User login
Cardiogenic shock: From ECMO to Impella and beyond
A 43-year-old man presented to a community hospital with acute chest pain and shortness of breath and was diagnosed with anterior ST-elevation myocardial infarction. He was a smoker with a history of alcohol abuse, hypertension, and hyperlipidemia, and in the past he had undergone percutaneous coronary interventions to the right coronary artery and the first obtuse marginal artery.
Angiography showed total occlusion in the left anterior descending artery, 90% stenosis in the right coronary artery, and mild disease in the left circumflex artery. A drug-eluting stent was placed in the left anterior descending artery, resulting in good blood flow.
However, his left ventricle continued to have severe dysfunction. An intra-aortic balloon pump was inserted. Afterward, computed tomography showed subsegmental pulmonary embolism with congestion. His mean arterial pressure was 60 mm Hg (normal 70–110), central venous pressure 12 mm Hg (3–8), pulmonary artery pressure 38/26 mm Hg (15–30/4–12), pulmonary capillary wedge pressure 24 mm Hg (2–15), and cardiac index 1.4 L/min (2.5–4).
The patient was started on dobutamine and norepinephrine and transferred to Cleveland Clinic on day 2. Over the next day, he had runs of ventricular tachycardia, for which he was given amiodarone and lidocaine. His urine output was low, and his serum creatinine was elevated at 1.65 mg/dL (baseline 1.2, normal 0.5–1.5). Liver function tests were also elevated, with aspartate aminotransferase at 115 U/L(14–40) and alanine aminotransferase at 187 U/L (10–54).
Poor oxygenation was evident: his arterial partial pressure of oxygen was 64 mm Hg (normal 75–100). He was intubated and given 100% oxygen with positive end-expiratory pressure of 12 cm H2O.
Echocardiography showed a left ventricular ejection fraction of 15% (normal 55%–70%) and mild right ventricular dysfunction.
ECMO and then Impella placement
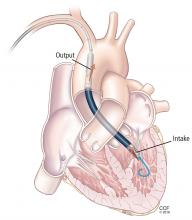
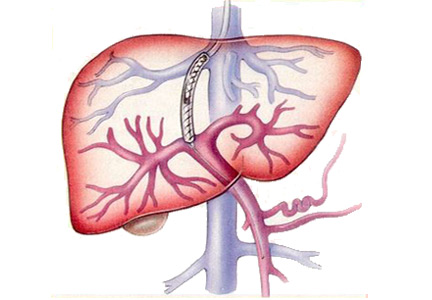
On his third hospital day, a venoarterial extracorporeal membrane oxygenation (ECMO) device was placed peripherally (Figure 1).
His hemodynamic variables stabilized, and he was weaned off dobutamine and norepinephrine. Results of liver function tests normalized, his urinary output increased, and his serum creatinine dropped to a normal 1.0 mg/dL. However, a chest radiograph showed pulmonary congestion, and echocardiography now showed severe left ventricular dysfunction.
On hospital day 5, the patient underwent surgical placement of an Impella 5.0 device (Abiomed, Danvers, MA) through the right axillary artery in an effort to improve his pulmonary edema. The ECMO device was removed. Placement of a venovenous ECMO device was deemed unnecessary when oxygenation improved with the Impella.
Three days after Impella placement, radiography showed improved edema with some remaining pleural effusion.
ACUTE CARDIOGENIC SHOCK
Cardiogenic shock remains a challenging clinical problem: patients with it are among the sickest in the hospital, and many of them die. ECMO was once the only therapy available and is still widely used. However, it is a 2-edged sword; complications such as bleeding, infection, and thrombosis are almost inevitable if it is used for long. Importantly, patients are usually kept intubated and bedridden.
In recent years, new devices have become available that are easier to place (some in the catheterization laboratory or even at the bedside) and allow safer bridging to recovery, transplant, or other therapies.
This case illustrates the natural history of cardiogenic shock and the preferred clinical approach: ie, ongoing evaluation that permits rapid response to evolving challenges.
In general, acute cardiogenic shock occurs within 24 to 48 hours after the initial insult, so even if a procedure succeeds, the patient may develop progressive hypotension and organ dysfunction. Reduced cardiac output causes a downward spiral with multiple systemic and inflammatory processes as well as increased nitric oxide synthesis, leading to progressive decline and eventual end-organ dysfunction.
Continuously evaluate
The cardiac team should continuously assess the acuity and severity of a patient’s condition, with the goals of maintaining end-organ perfusion and identifying the source of problems. Refractory cardiogenic shock, with tissue hypoperfusion despite vasoactive medications and treatment of the underlying cause, is associated with in-hospital mortality rates ranging from 30% to 50%.1,2 The rates have actually increased over the past decade, as sicker patients are being treated.
When a patient presents with cardiogenic shock, we first try a series of vasoactive drugs and usually an intra-aortic balloon pump (Figure 2). We then tailor treatment depending on etiology. For example, a patient may have viral myocarditis and may even require a biopsy.
If cardiogenic shock is refractory, mechanical circulatory support devices can be a short-term bridge to either recovery or a new decision. A multidisciplinary team should be consulted to consider transplant, a long-term device, or palliative care. Sometimes a case requires “bridging to a bridge,” with several devices used short-term in turn.
Prognostic factors in cardiogenic shock
Several tools help predict outcome in a severely ill patient. End-organ function, indicated by blood lactate levels and estimated glomerular filtration rate, is perhaps the most informative and should be monitored serially.
CardShock3 is a simple scoring system based on age, mental status at presentation, laboratory values, and medical history. Patients receive 1 point for each of the following factors:
- Age > 75
- Confusion at presentation
- Previous myocardial infarction or coronary artery bypass grafting
- Acute coronary syndrome etiology
- Left ventricular ejection fraction < 40%
- Blood lactate level between 2 and 4 mmol/L, inclusively (2 points for lactate levels > 4 mmol/L)
- Estimated glomerular filtration rate between 30 and 60 mL/min/1.73 m2, inclusively (2 points if < 30 mL/min/1.73 m2).
Thus, scores range from 0 (best) to 9 (worst). A score of 0 to 3 points was associated with a 9% risk of death in the hospital, a score of 4 or 5 with a risk of 36%, and a score of 6 through 9 with a risk of 77%.3
The Survival After Veno-arterial ECMO (SAVE) score (www.save-score.com) is a prediction tool derived from a large international ECMO registry.4 It is based on patient age, diagnosis, and indicators of end-organ dysfunction. Scores range from –35 (worst) to +7 (best).
The mortality rate associated with postcardiotomy cardiogenic shock increases with the amount of inotropic support provided. In a 1996–1999 case series of patients who underwent open-heart surgery,5 the hospital mortality rate was 40% in those who received 2 inotropes in high doses and 80% in those who received 3. A strategy of early implementation of mechanical support is critical.
Selection criteria for destination therapy
Deciding whether a patient should receive a long-term device is frequently a challenge. The decision often must be based on limited information about not only the medical indications but also psychosocial factors that influence long-term success.
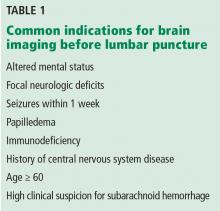
The Centers for Medicare and Medicaid Services have established criteria for candidates for left ventricular assist devices (LVADs) as destination therapy.6 Contraindications established for heart transplant should also be considered (Table 1).
CASE REVISITED
Several factors argued against LVAD placement in our patient. He had no health insurance and had been off medications. He smoked and said he consumed 3 hard liquor drinks per week. His Stanford Integrated Psychosocial Assessment for Transplantation score was 30 (minimally acceptable). He had hypoxia with subsegmental pulmonary edema, a strong contraindication to immediate transplant.
On the other hand, he had only mild right ventricular dysfunction. His CardShock score was 4 (intermediate risk, based on lactate 1.5 mmol/L and estimated glomerular filtration rate 52 mL/min/1.73 m2). His SAVE score was –9 (class IV), which overall is associated with a 30% risk of death (low enough to consider treatment).
During the patient’s time on temporary support, the team had the opportunity to better understand him and assess his family support and his ability to handle a permanent device. His surviving the acute course bolstered the team’s confidence that he could enjoy long-term survival with destination therapy.
CATHETERIZATION LABORATORY DEVICE CAPABILITIES
Although most implantation procedures are done in the operating room, they are often done in the catheterization laboratory because patients undergoing catheterization may not be stable enough for transfer, or an emergency intervention may be required during the night. Catheterization interventionists are also an important part of the team to help determine the best approach for long-term therapy.
The catheterization laboratory has multiple acute intervention options. Usually, decisions must be made quickly. In general, patients needing mechanical support are managed as follows:
- Those who need circulation support and oxygenation receive ECMO
- Those who need circulation support alone because of mechanical issues (eg, myocardial infarction) are considered for an intra-aortic balloon pump, Impella, or TandemHeart pump (Cardiac Assist, Pittsburgh, PA).
Factors that guide the selection of a temporary pump include:
- Left ventricular function
- Right ventricular function
- Aortic valve stenosis (some devices cannot be inserted through critical aortic stenosis)
- Aortic regurgitation (can affect some devices)
- Peripheral artery disease (some devices are large and must be placed percutaneously).
CHOOSING AMONG PERCUTANEOUS DEVICES
Circulatory support in cardiogenic shock improves outcomes, and devices play an important role in supporting high-risk procedures. The goal is not necessarily to use the device throughout the hospital stay. Acute stabilization is most important initially; a more considered decision about long-term therapy can be made when more is known about the patient.
Patient selection is the most important component of success. However, randomized data to support outcomes with the various devices are sparse and complicated by the critically ill state of the patient population.
SHORT-TERM CIRCULATORY SUPPORT: ECMO, IMPELLA, TANDEMHEART
A menu of options is available for temporary mechanical support. Options differ by their degree of circulatory support and ease of insertion (Table 2).
ECMO: A fast option with many advantages
ECMO has evolved and now can be placed quickly. A remote diagnostic platform such as CardioHub permits management at the bedside, in the medical unit, or in the cardiac intensive care unit.7
ECMO has several advantages. It can be used during cardiopulmonary bypass, it provides oxygenation, it is the only option in the setting of lung injury, it can be placed peripherally (without thoracotomy), and it is the only percutaneous option for biventricular support.
ECMO also has significant disadvantages
ECMO is a good device for acute resuscitation of a patient in shock, as it offers quick placement and resuscitation. But it is falling out of favor because of significant disadvantages.
Its major drawback is that it provides no left ventricular unloading. Although in a very unstable patient ECMO can stabilize end organs and restore their function, the lack of left ventricular unloading and reduced ventricular work threaten the myocardium. It creates extremely high afterload; therefore, in a left ventricle with poor function, wall tension and myocardial oxygen demand increase. Multiple studies have shown that coronary perfusion worsens, especially if the patient is cannulated peripherally. Because relative cerebral hypoxia occurs in many situations, it is imperative to check blood saturations at multiple sites to determine if perfusion is adequate everywhere.
Ineffective left ventricular unloading with venoarterial ECMO is managed in several ways. Sometimes left ventricular distention is slight and the effects are subtle. Left ventricular distention causing pulmonary edema can be addressed with:
- Inotropes (in moderate doses)
- Anticoagulation to prevent left ventricular thrombus formation
- An intra-aortic balloon pump. Most patients on ECMO already have an intra-aortic balloon pump in place, and it should be left in to provide additional support. For those who do not have one, it should be placed via the contralateral femoral artery.
If problems persist despite these measures, apical cannulation or left ventricular septostomy can be performed.
Outcomes with ECMO have been disappointing. Studies show that whether ECMO was indicated for cardiac failure or for respiratory failure, survival is only about 25% at 5 years. Analyzing data only for arteriovenous ECMO, survival was 48% in bridged patients and 41% in patients who were weaned.
The Extracorporeal Life Support Organization Registry, in their international summary from 2010, found that 34% of cardiac patients on ECMO survived to discharge or transfer. Most of these patients had cardiogenic shock from acute myocardial infarction. Outcomes are so poor because of complications endemic to ECMO, eg, dialysis-dependent renal failure (about 40%) and neurologic complications (about 30%), often involving ischemic or hemorrhagic stroke.
Limb and pump complications were also significant in the past. These have been reduced with the new reperfusion cannula and the Quadrox oxygenator.
Complications unique to ECMO should be understood and anticipated so that they can be avoided. Better tools are available, ie, Impella and TandemHeart.
Left-sided Impella: A longer-term temporary support
ECMO is a temporary fix that is usually used only for a few days. If longer support is needed, axillary placement of an Impella should be used as a bridge to recovery, transplant, or a durable LVAD.
The Impella device (Figure 3) is a miniature rotary blood pump increasingly used to treat cardiogenic shock. It is inserted retrograde across the aortic valve to provide short-term ventricular support. Most devices are approved by the US Food and Drug Administration (FDA) for less than 7 days of use, but we have experience using them up to 30 days. They are very hemocompatible, involving minimal hemolysis. Axillary placement allows early extubation and ambulation and is more stable than groin placement.
Several models are available: the 2.5 and 3.5 L/min devices can be placed percutaneously, while the 5 L/min model must be surgically placed in the axillary or groin region. Heparin is required with their use. They can replace ECMO. A right ventricular assist device (RVAD), Impella RP, is also available.
Physiologic impact of the Impella
The Impella fully unloads the left ventricle, reducing myocardial oxygen demand and increasing myocardial blood flow. It reduces end-diastolic volume and pressure, the mechanical work of the heart, and wall tension. Microvascular resistance is reduced, allowing increased coronary flow. Cardiac output and power are increased by multiple means.8–11
The RECOVER 1 trial evaluated the 5L Impella placed after cardiac surgery. The cardiac index increased in all the patients, and the systemic vascular resistance and wedge pressure decreased.12
Unloading the ventricle is critical. Meyns and colleagues13 found a fivefold reduction in infarct size from baseline in a left anterior descending occlusion model in pigs after off-loading the ventricle.
Impella has the advantage of simple percutaneous insertion (the 2.5 and CP models). It also tests right ventricular tolerance: if the right ventricle is doing well, one can predict with high certainty that it will tolerate an LVAD (eg, HeartWare, HeartMate 2 (Pleasanton, CA), or HeartMate 3 when available).
Disadvantages include that it provides only left ventricular support, although a right ventricular device can be inserted for dual support. Placement requires fluoroscopic or echocardiographic guidance.
TandemHeart requires septal puncture
The TandemHeart is approved for short-term and biventricular use. It consists of an extracorporeal centrifugal pump that withdraws blood from the left atrium via a trans-septal cannula placed through the femoral vein (Figure 4) and returns it to one or both femoral arteries. The blood is pumped at up to 5 L/min.
It is designed to reduce the pulmonary capillary wedge pressure, ventricular work, and myocardial oxygen demand and increase cardiac output and mean arterial pressure. It has the advantages of percutaneous placement and the ability to provide biventricular support with 2 devices. It can be used for up to 3 weeks. It can easily be converted to ECMO by either splicing in an oxygenator or adding another cannula.
Although the TandemHeart provides significant support, it is no longer often used. A 21F venous cannula must be passed to the left atrium by trans-septal puncture, which requires advanced skill and must be done in the catheterization laboratory. Insertion can take too much time and cause bleeding in patients taking an anticoagulant. Insertion usually destroys the septum, and removal requires a complete patch of the entire septum. Systemic anticoagulation is required. Other disadvantages are risks of hemolysis, limb ischemia, and infection with longer support times.
The CentriMag (Levitronix LLC; Framingham, MA) is an improved device that requires only 1 cannula instead of 2 to cover both areas.
DEVICES FOR RIGHT-SIDED SUPPORT
Most early devices were designed for left-sided support. The right heart, especially in failure, has been more difficult to manage. Previously the only option for a patient with right ventricular failure was venoarterial ECMO. This is more support than needed for a patient with isolated right ventricular failure and involves the risk of multiple complications from the device.
