User login
Fungal folliculitis masquerading as acute exanthematous pustulosis
THE DIFFERENTIAL DIAGNOSIS
The appearance of sterile pustules after starting a new antibiotic raised suspicion for acute localized exanthematous pustulosis, a variant of acute generalized exanthematous pustulosis. It is a serious but uncommon adverse drug reaction, with a frequency of one to five cases per million per year. The eruption of erythematous plaques studded with sterile pustules classically appears 1 to 5 days after starting a drug.1 Piperacillin-tazobactam has been infrequently reported in association with acute exanthematous pustulosis, but antibiotics in general are among the most commonly reported culprits.2–4
Clues to the correct diagnosis
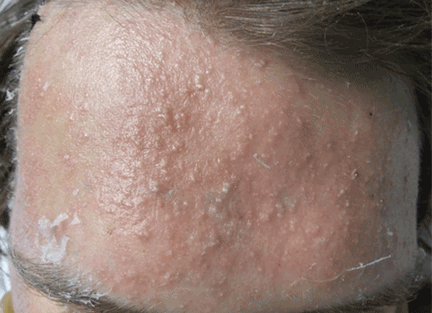
Although our concern for acute localized exanthematous pustulosis was warranted, the morphology and distribution of this patient’s exanthem also raised suspicion of fungal folliculitis, which is more common.
Malassezia folliculitis appears as a monomorphic papular and pustular eruption on the chest, back, and face,5 as in our patient. Differentiating fungal folliculitis from pustulosis is important, as each condition is treated differently: Malassezia folliculitis is treated with antifungals,5 and acute localized exanthematous pustulosis is managed with cessation of the offending drug, supportive care, and systemic or topical steroids.4
Take-home point
Our experience with this patient was a reminder to consider fungal folliculitis in the differential diagnosis of a pustular eruption, so as to allow appropriate management and to avert discontinuation of potentially life-saving medications.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol 2012; 53:87–92.
- Talati S, Lala M, Kapupara H, Thet Z. Acute generalized exanthematous pustulosis: a rare clinical entity with use of piperacillin/tazobactam. Am J Ther 2009; 16:591–592.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007; 157:989–996.
- Huilaja L, Kallioinen M, Soronen M, Riekki R, Tasanen K. Acute localized exanthematous pustulosis on inguinal area secondary to piperacillin/tazobactam. Acta Derm Venereol 2014; 94:106–107.
- Rubenstein RM, Malerich SA. Malassezia (pityrosporum) folliculitis. J Clin Aesthet Dermatol 2014; 73:37–41.
THE DIFFERENTIAL DIAGNOSIS
The appearance of sterile pustules after starting a new antibiotic raised suspicion for acute localized exanthematous pustulosis, a variant of acute generalized exanthematous pustulosis. It is a serious but uncommon adverse drug reaction, with a frequency of one to five cases per million per year. The eruption of erythematous plaques studded with sterile pustules classically appears 1 to 5 days after starting a drug.1 Piperacillin-tazobactam has been infrequently reported in association with acute exanthematous pustulosis, but antibiotics in general are among the most commonly reported culprits.2–4
Clues to the correct diagnosis
Although our concern for acute localized exanthematous pustulosis was warranted, the morphology and distribution of this patient’s exanthem also raised suspicion of fungal folliculitis, which is more common.
Malassezia folliculitis appears as a monomorphic papular and pustular eruption on the chest, back, and face,5 as in our patient. Differentiating fungal folliculitis from pustulosis is important, as each condition is treated differently: Malassezia folliculitis is treated with antifungals,5 and acute localized exanthematous pustulosis is managed with cessation of the offending drug, supportive care, and systemic or topical steroids.4
Take-home point
Our experience with this patient was a reminder to consider fungal folliculitis in the differential diagnosis of a pustular eruption, so as to allow appropriate management and to avert discontinuation of potentially life-saving medications.
THE DIFFERENTIAL DIAGNOSIS
The appearance of sterile pustules after starting a new antibiotic raised suspicion for acute localized exanthematous pustulosis, a variant of acute generalized exanthematous pustulosis. It is a serious but uncommon adverse drug reaction, with a frequency of one to five cases per million per year. The eruption of erythematous plaques studded with sterile pustules classically appears 1 to 5 days after starting a drug.1 Piperacillin-tazobactam has been infrequently reported in association with acute exanthematous pustulosis, but antibiotics in general are among the most commonly reported culprits.2–4
Clues to the correct diagnosis
Although our concern for acute localized exanthematous pustulosis was warranted, the morphology and distribution of this patient’s exanthem also raised suspicion of fungal folliculitis, which is more common.
Malassezia folliculitis appears as a monomorphic papular and pustular eruption on the chest, back, and face,5 as in our patient. Differentiating fungal folliculitis from pustulosis is important, as each condition is treated differently: Malassezia folliculitis is treated with antifungals,5 and acute localized exanthematous pustulosis is managed with cessation of the offending drug, supportive care, and systemic or topical steroids.4
Take-home point
Our experience with this patient was a reminder to consider fungal folliculitis in the differential diagnosis of a pustular eruption, so as to allow appropriate management and to avert discontinuation of potentially life-saving medications.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol 2012; 53:87–92.
- Talati S, Lala M, Kapupara H, Thet Z. Acute generalized exanthematous pustulosis: a rare clinical entity with use of piperacillin/tazobactam. Am J Ther 2009; 16:591–592.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007; 157:989–996.
- Huilaja L, Kallioinen M, Soronen M, Riekki R, Tasanen K. Acute localized exanthematous pustulosis on inguinal area secondary to piperacillin/tazobactam. Acta Derm Venereol 2014; 94:106–107.
- Rubenstein RM, Malerich SA. Malassezia (pityrosporum) folliculitis. J Clin Aesthet Dermatol 2014; 73:37–41.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol 2012; 53:87–92.
- Talati S, Lala M, Kapupara H, Thet Z. Acute generalized exanthematous pustulosis: a rare clinical entity with use of piperacillin/tazobactam. Am J Ther 2009; 16:591–592.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007; 157:989–996.
- Huilaja L, Kallioinen M, Soronen M, Riekki R, Tasanen K. Acute localized exanthematous pustulosis on inguinal area secondary to piperacillin/tazobactam. Acta Derm Venereol 2014; 94:106–107.
- Rubenstein RM, Malerich SA. Malassezia (pityrosporum) folliculitis. J Clin Aesthet Dermatol 2014; 73:37–41.
Common neurologic emergencies for nonneurologists: When minutes count
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
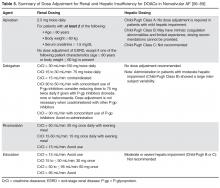
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
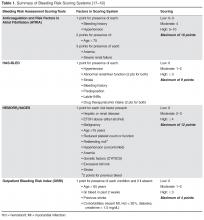
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
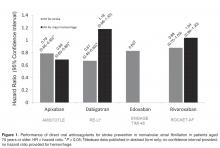
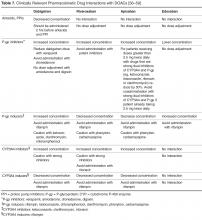
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
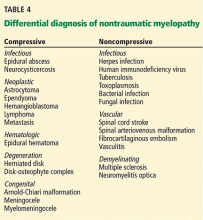
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
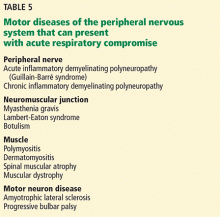
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
KEY POINTS
- Patients with possible acute ischemic stroke should be assessed quickly to see if they should receive tissue plasminogen activator, which should be started within 3 hours of stroke onset. Computed tomography (CT) of the head without contrast should be done immediately to rule out acute hemorrhagic stroke.
- Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy, and sometimes intracranial pressure control.
- If the clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT was negative, lumbar puncture is mandatory.
- Hyperosmolar therapy is the mainstay of emergency medical treatment of intracranial hypertension.
- Seizure activity must be treated aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.
Alcohol withdrawal syndrome in medical patients
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
KEY POINTS
- Patients diagnosed with or suspected of having alcohol withdrawal syndrome need a thorough history and physical examination, appropriate laboratory tests, and monitoring using the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) or a similar scale.
- For most patients, benzodiazepines should be given in a symptom-triggered fashion, using the CIWA-Ar score as a monitoring tool. Alternatively, scheduled benzodiazepine dosing should be considered for patients with a history of alcohol withdrawal delirium or for patients in whom withdrawal symptoms cannot be easily assessed.
- The choice of benzodiazepine should be individualized, based on the half-life of the drug, comorbid diseases, and monitoring plans.
- Many patients with alcohol withdrawal syndrome require fluid and electrolyte replacement, as well as adjunctive therapies such as haloperidol for delirium and antihypertensives for cardiac or adrenergic symptoms. No standard currently exists for drug dosing, administration, and assessment protocols in these patients. Therefore, clinicians are adapting study designs and assessment scales to meet patients’ individual needs.
Selecting a Direct Oral Anticoagulant for the Geriatric Patient with Nonvalvular Atrial Fibrillation
From the Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ.
Abstract
- Objective: To provide a clinical summary of the available data evaluating the use of direct oral anticoagulants (DOACs) in geriatric patients with nonvalvular atrial fibrillation.
- Methods: MEDLINE, Web of Science, and Google Scholar were used to identify pertinent systematic reviews, randomized controlled trials, observational studies, and pharmacokinetic studies evaluating use of DOACs in the geriatric population.
- Results: A total of 8 systemic reviews, 5 randomized controlled trials, 2 observational trials, and 5 pharmacokinetic studies of relevance were identified for inclusion in this review. The landscape of anticoagulation has dramatically changed over the past 5 years beginning with the development and marketing of an oral direct thrombin inhibitor and followed by 3 oral direct factor Xa inhibitors. Despite significant advances in this oral anticoagulation arena, many questions remain as to the best therapeutic approach in the geriatric population as the literature is lacking. This population has a higher risk of stroke; however, due to the increased risk of bleeding clinicians may often defer anticoagulant therapy due to the fear of hemorrhagic complications. Clinicians must consider the risk-benefit ratio and the associated outcomes in geriatric patients compared to other patient populations.
- Conclusions: Interpreting the available literature and understanding the benefits and limitations of the DOACs is critical when selecting the most appropriate pharmacologic strategy in geriatric patients.
Anticoagulants are among the top 5 drug classes associated with patient harm in the US [1] and are commonly reported as contributing to hospitalizations [2]. In just one quarter in 2012 alone, warfarin, dabigatran, and rivaroxaban accounted for 1734 of 50,289 adverse events reported to the Food and Drug Administration (FDA), including 233 deaths [3]. Appropriate use of anticoagulant agents and consideration of individual patient risk factors are essential to mitigate the occurrence of adverse consequences, especially in the geriatric population. This population is more likely to have risk factors for adverse drug events, for example, polypharmacy, age-related changes in pharmacokinetics and pharmacodynamics, and diminished organ function (ie, renal and hepatic) [4,5]. Another important consideration is the lack of consensus on the definition of a “geriatric” or “elderly” patient. Although many consider a chronological age of > 65 years as the defining variable for a geriatric individual, this definition does not account for overall health status [6,7]. Clinicians should consider this shortcoming when evaluating the quality of geriatric studies. For example, a study claiming to evaluate the pharmacokinetics of a drug in a geriatric population enrolling healthy subjects aged > 65 years may result in data that do not translate to clinical practice.
