity to lower the seizure threshold, and patients with synthetic stimulant toxicity are already at increased risk of seizure.
A psychiatric consult should be considered in the event of any suspected toxicity or for any patient whose behavior is difficult to manage.
Restraints may be needed in some circumstances when agitation cannot be controlled with benzodiazepines alone, to ensure safety for the patient as well as that of others in the emergency department.
Routine laboratory tests should be part of the workup of patients suspected of being under the influence of SLIDs. These include a complete blood cell count, complete metabolic panel, and urine toxicology (Table 3).23,25 A routine urine toxicology study will likely be negative, but either the patient or collateral information may give you a general idea of what the patient used, in which case the sample could be sent out for special tests for the more common substances found in herbal incense or bath salt products.
Electroencephalography may be indicated if there is any question as to whether the patient may have suffered a seizure. There should be a low threshold to order electrocardiography, especially in the case of synthetic stimulant intoxication.
Serial cardiac enzymes may be warranted if a patient with synthetic-stimulant intoxicated has chest pain.
Education, addiction treatment. Much is unknown about the risk of SLIDs, but given the adverse events reported in the literature, it seems likely that those with underlying cardiac or psychiatric issues may be at higher risk for the most serious drug-related consequences. With regard to synthetic stimulants, Winstock et al20 recommend a harm-reduction approach involving educating patients about avoiding the development of tolerance, not engaging in polydrug use, not injecting, and paying special attention to remaining cool and well hydrated.
Experience shows that once SLID patients get through their acute crisis and are no longer psychotic, they tend to be forthright in divulging what they used to get high. At that point, consideration should be given to consulting an addiction treatment specialist for further evaluation of the patient’s drug use history and for formulation of a treatment plan to help ensure that the patient doesn’t return to using these drugs.
SLIDs POSE A REAL CHALLENGE
SLIDs present a real challenge to law enforcement, governments, the public, and the addiction treatment community. There is currently no way to routinely test for these substances. Furthermore, any tests that are developed or laws that are enacted will be easily evaded, as there are many more synthetic substances waiting in the wings to be released.
Don’t be lulled into thinking that SLIDs are gone with the recent bans against some of the more common substances. More SLIDs are coming, and more morbidity should be expected in medical settings.
Doctors in emergency departments and other settings need to be prepared for the agitated and often psychotic presentation of SLID-intoxicated patients and should be ready with benzodiazepines, restraints, and a calm and reassuring manner. And for patients who present with psychotic symptoms, medical staff should also be ready to consider involuntary short-term commitment to an inpatient psychiatric unit.
Once they recover, patients need to be educated about the dangers of substances such as SLIDs that, because of their novelty, may be perceived as less dangerous alternatives to traditional illicit drugs.
- Wehrman J. Fake marijuana spurs more than 4,500 calls to US poison centers. American Association of Poison Control Centers (AAPCC), May 12, 2011. http://www.aapcc.org/dnn/Portals/0/prrel/updatedk2-may112011.pdf. Accessed February 20, 2012.
- Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan, November 13, 2010–March 31, 2011. MMWR Morb Mortal Wkly Rep 2011; 60( 19):624–627.
- Canton L. Poison control centers applaud DEA’s ban of bath salts. American Association of Poison Control Centers (AAPCC). September 8, 2011. http://www.mc.vanderbilt.edu/root/vumc.php?site=poisoncenter&doc=36028. Accessed February 20, 2012.
- Banta-Green C. “Club drug” use patterns and related behaviors in Seattle, King County. Survey data collected for STEPS (Stemming the Tide of Ecstasy through Prevention Strategies). Report to public health-Seattle, King County, Feb. 9, 2004.
- Erowid EF, Erowid F. Spice & spin-offs: prohibition’s high-tech cannabis substitutes. June 2009. http://www.erowid.org/chemicals/spice_product/spice_product_article1.shtml. Accessed February 20, 2012.
- Cary P. Spice, K2 and the problem of synthetic cannabinoids. Drug Court Practitioner Fact Sheet 2010; 6:2–3.
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA 2009 thematic paper—understanding the ‘Spice’ phenomenon. Luxembourg: Office for Official Publications of the European Communities, 2009.
- Rannazzi T. The dangers of synthetic cannabinoids and stimulants. Testimony before the Senate Caucus on International Narcotics Control, United States Senate. April 6, 2011. http://www.justice.gov/dea/speeches/110412_testimony.pdf. Accessed February 20, 2012.