With more options available for the right heart (Table 3), we can choose the most appropriate device according to the underlying cause of right heart failure (eg, right ventricular infarct, pulmonary hypertension), the likelihood of recovery, and the expected time to recovery.
The ideal RVAD would be easy to implant, maintain, and remove. It would allow for chest closure and patient ambulation. It would be durable and biocompatible, so that it could remain implanted for months if necessary. It would cause little blood trauma, have the capability for adding an oxygenator for pulmonary support, and be cost-effective.
Although no single system has all these qualities, each available device fulfills certain combinations of these criteria, so the best one can be selected for each patient’s needs.
ECMO Rotaflow centrifugal pump: Fast, simple, inexpensive
A recent improvement to ECMO is the Rotaflow centrifugal pump (Maquet, Wayne, NJ), which is connected by sewing an 8-mm graft onto the pulmonary artery and placing a venous cannula in the femoral vein. If the patient is not bleeding, the chest can then be closed. This creates a fast, simple, and inexpensive temporary RVAD system. When the patient is ready to be weaned, the outflow graft can be disconnected at the bedside without reopening the chest.
The disadvantage is that the Rotaflow system contains a sapphire bearing. Although it is magnetically coupled, it generates heat and is a nidus for thrombus formation, which can lead to pump failure and embolization. This system can be used for patients who are expected to need support for less than 5 to 7 days. Beyond this duration, the incidence of complications increases.
CentriMag Ventricular Assist System offers right, left, or bilateral support
The CentriMag Ventricular Assist System is a fully magnetically levitated pump containing no bearings or seals, and with the same technology as is found in many of the durable devices such as HeartMate 3. It is coupled with a reusable motor and is easy to use.
CentriMag offers versatility, allowing for right, left, or bilateral ventricular support. An oxygenator can be added for pulmonary edema and additional support. It is the most biocompatible device and is FDA-approved for use for 4 weeks, although it has been used successfully for much longer. It allows for chest closure and ambulation. It is especially important as a bridge to transplant. The main disadvantage is that insertion and removal require sternotomy.
Impella RP: One size does not fit all
The Impella RP (Figure 5) has an 11F catheter diameter, 23F pump, and a maximum flow rate of more than 4 L/minute. It has a unique 3-dimensional cannula design based on computed tomography 3-dimensional reconstructions from hundreds of patients.
The device is biocompatible and can be used for support for more than 7 days, although most patients require only 3 or 4 days. There is almost no priming volume, so there is no hemodilution.
The disadvantages are that it is more challenging to place than other devices, and some patients cannot use it because the cannula does not fit. It also does not provide pulmonary support. Finally, it is the most expensive of the 3 right-sided devices.
CASE REVISITED
The patient described at the beginning of this article was extubated on day 12 but was then reintubated. On day 20, a tracheotomy tube was placed. By day 24, he had improved so little that his family signed a “do-not-resuscitate–comfort-care-arrest” order (ie, if the patient’s heart or breathing stops, only comfort care is to be provided).
But slowly he got better, and the Impella was removed on day 30. Afterward, serum creatinine and liver function tests began rising again, requiring dobutamine for heart support.
On day 34, his family reversed the do-not-resuscitate order, and he was reevaluated for an LVAD as destination therapy. At this point, echocardiography showed a left ventricular ejection fraction of 10%, normal right ventricular function, with a normal heartbeat and valves. On day 47, a HeartMate II LVAD was placed.
On postoperative day 18, he was transferred out of the intensive care unit, then discharged to an acute rehabilitation facility 8 days later (hospital day 73). He was subsequently discharged.
At a recent follow-up appointment, the patient said that he was feeling “pretty good” and walked with no shortness of breath.
- Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol 2016; 13:481–492.
- Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI registry. JACC Cardiovasc Interv 2016; 9:341–351.
- Harjola VP, Lassus J, Sionis A, et al; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17:501–509.
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36:2246–2256.
- Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg 1999; 14:288–293.
- Centers for Medicare & Medicaid Services. Decision memo for ventricular assist devices as destination therapy (CAG-00119R2). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=243&ver=9&NcaName=Ventricular+Assist+Devices+as+Destination+Therapy+(2nd+Recon)&bc=BEAAAAAAEAAA&&fromdb=true. Accessed March 10, 2017.
- Kulkarni T, Sharma NS, Diaz-Guzman E. Extracorporeal membrane oxygenation in adults: a practical guide for internists. Cleve Clin J Med 2016; 83:373–384.
- Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella Recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 2007; 70:532–537.
- Aqel RA, Hage FG, Iskandrian AE. Improvement of myocardial perfusion with a percutaneously inserted left ventricular assist device. J Nucl Cardiol 2010; 17:158–160.
- Sarnoff SJ, Braunwald E, Welch Jr GH, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol 1957; 192:148–156.
- Braunwald E. 50th anniversary historical article. Myocardial oxygen consumption: the quest for its determinants and some clinical fallout. J Am Coll Cardiol 1999; 34:1365–1368.
- Griffith BP, Anderson MB, Samuels LE, Pae WE Jr, Naka Y, Frazier OH. The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg 2013; 145:548–554
- Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by cathteter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003; 41:1087–1095.
A 43-year-old man presented to a community hospital with acute chest pain and shortness of breath and was diagnosed with anterior ST-elevation myocardial infarction. He was a smoker with a history of alcohol abuse, hypertension, and hyperlipidemia, and in the past he had undergone percutaneous coronary interventions to the right coronary artery and the first obtuse marginal artery.
Angiography showed total occlusion in the left anterior descending artery, 90% stenosis in the right coronary artery, and mild disease in the left circumflex artery. A drug-eluting stent was placed in the left anterior descending artery, resulting in good blood flow.
However, his left ventricle continued to have severe dysfunction. An intra-aortic balloon pump was inserted. Afterward, computed tomography showed subsegmental pulmonary embolism with congestion. His mean arterial pressure was 60 mm Hg (normal 70–110), central venous pressure 12 mm Hg (3–8), pulmonary artery pressure 38/26 mm Hg (15–30/4–12), pulmonary capillary wedge pressure 24 mm Hg (2–15), and cardiac index 1.4 L/min (2.5–4).
The patient was started on dobutamine and norepinephrine and transferred to Cleveland Clinic on day 2. Over the next day, he had runs of ventricular tachycardia, for which he was given amiodarone and lidocaine. His urine output was low, and his serum creatinine was elevated at 1.65 mg/dL (baseline 1.2, normal 0.5–1.5). Liver function tests were also elevated, with aspartate aminotransferase at 115 U/L(14–40) and alanine aminotransferase at 187 U/L (10–54).
Poor oxygenation was evident: his arterial partial pressure of oxygen was 64 mm Hg (normal 75–100). He was intubated and given 100% oxygen with positive end-expiratory pressure of 12 cm H2O.
Echocardiography showed a left ventricular ejection fraction of 15% (normal 55%–70%) and mild right ventricular dysfunction.
ECMO and then Impella placement
On his third hospital day, a venoarterial extracorporeal membrane oxygenation (ECMO) device was placed peripherally (Figure 1).
His hemodynamic variables stabilized, and he was weaned off dobutamine and norepinephrine. Results of liver function tests normalized, his urinary output increased, and his serum creatinine dropped to a normal 1.0 mg/dL. However, a chest radiograph showed pulmonary congestion, and echocardiography now showed severe left ventricular dysfunction.
On hospital day 5, the patient underwent surgical placement of an Impella 5.0 device (Abiomed, Danvers, MA) through the right axillary artery in an effort to improve his pulmonary edema. The ECMO device was removed. Placement of a venovenous ECMO device was deemed unnecessary when oxygenation improved with the Impella.
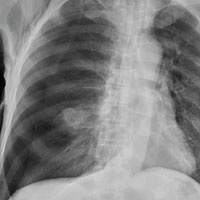
Three days after Impella placement, radiography showed improved edema with some remaining pleural effusion.
ACUTE CARDIOGENIC SHOCK
Cardiogenic shock remains a challenging clinical problem: patients with it are among the sickest in the hospital, and many of them die. ECMO was once the only therapy available and is still widely used. However, it is a 2-edged sword; complications such as bleeding, infection, and thrombosis are almost inevitable if it is used for long. Importantly, patients are usually kept intubated and bedridden.
In recent years, new devices have become available that are easier to place (some in the catheterization laboratory or even at the bedside) and allow safer bridging to recovery, transplant, or other therapies.
This case illustrates the natural history of cardiogenic shock and the preferred clinical approach: ie, ongoing evaluation that permits rapid response to evolving challenges.
In general, acute cardiogenic shock occurs within 24 to 48 hours after the initial insult, so even if a procedure succeeds, the patient may develop progressive hypotension and organ dysfunction. Reduced cardiac output causes a downward spiral with multiple systemic and inflammatory processes as well as increased nitric oxide synthesis, leading to progressive decline and eventual end-organ dysfunction.
Continuously evaluate
The cardiac team should continuously assess the acuity and severity of a patient’s condition, with the goals of maintaining end-organ perfusion and identifying the source of problems. Refractory cardiogenic shock, with tissue hypoperfusion despite vasoactive medications and treatment of the underlying cause, is associated with in-hospital mortality rates ranging from 30% to 50%.1,2 The rates have actually increased over the past decade, as sicker patients are being treated.
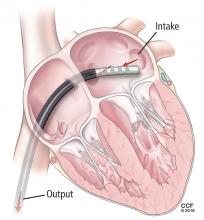
When a patient presents with cardiogenic shock, we first try a series of vasoactive drugs and usually an intra-aortic balloon pump (Figure 2). We then tailor treatment depending on etiology. For example, a patient may have viral myocarditis and may even require a biopsy.
If cardiogenic shock is refractory, mechanical circulatory support devices can be a short-term bridge to either recovery or a new decision. A multidisciplinary team should be consulted to consider transplant, a long-term device, or palliative care. Sometimes a case requires “bridging to a bridge,” with several devices used short-term in turn.
Prognostic factors in cardiogenic shock
Several tools help predict outcome in a severely ill patient. End-organ function, indicated by blood lactate levels and estimated glomerular filtration rate, is perhaps the most informative and should be monitored serially.
CardShock3 is a simple scoring system based on age, mental status at presentation, laboratory values, and medical history. Patients receive 1 point for each of the following factors:
- Age > 75
- Confusion at presentation
- Previous myocardial infarction or coronary artery bypass grafting
- Acute coronary syndrome etiology
- Left ventricular ejection fraction < 40%
- Blood lactate level between 2 and 4 mmol/L, inclusively (2 points for lactate levels > 4 mmol/L)
- Estimated glomerular filtration rate between 30 and 60 mL/min/1.73 m2, inclusively (2 points if < 30 mL/min/1.73 m2).
Thus, scores range from 0 (best) to 9 (worst). A score of 0 to 3 points was associated with a 9% risk of death in the hospital, a score of 4 or 5 with a risk of 36%, and a score of 6 through 9 with a risk of 77%.3
The Survival After Veno-arterial ECMO (SAVE) score (www.save-score.com) is a prediction tool derived from a large international ECMO registry.4 It is based on patient age, diagnosis, and indicators of end-organ dysfunction. Scores range from –35 (worst) to +7 (best).
The mortality rate associated with postcardiotomy cardiogenic shock increases with the amount of inotropic support provided. In a 1996–1999 case series of patients who underwent open-heart surgery,5 the hospital mortality rate was 40% in those who received 2 inotropes in high doses and 80% in those who received 3. A strategy of early implementation of mechanical support is critical.
Selection criteria for destination therapy
Deciding whether a patient should receive a long-term device is frequently a challenge. The decision often must be based on limited information about not only the medical indications but also psychosocial factors that influence long-term success.
The Centers for Medicare and Medicaid Services have established criteria for candidates for left ventricular assist devices (LVADs) as destination therapy.6 Contraindications established for heart transplant should also be considered (Table 1).
CASE REVISITED
Several factors argued against LVAD placement in our patient. He had no health insurance and had been off medications. He smoked and said he consumed 3 hard liquor drinks per week. His Stanford Integrated Psychosocial Assessment for Transplantation score was 30 (minimally acceptable). He had hypoxia with subsegmental pulmonary edema, a strong contraindication to immediate transplant.
On the other hand, he had only mild right ventricular dysfunction. His CardShock score was 4 (intermediate risk, based on lactate 1.5 mmol/L and estimated glomerular filtration rate 52 mL/min/1.73 m2). His SAVE score was –9 (class IV), which overall is associated with a 30% risk of death (low enough to consider treatment).
During the patient’s time on temporary support, the team had the opportunity to better understand him and assess his family support and his ability to handle a permanent device. His surviving the acute course bolstered the team’s confidence that he could enjoy long-term survival with destination therapy.
CATHETERIZATION LABORATORY DEVICE CAPABILITIES
Although most implantation procedures are done in the operating room, they are often done in the catheterization laboratory because patients undergoing catheterization may not be stable enough for transfer, or an emergency intervention may be required during the night. Catheterization interventionists are also an important part of the team to help determine the best approach for long-term therapy.
The catheterization laboratory has multiple acute intervention options. Usually, decisions must be made quickly. In general, patients needing mechanical support are managed as follows:
- Those who need circulation support and oxygenation receive ECMO
- Those who need circulation support alone because of mechanical issues (eg, myocardial infarction) are considered for an intra-aortic balloon pump, Impella, or TandemHeart pump (Cardiac Assist, Pittsburgh, PA).
Factors that guide the selection of a temporary pump include:
- Left ventricular function
- Right ventricular function
- Aortic valve stenosis (some devices cannot be inserted through critical aortic stenosis)
- Aortic regurgitation (can affect some devices)
- Peripheral artery disease (some devices are large and must be placed percutaneously).
CHOOSING AMONG PERCUTANEOUS DEVICES
Circulatory support in cardiogenic shock improves outcomes, and devices play an important role in supporting high-risk procedures. The goal is not necessarily to use the device throughout the hospital stay. Acute stabilization is most important initially; a more considered decision about long-term therapy can be made when more is known about the patient.
Patient selection is the most important component of success. However, randomized data to support outcomes with the various devices are sparse and complicated by the critically ill state of the patient population.
SHORT-TERM CIRCULATORY SUPPORT: ECMO, IMPELLA, TANDEMHEART
A menu of options is available for temporary mechanical support. Options differ by their degree of circulatory support and ease of insertion (Table 2).
ECMO: A fast option with many advantages
ECMO has evolved and now can be placed quickly. A remote diagnostic platform such as CardioHub permits management at the bedside, in the medical unit, or in the cardiac intensive care unit.7
ECMO has several advantages. It can be used during cardiopulmonary bypass, it provides oxygenation, it is the only option in the setting of lung injury, it can be placed peripherally (without thoracotomy), and it is the only percutaneous option for biventricular support.
ECMO also has significant disadvantages
ECMO is a good device for acute resuscitation of a patient in shock, as it offers quick placement and resuscitation. But it is falling out of favor because of significant disadvantages.
Its major drawback is that it provides no left ventricular unloading. Although in a very unstable patient ECMO can stabilize end organs and restore their function, the lack of left ventricular unloading and reduced ventricular work threaten the myocardium. It creates extremely high afterload; therefore, in a left ventricle with poor function, wall tension and myocardial oxygen demand increase. Multiple studies have shown that coronary perfusion worsens, especially if the patient is cannulated peripherally. Because relative cerebral hypoxia occurs in many situations, it is imperative to check blood saturations at multiple sites to determine if perfusion is adequate everywhere.
Ineffective left ventricular unloading with venoarterial ECMO is managed in several ways. Sometimes left ventricular distention is slight and the effects are subtle. Left ventricular distention causing pulmonary edema can be addressed with:
- Inotropes (in moderate doses)
- Anticoagulation to prevent left ventricular thrombus formation
- An intra-aortic balloon pump. Most patients on ECMO already have an intra-aortic balloon pump in place, and it should be left in to provide additional support. For those who do not have one, it should be placed via the contralateral femoral artery.
