User login
The Man With No Medical History
ANSWER
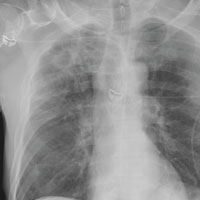
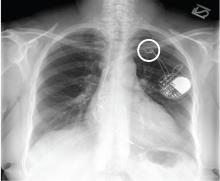
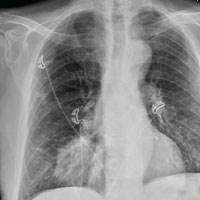
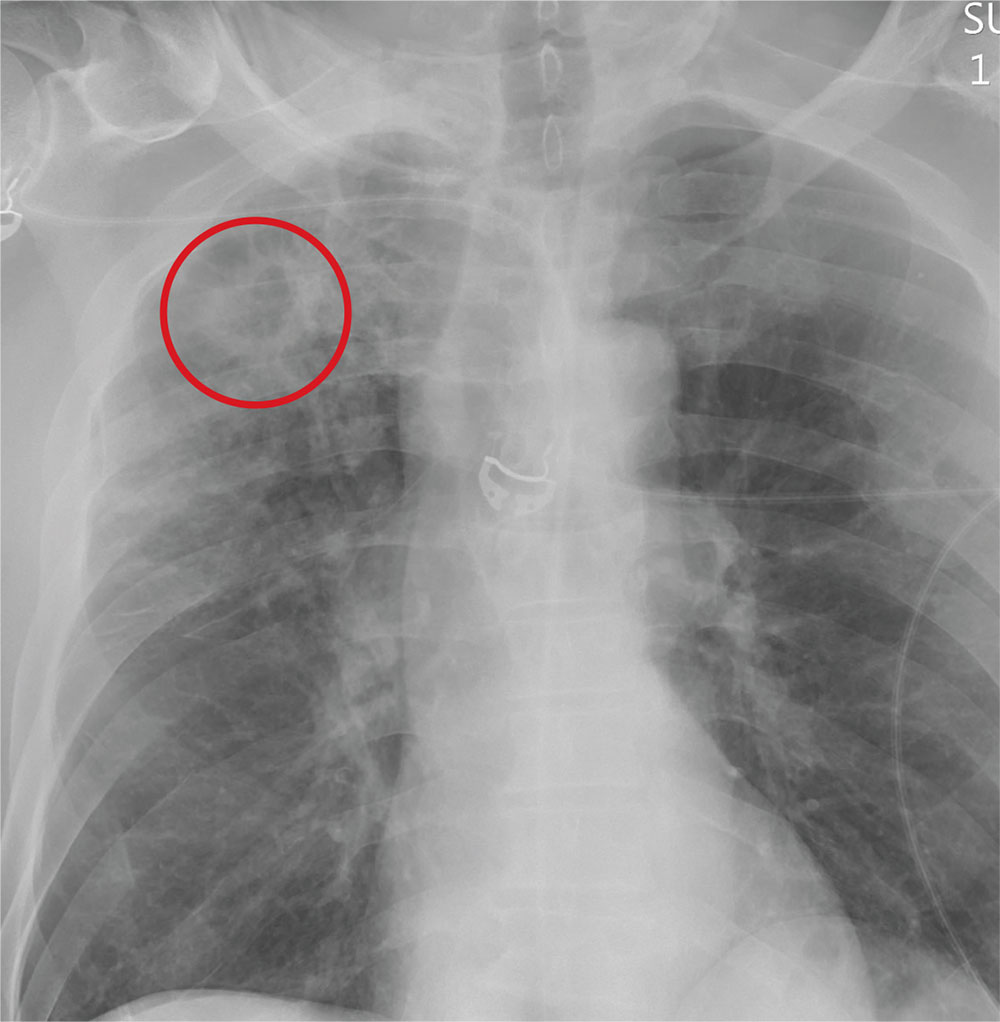
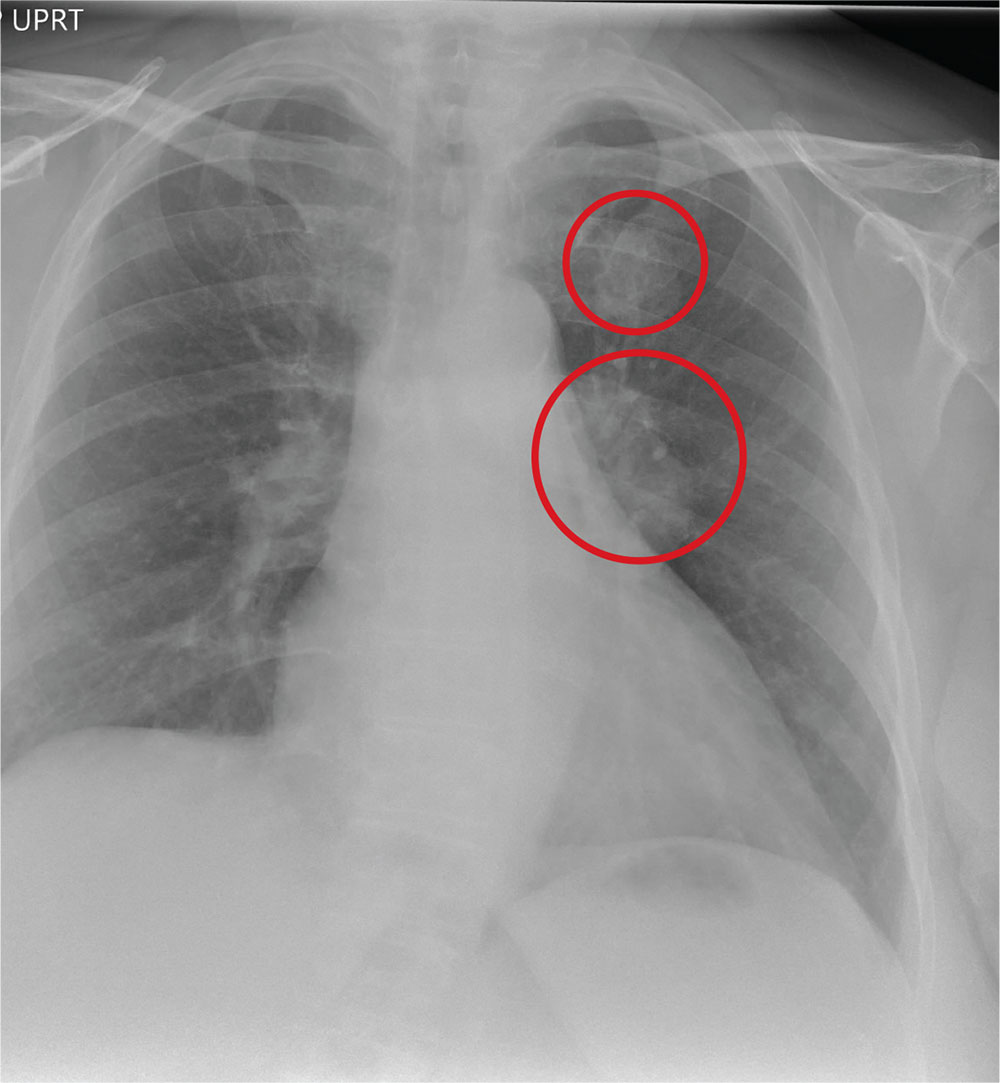
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.

ANSWER
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.

ANSWER
The radiograph demonstrates no acute fractures or pneumothorax. Of note is a right upper lobe infiltrate, which is a rounded cavitary lesion measuring approximately 4 cm. The differential includes pulmonary malignancy, active or previous pulmonary infection (eg, tuberculosis), or pneumatocele. Further evaluation with CT and a pulmonary consultation was coordinated.
Following a motor vehicle collision, a 60-year-old man is brought to the emergency department via ambulance. He was an unrestrained front-seat passenger in a vehicle that lost control on the roadway and went into a ditch. The patient complains of headache, chest wall pain, and left arm pain. He does not believe he lost consciousness.
He denies any medical history and adds that he does not seek regular medical treatment. He admits to tobacco use and frequent alcohol use.
On physical exam, you note an elderly-appearing male in no obvious distress with a Glasgow Coma Scale score of 15. His vital signs are all within normal limits. Other than slight swelling on the left side of his head, tenderness in the anterior chest wall, and pain in his left humerus, his exam is normal.