Compounding the concern for iatrogenic events is the frequency of anticoagulant use in the geriatric population, as several indications are found more commonly in this age-group. Stroke prevention in nonvalvular atrial fibrillation (AF), the most common arrhythmia in the elderly, is a common indication for long-term anticoagulation [8]. The prevalence of AF increases with age and is usually higher in men than in women [9,10]. AF is generally uncommon before 60 years of age, but the prevalence increases noticeably thereafter, affecting approximately 10% of the overall population by 80 years of age [11]. The median age of patients who have AF is 75 years with approximately 70% of patients between 65 and 85 years of age [8,12]. Currently in the United States, an estimated 2.3 million people are diagnosed with AF [8]. In 2020, the AF population is predicted to increase to 7.5 million individuals with an expected prevalence of 13.5% among individuals ≥ 75 years of age, and 18.2% for those ≥ 85 years of age [13]. These data underscore the importance of considering the influence of age on the balance between efficacy and safety of anticoagulant therapy.
Direct oral anticoagulants (DOACs) represent the first alternatives to warfarin in over 6 decades. Currently available products in US include apixaban, dabigatran, edoxaban, and rivaroxaban. DOACs possess many of the characteristics of an ideal anticoagulant, including predictable pharmacokinetics, a wider therapeutic window compared to warfarin, minimal drug interactions, a fixed dose, and no need for routine evaluation of coagulation parameters. The safety and efficacy of the DOACs for stroke prevention in nonvalvular AF have been substantiated in several landmark clinical trials [14–16]. Yet there are several important questions that need to be addressed, such as management of excessive anticoagulation, clinical outcome data with renally adjusted doses (an exclusion criterion in many landmark studies was a creatinine clearance of < 25–30 mL/min), whether monitoring of coagulation parameters could enhance efficacy and safety, and optimal dosing strategies in geriatric patients. This review provides clinicians a summary of data from landmark studies, post-marketing surveillance, and pharmacokinetic evaluations to support DOAC selection in the geriatric population.
Evaluating Bleeding Risk
These tools have been extensively evaluated with warfarin therapy, but their performance in predicting DOAC-related bleeding has not been definitively established. Nonetheless, until tools evaluated specifically for DOACs are developed, it is reasonable to use these for risk-prediction in combination with clinical judgment. As an example, the European Society of Cardiology guideline on the use of non–vitamin K antagonist (VKA) anticoagulants in patients with nonvalvular AF suggests that the HAS-BLED score may be used to identify risk factors for bleeding and correct those that are modifiable [20]. The HAS-BLED score is validated for VKA and non-VKA anticoagulants (early-generation oral direct thrombin inhibitor ximelgatran) [21] and is the only bleeding risk score predictive for intracranial hemorrhage [19]. In a 2013 “real world” comparison, HAS-BLED was easier to use and had better predictive accuracy that ATRIA [22].
One of the major challenges in geriatric patients is that those at highest risk for bleeding are those who would have the greatest benefit from anticoagulation [23]. The prediction scores can help clinicians balance the risk-benefit ratio for anticoagulation on a case by case basis. Although the scoring systems take into consideration several factors, including medical conditions that have been shown to significantly increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes, renal insufficiency, alcoholism and liver disease, not all are included in every scoring scheme [23]. These conditions are more common among elderly patients, and this should be taken into account when estimating the risk-benefit ratio of oral anticoagulation [15]. Patients’ preferences should also be taken into account. It is essential for clinicians to clearly discuss treatment options with patients as data suggest that clinician and patient perceptions of anticoagulation are often mismatched [24–26].
Performance of TSOACS in Landmark Studies
Some specific differences in outcomes seen in landmark studies that may facilitate selection among the DOACs include the risk of major bleeding, risk of gastrointestinal bleeding, risk of acute coronary syndrome, exclusion of valvular heart disease, and noninferiority versus superiority as the primary endpoint when compared to warfarin.
Major Bleeding
Gastrointestinal Bleeding
Among all of the DOACs, gastrointestinal (GI) bleeding was significantly greater with dabigatran, edoxaban, and rivaroxaban when compared to warfarin (HR, 1.49; 95% CI, 1.21–1.84; HR, 1.23; 95% CI, 1.02–1.50; and HR, 1.61; 95% CI, 1.30–1.99, respectively; P < 0.05 for all) [14–16] in landmark studies. Based on these data, clinicians may consider the selection of apixaban in patients with a previous history of GI pathology. GI bleeding may be more common in elderly patients due to the potential for preexisting GI pathology and high local concentrations of drug [29]. Clemens and colleagues suggested an “anticoagulation GI stress test” may predict GI malignancy [33]. They found that patients on DOACs that presented with a GI bleed were more likely to present with a GI malignancy. As such, it is reasonable to screen patients with a fecal occult blood test within the first month after initiating TSOAC treatment and then annually.
Acute Coronary Syndrome
A higher rate of myocardial infarction was observed with dabigatran 150 mg versus warfarin (0.74% vs 0.53% per year; P = 0.048) in the RE-LY study [16]. Whether the increase in myocardial infarction was due to dabigatran as a causative agent or warfarin’s ability to reduce the risk of myocardial infarction to a larger extent compared with dabigatran is unknown. Nonetheless, it may be prudent to use an alternative therapy in patients with a history of acute coronary syndrome.
Valvular Heart Disease
The risk of stroke and systemic embolism is higher in patients with valvular heart disease [34]. Patients with moderate to severe mitral stenosis or mechanical prosthetic heart valves were excluded from the DOAC landmark studies. Dabigatran was evaluated for prevention of stroke and systemic embolism in patients with valvular heart disease in the RE-ALIGN study [35,36]. Patients were randomized to warfarin titrated to a target INR of 2 to 3 or 2.5 to 3.5 on the basis of thromboembolic risk or dabigatran 150 mg, 220 mg, or 300 mg twice daily adjusted to a targeted trough of ≥ 50 ng/mL. The trial was terminated early due to a worse primary outcome (composite of stroke, systemic embolism, myocardial infarction, and death) with dabigatran versus warfarin (HR, 3.37, 95% CI, 0.76–14.95; P = 0.11). In addition, bleeding rates (any bleeding) was significantly greater with dabigatran (27%) versus warfarin (12%) (P = 0.01). Based on these data and the lack of data with the other TSOACs, warfarin remains the standard of care for valvular heart disease [37]. In patients with a previous bioprosthetic valve with AF, patients with mitral insufficiency, or aortic stensosis, TSOACs may be considered [37].
Landmark Study Efficacy Endpoints
The primary endpoint in each of the landmark studies was a composite of stroke (ischemic or hemorrhagic) and systemic embolism. For the primary endpoint only dabigatran 150 mg twice daily and apixaban 5 mg twice daily were found to be superior to warfarin for the prevention of stroke or systemic embolism in nonvalvular AF (HR, 0.66; 95% CI, 0.53–0.82; P < 0.001 and HR, 0.66; 95% CI, 0.66–0.95; P = 0.01, respectively). Both edoxaban (60 mg and 30 mg daily) and rivaroxaban were noninferior to warfarin for the primary endpoint. In terms of ischemic stroke, only dabigatran 150 mg twice daily was superior to warfarin for the reduction in ischemic stroke in patients with nonvalvular AF (HR, 0.76; 95% CI, 0.60–0.98; P = 0.03) [19]. All of the DOACs demonstrated a reduction in hemorrhagic stroke.
TSOAC Use in Elderly Patitents
Pharmacokinetic Evaluations
Several pharmacokinetic studies have evaluated the influence of age on DOAC disposition. In a study evaluating the influence of age on apixaban disposition, the area under the concentration-time curve to infinity was 32% higher in the elderly (aged 65 years or older) compared to the younger subjects (< age 40 years) [38]. These data provide the rationale for dosage adjustment in individuals aged 80 years or older with either low body mass (weight less than or equal to 60 kg) or renal impairment (serum creatinine 1.5 mg/dL or higher). In a pharmacokinetic study evaluating dabigatran in patients > 65 years of age, the time to steady state ranged from 2 to 3 days, correlating to a half-life of 12 to 14 hours, and peak concentrations (256 ng/mL females, 255 ng/mL males) were reached after a median of 3 hours (range, 2.0–4.0 hours) [39]. These data suggest a 1.7- to twofold increase in bioavailability. The area under the curve of rivaroxaban was significantly higher in subjects > 75 years versus subjects 18-45 years, while total and renal clearance were decreased [40].However, the time to maximum factor Xa inhibition and Cmax were not influenced by age.
Clinical Evaluations
Dabigatran
In a post-hoc analysis of the RE-LY trial, Eikelboom and colleagues found that patients 75 years of age and older treated with dabigatran 150 mg twice daily had a greater incidence of GI bleeding irrespective of renal function compared with those on warfarin (1.85%/year vs. 1.25%/year; P < 0.001) [29]. A higher risk in major bleeding also was seen in dabigatran patients (5.10% versus 4.37%; P = 0.07). As a result, the 2012 Beer’s Criteria lists dabigatran as a potentially inappropriate medication. An analysis was conducted of 134,414 elderly Medicare patients (defined as age > 65 years) with 37,587 person-years of follow-up who were treated with dabigatran or warfarin [44]. Approximately 60% of patients included in the analysis were over age 75 years. Dabigatran was associated with a significant reduction in ischemic stroke: HR 0.80 (CI 0.67–0.96); intracranial hemorrhage: HR 0.34 (CI 0.26–0.46); and death: HR 0.86 (CI 0.77–0.96) when compared with warfarin. As in the Eikelboom study, major gastrointestinal bleeding was significantly increased with dabigatran (HR, 1.28 [95% CI, 1.14–1.44]).
Rivaroxaban
For rivaroxaban, a subgroup analysis of patients ≥ 75 years in the ROCKET-AF trial reported similar rates of major bleeding (HR, 1.11; 95% CI, 0.92–1.34) with rivaroxaban compared with warfarin [31]. Clinically relevant non-major bleeding was significantly higher for patients aged ≥ 75 years compared with patients aged < 75 years (P = 0.01).
Apixaban
Halvorsen and colleagues found that age did not influence the benefits of apixaban in terms of efficacy and safety [47]. In the cohort of patients aged 75 years or older, major bleeding was significantly reduced compared to warfarin (HR, 0.64; 95% CI, 0.52–0.79). The safety benefits persisted even in the setting of age greater than 75 years and renal impairment. A significant reduction in major bleeding (HR, 0.35; 95% CI, 0.14–0.86) was seen in elderly patients with a CrCl; ≤ 30 mL/min (n = 221) treated with apixaban versus warfarin. Similarly, in elderly patients with a CrCl 30 to 50 mL/min (n = 1898) a significant reduction in major bleeding was reported (HR, 0.53; 95% CI, 0.37–0.76). These data are consistent with a meta-regression analysis that found a linear relationship between the relative risk of major bleeding and the magnitude of renal excretion for the DOACs (r2=0.66, P = 0.03) [48]. In this analysis, apixaban had the most favorable outcomes in terms of major bleeding compared to the other DOACs and also has the least dependence on renal function for clearance. In a pooled analysis of data from landmark trials, Ng and colleagues found that in elderly patients (defined as age > 75 years) with nonvalvular AF, only apixaban was associated with a significant reduction in both stroke and major hemorrhage (Figure 1) [49,50].