If problems persist despite these measures, apical cannulation or left ventricular septostomy can be performed.
Outcomes with ECMO have been disappointing. Studies show that whether ECMO was indicated for cardiac failure or for respiratory failure, survival is only about 25% at 5 years. Analyzing data only for arteriovenous ECMO, survival was 48% in bridged patients and 41% in patients who were weaned.
The Extracorporeal Life Support Organization Registry, in their international summary from 2010, found that 34% of cardiac patients on ECMO survived to discharge or transfer. Most of these patients had cardiogenic shock from acute myocardial infarction. Outcomes are so poor because of complications endemic to ECMO, eg, dialysis-dependent renal failure (about 40%) and neurologic complications (about 30%), often involving ischemic or hemorrhagic stroke.
Limb and pump complications were also significant in the past. These have been reduced with the new reperfusion cannula and the Quadrox oxygenator.
Complications unique to ECMO should be understood and anticipated so that they can be avoided. Better tools are available, ie, Impella and TandemHeart.
Left-sided Impella: A longer-term temporary support
ECMO is a temporary fix that is usually used only for a few days. If longer support is needed, axillary placement of an Impella should be used as a bridge to recovery, transplant, or a durable LVAD.
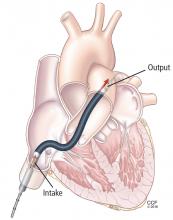
The Impella device (Figure 3) is a miniature rotary blood pump increasingly used to treat cardiogenic shock. It is inserted retrograde across the aortic valve to provide short-term ventricular support. Most devices are approved by the US Food and Drug Administration (FDA) for less than 7 days of use, but we have experience using them up to 30 days. They are very hemocompatible, involving minimal hemolysis. Axillary placement allows early extubation and ambulation and is more stable than groin placement.
Several models are available: the 2.5 and 3.5 L/min devices can be placed percutaneously, while the 5 L/min model must be surgically placed in the axillary or groin region. Heparin is required with their use. They can replace ECMO. A right ventricular assist device (RVAD), Impella RP, is also available.
Physiologic impact of the Impella
The Impella fully unloads the left ventricle, reducing myocardial oxygen demand and increasing myocardial blood flow. It reduces end-diastolic volume and pressure, the mechanical work of the heart, and wall tension. Microvascular resistance is reduced, allowing increased coronary flow. Cardiac output and power are increased by multiple means.8–11
The RECOVER 1 trial evaluated the 5L Impella placed after cardiac surgery. The cardiac index increased in all the patients, and the systemic vascular resistance and wedge pressure decreased.12
Unloading the ventricle is critical. Meyns and colleagues13 found a fivefold reduction in infarct size from baseline in a left anterior descending occlusion model in pigs after off-loading the ventricle.
Impella has the advantage of simple percutaneous insertion (the 2.5 and CP models). It also tests right ventricular tolerance: if the right ventricle is doing well, one can predict with high certainty that it will tolerate an LVAD (eg, HeartWare, HeartMate 2 (Pleasanton, CA), or HeartMate 3 when available).
Disadvantages include that it provides only left ventricular support, although a right ventricular device can be inserted for dual support. Placement requires fluoroscopic or echocardiographic guidance.
TandemHeart requires septal puncture
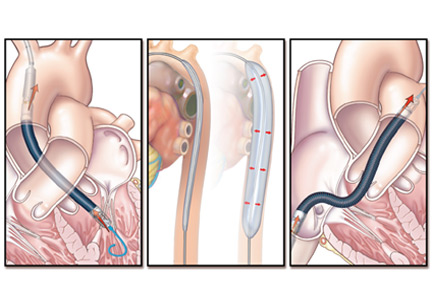
The TandemHeart is approved for short-term and biventricular use. It consists of an extracorporeal centrifugal pump that withdraws blood from the left atrium via a trans-septal cannula placed through the femoral vein (Figure 4) and returns it to one or both femoral arteries. The blood is pumped at up to 5 L/min.
It is designed to reduce the pulmonary capillary wedge pressure, ventricular work, and myocardial oxygen demand and increase cardiac output and mean arterial pressure. It has the advantages of percutaneous placement and the ability to provide biventricular support with 2 devices. It can be used for up to 3 weeks. It can easily be converted to ECMO by either splicing in an oxygenator or adding another cannula.
Although the TandemHeart provides significant support, it is no longer often used. A 21F venous cannula must be passed to the left atrium by trans-septal puncture, which requires advanced skill and must be done in the catheterization laboratory. Insertion can take too much time and cause bleeding in patients taking an anticoagulant. Insertion usually destroys the septum, and removal requires a complete patch of the entire septum. Systemic anticoagulation is required. Other disadvantages are risks of hemolysis, limb ischemia, and infection with longer support times.
The CentriMag (Levitronix LLC; Framingham, MA) is an improved device that requires only 1 cannula instead of 2 to cover both areas.
DEVICES FOR RIGHT-SIDED SUPPORT
Most early devices were designed for left-sided support. The right heart, especially in failure, has been more difficult to manage. Previously the only option for a patient with right ventricular failure was venoarterial ECMO. This is more support than needed for a patient with isolated right ventricular failure and involves the risk of multiple complications from the device.
With more options available for the right heart (Table 3), we can choose the most appropriate device according to the underlying cause of right heart failure (eg, right ventricular infarct, pulmonary hypertension), the likelihood of recovery, and the expected time to recovery.
The ideal RVAD would be easy to implant, maintain, and remove. It would allow for chest closure and patient ambulation. It would be durable and biocompatible, so that it could remain implanted for months if necessary. It would cause little blood trauma, have the capability for adding an oxygenator for pulmonary support, and be cost-effective.
Although no single system has all these qualities, each available device fulfills certain combinations of these criteria, so the best one can be selected for each patient’s needs.
ECMO Rotaflow centrifugal pump: Fast, simple, inexpensive
A recent improvement to ECMO is the Rotaflow centrifugal pump (Maquet, Wayne, NJ), which is connected by sewing an 8-mm graft onto the pulmonary artery and placing a venous cannula in the femoral vein. If the patient is not bleeding, the chest can then be closed. This creates a fast, simple, and inexpensive temporary RVAD system. When the patient is ready to be weaned, the outflow graft can be disconnected at the bedside without reopening the chest.
The disadvantage is that the Rotaflow system contains a sapphire bearing. Although it is magnetically coupled, it generates heat and is a nidus for thrombus formation, which can lead to pump failure and embolization. This system can be used for patients who are expected to need support for less than 5 to 7 days. Beyond this duration, the incidence of complications increases.
CentriMag Ventricular Assist System offers right, left, or bilateral support
The CentriMag Ventricular Assist System is a fully magnetically levitated pump containing no bearings or seals, and with the same technology as is found in many of the durable devices such as HeartMate 3. It is coupled with a reusable motor and is easy to use.
CentriMag offers versatility, allowing for right, left, or bilateral ventricular support. An oxygenator can be added for pulmonary edema and additional support. It is the most biocompatible device and is FDA-approved for use for 4 weeks, although it has been used successfully for much longer. It allows for chest closure and ambulation. It is especially important as a bridge to transplant. The main disadvantage is that insertion and removal require sternotomy.
Impella RP: One size does not fit all
The Impella RP (Figure 5) has an 11F catheter diameter, 23F pump, and a maximum flow rate of more than 4 L/minute. It has a unique 3-dimensional cannula design based on computed tomography 3-dimensional reconstructions from hundreds of patients.
The device is biocompatible and can be used for support for more than 7 days, although most patients require only 3 or 4 days. There is almost no priming volume, so there is no hemodilution.
The disadvantages are that it is more challenging to place than other devices, and some patients cannot use it because the cannula does not fit. It also does not provide pulmonary support. Finally, it is the most expensive of the 3 right-sided devices.
CASE REVISITED
The patient described at the beginning of this article was extubated on day 12 but was then reintubated. On day 20, a tracheotomy tube was placed. By day 24, he had improved so little that his family signed a “do-not-resuscitate–comfort-care-arrest” order (ie, if the patient’s heart or breathing stops, only comfort care is to be provided).
But slowly he got better, and the Impella was removed on day 30. Afterward, serum creatinine and liver function tests began rising again, requiring dobutamine for heart support.
On day 34, his family reversed the do-not-resuscitate order, and he was reevaluated for an LVAD as destination therapy. At this point, echocardiography showed a left ventricular ejection fraction of 10%, normal right ventricular function, with a normal heartbeat and valves. On day 47, a HeartMate II LVAD was placed.
On postoperative day 18, he was transferred out of the intensive care unit, then discharged to an acute rehabilitation facility 8 days later (hospital day 73). He was subsequently discharged.
At a recent follow-up appointment, the patient said that he was feeling “pretty good” and walked with no shortness of breath.
A 43-year-old man presented to a community hospital with acute chest pain and shortness of breath and was diagnosed with anterior ST-elevation myocardial infarction. He was a smoker with a history of alcohol abuse, hypertension, and hyperlipidemia, and in the past he had undergone percutaneous coronary interventions to the right coronary artery and the first obtuse marginal artery.
Angiography showed total occlusion in the left anterior descending artery, 90% stenosis in the right coronary artery, and mild disease in the left circumflex artery. A drug-eluting stent was placed in the left anterior descending artery, resulting in good blood flow.
However, his left ventricle continued to have severe dysfunction. An intra-aortic balloon pump was inserted. Afterward, computed tomography showed subsegmental pulmonary embolism with congestion. His mean arterial pressure was 60 mm Hg (normal 70–110), central venous pressure 12 mm Hg (3–8), pulmonary artery pressure 38/26 mm Hg (15–30/4–12), pulmonary capillary wedge pressure 24 mm Hg (2–15), and cardiac index 1.4 L/min (2.5–4).
The patient was started on dobutamine and norepinephrine and transferred to Cleveland Clinic on day 2. Over the next day, he had runs of ventricular tachycardia, for which he was given amiodarone and lidocaine. His urine output was low, and his serum creatinine was elevated at 1.65 mg/dL (baseline 1.2, normal 0.5–1.5). Liver function tests were also elevated, with aspartate aminotransferase at 115 U/L(14–40) and alanine aminotransferase at 187 U/L (10–54).
Poor oxygenation was evident: his arterial partial pressure of oxygen was 64 mm Hg (normal 75–100). He was intubated and given 100% oxygen with positive end-expiratory pressure of 12 cm H2O.
Echocardiography showed a left ventricular ejection fraction of 15% (normal 55%–70%) and mild right ventricular dysfunction.
ECMO and then Impella placement
On his third hospital day, a venoarterial extracorporeal membrane oxygenation (ECMO) device was placed peripherally (Figure 1).
His hemodynamic variables stabilized, and he was weaned off dobutamine and norepinephrine. Results of liver function tests normalized, his urinary output increased, and his serum creatinine dropped to a normal 1.0 mg/dL. However, a chest radiograph showed pulmonary congestion, and echocardiography now showed severe left ventricular dysfunction.
On hospital day 5, the patient underwent surgical placement of an Impella 5.0 device (Abiomed, Danvers, MA) through the right axillary artery in an effort to improve his pulmonary edema. The ECMO device was removed. Placement of a venovenous ECMO device was deemed unnecessary when oxygenation improved with the Impella.
Three days after Impella placement, radiography showed improved edema with some remaining pleural effusion.
ACUTE CARDIOGENIC SHOCK
Cardiogenic shock remains a challenging clinical problem: patients with it are among the sickest in the hospital, and many of them die. ECMO was once the only therapy available and is still widely used. However, it is a 2-edged sword; complications such as bleeding, infection, and thrombosis are almost inevitable if it is used for long. Importantly, patients are usually kept intubated and bedridden.
In recent years, new devices have become available that are easier to place (some in the catheterization laboratory or even at the bedside) and allow safer bridging to recovery, transplant, or other therapies.
This case illustrates the natural history of cardiogenic shock and the preferred clinical approach: ie, ongoing evaluation that permits rapid response to evolving challenges.
In general, acute cardiogenic shock occurs within 24 to 48 hours after the initial insult, so even if a procedure succeeds, the patient may develop progressive hypotension and organ dysfunction. Reduced cardiac output causes a downward spiral with multiple systemic and inflammatory processes as well as increased nitric oxide synthesis, leading to progressive decline and eventual end-organ dysfunction.
Continuously evaluate
The cardiac team should continuously assess the acuity and severity of a patient’s condition, with the goals of maintaining end-organ perfusion and identifying the source of problems. Refractory cardiogenic shock, with tissue hypoperfusion despite vasoactive medications and treatment of the underlying cause, is associated with in-hospital mortality rates ranging from 30% to 50%.1,2 The rates have actually increased over the past decade, as sicker patients are being treated.
When a patient presents with cardiogenic shock, we first try a series of vasoactive drugs and usually an intra-aortic balloon pump (Figure 2). We then tailor treatment depending on etiology. For example, a patient may have viral myocarditis and may even require a biopsy.
If cardiogenic shock is refractory, mechanical circulatory support devices can be a short-term bridge to either recovery or a new decision. A multidisciplinary team should be consulted to consider transplant, a long-term device, or palliative care. Sometimes a case requires “bridging to a bridge,” with several devices used short-term in turn.
Prognostic factors in cardiogenic shock
Several tools help predict outcome in a severely ill patient. End-organ function, indicated by blood lactate levels and estimated glomerular filtration rate, is perhaps the most informative and should be monitored serially.
CardShock3 is a simple scoring system based on age, mental status at presentation, laboratory values, and medical history. Patients receive 1 point for each of the following factors:
- Age > 75
- Confusion at presentation
- Previous myocardial infarction or coronary artery bypass grafting
- Acute coronary syndrome etiology
- Left ventricular ejection fraction < 40%
- Blood lactate level between 2 and 4 mmol/L, inclusively (2 points for lactate levels > 4 mmol/L)
- Estimated glomerular filtration rate between 30 and 60 mL/min/1.73 m2, inclusively (2 points if < 30 mL/min/1.73 m2).
Thus, scores range from 0 (best) to 9 (worst). A score of 0 to 3 points was associated with a 9% risk of death in the hospital, a score of 4 or 5 with a risk of 36%, and a score of 6 through 9 with a risk of 77%.3
The Survival After Veno-arterial ECMO (SAVE) score (www.save-score.com) is a prediction tool derived from a large international ECMO registry.4 It is based on patient age, diagnosis, and indicators of end-organ dysfunction. Scores range from –35 (worst) to +7 (best).
The mortality rate associated with postcardiotomy cardiogenic shock increases with the amount of inotropic support provided. In a 1996–1999 case series of patients who underwent open-heart surgery,5 the hospital mortality rate was 40% in those who received 2 inotropes in high doses and 80% in those who received 3. A strategy of early implementation of mechanical support is critical.
Selection criteria for destination therapy
Deciding whether a patient should receive a long-term device is frequently a challenge. The decision often must be based on limited information about not only the medical indications but also psychosocial factors that influence long-term success.
The Centers for Medicare and Medicaid Services have established criteria for candidates for left ventricular assist devices (LVADs) as destination therapy.6 Contraindications established for heart transplant should also be considered (Table 1).
CASE REVISITED
Several factors argued against LVAD placement in our patient. He had no health insurance and had been off medications. He smoked and said he consumed 3 hard liquor drinks per week. His Stanford Integrated Psychosocial Assessment for Transplantation score was 30 (minimally acceptable). He had hypoxia with subsegmental pulmonary edema, a strong contraindication to immediate transplant.
On the other hand, he had only mild right ventricular dysfunction. His CardShock score was 4 (intermediate risk, based on lactate 1.5 mmol/L and estimated glomerular filtration rate 52 mL/min/1.73 m2). His SAVE score was –9 (class IV), which overall is associated with a 30% risk of death (low enough to consider treatment).
During the patient’s time on temporary support, the team had the opportunity to better understand him and assess his family support and his ability to handle a permanent device. His surviving the acute course bolstered the team’s confidence that he could enjoy long-term survival with destination therapy.