You order trauma lab tests and appropriate radiographic studies; a portable chest radiograph is completed (shown). What is your impression?
Is there a doctor on board? In-flight medical emergencies
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
KEY POINTS
- The exact incidence of medical emergencies aboard airplanes is unknown, but they occurred in 1 in 604 flights in 1 study, which is likely an underestimate.
- The relatively low air pressure in the cabin can contribute to the development of acute medical issues.
- In the United States, the Federal Aviation Administration mandates that airlines carry a limited set of medical resources.
- The Aviation Medical Assistance Act protects responding providers against liability except in cases of “gross negligence.”
- You the physician can recommend that the flight be diverted to the closest airport, but only the captain can make the actual decision.
Woman’s Weakness is Worsening
ANSWER
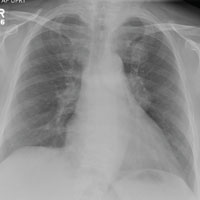
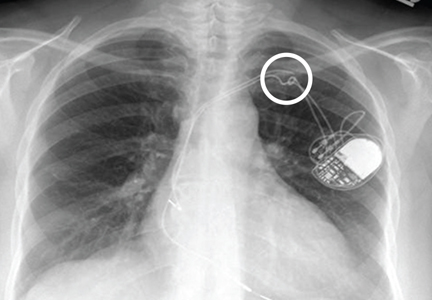
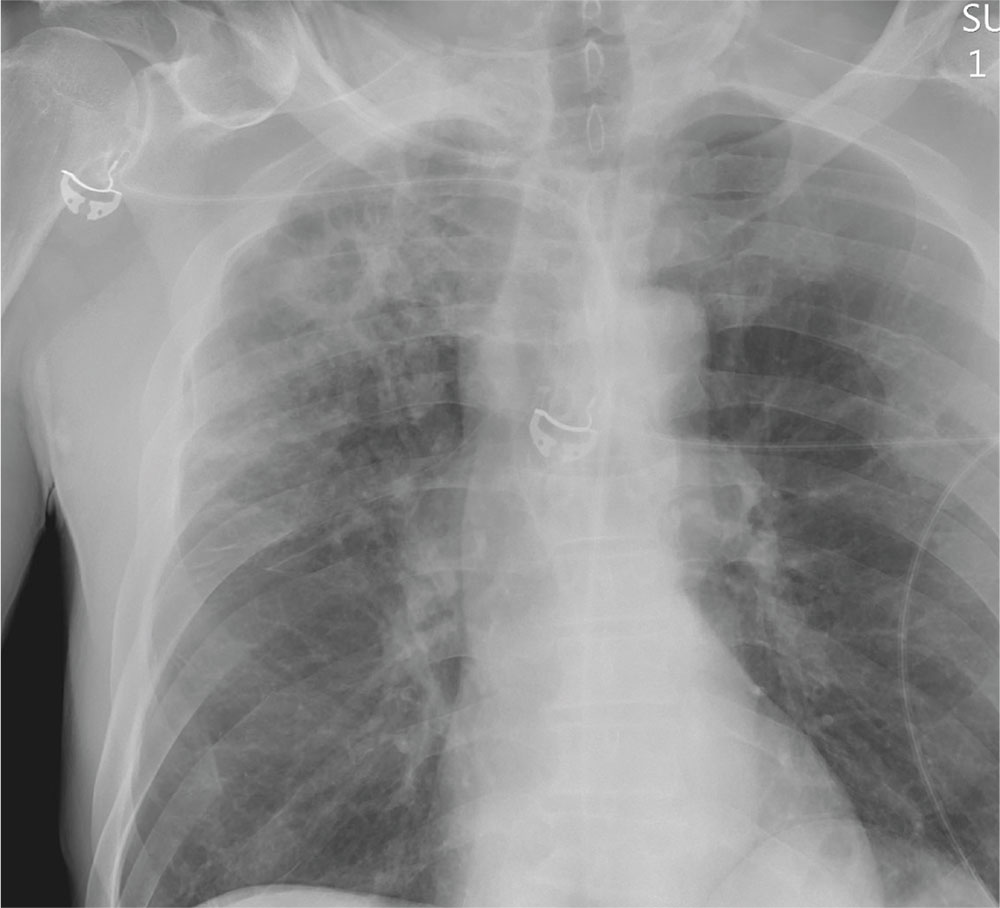
The radiograph shows a large, hyperdense mass within the left hilum. A second hyperdense mass is seen within the left upper lobe. Both are concerning for neoplastic processes and warrant further evaluation with contrast-enhanced CT.
Although thorough work-up and biopsy is needed, the presumptive diagnosis is a primary lung mass with likely metastasis to the brain.

ANSWER
The radiograph shows a large, hyperdense mass within the left hilum. A second hyperdense mass is seen within the left upper lobe. Both are concerning for neoplastic processes and warrant further evaluation with contrast-enhanced CT.
Although thorough work-up and biopsy is needed, the presumptive diagnosis is a primary lung mass with likely metastasis to the brain.

ANSWER
The radiograph shows a large, hyperdense mass within the left hilum. A second hyperdense mass is seen within the left upper lobe. Both are concerning for neoplastic processes and warrant further evaluation with contrast-enhanced CT.
Although thorough work-up and biopsy is needed, the presumptive diagnosis is a primary lung mass with likely metastasis to the brain.

A 65-year-old woman is transferred to your facility for evaluation of left-side weakness she has been experiencing for more than two months. She states that it is worsening with time but denies any other symptoms. Outpatient MRI of the brain, obtained by the referring provider, is reported to show a right parietal mass with surrounding edema.
Medical history is significant for hypertension, diabetes, and hypercholesterolemia, which are controlled with medication. The patient reports smoking nearly two packs of cigarettes daily for at least 30 years.
Physical examination reveals normal vital signs and no apparent distress. The patient does have left hemiparesis; her left upper extremity is approximately 4/5 throughout, and her left lower extremity is approximately 2/5 throughout. The exam is otherwise normal.
As you review her admission lab results, you note that a chest radiograph was obtained (shown). What is your impression?
Case Studies in Toxicology: Angioedema Post-tPA: Hemorrhage Is Not the Only Risk Factor
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
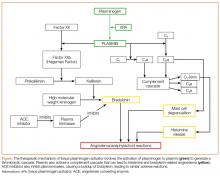
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
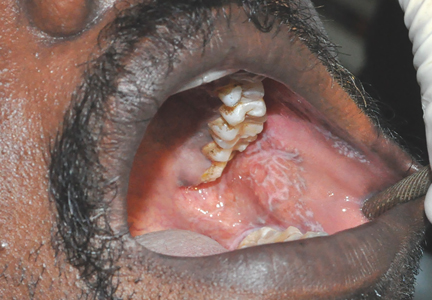
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
A broken pacemaker lead in a 69-year-old woman
A 69-year-old woman presented with fatigue, cough, and lightheadedness. She had a history of atrial fibrillation and complete heart block, for which she had a pacemaker (dual-pacing, dual-sensing, dual-response, and rate-adaptive mode) inserted in 2005. Her heart rate was 30 beats per minute.
A chest radiograph showed a fractured right ventricular pacemaker lead (Figure 1). Electrocardiography showed sinus rhythm with a high-grade atrioventricular block (Figure 2). Pacemaker interrogation confirmed the diagnosis of lead fracture. A new lead was placed, and the old lead was abandoned.
HOW LEADS BREAK
The rate of lead fracture ranges from 0.1% to 4.2% per patient-year, and the annual failure rate increases progressively with time after implantation.1,2
Extrinsic pressure on the lead can eventually break it. This can happen between the first rib and clavicle, in “subclavian crush” injury, or with any anatomical abnormality that narrows the thoracic outlet. Typically, classic subclavian crush results from entrapment of the pacemaker leads by the subclavius muscle or the costoclavicular ligament as the lead follows the needle course of the antecedent access puncture of the subclavian vein. This results in intermittent flexing of the lead and potential lead fracture3 and was likely the cause of lead fracture in our patient.