Edoxaban
Kato and colleagues performed a subgroup analysis of patients aged 75 years or older enrolled in the ENGAGE TIMI 48 study [50]. Currently the results are only published in abstract form. Regardless of treatment, the risk of major bleeding and stroke significantly increased with age (P < 0.001). An absolute risk reduction in major bleeding was reported with both 60 mg and 30 mg of edoxaban versus warfarin (4.0%/year and 2.2%/year versus 4.8%/year, respectively; no P value provided).
Therapeuti Drug Monitoring
Collectively, the data on assessment of the anticoagulant activity of DOACs using coagulation assays is evolving. These tests include but are not limited to prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin clotting time (TT), dilute TT, activated clotting time (ACT), anti factor Xa, and ecarin clotting time (ECT) assays. Although routine monitoring is not desirable, the ability to assess degree of anticoagulation in select patient populations may prove beneficial. Future studies are essential to confirm whether assessing DOAC activity using coagulation assays in vulnerable populations such as the elderly improves clinical outcomes. Several reviews on this subject matter have been published [51–55]. The reader is encouraged to review these data as there are significant limitations to currently available assays and incorrect interpretation may lead to suboptimal treatment decisions.
Renal and Hepatic Dysfunction
Depending on the specific agents, DOACs renal clearance varies from 27% to 80% [56–59]. Clinical trials often use the Cockcroft-Gault formula (CG) based on actual body weight to estimate renal function. Landmark trials evaluating the DOACs differed in their strategy for estimation of renal function using CG. For example, RE-LY and ROCKET-AF used actual body weight for the estimation of renal function, while ARISTOTLE did not specify which body weight to use. Estimation of renal function or glomerular filtration rate (GFR) by CG is frequently in discordance with actual renal function in the elderly [60]. MDRD (modification of diet in renal disease) and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) are also common estimations that provide an estimate of GFR. In a cross-sectional study, comparing the CG, MDRD, and EPI formulas in a clinical setting, data from potential kidney donors and adult patients who underwent a GFR measurement revealed that MDRD has the smallest mean bias. The influence of age was the absolute bias for estimation of renal function for all formulas. CG is additionally influenced by body weight and body mass index. When compared to CG, MDRD actually reported more accurate predictor of GFR in adults < 70 years old [61]. However, package inserts recommend dose adjustments based on estimation of CrCl using CG formula. This poses a problem in adjusting DOAC doses in elderly patients who are subject to overestimation of renal function with this antiquated equation. Among elderly patients with renal impairment, discordance between estimated and actual renal function was higher for dabigatran and rivaroxaban than for apixaban dosages [61].
Renal excretion of unchanged dabigatran is the predominant pathway for elimination (~80%) [58]. The FDA-approved dosing strategy in the US for dabigatran is 150 mg twice daily in patients with a CrCl ≥ 30 mL/min, 75 mg twice daily in patients with severe renal impairment (CrCl 15–30 mL/min), and is contraindicated in patients with a CrCl < 15 mL/min [58]. By comparison, the Canadian and the European Medicines Agency have listed patients with a CrCl < 30 mL/min (severe renal impairment) as a contraindication for use. The US-approved dosage for severe renal impairment was derived during the approval phase of dabigatran using a simulation pharmacokinetic model [62,63]. The dosage was estimated by pharmacokinetic simulation to provide similar Cmax and Cmin concentrations compared to the 150 mg twice-daily dosage in moderate renal impairment. Compared to patients with CrCl ≥ 80 mL/min, there was a 1.29- and a 1.47-fold increase in dabigatran trough plasma concentration in the CrCl 50–80 mL/min patients and the CrCl 30–50 mL/min patients, respectively. There have been many postmarketing reports of hemorrhage with dabigatran [36,84,85]. Although reporting bias is likely due to the novelty of the agent, clinicians may take key clinical pearls away from these reports. Patients often had risk factors, including low body weight, renal impairment, and polypharmacy with interacting drugs (eg, amiodarone). These risk factors are also important with the other DOACs.
A subgroup analysis of ROCKET-AF evaluating rivaroxaban 15 mg daily in patients with a CrCl of 30–49 mL/min did not identify any differences in endpoints with the exception of fatal bleeding, which occurred less often with rivaroxaban (0.28%/yr vs. 0.74%/yr; P = 0.047) [64].
Monitoring of renal function is essential to mitigate the risk of drug accumulation. Clinicians should consider obtaining a baseline renal assessment with annual reassessments in patients with normal (CrCl ≥ 80 mL/min) or mild (CrCl 50–79 mL/min) renal impairment, and 2 to 3 times per year in patients with moderate (CrCl 30–49 mL/min) renal impairment [65]. A summary of renal dose adjustments for DOAC therapy may be found in Table 5 [56–59].
In addition to renal function, hepatic impairment can also affect the metabolism of anticoagulants. Severe hepatic impairment can lead to prolonged PT. Therefore, patients who have liver dysfunction and are treated with anticoagulation have increased risk of hemorrhagic events. Large pivotal trials on the key indications of dabigatran, apixaban, and rivaroxaban excluded patients with significant signs of hepatic impairment. Table 5 provides dosing recommendations for the different DOACs in the setting of hepatic impairment [56–59].
Polypharmacy And The Potential For Adverse Consequences
Costs And Cost-Effectiveness of DOACS
With the high burden of AF and the aging population, analysis of cost and value is an important consideration [76]. There are limited publications comparing the cost-effectiveness between the anticoagulation options. However, numerous cost-effectiveness studies have evaluated the individual DOACs [71–79]. Overall, the studies suggest that the DOACs are a cost-effective alternative to warfarin in the general and elderly populations. One analysis reported that dabigatran may not be cost-effective in patients with a low CHADS2 score (≤ 2) [71].
Harrington et al [80] compared the cost-effectiveness of dabigatran, rivaroxaban, and apixaban versus warfarin. This cost-effectiveness study used published clinical trial data to build a decision model, and results indicated that for patients ≥ 70 years of age with an increased risk for stroke, normal renal function, and no previous contraindications to anticoagulant therapy, apixaban 5 mg, dabigatran 150 mg, and rivaroxaban 20 mg were cost-effective substitutes for warfarin for the prevention of stroke in nonvalvular AF [80]. Apixaban was the preferred anticoagulant for their hypothetical cohort of 70-year-old patients with nonvalvular AF, as it was most likely to be the cost-effective treatment option at all willing-to-pay thresholds > $40,000 per quality-adjusted life-year gained [76,81].
Prescription costs may vary depending on payor and level of insurance. If a patient does not have prescription insurance, the annual price of generic warfarin is roughly $200 to $360, depending on dosage. Approximate annual costs for the DOACs are greater than 20 times the cost of warfarin (apixaban $4500, dabigatran $4500, and rivaroxaban $4800) [82]. However, most patients on these medications are over 65 years old and have prescription coverage through Medicare Part D. Of note, patients may have more of a burden if or when they reach the “donut hole” coverage gap. Currently, once patients spend $2960 (for 2015) and $3310 (for 2016) on covered drugs they will fall into the donut hole unless they qualify for additional assistance. At this point Medicare Part D will reimburse 45% of the cost of the newer anticoagulants since generics are currently unavailable. As a result, individual affordability may become an issue. Further complicating the scenario is the inability to apply coupon and rebate cards in the setting of government-funded prescription coverage. Clinicians should discuss these issues with their patients to help select the most valuable therapy.
Conclusions And Recommendations
Corresponding author: Luigi Brunetti, PharmD, MPH, Rutgers University, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
1. Fanikos J, Stapinski C, Koo S, et al. Medication errors associated with anticoagulant therapy in the hospital. Am J Cardiol 2004;94:532–5.
2. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med2011;365:2002–12.
3. Institute for Safe Medication Practices. QuarterWatch. 9 January 2013. Available at http://www.ismp.org/quarterwatch/pdfs/2012Q2.pdf.
4. Hajjar ER, Hanlon JT, Artz MB, Let al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother 2003;1:82–9.
5. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107–16.
6. Singh S. Defining ‘elderly’ in clinical practice guidelines for pharmacotherapy. Pharm Pract 2014;12:489.
7. Singh S, Bajorek B. Pharmacotherapy in the aging patient: The impact of age per se (a review). Ageing Res Rev 2015 Jul 28. pii: S1568-1637(15)30008-8.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA 2001;285:2370–5.
9. Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe Chest 2012;142:1489–98.
10. Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association Europace 2012;14:1385–413.
11. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17–40.
12. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden 2010 study. Circulation 2014;129:837-47.
13. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25.
14. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51.
15. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91.
16. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92.
17. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED Bleeding Risk–Prediction Scores in Patients With Atrial Fibrillation Undergoing Anticoagulation: The AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol 2012;60:861–7.
18. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
19. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138:1093–100.
20. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507.
21. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173–80.
22. Roldán V, Marín F, Fernández H, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a "real-world" population with atrial fibrillation receiving anticoagulant therapy. Chest 2013;43:179–84.
23. Robert-Ebadi H, Le Gal G, Righini M. Use of anticoagulants in elderly patients: practical recommendations. Clin Interv Aging 2009;4:165–77.
24. Barcellona D, Contu P, Sorano GG, et al. The management of oral anticoagulant therapy: the patient's point of view. Thromb Haemost 2000;83:49–53.
25. Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991;151:1944–9.
26. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22.
27. Giugliano RP, Ruff CT, Braunwald E, Murphy SA. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104.
28. Barco S, Cheung YW, Eikelboom JW, Coppens M. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol 2013;26:215–24
29. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–72.
30. Coppens M, Eikelboom JW, Ezekowitz M, et al. Dabigatran versus warfarin in very elderly patients with atrial fibrillation: results from the RE-LY trial. Abstract. Circulation 2012;126:A15l537.
31. Halperin JL, Wojdyla D, Piccini JP, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the ROCKET-AF trial. Abstract. Stroke 2012;43:A148.
32. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62
33. Clemens A, Strack A, Noack H, et al. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014;46:672–8.
34. Petty GW, Khandheria BK, Whisnant JP, et al. Predictors of cerebrovascular events and death among patients with valvular heart disease: A population-based study. Stroke 2000;31:2628–35.
35. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14.
36. Schomburg JL, Medina EM, Lahti MT, Bianco RW. Dabigatran versus warfarin after mechanical mitral valve replacement in the swine model. J Invest Surg 2012;25:150–5.
37. Douketis J, Bell AD, Eikelboom J, Liew A. Approach to the new oral anticoagulants in family practice: part 2: addressing frequently asked questions. Can Fam Physician 2014;60:997–1001.