CATHETERIZATION LABORATORY DEVICE CAPABILITIES
Although most implantation procedures are done in the operating room, they are often done in the catheterization laboratory because patients undergoing catheterization may not be stable enough for transfer, or an emergency intervention may be required during the night. Catheterization interventionists are also an important part of the team to help determine the best approach for long-term therapy.
The catheterization laboratory has multiple acute intervention options. Usually, decisions must be made quickly. In general, patients needing mechanical support are managed as follows:
- Those who need circulation support and oxygenation receive ECMO
- Those who need circulation support alone because of mechanical issues (eg, myocardial infarction) are considered for an intra-aortic balloon pump, Impella, or TandemHeart pump (Cardiac Assist, Pittsburgh, PA).
Factors that guide the selection of a temporary pump include:
- Left ventricular function
- Right ventricular function
- Aortic valve stenosis (some devices cannot be inserted through critical aortic stenosis)
- Aortic regurgitation (can affect some devices)
- Peripheral artery disease (some devices are large and must be placed percutaneously).
CHOOSING AMONG PERCUTANEOUS DEVICES
Circulatory support in cardiogenic shock improves outcomes, and devices play an important role in supporting high-risk procedures. The goal is not necessarily to use the device throughout the hospital stay. Acute stabilization is most important initially; a more considered decision about long-term therapy can be made when more is known about the patient.
Patient selection is the most important component of success. However, randomized data to support outcomes with the various devices are sparse and complicated by the critically ill state of the patient population.
SHORT-TERM CIRCULATORY SUPPORT: ECMO, IMPELLA, TANDEMHEART
A menu of options is available for temporary mechanical support. Options differ by their degree of circulatory support and ease of insertion (Table 2).
ECMO: A fast option with many advantages
ECMO has evolved and now can be placed quickly. A remote diagnostic platform such as CardioHub permits management at the bedside, in the medical unit, or in the cardiac intensive care unit.7
ECMO has several advantages. It can be used during cardiopulmonary bypass, it provides oxygenation, it is the only option in the setting of lung injury, it can be placed peripherally (without thoracotomy), and it is the only percutaneous option for biventricular support.
ECMO also has significant disadvantages
ECMO is a good device for acute resuscitation of a patient in shock, as it offers quick placement and resuscitation. But it is falling out of favor because of significant disadvantages.
Its major drawback is that it provides no left ventricular unloading. Although in a very unstable patient ECMO can stabilize end organs and restore their function, the lack of left ventricular unloading and reduced ventricular work threaten the myocardium. It creates extremely high afterload; therefore, in a left ventricle with poor function, wall tension and myocardial oxygen demand increase. Multiple studies have shown that coronary perfusion worsens, especially if the patient is cannulated peripherally. Because relative cerebral hypoxia occurs in many situations, it is imperative to check blood saturations at multiple sites to determine if perfusion is adequate everywhere.
Ineffective left ventricular unloading with venoarterial ECMO is managed in several ways. Sometimes left ventricular distention is slight and the effects are subtle. Left ventricular distention causing pulmonary edema can be addressed with:
- Inotropes (in moderate doses)
- Anticoagulation to prevent left ventricular thrombus formation
- An intra-aortic balloon pump. Most patients on ECMO already have an intra-aortic balloon pump in place, and it should be left in to provide additional support. For those who do not have one, it should be placed via the contralateral femoral artery.
If problems persist despite these measures, apical cannulation or left ventricular septostomy can be performed.
Outcomes with ECMO have been disappointing. Studies show that whether ECMO was indicated for cardiac failure or for respiratory failure, survival is only about 25% at 5 years. Analyzing data only for arteriovenous ECMO, survival was 48% in bridged patients and 41% in patients who were weaned.
The Extracorporeal Life Support Organization Registry, in their international summary from 2010, found that 34% of cardiac patients on ECMO survived to discharge or transfer. Most of these patients had cardiogenic shock from acute myocardial infarction. Outcomes are so poor because of complications endemic to ECMO, eg, dialysis-dependent renal failure (about 40%) and neurologic complications (about 30%), often involving ischemic or hemorrhagic stroke.
Limb and pump complications were also significant in the past. These have been reduced with the new reperfusion cannula and the Quadrox oxygenator.
Complications unique to ECMO should be understood and anticipated so that they can be avoided. Better tools are available, ie, Impella and TandemHeart.
Left-sided Impella: A longer-term temporary support
ECMO is a temporary fix that is usually used only for a few days. If longer support is needed, axillary placement of an Impella should be used as a bridge to recovery, transplant, or a durable LVAD.
The Impella device (Figure 3) is a miniature rotary blood pump increasingly used to treat cardiogenic shock. It is inserted retrograde across the aortic valve to provide short-term ventricular support. Most devices are approved by the US Food and Drug Administration (FDA) for less than 7 days of use, but we have experience using them up to 30 days. They are very hemocompatible, involving minimal hemolysis. Axillary placement allows early extubation and ambulation and is more stable than groin placement.
Several models are available: the 2.5 and 3.5 L/min devices can be placed percutaneously, while the 5 L/min model must be surgically placed in the axillary or groin region. Heparin is required with their use. They can replace ECMO. A right ventricular assist device (RVAD), Impella RP, is also available.
Physiologic impact of the Impella
The Impella fully unloads the left ventricle, reducing myocardial oxygen demand and increasing myocardial blood flow. It reduces end-diastolic volume and pressure, the mechanical work of the heart, and wall tension. Microvascular resistance is reduced, allowing increased coronary flow. Cardiac output and power are increased by multiple means.8–11
The RECOVER 1 trial evaluated the 5L Impella placed after cardiac surgery. The cardiac index increased in all the patients, and the systemic vascular resistance and wedge pressure decreased.12
Unloading the ventricle is critical. Meyns and colleagues13 found a fivefold reduction in infarct size from baseline in a left anterior descending occlusion model in pigs after off-loading the ventricle.
Impella has the advantage of simple percutaneous insertion (the 2.5 and CP models). It also tests right ventricular tolerance: if the right ventricle is doing well, one can predict with high certainty that it will tolerate an LVAD (eg, HeartWare, HeartMate 2 (Pleasanton, CA), or HeartMate 3 when available).
Disadvantages include that it provides only left ventricular support, although a right ventricular device can be inserted for dual support. Placement requires fluoroscopic or echocardiographic guidance.
TandemHeart requires septal puncture
The TandemHeart is approved for short-term and biventricular use. It consists of an extracorporeal centrifugal pump that withdraws blood from the left atrium via a trans-septal cannula placed through the femoral vein (Figure 4) and returns it to one or both femoral arteries. The blood is pumped at up to 5 L/min.
It is designed to reduce the pulmonary capillary wedge pressure, ventricular work, and myocardial oxygen demand and increase cardiac output and mean arterial pressure. It has the advantages of percutaneous placement and the ability to provide biventricular support with 2 devices. It can be used for up to 3 weeks. It can easily be converted to ECMO by either splicing in an oxygenator or adding another cannula.
Although the TandemHeart provides significant support, it is no longer often used. A 21F venous cannula must be passed to the left atrium by trans-septal puncture, which requires advanced skill and must be done in the catheterization laboratory. Insertion can take too much time and cause bleeding in patients taking an anticoagulant. Insertion usually destroys the septum, and removal requires a complete patch of the entire septum. Systemic anticoagulation is required. Other disadvantages are risks of hemolysis, limb ischemia, and infection with longer support times.
The CentriMag (Levitronix LLC; Framingham, MA) is an improved device that requires only 1 cannula instead of 2 to cover both areas.
DEVICES FOR RIGHT-SIDED SUPPORT
Most early devices were designed for left-sided support. The right heart, especially in failure, has been more difficult to manage. Previously the only option for a patient with right ventricular failure was venoarterial ECMO. This is more support than needed for a patient with isolated right ventricular failure and involves the risk of multiple complications from the device.
With more options available for the right heart (Table 3), we can choose the most appropriate device according to the underlying cause of right heart failure (eg, right ventricular infarct, pulmonary hypertension), the likelihood of recovery, and the expected time to recovery.
The ideal RVAD would be easy to implant, maintain, and remove. It would allow for chest closure and patient ambulation. It would be durable and biocompatible, so that it could remain implanted for months if necessary. It would cause little blood trauma, have the capability for adding an oxygenator for pulmonary support, and be cost-effective.
Although no single system has all these qualities, each available device fulfills certain combinations of these criteria, so the best one can be selected for each patient’s needs.
ECMO Rotaflow centrifugal pump: Fast, simple, inexpensive
A recent improvement to ECMO is the Rotaflow centrifugal pump (Maquet, Wayne, NJ), which is connected by sewing an 8-mm graft onto the pulmonary artery and placing a venous cannula in the femoral vein. If the patient is not bleeding, the chest can then be closed. This creates a fast, simple, and inexpensive temporary RVAD system. When the patient is ready to be weaned, the outflow graft can be disconnected at the bedside without reopening the chest.
The disadvantage is that the Rotaflow system contains a sapphire bearing. Although it is magnetically coupled, it generates heat and is a nidus for thrombus formation, which can lead to pump failure and embolization. This system can be used for patients who are expected to need support for less than 5 to 7 days. Beyond this duration, the incidence of complications increases.
CentriMag Ventricular Assist System offers right, left, or bilateral support
The CentriMag Ventricular Assist System is a fully magnetically levitated pump containing no bearings or seals, and with the same technology as is found in many of the durable devices such as HeartMate 3. It is coupled with a reusable motor and is easy to use.
CentriMag offers versatility, allowing for right, left, or bilateral ventricular support. An oxygenator can be added for pulmonary edema and additional support. It is the most biocompatible device and is FDA-approved for use for 4 weeks, although it has been used successfully for much longer. It allows for chest closure and ambulation. It is especially important as a bridge to transplant. The main disadvantage is that insertion and removal require sternotomy.
Impella RP: One size does not fit all
The Impella RP (Figure 5) has an 11F catheter diameter, 23F pump, and a maximum flow rate of more than 4 L/minute. It has a unique 3-dimensional cannula design based on computed tomography 3-dimensional reconstructions from hundreds of patients.
The device is biocompatible and can be used for support for more than 7 days, although most patients require only 3 or 4 days. There is almost no priming volume, so there is no hemodilution.
The disadvantages are that it is more challenging to place than other devices, and some patients cannot use it because the cannula does not fit. It also does not provide pulmonary support. Finally, it is the most expensive of the 3 right-sided devices.
CASE REVISITED
The patient described at the beginning of this article was extubated on day 12 but was then reintubated. On day 20, a tracheotomy tube was placed. By day 24, he had improved so little that his family signed a “do-not-resuscitate–comfort-care-arrest” order (ie, if the patient’s heart or breathing stops, only comfort care is to be provided).
But slowly he got better, and the Impella was removed on day 30. Afterward, serum creatinine and liver function tests began rising again, requiring dobutamine for heart support.
On day 34, his family reversed the do-not-resuscitate order, and he was reevaluated for an LVAD as destination therapy. At this point, echocardiography showed a left ventricular ejection fraction of 10%, normal right ventricular function, with a normal heartbeat and valves. On day 47, a HeartMate II LVAD was placed.
On postoperative day 18, he was transferred out of the intensive care unit, then discharged to an acute rehabilitation facility 8 days later (hospital day 73). He was subsequently discharged.
At a recent follow-up appointment, the patient said that he was feeling “pretty good” and walked with no shortness of breath.
- Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol 2016; 13:481–492.
- Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI registry. JACC Cardiovasc Interv 2016; 9:341–351.
- Harjola VP, Lassus J, Sionis A, et al; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17:501–509.
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36:2246–2256.
- Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg 1999; 14:288–293.
- Centers for Medicare & Medicaid Services. Decision memo for ventricular assist devices as destination therapy (CAG-00119R2). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=243&ver=9&NcaName=Ventricular+Assist+Devices+as+Destination+Therapy+(2nd+Recon)&bc=BEAAAAAAEAAA&&fromdb=true. Accessed March 10, 2017.
- Kulkarni T, Sharma NS, Diaz-Guzman E. Extracorporeal membrane oxygenation in adults: a practical guide for internists. Cleve Clin J Med 2016; 83:373–384.
- Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella Recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 2007; 70:532–537.
- Aqel RA, Hage FG, Iskandrian AE. Improvement of myocardial perfusion with a percutaneously inserted left ventricular assist device. J Nucl Cardiol 2010; 17:158–160.
- Sarnoff SJ, Braunwald E, Welch Jr GH, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol 1957; 192:148–156.
- Braunwald E. 50th anniversary historical article. Myocardial oxygen consumption: the quest for its determinants and some clinical fallout. J Am Coll Cardiol 1999; 34:1365–1368.
- Griffith BP, Anderson MB, Samuels LE, Pae WE Jr, Naka Y, Frazier OH. The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg 2013; 145:548–554
- Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by cathteter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003; 41:1087–1095.
- Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol 2016; 13:481–492.
- Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI registry. JACC Cardiovasc Interv 2016; 9:341–351.
- Harjola VP, Lassus J, Sionis A, et al; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17:501–509.
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36:2246–2256.
- Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg 1999; 14:288–293.
- Centers for Medicare & Medicaid Services. Decision memo for ventricular assist devices as destination therapy (CAG-00119R2). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=243&ver=9&NcaName=Ventricular+Assist+Devices+as+Destination+Therapy+(2nd+Recon)&bc=BEAAAAAAEAAA&&fromdb=true. Accessed March 10, 2017.
- Kulkarni T, Sharma NS, Diaz-Guzman E. Extracorporeal membrane oxygenation in adults: a practical guide for internists. Cleve Clin J Med 2016; 83:373–384.
- Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella Recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 2007; 70:532–537.
- Aqel RA, Hage FG, Iskandrian AE. Improvement of myocardial perfusion with a percutaneously inserted left ventricular assist device. J Nucl Cardiol 2010; 17:158–160.
- Sarnoff SJ, Braunwald E, Welch Jr GH, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol 1957; 192:148–156.
- Braunwald E. 50th anniversary historical article. Myocardial oxygen consumption: the quest for its determinants and some clinical fallout. J Am Coll Cardiol 1999; 34:1365–1368.
- Griffith BP, Anderson MB, Samuels LE, Pae WE Jr, Naka Y, Frazier OH. The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg 2013; 145:548–554
- Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by cathteter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003; 41:1087–1095.
KEY POINTS
- ECMO is the fastest way to stabilize a patient in acute cardiogenic shock and prevent end-organ failure, but it should likely be used for a short time and does not reduce the work of (“unload”) the left ventricle.
- An intra-aortic balloon pump may provide diastolic filling in a patient on ECMO.
- The TandemHeart provides significant support, but its insertion requires puncture of the atrial septum.
- The Impella fully unloads the left ventricle, critically reducing the work of the heart.
- Options for right-ventricular support include the ECMO Rotaflow circuit, CentriMag, and Impella RP.
- The CentriMag is the most versatile device, allowing right, left, or biventricular support, but placement requires sternotomy.
Confusion and hypercalcemia in an 80-year-old man
A retired 80-year-old man presented to the emergency department after 10 days of increasing polydipsia, polyuria, dry mouth, confusion, and slurred speech. He also reported that he had gradually and unintentionally lost 20 pounds and had loss of appetite, constipation, and chronic itching. He denied fevers, chills, night sweats, nausea, vomiting, and abdominal pain.
Medical history. He had type 2 diabetes mellitus that was well controlled by oral hypoglycemics, hypothyroidism treated with levothyroxine in stable doses, and chronic hepatitis C complicated by liver cirrhosis without focal hepatic lesions. He also had hypertension, well controlled with hydrochlorothiazide and losartan. For his long-standing pruritus he had tried prescription drugs including gabapentin and pregabalin without improvement. He had also seen a naturopathic practitioner, who had prescribed supplements that relieved the symptoms.