The risk of fracture is higher in patients under the age of 50, those who perform intense physical activity, women, and patients with greater left ventricular ejection fraction.4,5 Certain leads are prone to fracture due to design flaws. One of these was the Medtronic Sprint Fidelis cardioverter defibrillator lead, which was recalled in 2007.5
DETECTING LEAD FRACTURE
Symptoms of lead fracture vary, depending on the patient’s pacemaker-dependency and on the degree of loss of capture (ie, the degree to which the heart fails to respond to the pacemaker’s signals), and may include lightheadedness, syncope, and extracardiac stimulation.
The electrical integrity of a lead can be tested by measuring the circuit impedance, which normally ranges from 300 to 1,000 ohms.6 An insulation failure results in very low impedance, while a disrupted circuit due to lead fracture commonly causes a sudden rather than gradual increase in impedance.6
Simple imaging studies such as chest radiography or fluoroscopy may establish the diagnosis of lead fracture. One should carefully trace every lead along its entire course and look for any conductor discontinuity, kinks, or sharp bends.6
REMOVE THE OLD LEAD, OR LEAVE IT IN PLACE?
The treatment for lead fracture is usually to put in a new lead, with or without extracting the old one.
In view of the potential complications of lead removal such as cardiac perforation or vascular tear, lead abandonment with placement of a new lead may be performed.7 There are no controlled clinical studies comparing lead abandonment vs lead extraction.8 However, extraction is currently recommended only in patients in whom the old lead causes life-threatening arrhythmias, interferes with the operation of implanted cardiac devices, interferes with radiation therapy or needed surgery, or, due to its design or failure, poses an immediate threat to the patient if left in place.7 Lead removal is reasonable in patients who require specific imaging studies such as magnetic resonance imaging with no available imaging alternative for the diagnosis.7
In our patient, a new lead was placed without removing the fractured lead, with no complications. Afterward, the patient’s heart rhythm was observed to be appropriately paced, and she was discharged home the following day.
- Alt E, Völker R, Blömer H. Lead fracture in pacemaker patients. Thorac Cardiovasc Surg 1987; 35:101–104.
- Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of > 10 years. Circulation 2007; 115:2474–2480.
- Magney JE, Flynn DM, Parsons JA, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol 1993; 16:445–457.
- Farwell D, Green MS, Lemery R, Gollob MH, Birnie DH. Accelerating risk of Fidelis lead fracture. Heart Rhythm 2008; 5:1375–1379.
- Morrison TB, Rea RF, Hodge DO, et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol 2010; 21:671–677.
- Swerdlow CD, Ellenbogen KA. Implantable cardioverter-defibrillator leads: design, diagnostics, and management. Circulation 2013; 128:2062–2071.
- Wilkoff BL, Love CJ, Byrd CL, et al; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6:1085–1104.
- Maytin M, Epstein LM, Henrikson CA. Lead extraction is preferred for lead revisions and system upgrades: when less is more. Circ Arrhythm Electrophysiol 2010; 3:413–424.
A 69-year-old woman presented with fatigue, cough, and lightheadedness. She had a history of atrial fibrillation and complete heart block, for which she had a pacemaker (dual-pacing, dual-sensing, dual-response, and rate-adaptive mode) inserted in 2005. Her heart rate was 30 beats per minute.
A chest radiograph showed a fractured right ventricular pacemaker lead (Figure 1). Electrocardiography showed sinus rhythm with a high-grade atrioventricular block (Figure 2). Pacemaker interrogation confirmed the diagnosis of lead fracture. A new lead was placed, and the old lead was abandoned.
HOW LEADS BREAK
The rate of lead fracture ranges from 0.1% to 4.2% per patient-year, and the annual failure rate increases progressively with time after implantation.1,2
Extrinsic pressure on the lead can eventually break it. This can happen between the first rib and clavicle, in “subclavian crush” injury, or with any anatomical abnormality that narrows the thoracic outlet. Typically, classic subclavian crush results from entrapment of the pacemaker leads by the subclavius muscle or the costoclavicular ligament as the lead follows the needle course of the antecedent access puncture of the subclavian vein. This results in intermittent flexing of the lead and potential lead fracture3 and was likely the cause of lead fracture in our patient.
The risk of fracture is higher in patients under the age of 50, those who perform intense physical activity, women, and patients with greater left ventricular ejection fraction.4,5 Certain leads are prone to fracture due to design flaws. One of these was the Medtronic Sprint Fidelis cardioverter defibrillator lead, which was recalled in 2007.5
DETECTING LEAD FRACTURE
Symptoms of lead fracture vary, depending on the patient’s pacemaker-dependency and on the degree of loss of capture (ie, the degree to which the heart fails to respond to the pacemaker’s signals), and may include lightheadedness, syncope, and extracardiac stimulation.
The electrical integrity of a lead can be tested by measuring the circuit impedance, which normally ranges from 300 to 1,000 ohms.6 An insulation failure results in very low impedance, while a disrupted circuit due to lead fracture commonly causes a sudden rather than gradual increase in impedance.6
Simple imaging studies such as chest radiography or fluoroscopy may establish the diagnosis of lead fracture. One should carefully trace every lead along its entire course and look for any conductor discontinuity, kinks, or sharp bends.6
REMOVE THE OLD LEAD, OR LEAVE IT IN PLACE?
The treatment for lead fracture is usually to put in a new lead, with or without extracting the old one.
In view of the potential complications of lead removal such as cardiac perforation or vascular tear, lead abandonment with placement of a new lead may be performed.7 There are no controlled clinical studies comparing lead abandonment vs lead extraction.8 However, extraction is currently recommended only in patients in whom the old lead causes life-threatening arrhythmias, interferes with the operation of implanted cardiac devices, interferes with radiation therapy or needed surgery, or, due to its design or failure, poses an immediate threat to the patient if left in place.7 Lead removal is reasonable in patients who require specific imaging studies such as magnetic resonance imaging with no available imaging alternative for the diagnosis.7
In our patient, a new lead was placed without removing the fractured lead, with no complications. Afterward, the patient’s heart rhythm was observed to be appropriately paced, and she was discharged home the following day.
A 69-year-old woman presented with fatigue, cough, and lightheadedness. She had a history of atrial fibrillation and complete heart block, for which she had a pacemaker (dual-pacing, dual-sensing, dual-response, and rate-adaptive mode) inserted in 2005. Her heart rate was 30 beats per minute.
A chest radiograph showed a fractured right ventricular pacemaker lead (Figure 1). Electrocardiography showed sinus rhythm with a high-grade atrioventricular block (Figure 2). Pacemaker interrogation confirmed the diagnosis of lead fracture. A new lead was placed, and the old lead was abandoned.
HOW LEADS BREAK
The rate of lead fracture ranges from 0.1% to 4.2% per patient-year, and the annual failure rate increases progressively with time after implantation.1,2
Extrinsic pressure on the lead can eventually break it. This can happen between the first rib and clavicle, in “subclavian crush” injury, or with any anatomical abnormality that narrows the thoracic outlet. Typically, classic subclavian crush results from entrapment of the pacemaker leads by the subclavius muscle or the costoclavicular ligament as the lead follows the needle course of the antecedent access puncture of the subclavian vein. This results in intermittent flexing of the lead and potential lead fracture3 and was likely the cause of lead fracture in our patient.
The risk of fracture is higher in patients under the age of 50, those who perform intense physical activity, women, and patients with greater left ventricular ejection fraction.4,5 Certain leads are prone to fracture due to design flaws. One of these was the Medtronic Sprint Fidelis cardioverter defibrillator lead, which was recalled in 2007.5
DETECTING LEAD FRACTURE
Symptoms of lead fracture vary, depending on the patient’s pacemaker-dependency and on the degree of loss of capture (ie, the degree to which the heart fails to respond to the pacemaker’s signals), and may include lightheadedness, syncope, and extracardiac stimulation.
The electrical integrity of a lead can be tested by measuring the circuit impedance, which normally ranges from 300 to 1,000 ohms.6 An insulation failure results in very low impedance, while a disrupted circuit due to lead fracture commonly causes a sudden rather than gradual increase in impedance.6
Simple imaging studies such as chest radiography or fluoroscopy may establish the diagnosis of lead fracture. One should carefully trace every lead along its entire course and look for any conductor discontinuity, kinks, or sharp bends.6
REMOVE THE OLD LEAD, OR LEAVE IT IN PLACE?
The treatment for lead fracture is usually to put in a new lead, with or without extracting the old one.