38. Frost CE, Nepal S, Barrett YC, LaCreta F. Effects of age and gender on the singledose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban. Abstract. J Thromb Haemost 2009;7(Suppl 2):PP-MO-407..
39. Stangier J, Stahle H, Rathgen K et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47–59.
40. Kubitza D, Becka M, Mueck W. The effect of extreme age, and gender on the pharmacology and tolerability of rivaroxaban, an oral direct factor Xa inhibitor. Blood 2006;108: Abstract 905.
41. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J 2013;34:489–98.
42. Evans A, Kalra L. Are the results of randomized controlled trials on anticoagulation in patients with atrial fibrillation generalizable to clinical practice? Arch Intern Med 2001;161:1443–7.
43. Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012;366:864–6.
44. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64.
45. Avgil-Tsadok M, Jackevicius CA, Essebag V, et al. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost 2015;115(1).
46. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012;172:397–402.
47. Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–72.
48. Lega JC, Bertoletti L, Gremillet C, et al. Consistency of safety profile of new oral anticoagulants in patients with renal failure. J Thromb Haemost 2014;12:337–43.
49. Ng KH, Hart RG, Eikelboom JW. Anticoagulation in patients aged ≥ 75 years with atrial fibrillation: role of novel oral anticoagulants. Cardiol Ther 2013;2:135–49.
50. Kato ET, Guigliano RP, Ruff CT, et al. Efficacy and safety of edoxaban for the management of elderly patients with atrial fibrillation: Engage-AF TIMI 48. Circulation 2014;130:A16612.
51. Tripodi A. The laboratory and the new oral anticoagulants. Clin Chem 2013;59:353–62.
52. Tripodi A, Di Iorio G, Lippi G, et al. Position paper on laboratory testing for patients taking new oral anticoagulants. Consensus document of FCSA, SIMeL, SIBioC and CISMEL. Clin Chem Lab Med 2012;50:2137-40.
53. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51.
54. Chin PK, Wright DF, Patterson DM, et al. A proposal for dose-adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol 2014;78:599–609.
55. Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012;69:1473–84.
56. Xarelto [package insert]. Titusville, NJ. Janssen Pharmaceuticals. September 2014.
57. Eliquis [package insert]. Princeton, NJ: Bristol-Meyers Squibb. June 2015.
58. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals. October 2010.
59. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo. September 2015.
60. Michels WM, Grootendorst DC, Verduijn M, et al. Performance of the Cockcroft-Gault, MDRD, and new CKDEPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5: 1003–9.
61. Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs 2012;72:1739–53.
62. Hariharan S, Madabushi R. Clinical pharmacology basis of deriving dosing recommendations for dabigatran in patients with severe renal impairment. J Clin Pharmacol 2012;52:119S–25S.
63. Lehr T, Haertter S, Liesenfeld KH, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. J Clin Pharmacol 2012;52:1373–8.
64. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387–94.
65. Pengo V, Crippa L, Falanga A et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost 2011;106:868–76.
66. Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999;14:141–52.
67. Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867–78.
68. Skov J, Bladbjerg EM, Sidelmann J, et al. Plenty of pills: polypharmacy prevails in patients of a Danish anticoagulant clinic. Eur J Clin Pharmacol 2011;67:1169–74.
69. Ukena C, Bohm M, Schirmer SH. Hot topics in cardiology: data from IABP-SHOCK II, TRILOGY-ACS, WOEST, ALTIDUDE, FAME II and more. Clin Res Cardiol 2012;101):861–74.
70. Dewilde, Willem JM, Oirbans T, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet;381:1107–15.
71. Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:
2562–70.
72. Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908–19.
73. Adcock AK, Lee-Iannotti JK, Aguilar MI, et al. Is dabigatran cost effective compared with warfarin for stroke prevention in atrial fibrillation?: a critically appraised topic. Neurologist 2012;18:102–7.
74. Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 2012;43:881–3.
75. Langkilde LK, Bergholdt AM, Overgaard M. Cost-effectiveness of dabigatran etexilate for stroke prevention in non-valvular atrial fibrillation. J Med Econ 2012;15:695-703.
76. Kansal AR, Sorensen SV, Gani R, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012; 98:573–8.
77. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1–11.
78. Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343:d6333.
79. Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation-cost analysis. Age Ageing 2012;41:681–4.
80. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676–81.
81. Amin A, Lingohr-Smith M, Bruno A, et al. Economic evaluations of medical cost differences: use of targeted-specific oral anticoagulants vs. warfarin among patients with nonvalvular atrial fibrillation and venous thromboembolism in the US. J Hematol Thrombo Dis 2015;3:209.
82. Lexicomp, Lexi-Drugs. Hudson, OH: Lexi-Comp.
83. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Apixaban NDA 202155/S-002 approval letter. Jan 30 2014. Available at http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/202155Orig1s002ltr.pdf
84. Hinojar R, Jimenez-Natcher JJ, Fernandez-Golfin C, Zamorano JL. New oral anticoagulants: a practical guide for physicians. Eur Heart J Cardiovasc Pharmacother 2015;1:134-45.
85. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patiets with atrial fibrillation. N Engl J Med 2011;364;806–17.
From the Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ.
Abstract
- Objective: To provide a clinical summary of the available data evaluating the use of direct oral anticoagulants (DOACs) in geriatric patients with nonvalvular atrial fibrillation.
- Methods: MEDLINE, Web of Science, and Google Scholar were used to identify pertinent systematic reviews, randomized controlled trials, observational studies, and pharmacokinetic studies evaluating use of DOACs in the geriatric population.
- Results: A total of 8 systemic reviews, 5 randomized controlled trials, 2 observational trials, and 5 pharmacokinetic studies of relevance were identified for inclusion in this review. The landscape of anticoagulation has dramatically changed over the past 5 years beginning with the development and marketing of an oral direct thrombin inhibitor and followed by 3 oral direct factor Xa inhibitors. Despite significant advances in this oral anticoagulation arena, many questions remain as to the best therapeutic approach in the geriatric population as the literature is lacking. This population has a higher risk of stroke; however, due to the increased risk of bleeding clinicians may often defer anticoagulant therapy due to the fear of hemorrhagic complications. Clinicians must consider the risk-benefit ratio and the associated outcomes in geriatric patients compared to other patient populations.
- Conclusions: Interpreting the available literature and understanding the benefits and limitations of the DOACs is critical when selecting the most appropriate pharmacologic strategy in geriatric patients.
Anticoagulants are among the top 5 drug classes associated with patient harm in the US [1] and are commonly reported as contributing to hospitalizations [2]. In just one quarter in 2012 alone, warfarin, dabigatran, and rivaroxaban accounted for 1734 of 50,289 adverse events reported to the Food and Drug Administration (FDA), including 233 deaths [3]. Appropriate use of anticoagulant agents and consideration of individual patient risk factors are essential to mitigate the occurrence of adverse consequences, especially in the geriatric population. This population is more likely to have risk factors for adverse drug events, for example, polypharmacy, age-related changes in pharmacokinetics and pharmacodynamics, and diminished organ function (ie, renal and hepatic) [4,5]. Another important consideration is the lack of consensus on the definition of a “geriatric” or “elderly” patient. Although many consider a chronological age of > 65 years as the defining variable for a geriatric individual, this definition does not account for overall health status [6,7]. Clinicians should consider this shortcoming when evaluating the quality of geriatric studies. For example, a study claiming to evaluate the pharmacokinetics of a drug in a geriatric population enrolling healthy subjects aged > 65 years may result in data that do not translate to clinical practice.
Compounding the concern for iatrogenic events is the frequency of anticoagulant use in the geriatric population, as several indications are found more commonly in this age-group. Stroke prevention in nonvalvular atrial fibrillation (AF), the most common arrhythmia in the elderly, is a common indication for long-term anticoagulation [8]. The prevalence of AF increases with age and is usually higher in men than in women [9,10]. AF is generally uncommon before 60 years of age, but the prevalence increases noticeably thereafter, affecting approximately 10% of the overall population by 80 years of age [11]. The median age of patients who have AF is 75 years with approximately 70% of patients between 65 and 85 years of age [8,12]. Currently in the United States, an estimated 2.3 million people are diagnosed with AF [8]. In 2020, the AF population is predicted to increase to 7.5 million individuals with an expected prevalence of 13.5% among individuals ≥ 75 years of age, and 18.2% for those ≥ 85 years of age [13]. These data underscore the importance of considering the influence of age on the balance between efficacy and safety of anticoagulant therapy.
Direct oral anticoagulants (DOACs) represent the first alternatives to warfarin in over 6 decades. Currently available products in US include apixaban, dabigatran, edoxaban, and rivaroxaban. DOACs possess many of the characteristics of an ideal anticoagulant, including predictable pharmacokinetics, a wider therapeutic window compared to warfarin, minimal drug interactions, a fixed dose, and no need for routine evaluation of coagulation parameters. The safety and efficacy of the DOACs for stroke prevention in nonvalvular AF have been substantiated in several landmark clinical trials [14–16]. Yet there are several important questions that need to be addressed, such as management of excessive anticoagulation, clinical outcome data with renally adjusted doses (an exclusion criterion in many landmark studies was a creatinine clearance of < 25–30 mL/min), whether monitoring of coagulation parameters could enhance efficacy and safety, and optimal dosing strategies in geriatric patients. This review provides clinicians a summary of data from landmark studies, post-marketing surveillance, and pharmacokinetic evaluations to support DOAC selection in the geriatric population.
Evaluating Bleeding Risk
These tools have been extensively evaluated with warfarin therapy, but their performance in predicting DOAC-related bleeding has not been definitively established. Nonetheless, until tools evaluated specifically for DOACs are developed, it is reasonable to use these for risk-prediction in combination with clinical judgment. As an example, the European Society of Cardiology guideline on the use of non–vitamin K antagonist (VKA) anticoagulants in patients with nonvalvular AF suggests that the HAS-BLED score may be used to identify risk factors for bleeding and correct those that are modifiable [20]. The HAS-BLED score is validated for VKA and non-VKA anticoagulants (early-generation oral direct thrombin inhibitor ximelgatran) [21] and is the only bleeding risk score predictive for intracranial hemorrhage [19]. In a 2013 “real world” comparison, HAS-BLED was easier to use and had better predictive accuracy that ATRIA [22].
One of the major challenges in geriatric patients is that those at highest risk for bleeding are those who would have the greatest benefit from anticoagulation [23]. The prediction scores can help clinicians balance the risk-benefit ratio for anticoagulation on a case by case basis. Although the scoring systems take into consideration several factors, including medical conditions that have been shown to significantly increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes, renal insufficiency, alcoholism and liver disease, not all are included in every scoring scheme [23]. These conditions are more common among elderly patients, and this should be taken into account when estimating the risk-benefit ratio of oral anticoagulation [15]. Patients’ preferences should also be taken into account. It is essential for clinicians to clearly discuss treatment options with patients as data suggest that clinician and patient perceptions of anticoagulation are often mismatched [24–26].