- American Association of Poison Control Centers. Poison centers report calls about synthetic marijuana. www.AAPCC.org. Accessed February 22, 2012.
- Müller H, Sperling W, Körhrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 2010; 118:309–310.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011: 49;760–764.
- Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 2010; 197:157–162.
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. BMJ 2006; 332:172–175.
- Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328.
- Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend 2011; 117:152–157.
- Huffman JW, Thompson AL, Wilety JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem 2008; 16:322–335.
- Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int 2011; 210:195–200.
- Drug Enforcement Administration. 3,4-Methylenedioxypyrovalerone (MDPV). (Street names: “bath salts,” Ivory Wave,” “plant fertilizer,” “Vanilla Sky,” “Energy-1”). October 2011. www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf. Accessed February 20, 2012.
- Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol 1992; 70:77–86.
- Winstock AR, Marsen J, Mitcheson L. What should be done about mephedrone? BMJ 2010; 340:c1605.
- Saem de Burnaga Sanchez J. Sur un homologue de l’ éphédrine. Bulletin de la Societé Chimique de France 1929; 45:284–286.
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction 2011; 106:1991–1996.
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction 2011; 106:154–161.
- James D, Adams RD, Spears R, et al; National Poisons Information Service. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J 2011; 28:686–689.
- Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of 4-methylmethcathinone (4-MMC) with associated sympathomimetic toxicity. J Med Toxicol 2010; 6:327–330.
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J 2011; 28:1055–1058.
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J 2011; 28:280–282.
- Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol 2005; 59:574–581.
- Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: “bath salts.” Gen Hosp Psychiatry 2011; 33:525–526.
- Wehrman J. Fake marijuana spurs more than 4,500 calls to US poison centers. American Association of Poison Control Centers (AAPCC), May 12, 2011. http://www.aapcc.org/dnn/Portals/0/prrel/updatedk2-may112011.pdf. Accessed February 20, 2012.
- Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan, November 13, 2010–March 31, 2011. MMWR Morb Mortal Wkly Rep 2011; 60( 19):624–627.
- Canton L. Poison control centers applaud DEA’s ban of bath salts. American Association of Poison Control Centers (AAPCC). September 8, 2011. http://www.mc.vanderbilt.edu/root/vumc.php?site=poisoncenter&doc=36028. Accessed February 20, 2012.
- Banta-Green C. “Club drug” use patterns and related behaviors in Seattle, King County. Survey data collected for STEPS (Stemming the Tide of Ecstasy through Prevention Strategies). Report to public health-Seattle, King County, Feb. 9, 2004.
- Erowid EF, Erowid F. Spice & spin-offs: prohibition’s high-tech cannabis substitutes. June 2009. http://www.erowid.org/chemicals/spice_product/spice_product_article1.shtml. Accessed February 20, 2012.
- Cary P. Spice, K2 and the problem of synthetic cannabinoids. Drug Court Practitioner Fact Sheet 2010; 6:2–3.
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA 2009 thematic paper—understanding the ‘Spice’ phenomenon. Luxembourg: Office for Official Publications of the European Communities, 2009.
- Rannazzi T. The dangers of synthetic cannabinoids and stimulants. Testimony before the Senate Caucus on International Narcotics Control, United States Senate. April 6, 2011. http://www.justice.gov/dea/speeches/110412_testimony.pdf. Accessed February 20, 2012.
- American Association of Poison Control Centers. Poison centers report calls about synthetic marijuana. www.AAPCC.org. Accessed February 22, 2012.
- Müller H, Sperling W, Körhrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 2010; 118:309–310.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011: 49;760–764.
- Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 2010; 197:157–162.
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. BMJ 2006; 332:172–175.
- Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328.
- Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend 2011; 117:152–157.
- Huffman JW, Thompson AL, Wilety JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem 2008; 16:322–335.
- Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int 2011; 210:195–200.
- Drug Enforcement Administration. 3,4-Methylenedioxypyrovalerone (MDPV). (Street names: “bath salts,” Ivory Wave,” “plant fertilizer,” “Vanilla Sky,” “Energy-1”). October 2011. www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf. Accessed February 20, 2012.
- Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol 1992; 70:77–86.
- Winstock AR, Marsen J, Mitcheson L. What should be done about mephedrone? BMJ 2010; 340:c1605.
- Saem de Burnaga Sanchez J. Sur un homologue de l’ éphédrine. Bulletin de la Societé Chimique de France 1929; 45:284–286.
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction 2011; 106:1991–1996.
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction 2011; 106:154–161.