Examination. The patient was in no acute distress. He appeared thin, with a weight of 140 lb and a body mass index of 21 kg/m2. His temperature was 36.8°C (98.2°F), blood pressure 198/82 mm Hg, heart rate 72 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation 97%. His skin was without jaundice or rashes. The mucous membranes in the oropharynx were dry.
Neurologic examination revealed mild confusion, dysarthria, and ataxic gait. Sensation to light touch, pinprick, and vibration was intact. Generalized weakness was noted. Cranial nerves II through XII were intact. Deep tendon reflexes were symmetrically globally suppressed. Asterixis was absent. The remainder of the physical examination was unremarkable.
Laboratory values in the emergency department. We initially suspected he had symptomatic hyperglycemia, but a bedside blood glucose value of 113 mg/dL ruled this out. Other initial laboratory values:
- Blood urea nitrogen 31 mg/dL (reference range 9–24)
- Serum creatinine 1.7 mg/dL (0.73–1.22; an earlier value had been 1.0 mg/dL)
- Total serum calcium 14.4 mg/dL (8.6–10.0)
Complete blood cell counts were unremarkable. Computed tomography of the head was negative for acute pathology.
In view of the patient’s hypercalcemia, he was given aggressive intravenous fluid resuscitation (2 L of normal saline over 2 hours) and was admitted to the hospital. His laboratory values on admission are shown in Table 1. Fluid resuscitation was continued while the laboratory results were pending.
CAUSES OF HYPERCALCEMIA
1. Based on this information, which is the most likely cause of this patient’s hypercalcemia?
- Primary hyperparathyroidism
- Malignancy
- Hyperthyroidism
- Hypervitaminosis D
- Sarcoidosis
Traditionally, the workup for hypercalcemia in an outpatient starts with measuring the serum parathyroid hormone (PTH) level. Based on the results, a further evaluation of PTH-mediated vs PTH-independent causes of hypercalcemia would be initiated.
Primary hyperparathyroidism and malignancy account for 90% of all cases of hypercalcemia. The serum PTH concentration is usually high in primary hyperparathyroidism but low in malignancy, which helps distinguish the conditions from each other.1
Primary hyperparathyroidism
In primary hyperparathyroidism, there is overproduction of PTH, most commonly from a parathyroid adenoma, though parathyroid hyperplasia or, more rarely, parathyroid carcinoma can also overproduce the hormone.
PTH increases serum calcium levels through 3 primary mechanisms: increasing bone resorption, increasing intestinal absorption of calcium, and decreasing renal excretion of calcium. It also induces renal phosphorus excretion.
Typically, in primary hyperparathyroidism, the increases in serum calcium are small (with serum levels of total calcium rising to no higher than 11 mg/dL) and often intermittent.2 Our patient had extremely high serum calcium, low PTH, and high phosphorus levels—all of which are inconsistent with primary hyperparathyroidism.
Malignancy
In some solid tumors, the major mechanism of hypercalcemia is secretion of PTH-related peptide (PTHrP) through promotion of osteoclast function and also increased renal absorption of calcium.3 Hematologic malignancies (eg, multiple myeloma) produce osteoclast-activating factors such as RANK ligand, lymphotoxin, and interleukin 6. Direct tumor invasion of bone can cause osteolysis and subsequent hypercalcemia.4 These mechanisms are usually associated with a fall in PTH.
Less commonly, tumors can also increase levels of 1,25-dihydroxyvitamin D or produce PTH independently of the parathyroid gland.5 There have also been reports of severe hypercalcemia from hepatocellular carcinoma due to PTHrP production.6
Our patient is certainly at risk for malignancy, given his long-standing history of hepatitis C and cirrhosis. He also had a mildly elevated alpha fetoprotein level and suppressed PTH. However, his PTHrP level was normal, and ultrasonography done recently to screen for hepatocellular carcinoma (recommended every 6 months by the American Association for the Study of Liver Diseases in high-risk patients) was negative.7
Multiple myeloma screening involves testing with serum protein electrophoresis with immunofixation in combination with either a serum free light chain assay or 24-hour urine protein electrophoresis with immunofixation. This provides a 97% sensitivity.8 In this patient, these tests for multiple myeloma were negative.
Hyperthyroidism
As many as half of all patients with hyperthyroidism have elevated levels of ionized serum calcium.9 Increased osteoclastic activity is the likely mechanism. Hyperthyroid patients have increased levels of serum interleukin 6 and increased sensitivity of bone to this factor. This cytokine induces differentiation of monocytic cells into osteoclast precursors.10 These patients also have normal or low PTH levels.9
Our patient was receiving levothyroxine for hypothyroidism, but there was no evidence that the dosage was too high, as his thyroid-stimulating hormone level was within an acceptable range.
Hypervitaminosis D
Vitamin D precursors arise from the skin and from the diet. These precursors are hydroxylated in the liver and then the kidneys to biologically active 1,25-dihydroxyvitamin D (Figure 1).11 Vitamin D’s primary actions are in the intestines to increase absorption of calcium and in bone to induce osteoclast action. These actions raise the serum calcium level, which in turn lowers the PTH level through negative feedback on the parathyroid gland.
Most vitamin D supplements consist of the inactive precursor cholecalciferol (vitamin D3). To assess the degree of supplementation, 25-hydroxyvitamin D levels, which indicate the size of the body’s vitamin D reservoir, are measured.11,12
Our patient’s 25-hydroxyvitamin D level is extremely elevated, well beyond the 250-ng/mL upper limit that is considered safe.13 His low PTH level, lack of other likely causes, and history of supplement use point toward the diagnosis of hypervitaminosis D.
Sarcoidosis
Up to 10% of patients with sarcoidosis have hypercalcemia that is not mediated by PTH. Hypercalcemia in sarcoidosis has several potential mechanisms, including increased activity of the enzyme 1-alpha hydroxylase with a subsequent increase in physiologically active 1,25-dihydroxyvitamin D3 production.14
Our patient had elevated levels of 25-hydroxyvitamin D, but his biologically active 1,25-dihydroxyvitamin D level remained within the laboratory’s reference range.
LESS LIKELY CAUSES OF HYPERCALCEMIA
2. Which of the following would be least likely to cause hypercalcemia?
- Thiazide diuretics
- Over-the-counter antacid tablets
- Lithium
- Vitamin A supplementation
- Proton pump inhibitors
Thiazide diuretics
This class of drugs is well known to cause hypercalcemia. The most familiar of the mechanisms is a reduction in urinary calcium excretion. There is also an increase in intestinal absorption of dietary calcium. Evidence is increasing that most patients (as many as two-thirds) who develop hypercalcemia while using a thiazide diuretic have subclinical primary hyperparathyroidism that is uncovered with use of the diuretic.
Of importance, the hypercalcemia that thiazide diuretics cause is mild. In a series of 72 patients with thiazide-induced hypercalcemia, the average serum calcium level was 10.7 mg/dL.15
Our patient was receiving a thiazide diuretic but presented with severe hypercalcemia, which is inconsistent with thiazide-induced hypercalcemia.
Over-the-counter antacid tablets
Calcium carbonate, a popular over-the-counter antacid, can cause a milk-alkali syndrome that is defined by ingestion of excessive calcium and alkalotic substances, leading to metabolic alkalosis, hypercalcemia, and renal insufficiency. To induce this syndrome generally requires up to 4 g of calcium intake daily, but even lower levels (1.0 to 1.5 g) are known to cause it.16
Lithium
Lithium is known to cause hypercalcemia. Multiple mechanisms have been proposed, including direct action on renal tubules and the intestines leading to calcium reabsorption and stimulation of PTH release. Interestingly, parathyroid gland hyperplasia has been noted in long-term users of lithium. An often-proposed mechanism is that lithium increases the threshold at which the parathyroid glands slow their production of PTH, making them less sensitive to serum calcium levels.17
Vitamin A supplementation
Multiple case reports have linked hypercalcemia to ingestion of large doses of vitamin A. The mechanism is thought to be increased bone resorption.18.19
Although our patient reported supplement use, he denied taking vitamin A in any form.
Proton pump inhibitors
Proton pump inhibitors are not known to cause hypercalcemia. On the contrary, case reports suggest that prolonged use of proton pump inhibitors is associated with hypocalcemia and hypomagnesemia, although the mechanism is still not fully understood. A low magnesium level is known to reduce PTH secretion and also skeletal responsiveness to PTH, which can lead to profound hypocalcemia.20
CASE CONTINUED
On further questioning, the patient revealed that the supplement prescribed by his naturopathic practitioner contained vitamin D. Although he had been instructed to take 1 tablet weekly, he had begun taking it daily with his other routine medications, resulting in a daily dose in excess of 60,000 IU of cholecalciferol (vitamin D3). The recommended dose is no more than 4,000 IU/day.
The supplement was immediately discontinued. His hydrochlorothiazide was also held due to its known effect of reducing urinary calcium excretion.
INITIAL TREATMENT OF HYPERCALCEMIA
3. Which of the following treatments is not recommended as part of this patient’s initial treatment?
- Bisphosphonates
- Calcitonin
- Intravenous fluids
- Furosemide
Our patient met the criteria for the diagnosis of hypercalcemic crisis, usually defined as an albumin-corrected serum calcium level higher than 14 mg/dL associated with multiorgan dysfunction resulting from the hypercalcemia.21 The mnemonic “stones, bones, abdominal moans, and psychic groans” captures the renal, skeletal, gastrointestinal, and neurologic manifestations.1
Bisphosphonates
Bisphosphonates are analogues of pyrophosphonates, which are normally incorporated into bone. Unlike pyrophosphonates, bisphosphonates inhibit osteoclast function. They are often used to treat hypercalcemia of any cause, although they are currently approved by the US Food and Drug Administration for treating hypercalcemia of malignancy only. As intravenous monotherapy, they are superior to other forms of treatment and are among the first-line agents in management.
Two bisphosphonates shown to be effective in hypercalcemia are zoledronate and pamidronate. Pamidronate begins to lower serum calcium levels within 2 days, with a peak effect at around 6 days.22 However, in studies comparing the 2 drugs, zoledronate has been shown to be more effective in normalizing serum calcium, with the additional benefit of having a much more rapid infusion time.23 Zoledronate is contraindicated in patients with creatinine clearance less than 30 mL/min; however, pamidronate may continue to be used.24
Calcitonin
This hormone inhibits bone resorption and increases excretion of calcium in the kidneys. It is not recommended for use alone because of its short duration of action and tachyphylaxis, but it can be used in combination with other agents, particularly in hypercalcemic crisis.22 It has the most rapid onset (within 2 hours) of the available medications, and when used in combination with bisphosphonates it produces a more substantial and rapid reduction in serum calcium.25,26
In a patient such as ours, with severe hypercalcemia and evidence of neurologic consequences, calcitonin should be used for its rapid and effective action in lowering serum calcium as other interventions take effect.
Intravenous fluids
Like our patient, many patients with significant hypercalcemia have volume depletion as a result of calciuresis-induced polyuria. Many also have nephrogenic diabetes insipidus from the cytotoxic effect of calcium on renal cells, leading to further volume depletion.27
All management approaches call for fluid repletion as an initial step in hypercalcemia. However, for severe hypercalcemia, volume resuscitation alone is unlikely to completely correct the imbalance. In addition to correcting dehydration, giving fluids increases glomerular filtration, allowing for increased secretion of calcium at the distal tubule.28 The recommendation is 2.5 to 4 L of normal saline over the first 24 hours, with continued aggressive hydration until good urine output is established.21
Our patient, in addition to having acute kidney injury thought to be due to prerenal azotemia, appeared to be volume-depleted and was given aggressive intravenous hydration.
Furosemide
Furosemide inhibits calcium reabsorption at the thick ascending loop of Henle, but this effect depends on the glomerular filtration rate. While our patient would likely eventually benefit from furosemide, it should not be considered the first-line therapy, as diuretic use in the setting of volume depletion can cause circulatory collapse.29 A relative contraindication was his presentation with acute kidney injury.
LONG-TERM TREATMENT
4. In the continued management of a patient with vitamin D toxicity with severe hypercalcemia, which of the following provides prolonged benefit?
- Intravenous hydrocortisone
- Fluid repletion
- Pamidronate
- Calcium-restricted diet
Much has been postulated concerning the mechanism of vitamin D intoxication and subsequent hypercalcemia. Studies have shown it is not an increase in dietary calcium absorption that drives the hypercalcemia but rather an increase in bone resorption. As such, bisphosphonates such as pamidronate have been shown to have a dramatic and rapid effect on severe hypercalcemia from vitamin D toxicity. The duration of action varies but is typically between 1 and 2 weeks.22,30
Corticosteroids such as hydrocortisone are also indicated in situations of severe toxicity. They block the action of 1-alpha-hydroxylase, which converts inactive 25-hydroxyvitamin D to the active 1,25-dihydroxyvitamin D. Corticosteroids have also been shown to more directly reduce calcium resorption from bone and intestine in addition to increasing calciuresis.31 A small study in the United Kingdom noted that while bisphosphonates and steroids were equally effective in reducing serum calcium levels, bisphosphonates accomplished this reduction more rapidly, with a time to therapeutic effect of 9 days as opposed to 22 days.
Fluid hydration, though necessary, is unlikely to produce complete correction on its own, as previously discussed.
THE PATIENT RECOVERS
The patient was treated with intravenous fluids over 3 days and received 1 dose of pamidronate. Calcitonin was provided over the first 48 hours after presentation to more rapidly reduce his calcium levels. He was advised to avoid taking the supplements prescribed by his naturopathic practitioner.
On follow-up with an endocrinologist 1 week later, his symptoms had entirely resolved, and his calcium level was 10.5 mg/dL.
TAKE-AWAY POINTS
- A good medication history includes over-the-counter products such as vitamin D supplements, as more and more people are taking them.
- The level of 25-hydroxyvitamin D should be monitored within 3 to 4 months after initiating treatment for vitamin D deficiency.11
- Vitamin D toxicity can have profound consequences, which are usually seen when levels of 25-hydroxyvitamin D rise above 250 ng/mL.13
- The Institute of Medicine recommends that the dosage of vitamin D supplements be no more than 4,000 IU/day and that doses may need to be lowered to account for concurrent use of hypercalcemia-inducing drugs and other vitamin D-containing supplements.32
- Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician 2003; 67:1959–1966.
- al Zahrani A, Levine MA. Primary hyperparathyroidism. Lancet 1997; 349:1233–1238.
- Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol 2008; 19:672–675.
- Ratcliffe WA, Hutchesson AC, Bundred NJ, Ratcliffe JG. Role of assays for parathyroid-hormone-related protein in investigation of hypercalcaemia. Lancet 1992; 339:164–167.
- Hewison M, Kantorovich V, Liker HR, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res 2003; 18:579–582.
- Ghobrial MW, George J, Mannam S, Henien SR. Severe hypercalcemia as an initial presenting manifestation of hepatocellular carcinoma. Can J Gastroenterol 2002; 16:607–609.
- Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol 2016; 50:120–133.
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 2016; 91:101–119.
- Burman KD, Monchik JM, Earll JM, Wartofsky L. Ionized and total serum calcium and parathyroid hormone in hyperthyroidism. Ann Intern Med 1976; 84:668–671.
- Iqbal AA, Burgess EH, Gallina DL, Nanes MS, Cook CB. Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract 2003; 9:517–521.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281.
- Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol 2006; 54:301–317.
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88:582S–586S.
- Inui N, Murayama A, Sasaki S, et al. Correlation between 25-hydroxyvitamin D3 1 alpha-hydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med 2001; 110:687–693.
- Wermers RA, Kearns AE, Jenkins GD, Melton LJ 3rd. Incidence and clinical spectrum of thiazide-associated hypercalcemia. Am J Med 2007; 120:911.e9–e15.
- Patel AM, Goldfarb S. Got calcium? Welcome to the calcium-alkali syndrome. J Am Soc Nephrol 2010; 21:1440–1443.
- Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry 2015; 172:12–15.