In view of the potential complications of lead removal such as cardiac perforation or vascular tear, lead abandonment with placement of a new lead may be performed.7 There are no controlled clinical studies comparing lead abandonment vs lead extraction.8 However, extraction is currently recommended only in patients in whom the old lead causes life-threatening arrhythmias, interferes with the operation of implanted cardiac devices, interferes with radiation therapy or needed surgery, or, due to its design or failure, poses an immediate threat to the patient if left in place.7 Lead removal is reasonable in patients who require specific imaging studies such as magnetic resonance imaging with no available imaging alternative for the diagnosis.7
In our patient, a new lead was placed without removing the fractured lead, with no complications. Afterward, the patient’s heart rhythm was observed to be appropriately paced, and she was discharged home the following day.
- Alt E, Völker R, Blömer H. Lead fracture in pacemaker patients. Thorac Cardiovasc Surg 1987; 35:101–104.
- Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of > 10 years. Circulation 2007; 115:2474–2480.
- Magney JE, Flynn DM, Parsons JA, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol 1993; 16:445–457.
- Farwell D, Green MS, Lemery R, Gollob MH, Birnie DH. Accelerating risk of Fidelis lead fracture. Heart Rhythm 2008; 5:1375–1379.
- Morrison TB, Rea RF, Hodge DO, et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol 2010; 21:671–677.
- Swerdlow CD, Ellenbogen KA. Implantable cardioverter-defibrillator leads: design, diagnostics, and management. Circulation 2013; 128:2062–2071.
- Wilkoff BL, Love CJ, Byrd CL, et al; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6:1085–1104.
- Maytin M, Epstein LM, Henrikson CA. Lead extraction is preferred for lead revisions and system upgrades: when less is more. Circ Arrhythm Electrophysiol 2010; 3:413–424.
- Alt E, Völker R, Blömer H. Lead fracture in pacemaker patients. Thorac Cardiovasc Surg 1987; 35:101–104.
- Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of > 10 years. Circulation 2007; 115:2474–2480.
- Magney JE, Flynn DM, Parsons JA, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol 1993; 16:445–457.
- Farwell D, Green MS, Lemery R, Gollob MH, Birnie DH. Accelerating risk of Fidelis lead fracture. Heart Rhythm 2008; 5:1375–1379.
- Morrison TB, Rea RF, Hodge DO, et al. Risk factors for implantable defibrillator lead fracture in a recalled and a nonrecalled lead. J Cardiovasc Electrophysiol 2010; 21:671–677.
- Swerdlow CD, Ellenbogen KA. Implantable cardioverter-defibrillator leads: design, diagnostics, and management. Circulation 2013; 128:2062–2071.
- Wilkoff BL, Love CJ, Byrd CL, et al; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6:1085–1104.
- Maytin M, Epstein LM, Henrikson CA. Lead extraction is preferred for lead revisions and system upgrades: when less is more. Circ Arrhythm Electrophysiol 2010; 3:413–424.
A burning sensation in the mouth
A 38-year-old man presented with a burning sensation in the mouth for the previous 2 months. He was currently a smoker. He had no history of any dental procedure or new drug intake.
On examination, his oral hygiene was poor, and a whitish, striated lesion 3-by-3 cm was noted on the left buccal mucosa (Figure 1). Based on the appearance and pattern of the lesion, a clinical diagnosis of reticular-type oral lichen planus was made. The patient was advised to stop smoking. A thorough scaling of the teeth was done, and he was started on topical triamcinolone acetonide ointment for 2 weeks. At a 4-month follow-up visit, the symptoms had resolved.
ORAL LICHEN PLANUS
Oral lichen planus is a chronic inflammatory disease that affects the mucous membrane of the oral cavity. It is a T-cell mediated autoimmune disease in which the cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium.1 No risk factors have been identified, but the condition has been associated with smoking, chewing tobacco, dental materials such as amalgam, celiac disease, ulcerative colitis, stress, diabetes, and use of systemic drugs such as nonsteroidal anti-inflammatory drugs and beta-blockers.1,2 Women are affected more than men in a ratio of 1.4:1, frequently in the fourth decade.
The clinical forms are reticular, papular, plaque-like, erosive, atrophic, and bullous types.
Clinical presentation
The lesions are usually asymptomatic. Symptoms such as a burning sensation are associated mainly with the erosive and atrophic types,1–3 although in rare cases it occurs with the reticular form, as in our patient. Erosive and atrophic types carry a 1% to 2% risk of malignant transformation and require close follow-up.3
Oral mucosal lesions present alone or, in up to 15% to 20% of cases, with concomitant skin lesions.1,2 The most common oral sites are the buccal mucosa, tongue, and gingiva. Skin lesions present as violaceous flat-topped papules on the ankles, wrists, and genitalia.
DIAGNOSIS AND TREATMENT
The differential diagnosis of oral lichen planus includes cheek-frictional keratosis, lichenoid reaction, leukoplakia, pemphigus, oral candidiasis, and chronic ulcerative stomatitis.1,2
The diagnosis is clinical, but biopsy is indicated in the erosive or atrophic type and when a malignant lesion is suspected, especially in patients with a history of smoking and tobacco chewing. Oral lichen planus may be associated with oral candidiasis, especially in patients with poor oral hygiene and smoking, and this needs to be addressed before a diagnosis of oral lichen planus can be made.
The mainstay of management is oral or topical corticosteroids.4 Calcineurin inhibitors, retinoids, dapsone, hydroxychloroquine, mycophenolate mofetil, and enoxaparin have also been shown to be effective and are used when corticosteroids alone are ineffective or contraindicated.4
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res 2016; 308:539–551.
- Nogueira PA, Carneiro S, Ramos-e-Silva M. Oral lichen planus: an update on its pathogenesis. Int J Dermatol 2015; 54:1005–1010.
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc 2014; 145:45–56.
- Lodi G, Carrozzo M, Furness S, Thongprasom K. Interventions for treating oral lichen planus: a systematic review. Br J Dermatol 2012; 166:938–947.
A 38-year-old man presented with a burning sensation in the mouth for the previous 2 months. He was currently a smoker. He had no history of any dental procedure or new drug intake.
On examination, his oral hygiene was poor, and a whitish, striated lesion 3-by-3 cm was noted on the left buccal mucosa (Figure 1). Based on the appearance and pattern of the lesion, a clinical diagnosis of reticular-type oral lichen planus was made. The patient was advised to stop smoking. A thorough scaling of the teeth was done, and he was started on topical triamcinolone acetonide ointment for 2 weeks. At a 4-month follow-up visit, the symptoms had resolved.
ORAL LICHEN PLANUS
Oral lichen planus is a chronic inflammatory disease that affects the mucous membrane of the oral cavity. It is a T-cell mediated autoimmune disease in which the cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium.1 No risk factors have been identified, but the condition has been associated with smoking, chewing tobacco, dental materials such as amalgam, celiac disease, ulcerative colitis, stress, diabetes, and use of systemic drugs such as nonsteroidal anti-inflammatory drugs and beta-blockers.1,2 Women are affected more than men in a ratio of 1.4:1, frequently in the fourth decade.
The clinical forms are reticular, papular, plaque-like, erosive, atrophic, and bullous types.
Clinical presentation
The lesions are usually asymptomatic. Symptoms such as a burning sensation are associated mainly with the erosive and atrophic types,1–3 although in rare cases it occurs with the reticular form, as in our patient. Erosive and atrophic types carry a 1% to 2% risk of malignant transformation and require close follow-up.3
Oral mucosal lesions present alone or, in up to 15% to 20% of cases, with concomitant skin lesions.1,2 The most common oral sites are the buccal mucosa, tongue, and gingiva. Skin lesions present as violaceous flat-topped papules on the ankles, wrists, and genitalia.
DIAGNOSIS AND TREATMENT
The differential diagnosis of oral lichen planus includes cheek-frictional keratosis, lichenoid reaction, leukoplakia, pemphigus, oral candidiasis, and chronic ulcerative stomatitis.1,2
The diagnosis is clinical, but biopsy is indicated in the erosive or atrophic type and when a malignant lesion is suspected, especially in patients with a history of smoking and tobacco chewing. Oral lichen planus may be associated with oral candidiasis, especially in patients with poor oral hygiene and smoking, and this needs to be addressed before a diagnosis of oral lichen planus can be made.