- James D, Adams RD, Spears R, et al; National Poisons Information Service. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J 2011; 28:686–689.
- Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of 4-methylmethcathinone (4-MMC) with associated sympathomimetic toxicity. J Med Toxicol 2010; 6:327–330.
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J 2011; 28:1055–1058.
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J 2011; 28:280–282.
- Al-Motarreb A, Briancon S, Al-Jaber N, et al. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol 2005; 59:574–581.
- Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: “bath salts.” Gen Hosp Psychiatry 2011; 33:525–526.
KEY POINTS
- These products are sold under misleading names and deceptive labels to avoid regulation. Although several have recently been banned, many more are waiting to be brought to the market in a similar fashion.
- “Incense” products often contain synthetic cannabinoids; scientific research into their potential long-term effects in humans has been very limited.
- The potential for medical and psychiatric adverse events from synthetic cannabinoids may be heightened because of their full-agonist mechanism of action and because of the variable concentration and unregulated potency of these compounds in incense products.
- Bath salt intoxication, when encountered in the emergency department, may present as a psychiatric disorder or as a range of medical problems including cardiovascular issues, seizures, and hyperthermia.
Grand Rounds: Man, 30, With Traumatic Finger Amputations
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
A 30-year-old man sustained traumatic amputations of three of his left fingers while at work. A heavy object fell when a supporting chain snapped; although he moved quickly, three of his left distal fingers were caught under the object. He was flown to a hospital for definitive hand care.
During the preadmission history and physical, it was noted that the patient had mild right knee pain in addition to his finger injuries. He had experienced no head injury and no loss of consciousness or other complaints. He did not remember injuring his leg, although he said it might have been struck by the falling object; all he could remember was the injury to his fingers.
On physical exam, the only abnormality other than the man’s traumatic finger amputations was mild right knee edema and a small bruised area medially. Initially, he complained of mild pain on palpation and moderate pain with passive range of motion, but range of motion was intact. His pain was worse at the proximal, medial tibial area, and he had mild lateral mid-calf tenderness though no bruising. Distally, his right lower extremity motor and sensory function were intact, and he had no open wounds or skin breakdown. He had 2+ dorsalis pedis pulse and 1+ posterior tibial pulse. The toes were pink and warm with brisk capillary refill. All compartments were soft and compressible.
Upon review of his plain radiographs (three views of the right knee), the patient was noted to have a severely comminuted medial tibial plateau fracture that extended to the midline in the region of the tibial spine, with mild depression of the fracture fragments measuring about 6 mm (see Figures 1a, 1b, and 1c). This would translate into a Schatzker IV classification type1 fracture (see Figure 22,3).
The man was admitted and underwent emergent surgery on his injured left fingers that night. Further diagnostic knee testing was performed, including CT and MRI (see Figures 3 and 4). Three days after admission, the patient underwent open reduction and internal fixation (plating) of the right medial, proximal tibia (see Figure 5). He has done very well since without issue.
DISCUSSION
Fractures of the tibial plateau occur along the articular, or joint, surface of the proximal tibia. The plateau consists of lateral and medial condylar surfaces. These concave structures function as an articulation point for the cartilaginous menisci and the femoral condyles.4 The medial plateau and condyle are stronger than those of the lateral side, and therefore are less commonly fractured. An elevated intercondylar eminence divides the lateral and medial plateaus, providing an attachment site for the cruciate ligaments.3
The Schatzker classification system1 is most commonly used to describe the types of tibial plateau fractures (as seen in Figure 22,3). Schatzker et al1 divided these injuries into six categories, according to the impact of increased energy exerted onto the bone; the rising classification numbers indicate an increase in complexity and severity and usually a worsening prognosis.
The type I fracture represents a split fracture of the lateral plateau. Typically, a fracture of this type has depression or displacement measuring less than 4 mm.
Type II tibial plateau fractures, the most common Schatzker injury, are lateral plateau fractures with depression noted at the split. Not always evident on plain radiographs, this depression can often be overlooked, and the injury mistaken for a type I fracture. The depression is measured vertically from the lower edge of the medial plateau to the lowest depression point of the lateral plateau.5
Type III fractures, the least common among the Schatzker injuries, are described as pure depression fractures of the lateral plateau. These fractures do not have an appreciable “split” along the plateau and are usually found in older patients with osteopenia.2
The Schatzker type IV injury is a medial fracture with displacement or depression to a portion of the plateau. The fracture may be split or comminuted and may originate in the intercondylar area.