Performance of TSOACS in Landmark Studies
Some specific differences in outcomes seen in landmark studies that may facilitate selection among the DOACs include the risk of major bleeding, risk of gastrointestinal bleeding, risk of acute coronary syndrome, exclusion of valvular heart disease, and noninferiority versus superiority as the primary endpoint when compared to warfarin.
Major Bleeding
Gastrointestinal Bleeding
Among all of the DOACs, gastrointestinal (GI) bleeding was significantly greater with dabigatran, edoxaban, and rivaroxaban when compared to warfarin (HR, 1.49; 95% CI, 1.21–1.84; HR, 1.23; 95% CI, 1.02–1.50; and HR, 1.61; 95% CI, 1.30–1.99, respectively; P < 0.05 for all) [14–16] in landmark studies. Based on these data, clinicians may consider the selection of apixaban in patients with a previous history of GI pathology. GI bleeding may be more common in elderly patients due to the potential for preexisting GI pathology and high local concentrations of drug [29]. Clemens and colleagues suggested an “anticoagulation GI stress test” may predict GI malignancy [33]. They found that patients on DOACs that presented with a GI bleed were more likely to present with a GI malignancy. As such, it is reasonable to screen patients with a fecal occult blood test within the first month after initiating TSOAC treatment and then annually.
Acute Coronary Syndrome
A higher rate of myocardial infarction was observed with dabigatran 150 mg versus warfarin (0.74% vs 0.53% per year; P = 0.048) in the RE-LY study [16]. Whether the increase in myocardial infarction was due to dabigatran as a causative agent or warfarin’s ability to reduce the risk of myocardial infarction to a larger extent compared with dabigatran is unknown. Nonetheless, it may be prudent to use an alternative therapy in patients with a history of acute coronary syndrome.
Valvular Heart Disease
The risk of stroke and systemic embolism is higher in patients with valvular heart disease [34]. Patients with moderate to severe mitral stenosis or mechanical prosthetic heart valves were excluded from the DOAC landmark studies. Dabigatran was evaluated for prevention of stroke and systemic embolism in patients with valvular heart disease in the RE-ALIGN study [35,36]. Patients were randomized to warfarin titrated to a target INR of 2 to 3 or 2.5 to 3.5 on the basis of thromboembolic risk or dabigatran 150 mg, 220 mg, or 300 mg twice daily adjusted to a targeted trough of ≥ 50 ng/mL. The trial was terminated early due to a worse primary outcome (composite of stroke, systemic embolism, myocardial infarction, and death) with dabigatran versus warfarin (HR, 3.37, 95% CI, 0.76–14.95; P = 0.11). In addition, bleeding rates (any bleeding) was significantly greater with dabigatran (27%) versus warfarin (12%) (P = 0.01). Based on these data and the lack of data with the other TSOACs, warfarin remains the standard of care for valvular heart disease [37]. In patients with a previous bioprosthetic valve with AF, patients with mitral insufficiency, or aortic stensosis, TSOACs may be considered [37].
Landmark Study Efficacy Endpoints
The primary endpoint in each of the landmark studies was a composite of stroke (ischemic or hemorrhagic) and systemic embolism. For the primary endpoint only dabigatran 150 mg twice daily and apixaban 5 mg twice daily were found to be superior to warfarin for the prevention of stroke or systemic embolism in nonvalvular AF (HR, 0.66; 95% CI, 0.53–0.82; P < 0.001 and HR, 0.66; 95% CI, 0.66–0.95; P = 0.01, respectively). Both edoxaban (60 mg and 30 mg daily) and rivaroxaban were noninferior to warfarin for the primary endpoint. In terms of ischemic stroke, only dabigatran 150 mg twice daily was superior to warfarin for the reduction in ischemic stroke in patients with nonvalvular AF (HR, 0.76; 95% CI, 0.60–0.98; P = 0.03) [19]. All of the DOACs demonstrated a reduction in hemorrhagic stroke.
TSOAC Use in Elderly Patitents
Pharmacokinetic Evaluations
Several pharmacokinetic studies have evaluated the influence of age on DOAC disposition. In a study evaluating the influence of age on apixaban disposition, the area under the concentration-time curve to infinity was 32% higher in the elderly (aged 65 years or older) compared to the younger subjects (< age 40 years) [38]. These data provide the rationale for dosage adjustment in individuals aged 80 years or older with either low body mass (weight less than or equal to 60 kg) or renal impairment (serum creatinine 1.5 mg/dL or higher). In a pharmacokinetic study evaluating dabigatran in patients > 65 years of age, the time to steady state ranged from 2 to 3 days, correlating to a half-life of 12 to 14 hours, and peak concentrations (256 ng/mL females, 255 ng/mL males) were reached after a median of 3 hours (range, 2.0–4.0 hours) [39]. These data suggest a 1.7- to twofold increase in bioavailability. The area under the curve of rivaroxaban was significantly higher in subjects > 75 years versus subjects 18-45 years, while total and renal clearance were decreased [40].However, the time to maximum factor Xa inhibition and Cmax were not influenced by age.
Clinical Evaluations
Dabigatran
In a post-hoc analysis of the RE-LY trial, Eikelboom and colleagues found that patients 75 years of age and older treated with dabigatran 150 mg twice daily had a greater incidence of GI bleeding irrespective of renal function compared with those on warfarin (1.85%/year vs. 1.25%/year; P < 0.001) [29]. A higher risk in major bleeding also was seen in dabigatran patients (5.10% versus 4.37%; P = 0.07). As a result, the 2012 Beer’s Criteria lists dabigatran as a potentially inappropriate medication. An analysis was conducted of 134,414 elderly Medicare patients (defined as age > 65 years) with 37,587 person-years of follow-up who were treated with dabigatran or warfarin [44]. Approximately 60% of patients included in the analysis were over age 75 years. Dabigatran was associated with a significant reduction in ischemic stroke: HR 0.80 (CI 0.67–0.96); intracranial hemorrhage: HR 0.34 (CI 0.26–0.46); and death: HR 0.86 (CI 0.77–0.96) when compared with warfarin. As in the Eikelboom study, major gastrointestinal bleeding was significantly increased with dabigatran (HR, 1.28 [95% CI, 1.14–1.44]).
Rivaroxaban
For rivaroxaban, a subgroup analysis of patients ≥ 75 years in the ROCKET-AF trial reported similar rates of major bleeding (HR, 1.11; 95% CI, 0.92–1.34) with rivaroxaban compared with warfarin [31]. Clinically relevant non-major bleeding was significantly higher for patients aged ≥ 75 years compared with patients aged < 75 years (P = 0.01).
Apixaban
Halvorsen and colleagues found that age did not influence the benefits of apixaban in terms of efficacy and safety [47]. In the cohort of patients aged 75 years or older, major bleeding was significantly reduced compared to warfarin (HR, 0.64; 95% CI, 0.52–0.79). The safety benefits persisted even in the setting of age greater than 75 years and renal impairment. A significant reduction in major bleeding (HR, 0.35; 95% CI, 0.14–0.86) was seen in elderly patients with a CrCl; ≤ 30 mL/min (n = 221) treated with apixaban versus warfarin. Similarly, in elderly patients with a CrCl 30 to 50 mL/min (n = 1898) a significant reduction in major bleeding was reported (HR, 0.53; 95% CI, 0.37–0.76). These data are consistent with a meta-regression analysis that found a linear relationship between the relative risk of major bleeding and the magnitude of renal excretion for the DOACs (r2=0.66, P = 0.03) [48]. In this analysis, apixaban had the most favorable outcomes in terms of major bleeding compared to the other DOACs and also has the least dependence on renal function for clearance. In a pooled analysis of data from landmark trials, Ng and colleagues found that in elderly patients (defined as age > 75 years) with nonvalvular AF, only apixaban was associated with a significant reduction in both stroke and major hemorrhage (Figure 1) [49,50].
Edoxaban
Kato and colleagues performed a subgroup analysis of patients aged 75 years or older enrolled in the ENGAGE TIMI 48 study [50]. Currently the results are only published in abstract form. Regardless of treatment, the risk of major bleeding and stroke significantly increased with age (P < 0.001). An absolute risk reduction in major bleeding was reported with both 60 mg and 30 mg of edoxaban versus warfarin (4.0%/year and 2.2%/year versus 4.8%/year, respectively; no P value provided).
Therapeuti Drug Monitoring
Collectively, the data on assessment of the anticoagulant activity of DOACs using coagulation assays is evolving. These tests include but are not limited to prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin clotting time (TT), dilute TT, activated clotting time (ACT), anti factor Xa, and ecarin clotting time (ECT) assays. Although routine monitoring is not desirable, the ability to assess degree of anticoagulation in select patient populations may prove beneficial. Future studies are essential to confirm whether assessing DOAC activity using coagulation assays in vulnerable populations such as the elderly improves clinical outcomes. Several reviews on this subject matter have been published [51–55]. The reader is encouraged to review these data as there are significant limitations to currently available assays and incorrect interpretation may lead to suboptimal treatment decisions.
Renal and Hepatic Dysfunction
Depending on the specific agents, DOACs renal clearance varies from 27% to 80% [56–59]. Clinical trials often use the Cockcroft-Gault formula (CG) based on actual body weight to estimate renal function. Landmark trials evaluating the DOACs differed in their strategy for estimation of renal function using CG. For example, RE-LY and ROCKET-AF used actual body weight for the estimation of renal function, while ARISTOTLE did not specify which body weight to use. Estimation of renal function or glomerular filtration rate (GFR) by CG is frequently in discordance with actual renal function in the elderly [60]. MDRD (modification of diet in renal disease) and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) are also common estimations that provide an estimate of GFR. In a cross-sectional study, comparing the CG, MDRD, and EPI formulas in a clinical setting, data from potential kidney donors and adult patients who underwent a GFR measurement revealed that MDRD has the smallest mean bias. The influence of age was the absolute bias for estimation of renal function for all formulas. CG is additionally influenced by body weight and body mass index. When compared to CG, MDRD actually reported more accurate predictor of GFR in adults < 70 years old [61]. However, package inserts recommend dose adjustments based on estimation of CrCl using CG formula. This poses a problem in adjusting DOAC doses in elderly patients who are subject to overestimation of renal function with this antiquated equation. Among elderly patients with renal impairment, discordance between estimated and actual renal function was higher for dabigatran and rivaroxaban than for apixaban dosages [61].
Renal excretion of unchanged dabigatran is the predominant pathway for elimination (~80%) [58]. The FDA-approved dosing strategy in the US for dabigatran is 150 mg twice daily in patients with a CrCl ≥ 30 mL/min, 75 mg twice daily in patients with severe renal impairment (CrCl 15–30 mL/min), and is contraindicated in patients with a CrCl < 15 mL/min [58]. By comparison, the Canadian and the European Medicines Agency have listed patients with a CrCl < 30 mL/min (severe renal impairment) as a contraindication for use. The US-approved dosage for severe renal impairment was derived during the approval phase of dabigatran using a simulation pharmacokinetic model [62,63]. The dosage was estimated by pharmacokinetic simulation to provide similar Cmax and Cmin concentrations compared to the 150 mg twice-daily dosage in moderate renal impairment. Compared to patients with CrCl ≥ 80 mL/min, there was a 1.29- and a 1.47-fold increase in dabigatran trough plasma concentration in the CrCl 50–80 mL/min patients and the CrCl 30–50 mL/min patients, respectively. There have been many postmarketing reports of hemorrhage with dabigatran [36,84,85]. Although reporting bias is likely due to the novelty of the agent, clinicians may take key clinical pearls away from these reports. Patients often had risk factors, including low body weight, renal impairment, and polypharmacy with interacting drugs (eg, amiodarone). These risk factors are also important with the other DOACs.