- Farrington K, Miller P, Varghese Z, Baillod RA, Moorhead JF. Vitamin A toxicity and hypercalcaemia in chronic renal failure. Br Med J (Clin Res Ed) 1981; 282:1999–2002.
- Frame B, Jackson CE, Reynolds WA, Umphrey JE. Hypercalcemia and skeletal effects in chronic hypervitaminosis A. Ann Intern Med 1974; 80:44–48.
- Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol 2012; 1:151–154.
- Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med 2015; 128:239–245.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med 1993; 95:297–304.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19:558–567.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 2008; 74:1385–1393.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med 1992; 326:1196–1203.
- Ralston SH. Medical management of hypercalcaemia. Br J Clin Pharmacol 1992; 34:11–20.
- Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis 2005; 45:626–637.
- Hosking DJ, Cowley A, Bucknall CA. Rehydration in the treatment of severe hypercalcaemia. Q J Med 1981; 50:473–481.
- Suki WN, Yium JJ, Von Minden M, Saller-Hebert C, Eknoyan G, Martinez-Maldonado M. Acute treatment of hypercalcemia with furosemide. N Engl J Med 1970; 283:836–840.
- Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol 1995; 43:531–536.
- Davies M, Mawer EB, Freemont AJ. The osteodystrophy of hypervitaminosis D: a metabolic study. Q J Med 1986; 61:911–919.
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011.
A retired 80-year-old man presented to the emergency department after 10 days of increasing polydipsia, polyuria, dry mouth, confusion, and slurred speech. He also reported that he had gradually and unintentionally lost 20 pounds and had loss of appetite, constipation, and chronic itching. He denied fevers, chills, night sweats, nausea, vomiting, and abdominal pain.
Medical history. He had type 2 diabetes mellitus that was well controlled by oral hypoglycemics, hypothyroidism treated with levothyroxine in stable doses, and chronic hepatitis C complicated by liver cirrhosis without focal hepatic lesions. He also had hypertension, well controlled with hydrochlorothiazide and losartan. For his long-standing pruritus he had tried prescription drugs including gabapentin and pregabalin without improvement. He had also seen a naturopathic practitioner, who had prescribed supplements that relieved the symptoms.
Examination. The patient was in no acute distress. He appeared thin, with a weight of 140 lb and a body mass index of 21 kg/m2. His temperature was 36.8°C (98.2°F), blood pressure 198/82 mm Hg, heart rate 72 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation 97%. His skin was without jaundice or rashes. The mucous membranes in the oropharynx were dry.
Neurologic examination revealed mild confusion, dysarthria, and ataxic gait. Sensation to light touch, pinprick, and vibration was intact. Generalized weakness was noted. Cranial nerves II through XII were intact. Deep tendon reflexes were symmetrically globally suppressed. Asterixis was absent. The remainder of the physical examination was unremarkable.
Laboratory values in the emergency department. We initially suspected he had symptomatic hyperglycemia, but a bedside blood glucose value of 113 mg/dL ruled this out. Other initial laboratory values:
- Blood urea nitrogen 31 mg/dL (reference range 9–24)
- Serum creatinine 1.7 mg/dL (0.73–1.22; an earlier value had been 1.0 mg/dL)
- Total serum calcium 14.4 mg/dL (8.6–10.0)
Complete blood cell counts were unremarkable. Computed tomography of the head was negative for acute pathology.
In view of the patient’s hypercalcemia, he was given aggressive intravenous fluid resuscitation (2 L of normal saline over 2 hours) and was admitted to the hospital. His laboratory values on admission are shown in Table 1. Fluid resuscitation was continued while the laboratory results were pending.
CAUSES OF HYPERCALCEMIA
1. Based on this information, which is the most likely cause of this patient’s hypercalcemia?
- Primary hyperparathyroidism
- Malignancy
- Hyperthyroidism
- Hypervitaminosis D
- Sarcoidosis
Traditionally, the workup for hypercalcemia in an outpatient starts with measuring the serum parathyroid hormone (PTH) level. Based on the results, a further evaluation of PTH-mediated vs PTH-independent causes of hypercalcemia would be initiated.
Primary hyperparathyroidism and malignancy account for 90% of all cases of hypercalcemia. The serum PTH concentration is usually high in primary hyperparathyroidism but low in malignancy, which helps distinguish the conditions from each other.1
Primary hyperparathyroidism
In primary hyperparathyroidism, there is overproduction of PTH, most commonly from a parathyroid adenoma, though parathyroid hyperplasia or, more rarely, parathyroid carcinoma can also overproduce the hormone.
PTH increases serum calcium levels through 3 primary mechanisms: increasing bone resorption, increasing intestinal absorption of calcium, and decreasing renal excretion of calcium. It also induces renal phosphorus excretion.
Typically, in primary hyperparathyroidism, the increases in serum calcium are small (with serum levels of total calcium rising to no higher than 11 mg/dL) and often intermittent.2 Our patient had extremely high serum calcium, low PTH, and high phosphorus levels—all of which are inconsistent with primary hyperparathyroidism.
Malignancy
In some solid tumors, the major mechanism of hypercalcemia is secretion of PTH-related peptide (PTHrP) through promotion of osteoclast function and also increased renal absorption of calcium.3 Hematologic malignancies (eg, multiple myeloma) produce osteoclast-activating factors such as RANK ligand, lymphotoxin, and interleukin 6. Direct tumor invasion of bone can cause osteolysis and subsequent hypercalcemia.4 These mechanisms are usually associated with a fall in PTH.
Less commonly, tumors can also increase levels of 1,25-dihydroxyvitamin D or produce PTH independently of the parathyroid gland.5 There have also been reports of severe hypercalcemia from hepatocellular carcinoma due to PTHrP production.6
Our patient is certainly at risk for malignancy, given his long-standing history of hepatitis C and cirrhosis. He also had a mildly elevated alpha fetoprotein level and suppressed PTH. However, his PTHrP level was normal, and ultrasonography done recently to screen for hepatocellular carcinoma (recommended every 6 months by the American Association for the Study of Liver Diseases in high-risk patients) was negative.7
Multiple myeloma screening involves testing with serum protein electrophoresis with immunofixation in combination with either a serum free light chain assay or 24-hour urine protein electrophoresis with immunofixation. This provides a 97% sensitivity.8 In this patient, these tests for multiple myeloma were negative.
Hyperthyroidism
As many as half of all patients with hyperthyroidism have elevated levels of ionized serum calcium.9 Increased osteoclastic activity is the likely mechanism. Hyperthyroid patients have increased levels of serum interleukin 6 and increased sensitivity of bone to this factor. This cytokine induces differentiation of monocytic cells into osteoclast precursors.10 These patients also have normal or low PTH levels.9
Our patient was receiving levothyroxine for hypothyroidism, but there was no evidence that the dosage was too high, as his thyroid-stimulating hormone level was within an acceptable range.
Hypervitaminosis D
Vitamin D precursors arise from the skin and from the diet. These precursors are hydroxylated in the liver and then the kidneys to biologically active 1,25-dihydroxyvitamin D (Figure 1).11 Vitamin D’s primary actions are in the intestines to increase absorption of calcium and in bone to induce osteoclast action. These actions raise the serum calcium level, which in turn lowers the PTH level through negative feedback on the parathyroid gland.
Most vitamin D supplements consist of the inactive precursor cholecalciferol (vitamin D3). To assess the degree of supplementation, 25-hydroxyvitamin D levels, which indicate the size of the body’s vitamin D reservoir, are measured.11,12
Our patient’s 25-hydroxyvitamin D level is extremely elevated, well beyond the 250-ng/mL upper limit that is considered safe.13 His low PTH level, lack of other likely causes, and history of supplement use point toward the diagnosis of hypervitaminosis D.
Sarcoidosis
Up to 10% of patients with sarcoidosis have hypercalcemia that is not mediated by PTH. Hypercalcemia in sarcoidosis has several potential mechanisms, including increased activity of the enzyme 1-alpha hydroxylase with a subsequent increase in physiologically active 1,25-dihydroxyvitamin D3 production.14
Our patient had elevated levels of 25-hydroxyvitamin D, but his biologically active 1,25-dihydroxyvitamin D level remained within the laboratory’s reference range.
LESS LIKELY CAUSES OF HYPERCALCEMIA
2. Which of the following would be least likely to cause hypercalcemia?
- Thiazide diuretics
- Over-the-counter antacid tablets
- Lithium
- Vitamin A supplementation
- Proton pump inhibitors
Thiazide diuretics
This class of drugs is well known to cause hypercalcemia. The most familiar of the mechanisms is a reduction in urinary calcium excretion. There is also an increase in intestinal absorption of dietary calcium. Evidence is increasing that most patients (as many as two-thirds) who develop hypercalcemia while using a thiazide diuretic have subclinical primary hyperparathyroidism that is uncovered with use of the diuretic.
Of importance, the hypercalcemia that thiazide diuretics cause is mild. In a series of 72 patients with thiazide-induced hypercalcemia, the average serum calcium level was 10.7 mg/dL.15
Our patient was receiving a thiazide diuretic but presented with severe hypercalcemia, which is inconsistent with thiazide-induced hypercalcemia.
Over-the-counter antacid tablets
Calcium carbonate, a popular over-the-counter antacid, can cause a milk-alkali syndrome that is defined by ingestion of excessive calcium and alkalotic substances, leading to metabolic alkalosis, hypercalcemia, and renal insufficiency. To induce this syndrome generally requires up to 4 g of calcium intake daily, but even lower levels (1.0 to 1.5 g) are known to cause it.16
Lithium
Lithium is known to cause hypercalcemia. Multiple mechanisms have been proposed, including direct action on renal tubules and the intestines leading to calcium reabsorption and stimulation of PTH release. Interestingly, parathyroid gland hyperplasia has been noted in long-term users of lithium. An often-proposed mechanism is that lithium increases the threshold at which the parathyroid glands slow their production of PTH, making them less sensitive to serum calcium levels.17
Vitamin A supplementation
Multiple case reports have linked hypercalcemia to ingestion of large doses of vitamin A. The mechanism is thought to be increased bone resorption.18.19
Although our patient reported supplement use, he denied taking vitamin A in any form.
Proton pump inhibitors
Proton pump inhibitors are not known to cause hypercalcemia. On the contrary, case reports suggest that prolonged use of proton pump inhibitors is associated with hypocalcemia and hypomagnesemia, although the mechanism is still not fully understood. A low magnesium level is known to reduce PTH secretion and also skeletal responsiveness to PTH, which can lead to profound hypocalcemia.20
CASE CONTINUED
On further questioning, the patient revealed that the supplement prescribed by his naturopathic practitioner contained vitamin D. Although he had been instructed to take 1 tablet weekly, he had begun taking it daily with his other routine medications, resulting in a daily dose in excess of 60,000 IU of cholecalciferol (vitamin D3). The recommended dose is no more than 4,000 IU/day.
The supplement was immediately discontinued. His hydrochlorothiazide was also held due to its known effect of reducing urinary calcium excretion.
INITIAL TREATMENT OF HYPERCALCEMIA
3. Which of the following treatments is not recommended as part of this patient’s initial treatment?
- Bisphosphonates
- Calcitonin
- Intravenous fluids
- Furosemide
Our patient met the criteria for the diagnosis of hypercalcemic crisis, usually defined as an albumin-corrected serum calcium level higher than 14 mg/dL associated with multiorgan dysfunction resulting from the hypercalcemia.21 The mnemonic “stones, bones, abdominal moans, and psychic groans” captures the renal, skeletal, gastrointestinal, and neurologic manifestations.1
Bisphosphonates
Bisphosphonates are analogues of pyrophosphonates, which are normally incorporated into bone. Unlike pyrophosphonates, bisphosphonates inhibit osteoclast function. They are often used to treat hypercalcemia of any cause, although they are currently approved by the US Food and Drug Administration for treating hypercalcemia of malignancy only. As intravenous monotherapy, they are superior to other forms of treatment and are among the first-line agents in management.
Two bisphosphonates shown to be effective in hypercalcemia are zoledronate and pamidronate. Pamidronate begins to lower serum calcium levels within 2 days, with a peak effect at around 6 days.22 However, in studies comparing the 2 drugs, zoledronate has been shown to be more effective in normalizing serum calcium, with the additional benefit of having a much more rapid infusion time.23 Zoledronate is contraindicated in patients with creatinine clearance less than 30 mL/min; however, pamidronate may continue to be used.24
Calcitonin
This hormone inhibits bone resorption and increases excretion of calcium in the kidneys. It is not recommended for use alone because of its short duration of action and tachyphylaxis, but it can be used in combination with other agents, particularly in hypercalcemic crisis.22 It has the most rapid onset (within 2 hours) of the available medications, and when used in combination with bisphosphonates it produces a more substantial and rapid reduction in serum calcium.25,26
In a patient such as ours, with severe hypercalcemia and evidence of neurologic consequences, calcitonin should be used for its rapid and effective action in lowering serum calcium as other interventions take effect.
Intravenous fluids
Like our patient, many patients with significant hypercalcemia have volume depletion as a result of calciuresis-induced polyuria. Many also have nephrogenic diabetes insipidus from the cytotoxic effect of calcium on renal cells, leading to further volume depletion.27
All management approaches call for fluid repletion as an initial step in hypercalcemia. However, for severe hypercalcemia, volume resuscitation alone is unlikely to completely correct the imbalance. In addition to correcting dehydration, giving fluids increases glomerular filtration, allowing for increased secretion of calcium at the distal tubule.28 The recommendation is 2.5 to 4 L of normal saline over the first 24 hours, with continued aggressive hydration until good urine output is established.21
Our patient, in addition to having acute kidney injury thought to be due to prerenal azotemia, appeared to be volume-depleted and was given aggressive intravenous hydration.
Furosemide
Furosemide inhibits calcium reabsorption at the thick ascending loop of Henle, but this effect depends on the glomerular filtration rate. While our patient would likely eventually benefit from furosemide, it should not be considered the first-line therapy, as diuretic use in the setting of volume depletion can cause circulatory collapse.29 A relative contraindication was his presentation with acute kidney injury.
LONG-TERM TREATMENT
4. In the continued management of a patient with vitamin D toxicity with severe hypercalcemia, which of the following provides prolonged benefit?
- Intravenous hydrocortisone
- Fluid repletion
- Pamidronate
- Calcium-restricted diet
Much has been postulated concerning the mechanism of vitamin D intoxication and subsequent hypercalcemia. Studies have shown it is not an increase in dietary calcium absorption that drives the hypercalcemia but rather an increase in bone resorption. As such, bisphosphonates such as pamidronate have been shown to have a dramatic and rapid effect on severe hypercalcemia from vitamin D toxicity. The duration of action varies but is typically between 1 and 2 weeks.22,30
Corticosteroids such as hydrocortisone are also indicated in situations of severe toxicity. They block the action of 1-alpha-hydroxylase, which converts inactive 25-hydroxyvitamin D to the active 1,25-dihydroxyvitamin D. Corticosteroids have also been shown to more directly reduce calcium resorption from bone and intestine in addition to increasing calciuresis.31 A small study in the United Kingdom noted that while bisphosphonates and steroids were equally effective in reducing serum calcium levels, bisphosphonates accomplished this reduction more rapidly, with a time to therapeutic effect of 9 days as opposed to 22 days.
Fluid hydration, though necessary, is unlikely to produce complete correction on its own, as previously discussed.
THE PATIENT RECOVERS
The patient was treated with intravenous fluids over 3 days and received 1 dose of pamidronate. Calcitonin was provided over the first 48 hours after presentation to more rapidly reduce his calcium levels. He was advised to avoid taking the supplements prescribed by his naturopathic practitioner.
On follow-up with an endocrinologist 1 week later, his symptoms had entirely resolved, and his calcium level was 10.5 mg/dL.
TAKE-AWAY POINTS
- A good medication history includes over-the-counter products such as vitamin D supplements, as more and more people are taking them.