Type V fractures, also known as “bicondylar fractures,” affect both the lateral and medial plateau. An inverted “Y” pattern is frequently seen, and there may be additional involvement of the intercondylar eminence. Type V fractures differ from type VI injuries in that there is no disturbance of the metaphyseal-diaphyseal connection. Thus, type VI fractures also include a transverse component that separates the condyles (metaphysis) of the bone from the shaft (diaphysis). Wide variation is seen among type VI fractures.5
Assessment and Diagnosis
Originally termed “fender fractures” due to their frequent association with automobile injuries, fractures of the tibial plateau account for 1% of all fractures and 8% of fractures in elderly patients.6 Tibial plateau fractures occur when varus or valgus force is combined with axial loading. The fracture itself occurs when the femoral condyle is driven into the lateral or medial plateau. Bicondylar injuries occur when rigorous axial force is sustained in a fully extended knee.
Injuries may also include those of the ligaments or menisci, resulting in joint instability. Patients may present with generalized knee pain or difficulty bearing weight after sustaining injuries, such as being struck in a motor vehicle accident, being tackled, or falling from some height.4
Evaluation of a patient with a suspected tibial plateau fracture begins with a detailed history and thorough physical examination. Details regarding the mechanism of injury help to predict the pattern of the fracture and may indicate whether a more focused neurovascular exam is warranted. Low-energy injuries (often seen with Schatzker types I to III) or twisting injuries yield low suspicion for neurovascular injury or compartment syndrome. However, high-energy injuries (seen often with Schatzker types IV through VI) have a greater likelihood of resulting in complicated injuries that must be urgently or emergently treated.5
The popliteal artery is bound posteriorly and distally to the tibial plateau, and the peroneal nerve is located laterally and positioned around the fibular head. It is essential to assess for the popliteal pulse, as well as lateral lower-extremity sensation and the patient’s ability to dorsiflex. Along with motor and neurovascular injuries, presentation with a painful, strikingly swollen knee and difficulty bearing weight may indicate a hemarthrosis. Soft tissue injuries over the knee resulting from direct trauma may require a saline arthrogram to rule out communication into the joint. Furthermore, a thorough ligamentous exam of the knee is helpful in determining the extent of the injuries.3
Compartment syndrome is a serious, emergent complication that can occur with tibial plateau fractures, especially those sustained during high-energy trauma.7 The health care provider must perform serial exams of the lower extremity to assess for classic signs of compartment syndrome. Are the compartments tense or noncompressible? Does the patient have pain with passive stretch or with range of motion of the lower extremity? Is there pallor or paresthesia to the affected limb? Is the pulse weak or absent? Presence of any of the aforementioned symptoms should prompt a high suspicion for compartment syndrome, and the patient must be sent to an emergency department for urgent evaluation.5
Treatment/Rehabilitation
For Schatzker types I through III, intervention focuses on the articular cartilage examination and repair. Type IV injuries often include corresponding damage to the popliteal artery and/or peroneal nerve, and types V and VI often have such overlying soft tissue damage that temporary placement of an external fixation device is required before definitive surgical intervention can be performed.8
However, it should be noted that conservative versus surgical treatment is often debated among surgeons for treatment of Schatzker fractures. The management of a tibial plateau fracture depends on the physical demands and health of the patient, the severity of the fracture, the stability of the joint, and the surgeon’s skill set and preferences.4 Operative intervention is generally indicated for fractures with depressions greater than 2 mm (although some surgeons allow up to 1 cm of depression), fractures with joint instability, or open fractures. Injuries with concern for vascular injury or compartment syndrome are also treated both operatively and emergently. Postoperatively, patients will remain non–weight-bearing for eight to 12 weeks after surgery, and in the interim, depending on the surgeon’s preference, may or may not engage in active or passive range of motion of the knee.
Advocates of open reduction and internal fixation (ORIF) argue that this method allows for the fracture reduction and anatomic alignment to be directly examined, but they also acknowledge that this approach compromises a great deal of soft tissue surrounding the proximal tibia.9,10
In order to reduce soft tissue damage, some surgeons favor external fixation. Initial use of this surgical technique results in minimal soft tissue swelling and allows early range of motion. While the external fixation device is in place, there is a risk for pin site infection, and proper site care must be provided.6,11
Generally, the treatment of tibial plateau fractures is considered successful when the fracture reduction is sustained, the patient’s functional capacity and axial loading are restored, and the articular surface is reconstructed. As a rule, nonoperative treatment is reserved for tibial plateau fractures that are minimally depressed or nondisplaced, or for patients with advanced osteoporosis. Under these circumstances, after a non–weight-bearing period of four to eight weeks, patients will begin to perform protected and partial weight bearing using a hinged knee brace.2 Early active range of motion, along with isometric exercises to strengthen the quadriceps, is recommended.