A subgroup analysis of ROCKET-AF evaluating rivaroxaban 15 mg daily in patients with a CrCl of 30–49 mL/min did not identify any differences in endpoints with the exception of fatal bleeding, which occurred less often with rivaroxaban (0.28%/yr vs. 0.74%/yr; P = 0.047) [64].
Monitoring of renal function is essential to mitigate the risk of drug accumulation. Clinicians should consider obtaining a baseline renal assessment with annual reassessments in patients with normal (CrCl ≥ 80 mL/min) or mild (CrCl 50–79 mL/min) renal impairment, and 2 to 3 times per year in patients with moderate (CrCl 30–49 mL/min) renal impairment [65]. A summary of renal dose adjustments for DOAC therapy may be found in Table 5 [56–59].
In addition to renal function, hepatic impairment can also affect the metabolism of anticoagulants. Severe hepatic impairment can lead to prolonged PT. Therefore, patients who have liver dysfunction and are treated with anticoagulation have increased risk of hemorrhagic events. Large pivotal trials on the key indications of dabigatran, apixaban, and rivaroxaban excluded patients with significant signs of hepatic impairment. Table 5 provides dosing recommendations for the different DOACs in the setting of hepatic impairment [56–59].
Polypharmacy And The Potential For Adverse Consequences
Costs And Cost-Effectiveness of DOACS
With the high burden of AF and the aging population, analysis of cost and value is an important consideration [76]. There are limited publications comparing the cost-effectiveness between the anticoagulation options. However, numerous cost-effectiveness studies have evaluated the individual DOACs [71–79]. Overall, the studies suggest that the DOACs are a cost-effective alternative to warfarin in the general and elderly populations. One analysis reported that dabigatran may not be cost-effective in patients with a low CHADS2 score (≤ 2) [71].
Harrington et al [80] compared the cost-effectiveness of dabigatran, rivaroxaban, and apixaban versus warfarin. This cost-effectiveness study used published clinical trial data to build a decision model, and results indicated that for patients ≥ 70 years of age with an increased risk for stroke, normal renal function, and no previous contraindications to anticoagulant therapy, apixaban 5 mg, dabigatran 150 mg, and rivaroxaban 20 mg were cost-effective substitutes for warfarin for the prevention of stroke in nonvalvular AF [80]. Apixaban was the preferred anticoagulant for their hypothetical cohort of 70-year-old patients with nonvalvular AF, as it was most likely to be the cost-effective treatment option at all willing-to-pay thresholds > $40,000 per quality-adjusted life-year gained [76,81].
Prescription costs may vary depending on payor and level of insurance. If a patient does not have prescription insurance, the annual price of generic warfarin is roughly $200 to $360, depending on dosage. Approximate annual costs for the DOACs are greater than 20 times the cost of warfarin (apixaban $4500, dabigatran $4500, and rivaroxaban $4800) [82]. However, most patients on these medications are over 65 years old and have prescription coverage through Medicare Part D. Of note, patients may have more of a burden if or when they reach the “donut hole” coverage gap. Currently, once patients spend $2960 (for 2015) and $3310 (for 2016) on covered drugs they will fall into the donut hole unless they qualify for additional assistance. At this point Medicare Part D will reimburse 45% of the cost of the newer anticoagulants since generics are currently unavailable. As a result, individual affordability may become an issue. Further complicating the scenario is the inability to apply coupon and rebate cards in the setting of government-funded prescription coverage. Clinicians should discuss these issues with their patients to help select the most valuable therapy.
Conclusions And Recommendations
Corresponding author: Luigi Brunetti, PharmD, MPH, Rutgers University, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
From the Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ.
Abstract
- Objective: To provide a clinical summary of the available data evaluating the use of direct oral anticoagulants (DOACs) in geriatric patients with nonvalvular atrial fibrillation.
- Methods: MEDLINE, Web of Science, and Google Scholar were used to identify pertinent systematic reviews, randomized controlled trials, observational studies, and pharmacokinetic studies evaluating use of DOACs in the geriatric population.
- Results: A total of 8 systemic reviews, 5 randomized controlled trials, 2 observational trials, and 5 pharmacokinetic studies of relevance were identified for inclusion in this review. The landscape of anticoagulation has dramatically changed over the past 5 years beginning with the development and marketing of an oral direct thrombin inhibitor and followed by 3 oral direct factor Xa inhibitors. Despite significant advances in this oral anticoagulation arena, many questions remain as to the best therapeutic approach in the geriatric population as the literature is lacking. This population has a higher risk of stroke; however, due to the increased risk of bleeding clinicians may often defer anticoagulant therapy due to the fear of hemorrhagic complications. Clinicians must consider the risk-benefit ratio and the associated outcomes in geriatric patients compared to other patient populations.
- Conclusions: Interpreting the available literature and understanding the benefits and limitations of the DOACs is critical when selecting the most appropriate pharmacologic strategy in geriatric patients.
Anticoagulants are among the top 5 drug classes associated with patient harm in the US [1] and are commonly reported as contributing to hospitalizations [2]. In just one quarter in 2012 alone, warfarin, dabigatran, and rivaroxaban accounted for 1734 of 50,289 adverse events reported to the Food and Drug Administration (FDA), including 233 deaths [3]. Appropriate use of anticoagulant agents and consideration of individual patient risk factors are essential to mitigate the occurrence of adverse consequences, especially in the geriatric population. This population is more likely to have risk factors for adverse drug events, for example, polypharmacy, age-related changes in pharmacokinetics and pharmacodynamics, and diminished organ function (ie, renal and hepatic) [4,5]. Another important consideration is the lack of consensus on the definition of a “geriatric” or “elderly” patient. Although many consider a chronological age of > 65 years as the defining variable for a geriatric individual, this definition does not account for overall health status [6,7]. Clinicians should consider this shortcoming when evaluating the quality of geriatric studies. For example, a study claiming to evaluate the pharmacokinetics of a drug in a geriatric population enrolling healthy subjects aged > 65 years may result in data that do not translate to clinical practice.
Compounding the concern for iatrogenic events is the frequency of anticoagulant use in the geriatric population, as several indications are found more commonly in this age-group. Stroke prevention in nonvalvular atrial fibrillation (AF), the most common arrhythmia in the elderly, is a common indication for long-term anticoagulation [8]. The prevalence of AF increases with age and is usually higher in men than in women [9,10]. AF is generally uncommon before 60 years of age, but the prevalence increases noticeably thereafter, affecting approximately 10% of the overall population by 80 years of age [11]. The median age of patients who have AF is 75 years with approximately 70% of patients between 65 and 85 years of age [8,12]. Currently in the United States, an estimated 2.3 million people are diagnosed with AF [8]. In 2020, the AF population is predicted to increase to 7.5 million individuals with an expected prevalence of 13.5% among individuals ≥ 75 years of age, and 18.2% for those ≥ 85 years of age [13]. These data underscore the importance of considering the influence of age on the balance between efficacy and safety of anticoagulant therapy.
Direct oral anticoagulants (DOACs) represent the first alternatives to warfarin in over 6 decades. Currently available products in US include apixaban, dabigatran, edoxaban, and rivaroxaban. DOACs possess many of the characteristics of an ideal anticoagulant, including predictable pharmacokinetics, a wider therapeutic window compared to warfarin, minimal drug interactions, a fixed dose, and no need for routine evaluation of coagulation parameters. The safety and efficacy of the DOACs for stroke prevention in nonvalvular AF have been substantiated in several landmark clinical trials [14–16]. Yet there are several important questions that need to be addressed, such as management of excessive anticoagulation, clinical outcome data with renally adjusted doses (an exclusion criterion in many landmark studies was a creatinine clearance of < 25–30 mL/min), whether monitoring of coagulation parameters could enhance efficacy and safety, and optimal dosing strategies in geriatric patients. This review provides clinicians a summary of data from landmark studies, post-marketing surveillance, and pharmacokinetic evaluations to support DOAC selection in the geriatric population.
Evaluating Bleeding Risk
These tools have been extensively evaluated with warfarin therapy, but their performance in predicting DOAC-related bleeding has not been definitively established. Nonetheless, until tools evaluated specifically for DOACs are developed, it is reasonable to use these for risk-prediction in combination with clinical judgment. As an example, the European Society of Cardiology guideline on the use of non–vitamin K antagonist (VKA) anticoagulants in patients with nonvalvular AF suggests that the HAS-BLED score may be used to identify risk factors for bleeding and correct those that are modifiable [20]. The HAS-BLED score is validated for VKA and non-VKA anticoagulants (early-generation oral direct thrombin inhibitor ximelgatran) [21] and is the only bleeding risk score predictive for intracranial hemorrhage [19]. In a 2013 “real world” comparison, HAS-BLED was easier to use and had better predictive accuracy that ATRIA [22].
One of the major challenges in geriatric patients is that those at highest risk for bleeding are those who would have the greatest benefit from anticoagulation [23]. The prediction scores can help clinicians balance the risk-benefit ratio for anticoagulation on a case by case basis. Although the scoring systems take into consideration several factors, including medical conditions that have been shown to significantly increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes, renal insufficiency, alcoholism and liver disease, not all are included in every scoring scheme [23]. These conditions are more common among elderly patients, and this should be taken into account when estimating the risk-benefit ratio of oral anticoagulation [15]. Patients’ preferences should also be taken into account. It is essential for clinicians to clearly discuss treatment options with patients as data suggest that clinician and patient perceptions of anticoagulation are often mismatched [24–26].
Performance of TSOACS in Landmark Studies
Some specific differences in outcomes seen in landmark studies that may facilitate selection among the DOACs include the risk of major bleeding, risk of gastrointestinal bleeding, risk of acute coronary syndrome, exclusion of valvular heart disease, and noninferiority versus superiority as the primary endpoint when compared to warfarin.
Major Bleeding
Gastrointestinal Bleeding
Among all of the DOACs, gastrointestinal (GI) bleeding was significantly greater with dabigatran, edoxaban, and rivaroxaban when compared to warfarin (HR, 1.49; 95% CI, 1.21–1.84; HR, 1.23; 95% CI, 1.02–1.50; and HR, 1.61; 95% CI, 1.30–1.99, respectively; P < 0.05 for all) [14–16] in landmark studies. Based on these data, clinicians may consider the selection of apixaban in patients with a previous history of GI pathology. GI bleeding may be more common in elderly patients due to the potential for preexisting GI pathology and high local concentrations of drug [29]. Clemens and colleagues suggested an “anticoagulation GI stress test” may predict GI malignancy [33]. They found that patients on DOACs that presented with a GI bleed were more likely to present with a GI malignancy. As such, it is reasonable to screen patients with a fecal occult blood test within the first month after initiating TSOAC treatment and then annually.