- The level of 25-hydroxyvitamin D should be monitored within 3 to 4 months after initiating treatment for vitamin D deficiency.11
- Vitamin D toxicity can have profound consequences, which are usually seen when levels of 25-hydroxyvitamin D rise above 250 ng/mL.13
- The Institute of Medicine recommends that the dosage of vitamin D supplements be no more than 4,000 IU/day and that doses may need to be lowered to account for concurrent use of hypercalcemia-inducing drugs and other vitamin D-containing supplements.32
A retired 80-year-old man presented to the emergency department after 10 days of increasing polydipsia, polyuria, dry mouth, confusion, and slurred speech. He also reported that he had gradually and unintentionally lost 20 pounds and had loss of appetite, constipation, and chronic itching. He denied fevers, chills, night sweats, nausea, vomiting, and abdominal pain.
Medical history. He had type 2 diabetes mellitus that was well controlled by oral hypoglycemics, hypothyroidism treated with levothyroxine in stable doses, and chronic hepatitis C complicated by liver cirrhosis without focal hepatic lesions. He also had hypertension, well controlled with hydrochlorothiazide and losartan. For his long-standing pruritus he had tried prescription drugs including gabapentin and pregabalin without improvement. He had also seen a naturopathic practitioner, who had prescribed supplements that relieved the symptoms.
Examination. The patient was in no acute distress. He appeared thin, with a weight of 140 lb and a body mass index of 21 kg/m2. His temperature was 36.8°C (98.2°F), blood pressure 198/82 mm Hg, heart rate 72 beats per minute, respiratory rate 16 breaths per minute, and oxygen saturation 97%. His skin was without jaundice or rashes. The mucous membranes in the oropharynx were dry.
Neurologic examination revealed mild confusion, dysarthria, and ataxic gait. Sensation to light touch, pinprick, and vibration was intact. Generalized weakness was noted. Cranial nerves II through XII were intact. Deep tendon reflexes were symmetrically globally suppressed. Asterixis was absent. The remainder of the physical examination was unremarkable.
Laboratory values in the emergency department. We initially suspected he had symptomatic hyperglycemia, but a bedside blood glucose value of 113 mg/dL ruled this out. Other initial laboratory values:
- Blood urea nitrogen 31 mg/dL (reference range 9–24)
- Serum creatinine 1.7 mg/dL (0.73–1.22; an earlier value had been 1.0 mg/dL)
- Total serum calcium 14.4 mg/dL (8.6–10.0)
Complete blood cell counts were unremarkable. Computed tomography of the head was negative for acute pathology.
In view of the patient’s hypercalcemia, he was given aggressive intravenous fluid resuscitation (2 L of normal saline over 2 hours) and was admitted to the hospital. His laboratory values on admission are shown in Table 1. Fluid resuscitation was continued while the laboratory results were pending.
CAUSES OF HYPERCALCEMIA
1. Based on this information, which is the most likely cause of this patient’s hypercalcemia?
- Primary hyperparathyroidism
- Malignancy
- Hyperthyroidism
- Hypervitaminosis D
- Sarcoidosis
Traditionally, the workup for hypercalcemia in an outpatient starts with measuring the serum parathyroid hormone (PTH) level. Based on the results, a further evaluation of PTH-mediated vs PTH-independent causes of hypercalcemia would be initiated.
Primary hyperparathyroidism and malignancy account for 90% of all cases of hypercalcemia. The serum PTH concentration is usually high in primary hyperparathyroidism but low in malignancy, which helps distinguish the conditions from each other.1
Primary hyperparathyroidism
In primary hyperparathyroidism, there is overproduction of PTH, most commonly from a parathyroid adenoma, though parathyroid hyperplasia or, more rarely, parathyroid carcinoma can also overproduce the hormone.
PTH increases serum calcium levels through 3 primary mechanisms: increasing bone resorption, increasing intestinal absorption of calcium, and decreasing renal excretion of calcium. It also induces renal phosphorus excretion.
Typically, in primary hyperparathyroidism, the increases in serum calcium are small (with serum levels of total calcium rising to no higher than 11 mg/dL) and often intermittent.2 Our patient had extremely high serum calcium, low PTH, and high phosphorus levels—all of which are inconsistent with primary hyperparathyroidism.
Malignancy
In some solid tumors, the major mechanism of hypercalcemia is secretion of PTH-related peptide (PTHrP) through promotion of osteoclast function and also increased renal absorption of calcium.3 Hematologic malignancies (eg, multiple myeloma) produce osteoclast-activating factors such as RANK ligand, lymphotoxin, and interleukin 6. Direct tumor invasion of bone can cause osteolysis and subsequent hypercalcemia.4 These mechanisms are usually associated with a fall in PTH.
Less commonly, tumors can also increase levels of 1,25-dihydroxyvitamin D or produce PTH independently of the parathyroid gland.5 There have also been reports of severe hypercalcemia from hepatocellular carcinoma due to PTHrP production.6
Our patient is certainly at risk for malignancy, given his long-standing history of hepatitis C and cirrhosis. He also had a mildly elevated alpha fetoprotein level and suppressed PTH. However, his PTHrP level was normal, and ultrasonography done recently to screen for hepatocellular carcinoma (recommended every 6 months by the American Association for the Study of Liver Diseases in high-risk patients) was negative.7
Multiple myeloma screening involves testing with serum protein electrophoresis with immunofixation in combination with either a serum free light chain assay or 24-hour urine protein electrophoresis with immunofixation. This provides a 97% sensitivity.8 In this patient, these tests for multiple myeloma were negative.
Hyperthyroidism
As many as half of all patients with hyperthyroidism have elevated levels of ionized serum calcium.9 Increased osteoclastic activity is the likely mechanism. Hyperthyroid patients have increased levels of serum interleukin 6 and increased sensitivity of bone to this factor. This cytokine induces differentiation of monocytic cells into osteoclast precursors.10 These patients also have normal or low PTH levels.9
Our patient was receiving levothyroxine for hypothyroidism, but there was no evidence that the dosage was too high, as his thyroid-stimulating hormone level was within an acceptable range.
Hypervitaminosis D
Vitamin D precursors arise from the skin and from the diet. These precursors are hydroxylated in the liver and then the kidneys to biologically active 1,25-dihydroxyvitamin D (Figure 1).11 Vitamin D’s primary actions are in the intestines to increase absorption of calcium and in bone to induce osteoclast action. These actions raise the serum calcium level, which in turn lowers the PTH level through negative feedback on the parathyroid gland.
Most vitamin D supplements consist of the inactive precursor cholecalciferol (vitamin D3). To assess the degree of supplementation, 25-hydroxyvitamin D levels, which indicate the size of the body’s vitamin D reservoir, are measured.11,12
Our patient’s 25-hydroxyvitamin D level is extremely elevated, well beyond the 250-ng/mL upper limit that is considered safe.13 His low PTH level, lack of other likely causes, and history of supplement use point toward the diagnosis of hypervitaminosis D.
Sarcoidosis
Up to 10% of patients with sarcoidosis have hypercalcemia that is not mediated by PTH. Hypercalcemia in sarcoidosis has several potential mechanisms, including increased activity of the enzyme 1-alpha hydroxylase with a subsequent increase in physiologically active 1,25-dihydroxyvitamin D3 production.14
Our patient had elevated levels of 25-hydroxyvitamin D, but his biologically active 1,25-dihydroxyvitamin D level remained within the laboratory’s reference range.
LESS LIKELY CAUSES OF HYPERCALCEMIA
2. Which of the following would be least likely to cause hypercalcemia?
- Thiazide diuretics
- Over-the-counter antacid tablets
- Lithium
- Vitamin A supplementation
- Proton pump inhibitors
Thiazide diuretics
This class of drugs is well known to cause hypercalcemia. The most familiar of the mechanisms is a reduction in urinary calcium excretion. There is also an increase in intestinal absorption of dietary calcium. Evidence is increasing that most patients (as many as two-thirds) who develop hypercalcemia while using a thiazide diuretic have subclinical primary hyperparathyroidism that is uncovered with use of the diuretic.
Of importance, the hypercalcemia that thiazide diuretics cause is mild. In a series of 72 patients with thiazide-induced hypercalcemia, the average serum calcium level was 10.7 mg/dL.15
Our patient was receiving a thiazide diuretic but presented with severe hypercalcemia, which is inconsistent with thiazide-induced hypercalcemia.
Over-the-counter antacid tablets
Calcium carbonate, a popular over-the-counter antacid, can cause a milk-alkali syndrome that is defined by ingestion of excessive calcium and alkalotic substances, leading to metabolic alkalosis, hypercalcemia, and renal insufficiency. To induce this syndrome generally requires up to 4 g of calcium intake daily, but even lower levels (1.0 to 1.5 g) are known to cause it.16
Lithium
Lithium is known to cause hypercalcemia. Multiple mechanisms have been proposed, including direct action on renal tubules and the intestines leading to calcium reabsorption and stimulation of PTH release. Interestingly, parathyroid gland hyperplasia has been noted in long-term users of lithium. An often-proposed mechanism is that lithium increases the threshold at which the parathyroid glands slow their production of PTH, making them less sensitive to serum calcium levels.17
Vitamin A supplementation
Multiple case reports have linked hypercalcemia to ingestion of large doses of vitamin A. The mechanism is thought to be increased bone resorption.18.19
Although our patient reported supplement use, he denied taking vitamin A in any form.
Proton pump inhibitors
Proton pump inhibitors are not known to cause hypercalcemia. On the contrary, case reports suggest that prolonged use of proton pump inhibitors is associated with hypocalcemia and hypomagnesemia, although the mechanism is still not fully understood. A low magnesium level is known to reduce PTH secretion and also skeletal responsiveness to PTH, which can lead to profound hypocalcemia.20
CASE CONTINUED
On further questioning, the patient revealed that the supplement prescribed by his naturopathic practitioner contained vitamin D. Although he had been instructed to take 1 tablet weekly, he had begun taking it daily with his other routine medications, resulting in a daily dose in excess of 60,000 IU of cholecalciferol (vitamin D3). The recommended dose is no more than 4,000 IU/day.
The supplement was immediately discontinued. His hydrochlorothiazide was also held due to its known effect of reducing urinary calcium excretion.
INITIAL TREATMENT OF HYPERCALCEMIA
3. Which of the following treatments is not recommended as part of this patient’s initial treatment?
- Bisphosphonates
- Calcitonin
- Intravenous fluids
- Furosemide
Our patient met the criteria for the diagnosis of hypercalcemic crisis, usually defined as an albumin-corrected serum calcium level higher than 14 mg/dL associated with multiorgan dysfunction resulting from the hypercalcemia.21 The mnemonic “stones, bones, abdominal moans, and psychic groans” captures the renal, skeletal, gastrointestinal, and neurologic manifestations.1
Bisphosphonates
Bisphosphonates are analogues of pyrophosphonates, which are normally incorporated into bone. Unlike pyrophosphonates, bisphosphonates inhibit osteoclast function. They are often used to treat hypercalcemia of any cause, although they are currently approved by the US Food and Drug Administration for treating hypercalcemia of malignancy only. As intravenous monotherapy, they are superior to other forms of treatment and are among the first-line agents in management.
Two bisphosphonates shown to be effective in hypercalcemia are zoledronate and pamidronate. Pamidronate begins to lower serum calcium levels within 2 days, with a peak effect at around 6 days.22 However, in studies comparing the 2 drugs, zoledronate has been shown to be more effective in normalizing serum calcium, with the additional benefit of having a much more rapid infusion time.23 Zoledronate is contraindicated in patients with creatinine clearance less than 30 mL/min; however, pamidronate may continue to be used.24
Calcitonin
This hormone inhibits bone resorption and increases excretion of calcium in the kidneys. It is not recommended for use alone because of its short duration of action and tachyphylaxis, but it can be used in combination with other agents, particularly in hypercalcemic crisis.22 It has the most rapid onset (within 2 hours) of the available medications, and when used in combination with bisphosphonates it produces a more substantial and rapid reduction in serum calcium.25,26
In a patient such as ours, with severe hypercalcemia and evidence of neurologic consequences, calcitonin should be used for its rapid and effective action in lowering serum calcium as other interventions take effect.
Intravenous fluids
Like our patient, many patients with significant hypercalcemia have volume depletion as a result of calciuresis-induced polyuria. Many also have nephrogenic diabetes insipidus from the cytotoxic effect of calcium on renal cells, leading to further volume depletion.27
All management approaches call for fluid repletion as an initial step in hypercalcemia. However, for severe hypercalcemia, volume resuscitation alone is unlikely to completely correct the imbalance. In addition to correcting dehydration, giving fluids increases glomerular filtration, allowing for increased secretion of calcium at the distal tubule.28 The recommendation is 2.5 to 4 L of normal saline over the first 24 hours, with continued aggressive hydration until good urine output is established.21
Our patient, in addition to having acute kidney injury thought to be due to prerenal azotemia, appeared to be volume-depleted and was given aggressive intravenous hydration.
Furosemide
Furosemide inhibits calcium reabsorption at the thick ascending loop of Henle, but this effect depends on the glomerular filtration rate. While our patient would likely eventually benefit from furosemide, it should not be considered the first-line therapy, as diuretic use in the setting of volume depletion can cause circulatory collapse.29 A relative contraindication was his presentation with acute kidney injury.
LONG-TERM TREATMENT
4. In the continued management of a patient with vitamin D toxicity with severe hypercalcemia, which of the following provides prolonged benefit?
- Intravenous hydrocortisone
- Fluid repletion
- Pamidronate
- Calcium-restricted diet
Much has been postulated concerning the mechanism of vitamin D intoxication and subsequent hypercalcemia. Studies have shown it is not an increase in dietary calcium absorption that drives the hypercalcemia but rather an increase in bone resorption. As such, bisphosphonates such as pamidronate have been shown to have a dramatic and rapid effect on severe hypercalcemia from vitamin D toxicity. The duration of action varies but is typically between 1 and 2 weeks.22,30
Corticosteroids such as hydrocortisone are also indicated in situations of severe toxicity. They block the action of 1-alpha-hydroxylase, which converts inactive 25-hydroxyvitamin D to the active 1,25-dihydroxyvitamin D. Corticosteroids have also been shown to more directly reduce calcium resorption from bone and intestine in addition to increasing calciuresis.31 A small study in the United Kingdom noted that while bisphosphonates and steroids were equally effective in reducing serum calcium levels, bisphosphonates accomplished this reduction more rapidly, with a time to therapeutic effect of 9 days as opposed to 22 days.
Fluid hydration, though necessary, is unlikely to produce complete correction on its own, as previously discussed.
THE PATIENT RECOVERS
The patient was treated with intravenous fluids over 3 days and received 1 dose of pamidronate. Calcitonin was provided over the first 48 hours after presentation to more rapidly reduce his calcium levels. He was advised to avoid taking the supplements prescribed by his naturopathic practitioner.
On follow-up with an endocrinologist 1 week later, his symptoms had entirely resolved, and his calcium level was 10.5 mg/dL.
TAKE-AWAY POINTS
- A good medication history includes over-the-counter products such as vitamin D supplements, as more and more people are taking them.
- The level of 25-hydroxyvitamin D should be monitored within 3 to 4 months after initiating treatment for vitamin D deficiency.11
- Vitamin D toxicity can have profound consequences, which are usually seen when levels of 25-hydroxyvitamin D rise above 250 ng/mL.13
- The Institute of Medicine recommends that the dosage of vitamin D supplements be no more than 4,000 IU/day and that doses may need to be lowered to account for concurrent use of hypercalcemia-inducing drugs and other vitamin D-containing supplements.32
- Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician 2003; 67:1959–1966.
- al Zahrani A, Levine MA. Primary hyperparathyroidism. Lancet 1997; 349:1233–1238.
- Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol 2008; 19:672–675.
- Ratcliffe WA, Hutchesson AC, Bundred NJ, Ratcliffe JG. Role of assays for parathyroid-hormone-related protein in investigation of hypercalcaemia. Lancet 1992; 339:164–167.