Whether surgical or conservative treatment is chosen, complications of tibial plateau fractures include knee stiffness, wound breakdown and infection, malunion or nonunion, vascular or neurologic injury, prominent or painful hardware, or avascular necrosis of fragmented bone pieces.4
CONCLUSION
The primary care practitioner must never overlook patients’ complaints of knee pain, especially after varus or valgus stress injuries or axial loading injuries to the knee. The patient may be able to ambulate; however, ordering a radiograph is an easy method for evaluation and for ruling out tibial plateau injuries. If there is any question regarding the presence of fracture with plain radiographs and/or the clinical exam warrants it, CT is an appropriate second diagnostic intervention.
Should a tibial plateau fracture present in a primary care or urgent care setting, thorough examination of neurovascular status and risk for compartment syndrome must be done urgently, followed by a referral to an orthopedic surgeon or emergency department.
REFERENCES
1. Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience, 1968–1975. Clin Orthop Relat Res. 1979;(138): 94-104.
2. Marsh JL. Tibial plateau fractures. In: Bucholz RW, Court-Brown CM, Heckman HD, Tornetta P. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1780-1831.
3. Egol K, Koval KJ, Zuckerman JD. Tibial plateau. In: Egol K, Koval KJ, Zuckerman JD. Handbook of Fractures. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:455-463.
4. Fenton PP, Porter KK. Tibial plateau fractures: a review. Trauma. 2011;13(3):181-187.
5. Markhardt BK, Gross JM, Monu JU. Schatzker classification of tibial plateau fractures: use of CT and MR imaging improves assessment. Radiographics. 2009;29(2):585-597.
6. Lewis C. Does the mode of fixation of tibial plateau fractures, i.e. external fixation versus internal fixation, influence the time to union? A systematic review of the literature. Eur J Orthopaed Surg Traumatol. 2008;18(5):365-370.
7. Weinlein J, Schmidt A. Acute compartment syndrome in tibial plateau fractures—beware! J Knee Surg. 2010;31(1):9-16.
8. te Stroet MA, Holla M, Biert J, van Kampen A. The value of CT scan compared to plain radiographs for the classification and treatment plan in tibial plateau fractures. Emerg Radiol. 2011;18(4):279-283.
9. Musahl V, Tarkin I, Kobbe P, et al. New trends and techniques in open reduction and internal fixation of fractures of the tibial plateau. J Bone Joint Surg Br. 2009;91(4):426-433.
10. Toro-Arbelaez JB, Gardner MJ, Shindle MK, et al. Open reduction and internal fixation of intraarticular tibial plateau nonunions. Injury. 2007;38(3):378-383.
11. Marsh JL, Smith ST, Do TT. External fixation and limited internal fixation for complex fractures of the tibial plateau. J Bone Joint Surg Am. 1995;77(5):661-673.
Man Fell Off Roof
ANSWER
The radiograph shows a comminuted fracture of the os calcis, as well as a comminuted fracture of the navicular bone. CT on this patient was pending to further assess for additional fractures, and the patient will likely undergo open reduction and internal fixation for definitive treatment.
ANSWER
The radiograph shows a comminuted fracture of the os calcis, as well as a comminuted fracture of the navicular bone. CT on this patient was pending to further assess for additional fractures, and the patient will likely undergo open reduction and internal fixation for definitive treatment.
ANSWER
The radiograph shows a comminuted fracture of the os calcis, as well as a comminuted fracture of the navicular bone. CT on this patient was pending to further assess for additional fractures, and the patient will likely undergo open reduction and internal fixation for definitive treatment.
A 51-year-old man is brought to your facility for evaluation of right foot pain after sustaining a fall. He was working on top of his house when he lost his balance and fell approximately 15 to 20 feet. He states he landed on his feet and has the above complaint. His medical history is significant for hypertension and heart disease. He regularly works as a truck driver and smokes one to two packs of cigarettes per day. His exam shows a middle-aged male who is uncomfortable but in no obvious distress. Primary survey is normal. His right foot shows no obvious deformity. There is some swelling and moderate tenderness over the dorsal aspect, as well as in the area of the heel. Pulses are present, sensation is intact, and good capillary refill time is noted. Portable radiograph of the right foot is obtained. What is your impression?