Acute Coronary Syndrome
A higher rate of myocardial infarction was observed with dabigatran 150 mg versus warfarin (0.74% vs 0.53% per year; P = 0.048) in the RE-LY study [16]. Whether the increase in myocardial infarction was due to dabigatran as a causative agent or warfarin’s ability to reduce the risk of myocardial infarction to a larger extent compared with dabigatran is unknown. Nonetheless, it may be prudent to use an alternative therapy in patients with a history of acute coronary syndrome.
Valvular Heart Disease
The risk of stroke and systemic embolism is higher in patients with valvular heart disease [34]. Patients with moderate to severe mitral stenosis or mechanical prosthetic heart valves were excluded from the DOAC landmark studies. Dabigatran was evaluated for prevention of stroke and systemic embolism in patients with valvular heart disease in the RE-ALIGN study [35,36]. Patients were randomized to warfarin titrated to a target INR of 2 to 3 or 2.5 to 3.5 on the basis of thromboembolic risk or dabigatran 150 mg, 220 mg, or 300 mg twice daily adjusted to a targeted trough of ≥ 50 ng/mL. The trial was terminated early due to a worse primary outcome (composite of stroke, systemic embolism, myocardial infarction, and death) with dabigatran versus warfarin (HR, 3.37, 95% CI, 0.76–14.95; P = 0.11). In addition, bleeding rates (any bleeding) was significantly greater with dabigatran (27%) versus warfarin (12%) (P = 0.01). Based on these data and the lack of data with the other TSOACs, warfarin remains the standard of care for valvular heart disease [37]. In patients with a previous bioprosthetic valve with AF, patients with mitral insufficiency, or aortic stensosis, TSOACs may be considered [37].
Landmark Study Efficacy Endpoints
The primary endpoint in each of the landmark studies was a composite of stroke (ischemic or hemorrhagic) and systemic embolism. For the primary endpoint only dabigatran 150 mg twice daily and apixaban 5 mg twice daily were found to be superior to warfarin for the prevention of stroke or systemic embolism in nonvalvular AF (HR, 0.66; 95% CI, 0.53–0.82; P < 0.001 and HR, 0.66; 95% CI, 0.66–0.95; P = 0.01, respectively). Both edoxaban (60 mg and 30 mg daily) and rivaroxaban were noninferior to warfarin for the primary endpoint. In terms of ischemic stroke, only dabigatran 150 mg twice daily was superior to warfarin for the reduction in ischemic stroke in patients with nonvalvular AF (HR, 0.76; 95% CI, 0.60–0.98; P = 0.03) [19]. All of the DOACs demonstrated a reduction in hemorrhagic stroke.
TSOAC Use in Elderly Patitents
Pharmacokinetic Evaluations
Several pharmacokinetic studies have evaluated the influence of age on DOAC disposition. In a study evaluating the influence of age on apixaban disposition, the area under the concentration-time curve to infinity was 32% higher in the elderly (aged 65 years or older) compared to the younger subjects (< age 40 years) [38]. These data provide the rationale for dosage adjustment in individuals aged 80 years or older with either low body mass (weight less than or equal to 60 kg) or renal impairment (serum creatinine 1.5 mg/dL or higher). In a pharmacokinetic study evaluating dabigatran in patients > 65 years of age, the time to steady state ranged from 2 to 3 days, correlating to a half-life of 12 to 14 hours, and peak concentrations (256 ng/mL females, 255 ng/mL males) were reached after a median of 3 hours (range, 2.0–4.0 hours) [39]. These data suggest a 1.7- to twofold increase in bioavailability. The area under the curve of rivaroxaban was significantly higher in subjects > 75 years versus subjects 18-45 years, while total and renal clearance were decreased [40].However, the time to maximum factor Xa inhibition and Cmax were not influenced by age.
Clinical Evaluations
Dabigatran
In a post-hoc analysis of the RE-LY trial, Eikelboom and colleagues found that patients 75 years of age and older treated with dabigatran 150 mg twice daily had a greater incidence of GI bleeding irrespective of renal function compared with those on warfarin (1.85%/year vs. 1.25%/year; P < 0.001) [29]. A higher risk in major bleeding also was seen in dabigatran patients (5.10% versus 4.37%; P = 0.07). As a result, the 2012 Beer’s Criteria lists dabigatran as a potentially inappropriate medication. An analysis was conducted of 134,414 elderly Medicare patients (defined as age > 65 years) with 37,587 person-years of follow-up who were treated with dabigatran or warfarin [44]. Approximately 60% of patients included in the analysis were over age 75 years. Dabigatran was associated with a significant reduction in ischemic stroke: HR 0.80 (CI 0.67–0.96); intracranial hemorrhage: HR 0.34 (CI 0.26–0.46); and death: HR 0.86 (CI 0.77–0.96) when compared with warfarin. As in the Eikelboom study, major gastrointestinal bleeding was significantly increased with dabigatran (HR, 1.28 [95% CI, 1.14–1.44]).
Rivaroxaban
For rivaroxaban, a subgroup analysis of patients ≥ 75 years in the ROCKET-AF trial reported similar rates of major bleeding (HR, 1.11; 95% CI, 0.92–1.34) with rivaroxaban compared with warfarin [31]. Clinically relevant non-major bleeding was significantly higher for patients aged ≥ 75 years compared with patients aged < 75 years (P = 0.01).
Apixaban
Halvorsen and colleagues found that age did not influence the benefits of apixaban in terms of efficacy and safety [47]. In the cohort of patients aged 75 years or older, major bleeding was significantly reduced compared to warfarin (HR, 0.64; 95% CI, 0.52–0.79). The safety benefits persisted even in the setting of age greater than 75 years and renal impairment. A significant reduction in major bleeding (HR, 0.35; 95% CI, 0.14–0.86) was seen in elderly patients with a CrCl; ≤ 30 mL/min (n = 221) treated with apixaban versus warfarin. Similarly, in elderly patients with a CrCl 30 to 50 mL/min (n = 1898) a significant reduction in major bleeding was reported (HR, 0.53; 95% CI, 0.37–0.76). These data are consistent with a meta-regression analysis that found a linear relationship between the relative risk of major bleeding and the magnitude of renal excretion for the DOACs (r2=0.66, P = 0.03) [48]. In this analysis, apixaban had the most favorable outcomes in terms of major bleeding compared to the other DOACs and also has the least dependence on renal function for clearance. In a pooled analysis of data from landmark trials, Ng and colleagues found that in elderly patients (defined as age > 75 years) with nonvalvular AF, only apixaban was associated with a significant reduction in both stroke and major hemorrhage (Figure 1) [49,50].
Edoxaban
Kato and colleagues performed a subgroup analysis of patients aged 75 years or older enrolled in the ENGAGE TIMI 48 study [50]. Currently the results are only published in abstract form. Regardless of treatment, the risk of major bleeding and stroke significantly increased with age (P < 0.001). An absolute risk reduction in major bleeding was reported with both 60 mg and 30 mg of edoxaban versus warfarin (4.0%/year and 2.2%/year versus 4.8%/year, respectively; no P value provided).
Therapeuti Drug Monitoring
Collectively, the data on assessment of the anticoagulant activity of DOACs using coagulation assays is evolving. These tests include but are not limited to prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin clotting time (TT), dilute TT, activated clotting time (ACT), anti factor Xa, and ecarin clotting time (ECT) assays. Although routine monitoring is not desirable, the ability to assess degree of anticoagulation in select patient populations may prove beneficial. Future studies are essential to confirm whether assessing DOAC activity using coagulation assays in vulnerable populations such as the elderly improves clinical outcomes. Several reviews on this subject matter have been published [51–55]. The reader is encouraged to review these data as there are significant limitations to currently available assays and incorrect interpretation may lead to suboptimal treatment decisions.
Renal and Hepatic Dysfunction
Depending on the specific agents, DOACs renal clearance varies from 27% to 80% [56–59]. Clinical trials often use the Cockcroft-Gault formula (CG) based on actual body weight to estimate renal function. Landmark trials evaluating the DOACs differed in their strategy for estimation of renal function using CG. For example, RE-LY and ROCKET-AF used actual body weight for the estimation of renal function, while ARISTOTLE did not specify which body weight to use. Estimation of renal function or glomerular filtration rate (GFR) by CG is frequently in discordance with actual renal function in the elderly [60]. MDRD (modification of diet in renal disease) and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) are also common estimations that provide an estimate of GFR. In a cross-sectional study, comparing the CG, MDRD, and EPI formulas in a clinical setting, data from potential kidney donors and adult patients who underwent a GFR measurement revealed that MDRD has the smallest mean bias. The influence of age was the absolute bias for estimation of renal function for all formulas. CG is additionally influenced by body weight and body mass index. When compared to CG, MDRD actually reported more accurate predictor of GFR in adults < 70 years old [61]. However, package inserts recommend dose adjustments based on estimation of CrCl using CG formula. This poses a problem in adjusting DOAC doses in elderly patients who are subject to overestimation of renal function with this antiquated equation. Among elderly patients with renal impairment, discordance between estimated and actual renal function was higher for dabigatran and rivaroxaban than for apixaban dosages [61].
Renal excretion of unchanged dabigatran is the predominant pathway for elimination (~80%) [58]. The FDA-approved dosing strategy in the US for dabigatran is 150 mg twice daily in patients with a CrCl ≥ 30 mL/min, 75 mg twice daily in patients with severe renal impairment (CrCl 15–30 mL/min), and is contraindicated in patients with a CrCl < 15 mL/min [58]. By comparison, the Canadian and the European Medicines Agency have listed patients with a CrCl < 30 mL/min (severe renal impairment) as a contraindication for use. The US-approved dosage for severe renal impairment was derived during the approval phase of dabigatran using a simulation pharmacokinetic model [62,63]. The dosage was estimated by pharmacokinetic simulation to provide similar Cmax and Cmin concentrations compared to the 150 mg twice-daily dosage in moderate renal impairment. Compared to patients with CrCl ≥ 80 mL/min, there was a 1.29- and a 1.47-fold increase in dabigatran trough plasma concentration in the CrCl 50–80 mL/min patients and the CrCl 30–50 mL/min patients, respectively. There have been many postmarketing reports of hemorrhage with dabigatran [36,84,85]. Although reporting bias is likely due to the novelty of the agent, clinicians may take key clinical pearls away from these reports. Patients often had risk factors, including low body weight, renal impairment, and polypharmacy with interacting drugs (eg, amiodarone). These risk factors are also important with the other DOACs.
A subgroup analysis of ROCKET-AF evaluating rivaroxaban 15 mg daily in patients with a CrCl of 30–49 mL/min did not identify any differences in endpoints with the exception of fatal bleeding, which occurred less often with rivaroxaban (0.28%/yr vs. 0.74%/yr; P = 0.047) [64].