The mainstay of management is oral or topical corticosteroids.4 Calcineurin inhibitors, retinoids, dapsone, hydroxychloroquine, mycophenolate mofetil, and enoxaparin have also been shown to be effective and are used when corticosteroids alone are ineffective or contraindicated.4
A 38-year-old man presented with a burning sensation in the mouth for the previous 2 months. He was currently a smoker. He had no history of any dental procedure or new drug intake.
On examination, his oral hygiene was poor, and a whitish, striated lesion 3-by-3 cm was noted on the left buccal mucosa (Figure 1). Based on the appearance and pattern of the lesion, a clinical diagnosis of reticular-type oral lichen planus was made. The patient was advised to stop smoking. A thorough scaling of the teeth was done, and he was started on topical triamcinolone acetonide ointment for 2 weeks. At a 4-month follow-up visit, the symptoms had resolved.
ORAL LICHEN PLANUS
Oral lichen planus is a chronic inflammatory disease that affects the mucous membrane of the oral cavity. It is a T-cell mediated autoimmune disease in which the cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium.1 No risk factors have been identified, but the condition has been associated with smoking, chewing tobacco, dental materials such as amalgam, celiac disease, ulcerative colitis, stress, diabetes, and use of systemic drugs such as nonsteroidal anti-inflammatory drugs and beta-blockers.1,2 Women are affected more than men in a ratio of 1.4:1, frequently in the fourth decade.
The clinical forms are reticular, papular, plaque-like, erosive, atrophic, and bullous types.
Clinical presentation
The lesions are usually asymptomatic. Symptoms such as a burning sensation are associated mainly with the erosive and atrophic types,1–3 although in rare cases it occurs with the reticular form, as in our patient. Erosive and atrophic types carry a 1% to 2% risk of malignant transformation and require close follow-up.3
Oral mucosal lesions present alone or, in up to 15% to 20% of cases, with concomitant skin lesions.1,2 The most common oral sites are the buccal mucosa, tongue, and gingiva. Skin lesions present as violaceous flat-topped papules on the ankles, wrists, and genitalia.
DIAGNOSIS AND TREATMENT
The differential diagnosis of oral lichen planus includes cheek-frictional keratosis, lichenoid reaction, leukoplakia, pemphigus, oral candidiasis, and chronic ulcerative stomatitis.1,2
The diagnosis is clinical, but biopsy is indicated in the erosive or atrophic type and when a malignant lesion is suspected, especially in patients with a history of smoking and tobacco chewing. Oral lichen planus may be associated with oral candidiasis, especially in patients with poor oral hygiene and smoking, and this needs to be addressed before a diagnosis of oral lichen planus can be made.
The mainstay of management is oral or topical corticosteroids.4 Calcineurin inhibitors, retinoids, dapsone, hydroxychloroquine, mycophenolate mofetil, and enoxaparin have also been shown to be effective and are used when corticosteroids alone are ineffective or contraindicated.4
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res 2016; 308:539–551.
- Nogueira PA, Carneiro S, Ramos-e-Silva M. Oral lichen planus: an update on its pathogenesis. Int J Dermatol 2015; 54:1005–1010.
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc 2014; 145:45–56.
- Lodi G, Carrozzo M, Furness S, Thongprasom K. Interventions for treating oral lichen planus: a systematic review. Br J Dermatol 2012; 166:938–947.
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res 2016; 308:539–551.
- Nogueira PA, Carneiro S, Ramos-e-Silva M. Oral lichen planus: an update on its pathogenesis. Int J Dermatol 2015; 54:1005–1010.
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc 2014; 145:45–56.
- Lodi G, Carrozzo M, Furness S, Thongprasom K. Interventions for treating oral lichen planus: a systematic review. Br J Dermatol 2012; 166:938–947.
Serotonin syndrome
To the Editor: I enjoyed the article “Serotonin syndrome: Preventing, recognizing, and treating it.”1 I am a relatively new internal medicine physician, out of residency only 1 year, and sadly I felt that the psychiatric training I received was minimal at best. Therefore, I was very excited to read more about serotonin syndrome since such a large percentage of my patients are on selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors.
Could you speak to the time frame it takes for serotonin syndrome to develop? For instance, if someone is taking an SSRI and develops a terrible yeast infection, would 3 doses of fluconazole be enough to tip the scales? Or as-needed sumatriptan, with some ondansetron for migraine? The problem I have is that patients often require short doses of many medications that can interact, and I routinely sigh, briefly explain the possibility of serotonin syndrome, and then click through the flashing red warning signs on the electronic medical record and send patients out with their meds—though in honesty I do not know the likelihood of developing even mild symptoms of serotonin syndrome with short courses of interacting medications.
- Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med 2016; 83:810–817.
To the Editor: I enjoyed the article “Serotonin syndrome: Preventing, recognizing, and treating it.”1 I am a relatively new internal medicine physician, out of residency only 1 year, and sadly I felt that the psychiatric training I received was minimal at best. Therefore, I was very excited to read more about serotonin syndrome since such a large percentage of my patients are on selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors.
Could you speak to the time frame it takes for serotonin syndrome to develop? For instance, if someone is taking an SSRI and develops a terrible yeast infection, would 3 doses of fluconazole be enough to tip the scales? Or as-needed sumatriptan, with some ondansetron for migraine? The problem I have is that patients often require short doses of many medications that can interact, and I routinely sigh, briefly explain the possibility of serotonin syndrome, and then click through the flashing red warning signs on the electronic medical record and send patients out with their meds—though in honesty I do not know the likelihood of developing even mild symptoms of serotonin syndrome with short courses of interacting medications.
To the Editor: I enjoyed the article “Serotonin syndrome: Preventing, recognizing, and treating it.”1 I am a relatively new internal medicine physician, out of residency only 1 year, and sadly I felt that the psychiatric training I received was minimal at best. Therefore, I was very excited to read more about serotonin syndrome since such a large percentage of my patients are on selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors.
Could you speak to the time frame it takes for serotonin syndrome to develop? For instance, if someone is taking an SSRI and develops a terrible yeast infection, would 3 doses of fluconazole be enough to tip the scales? Or as-needed sumatriptan, with some ondansetron for migraine? The problem I have is that patients often require short doses of many medications that can interact, and I routinely sigh, briefly explain the possibility of serotonin syndrome, and then click through the flashing red warning signs on the electronic medical record and send patients out with their meds—though in honesty I do not know the likelihood of developing even mild symptoms of serotonin syndrome with short courses of interacting medications.
- Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med 2016; 83:810–817.
- Wang RZ, Vashistha V, Kaur S, Houchens NW. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med 2016; 83:810–817.
In reply: Serotonin syndrome
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
In Reply: The questions posed by Dr. Rose reflect critical issues primary care physicians encounter when prescribing medications for patients who are taking serotonergic agents. “Switching strategies” have been described for starting or discontinuing serotonergic antidepressants.1 Options range from conservative exchanges requiring 5 half-lives between discontinuation of 1 antidepressant and initiation of another vs a direct cross-taper exchange. Decisions regarding specific patients should take into account previous adverse effects from serotonergic medications and half-lives of discontinued antidepressants. To our knowledge, switching strategies have not been validated and are based on expert opinion. Scenarios are complicated further if patients have already been prescribed 2 or more antidepressants and 1 medication is exchanged or dose-adjusted while another is added. With this degree of complexity, we recommend referral to a psychiatrist.
Dr. Rose’s questions on prescribing nonpsychiatric serotonergic drugs concurrently with antidepressants broaches a topic with even less evidence. Some data exist about nonpsychiatric serotonergic drugs given in combination with triptans. Soldin et al2 reviewed the US Food and Drug Administration’s Adverse Event Reporting System and discovered 38 cases of serotonin syndrome in patients using triptans. Eleven of these patients were using triptans without concomitant antidepressants. Though definitive evidence is lacking for safe prescribing practice with triptans, the authors noted that most cases of triptan-induced serotonin toxicity occur within hours of triptan ingestion.2
The evidence on the risk of serotonin syndrome with other medications is limited to case reports. In regard to linezolid, a review suggested that when linezolid was administered to a patient on long-term citalopram, a prolonged serotonin syndrome was precipitated, which is not an issue with other antidepressants.3 The World Health Organization has issued warnings for serotonin toxicity with ondansetron and other 5-HT3 receptor antagonists based on case reports.4,5 No data are available for the appropriate prescribing of 5-HT3 antagonists with antidepressants. A review of cases suggests a link between fluconazole and severe serotonin toxicity in patients taking citalopram; however, no prescribing guidelines have been established for fluconazole either.6
Dr. Rose asks important clinical questions, but evidence-based answers are not available. We can only recommend that patients be advised to report symptoms immediately after starting any medication associated with serotonin syndrome. For patients on multiple antidepressants, psychiatric assistance is advised. An observational cohort study of patients using antidepressants while exposed to other suspect drugs may better delineate effects of several pharmaceuticals on the serotonergic axis. Only then may safe prescribing practices be validated with evidence.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr 2016; 39:76–83.
- Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network. Serotonin syndrome associated with triptan monotherapy (letter). N Engl J Med 2008; 15:2185–2186.
- Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Grau S. Linezolid-associated serotonin syndrome: what we can learn from cases reported so far. J Antimicrob Chemother 2005; 56:1176–1178.
- World Health Organization. Ondansetron and serotonin syndrome. WHO Pharmaceuticals Newsletter 2012; 3:16–21.
- Rojas-Fernandez CH. Can 5-HT3 antagonists really contribute to serotonin toxicity? A call for clarity and pharmacological law and order. Drugs Real World Outcomes 2014; 1:3–5.
- Levin TT, Cortes-Ladino A, Weiss M, Palomba ML. Life-threatening serotonin toxicity due to a citalopram-fluconazole drug interaction: case reports and discussion. Gen Hosp Psychiatry 2008; 30:372–377.
The Signs That Can’t Be Ignored
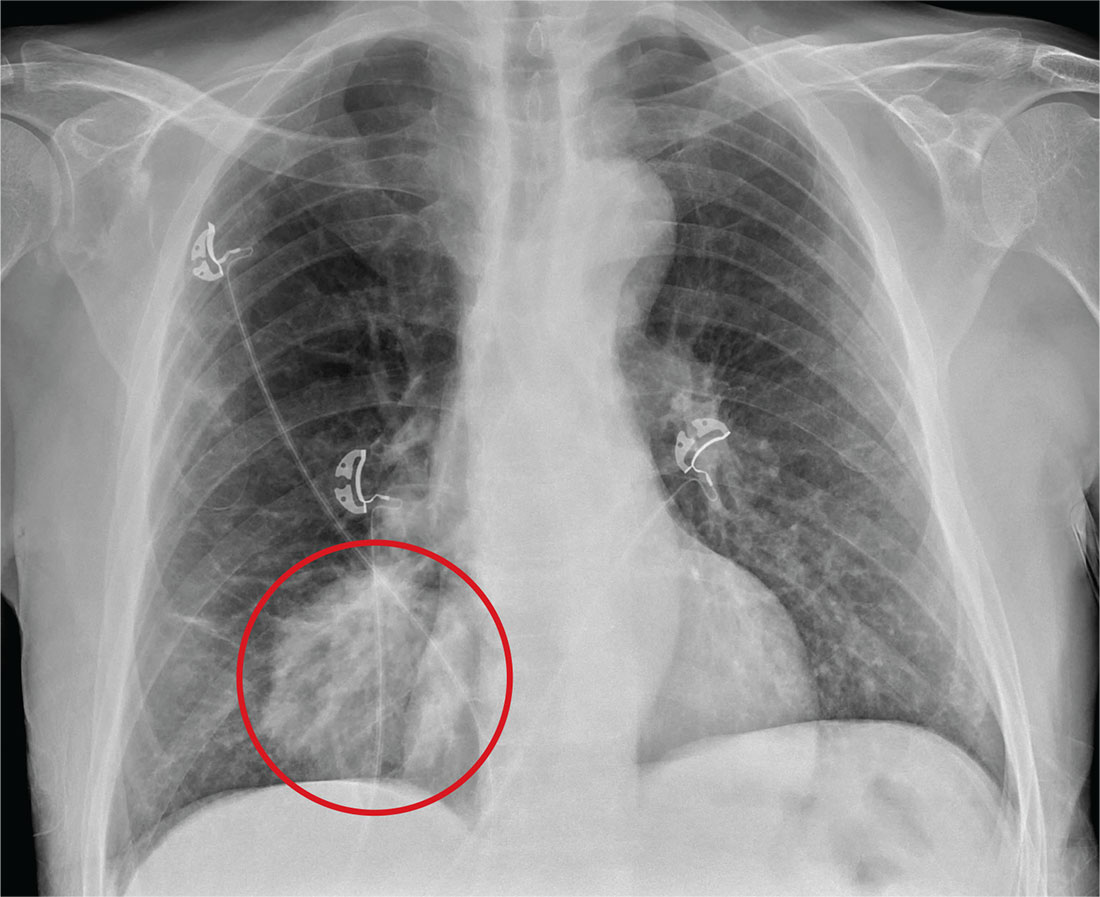
ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.

ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.

ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.
For several days, a 60-year-old man has been feeling weak. He has also noticed that he bruises easily, and he’s experienced black, tarry stools and episodic hemoptysis. He presents to the emergency department, where the triage team sends him for further evaluation.
The patient’s history is significant for a remote diagnosis of a deep venous thrombosis in one of his lower extremities, for which he takes warfarin. He does not recall his most recent INR level. He reports smoking up to one pack of cigarettes per day and consuming alcohol regularly.
Examination reveals an older appearing male in no obvious distress. His blood pressure is 90/60 mm Hg, and his heart rate is 110 beats/min. You note bruises on both arms. The rest of his physical exam is normal. Lung sounds are clear.
Labwork ordered by the triage team indicates a hemoglobin level of 8 g/dL and an INR of 9. In addition, his stool guaiac test came back positive.
You obtain a portable chest radiograph (shown). What is your impression?
Obese Man With Severe Pain and Swollen Hand
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).