- Hewison M, Kantorovich V, Liker HR, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res 2003; 18:579–582.
- Ghobrial MW, George J, Mannam S, Henien SR. Severe hypercalcemia as an initial presenting manifestation of hepatocellular carcinoma. Can J Gastroenterol 2002; 16:607–609.
- Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol 2016; 50:120–133.
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 2016; 91:101–119.
- Burman KD, Monchik JM, Earll JM, Wartofsky L. Ionized and total serum calcium and parathyroid hormone in hyperthyroidism. Ann Intern Med 1976; 84:668–671.
- Iqbal AA, Burgess EH, Gallina DL, Nanes MS, Cook CB. Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract 2003; 9:517–521.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281.
- Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol 2006; 54:301–317.
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88:582S–586S.
- Inui N, Murayama A, Sasaki S, et al. Correlation between 25-hydroxyvitamin D3 1 alpha-hydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med 2001; 110:687–693.
- Wermers RA, Kearns AE, Jenkins GD, Melton LJ 3rd. Incidence and clinical spectrum of thiazide-associated hypercalcemia. Am J Med 2007; 120:911.e9–e15.
- Patel AM, Goldfarb S. Got calcium? Welcome to the calcium-alkali syndrome. J Am Soc Nephrol 2010; 21:1440–1443.
- Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry 2015; 172:12–15.
- Farrington K, Miller P, Varghese Z, Baillod RA, Moorhead JF. Vitamin A toxicity and hypercalcaemia in chronic renal failure. Br Med J (Clin Res Ed) 1981; 282:1999–2002.
- Frame B, Jackson CE, Reynolds WA, Umphrey JE. Hypercalcemia and skeletal effects in chronic hypervitaminosis A. Ann Intern Med 1974; 80:44–48.
- Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol 2012; 1:151–154.
- Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med 2015; 128:239–245.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med 1993; 95:297–304.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19:558–567.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 2008; 74:1385–1393.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med 1992; 326:1196–1203.
- Ralston SH. Medical management of hypercalcaemia. Br J Clin Pharmacol 1992; 34:11–20.
- Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis 2005; 45:626–637.
- Hosking DJ, Cowley A, Bucknall CA. Rehydration in the treatment of severe hypercalcaemia. Q J Med 1981; 50:473–481.
- Suki WN, Yium JJ, Von Minden M, Saller-Hebert C, Eknoyan G, Martinez-Maldonado M. Acute treatment of hypercalcemia with furosemide. N Engl J Med 1970; 283:836–840.
- Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol 1995; 43:531–536.
- Davies M, Mawer EB, Freemont AJ. The osteodystrophy of hypervitaminosis D: a metabolic study. Q J Med 1986; 61:911–919.
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011.
- Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician 2003; 67:1959–1966.
- al Zahrani A, Levine MA. Primary hyperparathyroidism. Lancet 1997; 349:1233–1238.
- Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol 2008; 19:672–675.
- Ratcliffe WA, Hutchesson AC, Bundred NJ, Ratcliffe JG. Role of assays for parathyroid-hormone-related protein in investigation of hypercalcaemia. Lancet 1992; 339:164–167.
- Hewison M, Kantorovich V, Liker HR, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res 2003; 18:579–582.
- Ghobrial MW, George J, Mannam S, Henien SR. Severe hypercalcemia as an initial presenting manifestation of hepatocellular carcinoma. Can J Gastroenterol 2002; 16:607–609.
- Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol 2016; 50:120–133.
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 2016; 91:101–119.
- Burman KD, Monchik JM, Earll JM, Wartofsky L. Ionized and total serum calcium and parathyroid hormone in hyperthyroidism. Ann Intern Med 1976; 84:668–671.
- Iqbal AA, Burgess EH, Gallina DL, Nanes MS, Cook CB. Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract 2003; 9:517–521.
- Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281.
- Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol 2006; 54:301–317.
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88:582S–586S.
- Inui N, Murayama A, Sasaki S, et al. Correlation between 25-hydroxyvitamin D3 1 alpha-hydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med 2001; 110:687–693.
- Wermers RA, Kearns AE, Jenkins GD, Melton LJ 3rd. Incidence and clinical spectrum of thiazide-associated hypercalcemia. Am J Med 2007; 120:911.e9–e15.
- Patel AM, Goldfarb S. Got calcium? Welcome to the calcium-alkali syndrome. J Am Soc Nephrol 2010; 21:1440–1443.
- Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry 2015; 172:12–15.
- Farrington K, Miller P, Varghese Z, Baillod RA, Moorhead JF. Vitamin A toxicity and hypercalcaemia in chronic renal failure. Br Med J (Clin Res Ed) 1981; 282:1999–2002.
- Frame B, Jackson CE, Reynolds WA, Umphrey JE. Hypercalcemia and skeletal effects in chronic hypervitaminosis A. Ann Intern Med 1974; 80:44–48.
- Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol 2012; 1:151–154.
- Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med 2015; 128:239–245.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med 1993; 95:297–304.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19:558–567.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 2008; 74:1385–1393.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med 1992; 326:1196–1203.
- Ralston SH. Medical management of hypercalcaemia. Br J Clin Pharmacol 1992; 34:11–20.
- Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, Clark WF. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis 2005; 45:626–637.
- Hosking DJ, Cowley A, Bucknall CA. Rehydration in the treatment of severe hypercalcaemia. Q J Med 1981; 50:473–481.
- Suki WN, Yium JJ, Von Minden M, Saller-Hebert C, Eknoyan G, Martinez-Maldonado M. Acute treatment of hypercalcemia with furosemide. N Engl J Med 1970; 283:836–840.
- Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol 1995; 43:531–536.
- Davies M, Mawer EB, Freemont AJ. The osteodystrophy of hypervitaminosis D: a metabolic study. Q J Med 1986; 61:911–919.
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011.
Case Studies in Toxicology: Drink the Water, but Don’t Eat the Paint
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Bleeding esophageal varices: Who should receive a shunt?
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
Acid-base disturbances
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
In reply: Acid-base disturbances
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
When the Diagnosis Hurts
ANSWER
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.

ANSWER
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.

ANSWER
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.
A 60-year-old man presents to the emergency department for evaluation of chest pain that began a few hours ago. He denies injury and has no associated nausea or shortness of breath. Earlier today, he underwent biopsy of a recently discovered mass in his right lung. Otherwise, his medical history is only significant for hypertension. He is a former pack-a-day smoker but quit three months ago.
On physical exam, you note an uncomfortable male in no obvious distress. He is afebrile, with normal vital signs. His O2 saturation is 96% on room air. Breath sounds appear to be clear bilaterally, although the patient expresses some discomfort with inhalation. Heart sounds are normal as well.
While the nurse and tech place an IV, a portable chest radiograph is obtained. What is your impression?
Man’s best friend, fatal in the end
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
A previously healthy 59-year-old woman with a remote history of splenectomy following a motor vehicle accident presented to the emergency department with a chief complaint of fever. She had been in her usual state of health until the day before, when she developed chills and fever, with temperatures as high as 39.4°C (102.9°F). She also began to have nausea, vomiting, and diffuse body weakness and had to be brought to the emergency department in a wheelchair. She denied upper-respiratory or urinary symptoms, headache, stiff neck, recent travel, or sick contacts.
She had sustained a minor dog bite on her right hand 2 days before, but she denied swelling, erythema, or exudate. The dog, a family pet, was up to date on all of its vaccinations, including rabies.
Her temperature was 39.3°C (102.7°F), heart rate 121 beats per minute, and blood pressure 113/71 mm Hg. She had a clean, nonerythematous, healing, 1-cm laceration on her right thumb (Figure 1).
Initial laboratory values (Table 1) and a radiograph of her right thumb were unremarkable.
FEVER IN ASPLENIC PATIENTS
1. What is the appropriate next step in this patient’s management?
- Discharge her from the emergency department and have her follow up with her primary care physician within 48 hours
- Admit her for observation and defer antibiotic therapy
- Admit her and start empiric antibiotic therapy
- Admit but wait for culture results to come back before starting antibiotic therapy
The patient’s history of splenectomy and presentation with fever raise the concern that she may be going into sepsis. In addition to fever, patients with sepsis may present with flulike symptoms such as myalgias, headache, vomiting, diarrhea, and abdominal pain.1
Sepsis in asplenic patients, also known as overwhelming postsplenectomy infection, can have a sudden onset and fulminant course, with a mortality rate as high as 50%.2 It is important to recognize those who are susceptible, including patients without a spleen from splenectomy or congenital asplenia, as well as those with functional asplenia from diseases such as sickle cell disease. Without the spleen, the immune system cannot clear immunoglobulin G-coated bacteria and encapsulated bacteria that are not opsonized by antibodies or complement.3
Any asplenic patient presenting with fever or other symptoms of systemic infection warrants immediate antibiotic treatment, without delay for cultures or further testing.1
CASE CONTINUED: RAPID DETERIORATION
With no clear source of infection, the patient’s clinical presentation was presumed to be due to a viral infection, and antibiotics were deferred. She was admitted to the hospital for observation.
By the next morning, her mental status had declined. Her temperature at that time was 39.6°C (103.2°F), heart rate 115 per minute, and blood pressure 113/74 mm Hg. Her skin became mottled, and her lactate level increased from 1.9 mmol/L to 4.9 mmol/L (reference range 0.5–1.9 mmol/L) within 9 hours and continued to climb (Table 2).
EMPIRIC ANTIBIOTICS IN ASPLENIC SEPSIS
2. Which first-line antibiotics should have been started on initial presentation?
- Intravenous vancomycin and intravenous ceftriaxone
- Intravenous vancomycin and intravenous metronidazole
- Oral levofloxacin
- Oral amoxicillin
At initial presentation to the hospital, the most appropriate regimen for this patient would have been vancomycin and ceftriaxone or cefepime in meningitis-level (ie, high) doses.2,4
Due to impaired immunity, asplenic patients are highly susceptible to encapsulated gram-positive organisms such as Streptococcus pneumoniae and gram-negative organisms such as Haemophilus influenzae, Neisseria meningitidis, and Capnocytophaga canimorsus. These organisms are all susceptible to ceftriaxone, with the exception of methicillin-resistant S pneumoniae, which is best covered with vancomycin.1 Patients with beta-lactam hypersensitivity can be treated with moxifloxacin instead.4,5
Vancomycin and metronidazole alone would not be adequate. Oral levofloxacin or amoxicillin would be appropriate initial treatment if the patient did not have access to a hospital within 2 hours. Ideally, the patient would have had one of these medications on hand and taken it at the first sign of fever.4
CASE CONTINUED: TRANSFER TO ICU
The patient was empirically started on vancomycin and ceftriaxone and transferred to the intensive care unit. She required intubation for airway protection. She became hypotensive despite receiving intravenous fluids and multiple vasopressors. She continued to rapidly decline and developed lactic acidosis, which resulted in a severe anion gap metabolic acidosis with respiratory compensation. Her course was further complicated by disseminated intravascular coagulation, acute kidney failure, and ischemic hepatitis (“shock liver”) (Table 2).
CAUSES OF SEPSIS IN ASPLENIC PATIENTS
3. The patient’s septic shock is likely the result of which bacterial pathogen?
- S pneumoniae
- H influenzae
- C canimorsus
- N meningitidis
Encapsulated organisms including S pneumoniae, H influenzae, and N meningitidis account for almost 70% of infections in postsplenectomy patients, including those with overwhelming postsplenectomy infection.6 S pneumoniae is the most common culprit. However, the patient’s history of a recent dog bite suggests that the most likely cause was C canimorsus.
C canimorsus is a gram-negative bacillus commonly associated with exposure to dogs or cats through saliva, scratches, or bites.7,8 Even a seemingly small, benign-appearing wound, as seen in this case, can be a portal of entry for this organism. About 84 cases leading to fulminant sepsis were reported in the United States from 1990 to 2014.9 Patients infected with this organism can progress to fulminant sepsis with multiorgan failure with disseminated intravascular coagulation, anuria, and hypotension.10–12
CASE CONCLUDED
The patient died 40 hours after admission. Her blood cultures grew a slow-growing gram-negative rod within 2 days, subsequently identified as C canimorsus.
4. What is the best strategy for prevention of sepsis in an asplenic patient?
- Vaccinate against S pneumoniae (with PCV13 and PPSV23), H influenzae type b, and N meningitidis
- Prescribe antibiotics that the patient can take in case of fever
- Both of the above
- Prescribe lifelong daily antibiotic prophylaxis
- All of the above
Asplenic patients should receive pneumococcal, H influenzae type b, and meningococcal vaccines.13 Invasive bacterial infections, particularly with encapsulated organisms, occur 10 to 50 times more often in this population than in a healthy population and can be fatal.13 These vaccines have been shown to reduce the rate of life-threatening infections. Patients should receive the vaccines at least 2 weeks before an elective splenectomy or 2 weeks after a nonelective splenectomy.2
For the pneumococcal vaccines, PCV13 should be given first, followed by PPSV23 at least 8 weeks later. If the patient has already received PCV13, PPSV23 should be given at least 2 weeks after splenectomy. A second dose of PPSV23 should be given 5 years later.
The H influenzae type b vaccine should be administered if not already given.
For the meningococcal vaccine, the two-dose series should be administered with an interval of 8 to 12 weeks between doses. A booster meningococcal dose should be given every 5 years.
The patient should also receive the flu vaccine annually.2,14
Patients should also be given antibiotics (typically an antibiotic with activity against S pneumoniae, such as amoxicillin or levofloxacin) to carry with them. They should be told to take them if fever or chills develop and they cannot see a physician within 2 hours.2
Daily antibiotic prophylaxis with penicillin is typically given to patients younger than age 5, as studies have shown benefit in reducing pneumococcal sepsis. In adults, some experts recommend daily antibiotic prophylaxis for 1 year after splenectomy.2 However, there is a lack of data and expert consensus to recommend lifelong daily antibiotic prophylaxis for all asplenic patients. Thus, it is not recommended in adults unless the patient is immunocompromised or is a survivor of pneumococcal sepsis.4
KEY POINTS
- In an asplenic patient, fever can be an early sign of sepsis, which can have a rapid and fulminant course.
- Asplenic patients are particularly susceptible to infection by encapsulated organisms such as S pneumoniae, H influenzae, N meningitidis, and C canimorsus due to impaired immunity.
- If an asplenic patient has been exposed to a dog bite, scratch, or saliva, one should suspect C canimorsus.
- Asplenic patients who present with fever should be treated immediately with intravenous vancomycin and ceftriaxone without delay for laboratory tests or imaging.
- To help prevent fulminant sepsis, asplenic patients should receive vaccines (pneumococcal, meningococcal, and H influenzae type b) as well as a prescription for antibiotics (levofloxacin) to be used if they develop fever and cannot see a physician within 2 hours.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician 2001; 63:499–508.
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014; 371:349–356.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011; 378:86–97.
- Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection—an update. Crit Care Med 1999; 27:836–842.
- Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996; 10:693–707.
- Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. Br J Haematol 2015; 171:683–694.
- Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin Infect Dis 2003; 36:e42–e46.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–533.
- Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–1280.
- Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis 1996; 23:71–75.
- Chiappa V, Chang CY, Sellas MI, Pierce VM, Kradin RL. Case records of the Massachusetts General Hospital. Case 10-2014. A 45-year-old man with a rash. N Engl J Med 2014; 370:1238–1248.
- Martone WJ, Zuehl RW, Minson GE, Scheld WM. Postsplenectomy sepsis with DF-2: report of a case with isolation of the organism from the patient’s dog. Ann Intern Med 1980; 93:457–458.
- Centers for Disease Control and Prevention (CDC). Asplenia and adult vaccination. www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html. Accessed January 6, 2017.
- Rubin LG, Levin MJ, Ljungman P, et al; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
When should brain imaging precede lumbar puncture in cases of suspected bacterial meningitis?
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Ring-enhancing cerebral lesions
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.