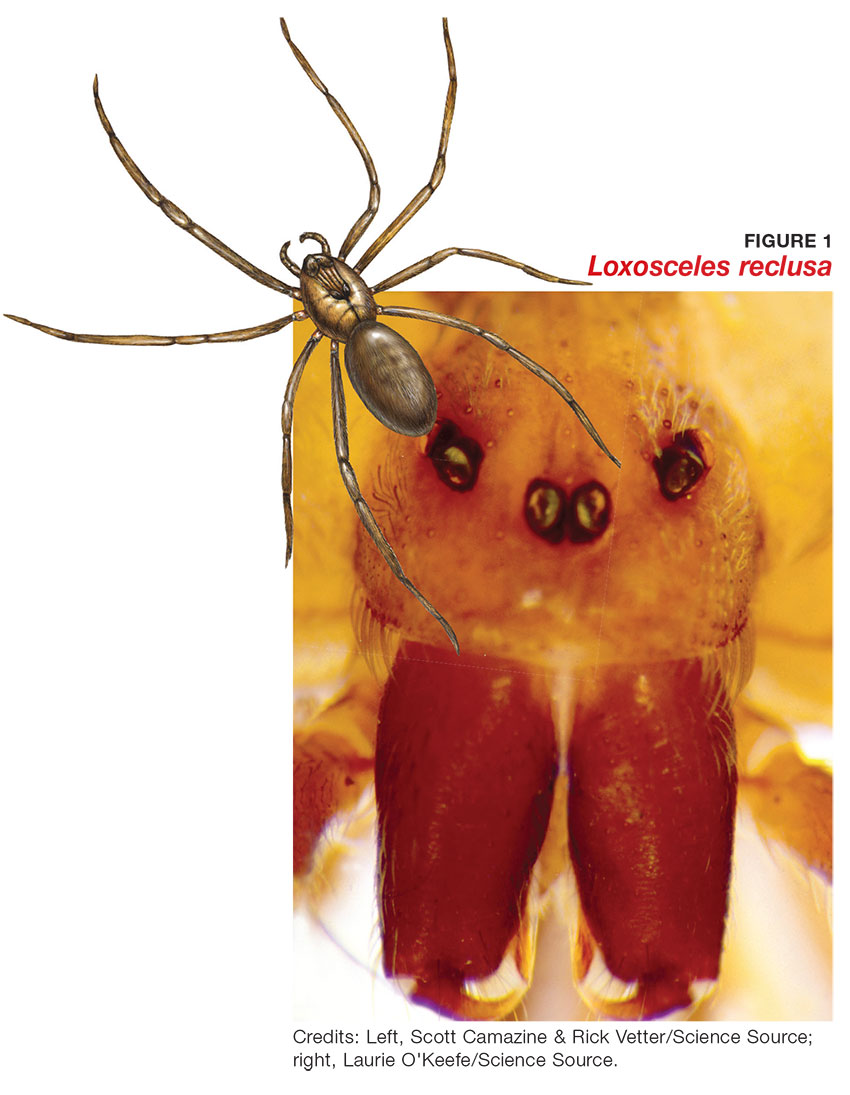
Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7
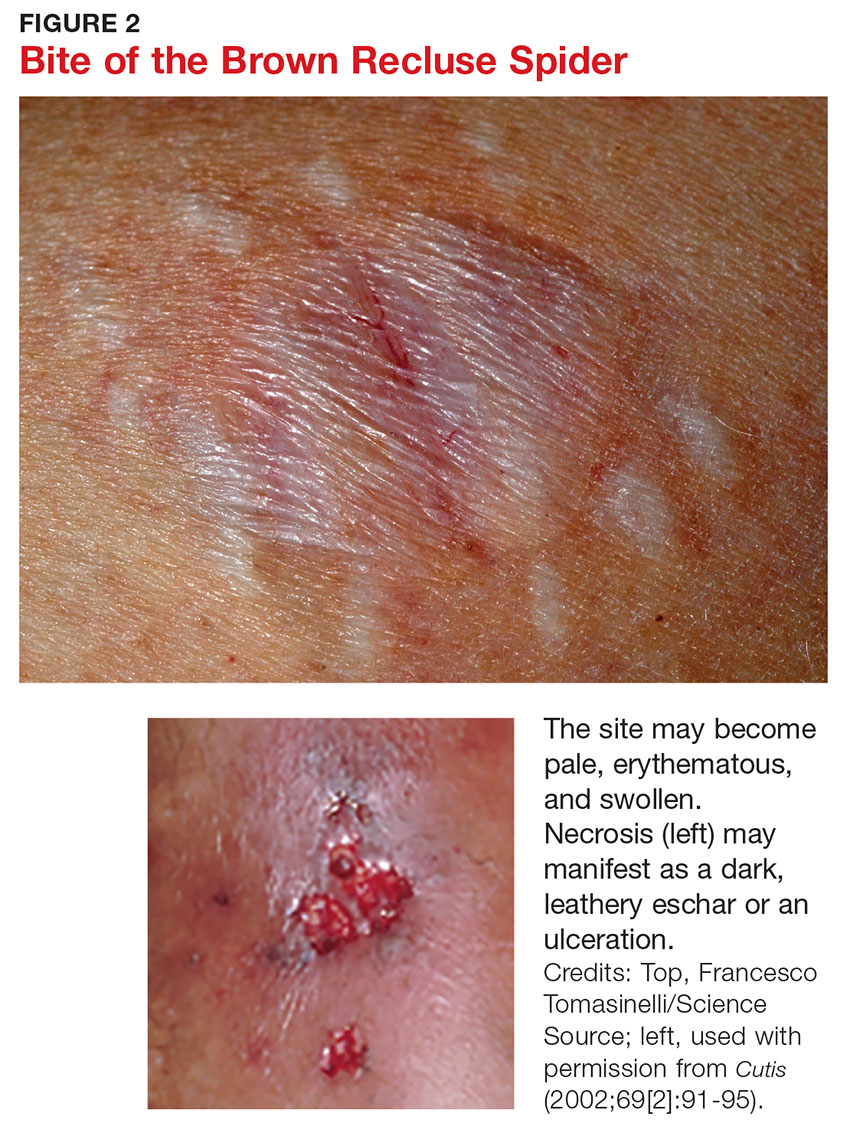
Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).

Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7

Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
IN THIS ARTICLE
- Diagnosis: questions to ask
- Treatment and management
- Follow-up care
An obese 43-year-old Hispanic man presents to the emergency department (ED) with complaints of severe pain and swelling in his right hand. The patient states that he felt a bite on his hand as he was planting flowers and laying down potting soil near a tree and decorative rocks in his yard. He did not seek immediate medical treatment because the pain was minimal.
As the hours passed, though, the pain increased, and he began to notice tightness in his hand. Twelve hours after the initial bite, the pain became intolerable and his hand swelled to double its normal size, such that he could no longer bend his fingers. He then sought treatment at the ED.
The patient denies previous drug use but indicates that he smokes 1.5 packs of cigarettes daily and drinks alcohol occasionally in social settings. He has no known drug or food allergies. His history is remarkable for hypertension and hyperlipidemia, treated with simvastatin (40 mg/d) and lisinopril (10 mg/d), respectively.
The physical examination reveals an arterial blood pressure of 152/84 mm Hg; heart rate, 76 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 99ºF. His height is 5 ft 8 in and weight, 297 lb. Cardiovascular examination reveals no irregular heart rhythm, and S1 and S2 are heard, with no murmurs or gallops. He denies chest pain and palpitations. Respiratory examination reveals clear breath sounds that are equal and unlabored. He denies shortness of breath or coughing. The patient states that he had nausea earlier that day, but it has subsided.
Dermatologic examination reveals severe erythema and 3+ edema in the patient’s right hand. A 3-cm, irregularly shaped, red, hemorrhagic blister is observed close to the thumb on the posterior side of the right hand. There are two small holes in the center and slight bruising around the lesion. The right hand is hard and warm to the touch upon palpation, and the patient rates his pain as severe (10 out of 10).
The symptoms of severe pain and swelling and the early observation of bruising and hemorrhagic blistering raise suspicion for venomous spider bite (ICD-10 code: T63.331A). Laboratory work-up, including complete blood count, electrolytes, kidney function studies, and urinalysis, is performed. The results are inconclusive, and the reported symptoms and objective assessment are used to make the diagnosis of spider bite.
DISCUSSION
The brown recluse spider (Loxosceles reclusa) is notorious for its bite, which can result in dermonecrosis within 24 to 48 hours. It inhabits the lower Midwest, south central, and southeastern regions of the United States and is not endemic in the West, Northeast, Mid-Atlantic, or Coastal South. Brown recluse spiders are nonaggressive and prefer warm, dark, dry habitats, dwelling under rocks, logs, woodpiles, and debris, as well as in attics, sheds, basements, boxes, travel bags, and motor vehicles.1,2 They can survive for months without food and can withstand temperatures ranging from 46.4°F to 109.4°F.3 They build irregular, cottony webs that serve as housing but are not used to capture prey.3 (Note that webs found strung along walls, ceilings, outdoor vegetation, and in other exposed areas are nearly always associated with other types of spiders.) The brown recluse is nocturnal, seeking insect prey, either alive or dead.
Brown recluse spiders range in size from 6 mm to 20 mm; they have a violin-shaped pattern on the cephalothorax and long legs that allow them to move quickly (see Figure 1). A distinguishing feature is their six eyes, arranged in three pairs (most spiders have eight eyes).

Venom production is influenced by the size and sex of the spider as well as ambient temperature.4 The venom contains at least eight enzyme and protein components, including the most active enzyme, sphingomyelinase D.3 This enzyme causes dermonecrosis, platelet aggregation, and complement-mediated hemolysis in vitro, and it may also be responsible for the ulcerating and systemic effects observed in humans.5 Sphingomyelinase D has been shown to induce grossly visible tissue necrosis in rabbit tissue within 24 hours after envenomation.3
CLINICAL PRESENTATION
The brown recluse spider bite may be imperceptible at the time of envenomation, requiring no medical attention. Depending on a person’s sensitivity level and the amount of venom injected, however, a mild stinging sensation at the site may be felt, which is usually accompanied by redness and inflammation that may disappear within seconds or last for a couple of hours.6
Within two to eight hours, severe pain may occur, progressing to a burning sensation.5 The bite site will become pale, due to venom-induced vasoconstriction, with increasing erythema and swelling in the surrounding tissue.5 This extreme pain could be due to absorption of the venom by the muscle tissues; if untreated, further tissue damage can occur. Within 12 to 24 hours, there is painful edema with induration and an irregular area of ecchymosis and ischemia.7 Occasionally, the site will develop red, white, and blue hemorrhagic blisters, with the blue ischemic portion centrally located and the red erythematous areas on the periphery.8 In almost half of all cases, the lesion is associated with nonspecific systemic symptoms, such as generalized pruritus and rash, headache, nausea, vomiting, and low-grade fever in the first 24 to 48 hours.7
Three days after envenomation, the wound will expand and deepen, with skin breakdown noted not sooner than 72 hours after the bite (see Figure 2).7,8 After five to seven days, the cutaneous lesion forms a dry necrotic eschar with a well-demarcated border. Within two to three weeks after the bite, the necrotic tissue should detach, and the wound should develop granulated tissue that indicates healing.8 Complete healing can take weeks or months, depending on the extent and depth of the wound, with scarring possible in severe cases.7

Severe systemic illness (ie, systemic loxoscelism)—rare in the US—is a potential complication of the brown recluse spider bite.4 It manifests with fever, malaise, vomiting, headache, and rash; in rare instances, it results in death.7
Diagnosis
Brown recluse bite is diagnosed based on history and clinical presentation and, when possible, identification of the spider. However, patients often do not realize they have been bitten before they develop symptoms, making it impossible to confirm the etiology of the lesion. It is often helpful to ask the following questions during the assessment
- Did you feel the bite take place?
- Did you see or capture the spider? If so, can you describe it?
- Where were you when the spider bit you?
- Did you recently clean any clutter or debris?
Furthermore, patients who recall seeing a spider after being bitten typically do not bring the arachnid to their health care facility. Another complicating factor is the numerous possible causes of necrotic skin lesions that can be mistaken for spider bites.5 The differential diagnosis can include allergic dermatitis, cellulitis, methicillin-resistant Staphylococcus aureus (MRSA) infection, skin abscesses, other arthropod bites, necrotizing fasciitis, or bee sting.
TREATMENT AND MANAGEMENT
One of the most important factors in successful treatment is timeliness of medical attention after the initial bite; because the most damaging tissue effects occur within the first three to six hours after envenomation, intervention during this time is imperative.8 Initial treatment of cutaneous brown recluse spider bite is often conservative, given the variation in clinical presentation, inability to predict the future extent of lesions, and lack of evidence-based treatment options.9 The goals of therapy are to ensure that skin integrity is maintained, infection is avoided, and circulation is preserved.10
Nonpharmacologic treatments for brown recluse spider bite consist of cleaning the wound, treating the bite area with “RICE” (rest-ice-compression-elevation) therapy during the first 72 hours to reduce tissue damage, and ensuring adequate hydration.1,10-13 The affected area should be cleaned thoroughly; infected wounds require topical antiseptics and sterile dressings. Applying a cold compress to the bite area at 20-minute intervals during the first 72 hours after envenomation has been shown to reduce tissue damage.10 Heat should not be applied to the area, as it may increase tissue damage.
Pharmacologic treatment. Patients who experience systemic symptoms such as nausea, vomiting, pain, fever, and pruritus should be provided antipyretics, hydration, and analgesics for symptomatic relief, as needed.9 Antihistamines and benzodiazepines have been found to be useful in relieving symptoms of anxiety and pruritus. To help manage mild pain, OTC NSAIDs are recommended.10
If the date of the last tetanus shot is unknown, a prophylactic tetanus booster (tetanus/diphtheria [Td] or TDaP) should be administered.10 The prophylactic use of cephalosporins to treat infection is indicated in patients with tissue breakdown.1
Among the more controversial treatment choices are use of corticosteroids and dapsone, prescribed frequently in the past. Use of oral corticosteroids for cutaneous forms of spider bite is not supported by current evidence.5,10,14 Research does, however, support their role in the treatment of bite-induced systemic illness, particularly for preventing kidney failure and hemolysis in children.1,15
Dapsone, prescribed for the necrotic lesions, may be useful in limiting the inflammatory response at the site of envenomation.1,3 However, human studies have shown conflicting results with dapsone administration, with some demonstrating no improvement in patient outcomes.8 The risks of dapsone’s many adverse effects, including dose-related hemolysis, sore throat, pallor, agranulocytosis, aplastic anemia, and cholestatic jaundice, may outweigh its benefits.1,12 Furthermore, dapsone treatment is restricted in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency because of their increased risk for hemolytic anemia.1 Accordingly, dapsone is recommended only for moderate-to-severe or rapidly progressing cases in adults.1
FOLLOW-UP CARE
A patient's follow-up care should be assessed individually, based on the nature of his/her reaction to the bite. In all instances, however, ask the patient to report worsening of symptoms and changes in the skin around the bite area; if systemic symptoms develop, patients should proceed to the ED. If, after six to eight weeks, the necrotic lesion is large and has stabilized in size, consider referring to a wound care clinic for surgical excision of the eschar.9
To avoid future spider bites, advise patients to clear all clutter, move beds away from the wall, remove bed skirts or ruffles, avoid using underbed storage containers, avoid leaving clothing on the floor in piles, and check shoes before dressing.5
OUTCOME FOR THE CASE PATIENT
Initial supportive treatment for this patient included cleaning the bite area with antiseptic soap and water. A cold compress was applied to the bite area at 20-minute intervals, and the right hand was elevated. Hydrocodone bitartrate/acetaminophen (5/325 mg qid) was administered to alleviate pain. The patient was also given a tetanus booster because the date of his last immunization was unknown.
After two hours of monitoring, the patient was no longer able to move his hand, swelling around the affected area increased, and the bite site began to appear necrotic. Cephalexin (500 mg bid) was ordered along with dapsone (100 mg/d). The patient was referred for consultation with wound care and infectious disease specialists because of possible tissue necrosis.
CONCLUSION
Brown recluse spider bites are uncommon, and most are unremarkable and self-healing. Patients who present following a brown recluse bite typically can be managed successfully with supportive care (RICE) and careful observation. In rare cases, however, bites may result in significant tissue necrosis or even death.
The diagnosis is typically based on thorough physical examination, with attention to the lesion characteristics and appropriate questions about the spider and the development of the lesion over time. Diagnosis through identification of the spider seldom occurs, since patients typically do not capture the spider and bring it with them for identification. The geographic region where the bite occurs is an important factor as well, since brown recluse envenomation is higher on the differential diagnosis of necrotic skin lesions in areas where these spiders are endemic (the lower Midwest, south central, and southeastern regions of the US).
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.
1. Andersen RJ, Campoli J, Johar SK, et al. Suspected brown recluse envenomation: a case report and review of different treatment modalities. J Emerg Med. 2011;41(2):e31-e37.
2. Vetter RS. Seasonality of brown recluse spiders, Loxosceles reclusa, submitted by the general public: implications for physicians regarding loxoscelism diagnoses. Toxicon. 2011;58(8):623-625.
3. Forks TP. Brown recluse spider bites. J Am Board Fam Pract. 2000;13(6):415-423.
4. Peterson ME. Brown spider envenomation. Clin Tech Small Anim Pract. 2006;21(4):191-193.
5. Vetter RS, Isbister GK. Medical aspects of spider bites. Ann Rev Entomol. 2008;53:409-429.
6. Szalay J. Brown recluse spiders: facts, bites & symptoms (2014). www.livescience.com/39996-brown-recluse-spiders.html. Accessed March 1, 2017.
7. Isbister GK, Fan HW. Spider bite. Lancet. 2011;378:2039-2047.
8. Hogan CJ, Barbaro KC, Winkel K. Loxoscelism: old obstacles, new directions. Ann Emerg Med. 2000;44:608-624.
9. Bernstein B, Ehrlich F. Brown recluse spider bites. J Emerg Med. 1986;4:457-462.
10. Rhoads J. Epidemiology of the brown recluse spider bite. J Am Acad Nurse Pract. 2007;19(2):79-85.
11. Carlson DS. Spider bite. Nursing. 2013;43(2):72.
12. Frundle TC. Management of spider bites. Air Med J. 2004; 23(4):24-26.
13. Sams HH, King LE Jr. Brown recluse spider bites. Dermatol Nurs. 1999;11(6):427-433.
14. Nunnelee JD. Brown recluse spider bites: a case report. J Perianesth Nurs. 2006;21(1):12-15.
15. Wendell RP. Brown recluse spiders: a review to help guide physicians in nonendemic areas. South Med J. 2003; 96(5):486-490.