Monitoring of renal function is essential to mitigate the risk of drug accumulation. Clinicians should consider obtaining a baseline renal assessment with annual reassessments in patients with normal (CrCl ≥ 80 mL/min) or mild (CrCl 50–79 mL/min) renal impairment, and 2 to 3 times per year in patients with moderate (CrCl 30–49 mL/min) renal impairment [65]. A summary of renal dose adjustments for DOAC therapy may be found in Table 5 [56–59].
In addition to renal function, hepatic impairment can also affect the metabolism of anticoagulants. Severe hepatic impairment can lead to prolonged PT. Therefore, patients who have liver dysfunction and are treated with anticoagulation have increased risk of hemorrhagic events. Large pivotal trials on the key indications of dabigatran, apixaban, and rivaroxaban excluded patients with significant signs of hepatic impairment. Table 5 provides dosing recommendations for the different DOACs in the setting of hepatic impairment [56–59].
Polypharmacy And The Potential For Adverse Consequences
Costs And Cost-Effectiveness of DOACS
With the high burden of AF and the aging population, analysis of cost and value is an important consideration [76]. There are limited publications comparing the cost-effectiveness between the anticoagulation options. However, numerous cost-effectiveness studies have evaluated the individual DOACs [71–79]. Overall, the studies suggest that the DOACs are a cost-effective alternative to warfarin in the general and elderly populations. One analysis reported that dabigatran may not be cost-effective in patients with a low CHADS2 score (≤ 2) [71].
Harrington et al [80] compared the cost-effectiveness of dabigatran, rivaroxaban, and apixaban versus warfarin. This cost-effectiveness study used published clinical trial data to build a decision model, and results indicated that for patients ≥ 70 years of age with an increased risk for stroke, normal renal function, and no previous contraindications to anticoagulant therapy, apixaban 5 mg, dabigatran 150 mg, and rivaroxaban 20 mg were cost-effective substitutes for warfarin for the prevention of stroke in nonvalvular AF [80]. Apixaban was the preferred anticoagulant for their hypothetical cohort of 70-year-old patients with nonvalvular AF, as it was most likely to be the cost-effective treatment option at all willing-to-pay thresholds > $40,000 per quality-adjusted life-year gained [76,81].
Prescription costs may vary depending on payor and level of insurance. If a patient does not have prescription insurance, the annual price of generic warfarin is roughly $200 to $360, depending on dosage. Approximate annual costs for the DOACs are greater than 20 times the cost of warfarin (apixaban $4500, dabigatran $4500, and rivaroxaban $4800) [82]. However, most patients on these medications are over 65 years old and have prescription coverage through Medicare Part D. Of note, patients may have more of a burden if or when they reach the “donut hole” coverage gap. Currently, once patients spend $2960 (for 2015) and $3310 (for 2016) on covered drugs they will fall into the donut hole unless they qualify for additional assistance. At this point Medicare Part D will reimburse 45% of the cost of the newer anticoagulants since generics are currently unavailable. As a result, individual affordability may become an issue. Further complicating the scenario is the inability to apply coupon and rebate cards in the setting of government-funded prescription coverage. Clinicians should discuss these issues with their patients to help select the most valuable therapy.
Conclusions And Recommendations
Corresponding author: Luigi Brunetti, PharmD, MPH, Rutgers University, 160 Frelinghuysen Rd, Piscataway, NJ 08854, [email protected].
1. Fanikos J, Stapinski C, Koo S, et al. Medication errors associated with anticoagulant therapy in the hospital. Am J Cardiol 2004;94:532–5.
2. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med2011;365:2002–12.
3. Institute for Safe Medication Practices. QuarterWatch. 9 January 2013. Available at http://www.ismp.org/quarterwatch/pdfs/2012Q2.pdf.
4. Hajjar ER, Hanlon JT, Artz MB, Let al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother 2003;1:82–9.
5. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107–16.
6. Singh S. Defining ‘elderly’ in clinical practice guidelines for pharmacotherapy. Pharm Pract 2014;12:489.
7. Singh S, Bajorek B. Pharmacotherapy in the aging patient: The impact of age per se (a review). Ageing Res Rev 2015 Jul 28. pii: S1568-1637(15)30008-8.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA 2001;285:2370–5.
9. Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe Chest 2012;142:1489–98.
10. Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association Europace 2012;14:1385–413.
11. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17–40.
12. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden 2010 study. Circulation 2014;129:837-47.
13. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25.
14. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51.
15. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91.
16. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92.
17. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED Bleeding Risk–Prediction Scores in Patients With Atrial Fibrillation Undergoing Anticoagulation: The AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol 2012;60:861–7.
18. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
19. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138:1093–100.
20. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507.
21. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173–80.
22. Roldán V, Marín F, Fernández H, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a "real-world" population with atrial fibrillation receiving anticoagulant therapy. Chest 2013;43:179–84.
23. Robert-Ebadi H, Le Gal G, Righini M. Use of anticoagulants in elderly patients: practical recommendations. Clin Interv Aging 2009;4:165–77.
24. Barcellona D, Contu P, Sorano GG, et al. The management of oral anticoagulant therapy: the patient's point of view. Thromb Haemost 2000;83:49–53.
25. Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991;151:1944–9.
26. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22.
27. Giugliano RP, Ruff CT, Braunwald E, Murphy SA. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104.
28. Barco S, Cheung YW, Eikelboom JW, Coppens M. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol 2013;26:215–24
29. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–72.
30. Coppens M, Eikelboom JW, Ezekowitz M, et al. Dabigatran versus warfarin in very elderly patients with atrial fibrillation: results from the RE-LY trial. Abstract. Circulation 2012;126:A15l537.
31. Halperin JL, Wojdyla D, Piccini JP, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the ROCKET-AF trial. Abstract. Stroke 2012;43:A148.
32. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62
33. Clemens A, Strack A, Noack H, et al. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014;46:672–8.
34. Petty GW, Khandheria BK, Whisnant JP, et al. Predictors of cerebrovascular events and death among patients with valvular heart disease: A population-based study. Stroke 2000;31:2628–35.
35. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14.
36. Schomburg JL, Medina EM, Lahti MT, Bianco RW. Dabigatran versus warfarin after mechanical mitral valve replacement in the swine model. J Invest Surg 2012;25:150–5.
37. Douketis J, Bell AD, Eikelboom J, Liew A. Approach to the new oral anticoagulants in family practice: part 2: addressing frequently asked questions. Can Fam Physician 2014;60:997–1001.
38. Frost CE, Nepal S, Barrett YC, LaCreta F. Effects of age and gender on the singledose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban. Abstract. J Thromb Haemost 2009;7(Suppl 2):PP-MO-407..
39. Stangier J, Stahle H, Rathgen K et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47–59.
40. Kubitza D, Becka M, Mueck W. The effect of extreme age, and gender on the pharmacology and tolerability of rivaroxaban, an oral direct factor Xa inhibitor. Blood 2006;108: Abstract 905.
41. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J 2013;34:489–98.
42. Evans A, Kalra L. Are the results of randomized controlled trials on anticoagulation in patients with atrial fibrillation generalizable to clinical practice? Arch Intern Med 2001;161:1443–7.
43. Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012;366:864–6.
44. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64.
45. Avgil-Tsadok M, Jackevicius CA, Essebag V, et al. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost 2015;115(1).
46. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012;172:397–402.
47. Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–72.
48. Lega JC, Bertoletti L, Gremillet C, et al. Consistency of safety profile of new oral anticoagulants in patients with renal failure. J Thromb Haemost 2014;12:337–43.
49. Ng KH, Hart RG, Eikelboom JW. Anticoagulation in patients aged ≥ 75 years with atrial fibrillation: role of novel oral anticoagulants. Cardiol Ther 2013;2:135–49.
50. Kato ET, Guigliano RP, Ruff CT, et al. Efficacy and safety of edoxaban for the management of elderly patients with atrial fibrillation: Engage-AF TIMI 48. Circulation 2014;130:A16612.
51. Tripodi A. The laboratory and the new oral anticoagulants. Clin Chem 2013;59:353–62.
52. Tripodi A, Di Iorio G, Lippi G, et al. Position paper on laboratory testing for patients taking new oral anticoagulants. Consensus document of FCSA, SIMeL, SIBioC and CISMEL. Clin Chem Lab Med 2012;50:2137-40.
53. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51.
54. Chin PK, Wright DF, Patterson DM, et al. A proposal for dose-adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol 2014;78:599–609.
55. Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012;69:1473–84.
56. Xarelto [package insert]. Titusville, NJ. Janssen Pharmaceuticals. September 2014.
57. Eliquis [package insert]. Princeton, NJ: Bristol-Meyers Squibb. June 2015.
58. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals. October 2010.
59. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo. September 2015.
60. Michels WM, Grootendorst DC, Verduijn M, et al. Performance of the Cockcroft-Gault, MDRD, and new CKDEPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5: 1003–9.
61. Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs 2012;72:1739–53.
62. Hariharan S, Madabushi R. Clinical pharmacology basis of deriving dosing recommendations for dabigatran in patients with severe renal impairment. J Clin Pharmacol 2012;52:119S–25S.
63. Lehr T, Haertter S, Liesenfeld KH, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. J Clin Pharmacol 2012;52:1373–8.
64. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387–94.
65. Pengo V, Crippa L, Falanga A et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost 2011;106:868–76.
66. Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999;14:141–52.
67. Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867–78.
68. Skov J, Bladbjerg EM, Sidelmann J, et al. Plenty of pills: polypharmacy prevails in patients of a Danish anticoagulant clinic. Eur J Clin Pharmacol 2011;67:1169–74.
69. Ukena C, Bohm M, Schirmer SH. Hot topics in cardiology: data from IABP-SHOCK II, TRILOGY-ACS, WOEST, ALTIDUDE, FAME II and more. Clin Res Cardiol 2012;101):861–74.
70. Dewilde, Willem JM, Oirbans T, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet;381:1107–15.
71. Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:
2562–70.
72. Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908–19.
73. Adcock AK, Lee-Iannotti JK, Aguilar MI, et al. Is dabigatran cost effective compared with warfarin for stroke prevention in atrial fibrillation?: a critically appraised topic. Neurologist 2012;18:102–7.
74. Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 2012;43:881–3.
75. Langkilde LK, Bergholdt AM, Overgaard M. Cost-effectiveness of dabigatran etexilate for stroke prevention in non-valvular atrial fibrillation. J Med Econ 2012;15:695-703.
76. Kansal AR, Sorensen SV, Gani R, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012; 98:573–8.
77. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1–11.
78. Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343:d6333.
79. Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation-cost analysis. Age Ageing 2012;41:681–4.
80. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676–81.
81. Amin A, Lingohr-Smith M, Bruno A, et al. Economic evaluations of medical cost differences: use of targeted-specific oral anticoagulants vs. warfarin among patients with nonvalvular atrial fibrillation and venous thromboembolism in the US. J Hematol Thrombo Dis 2015;3:209.
82. Lexicomp, Lexi-Drugs. Hudson, OH: Lexi-Comp.
83. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Apixaban NDA 202155/S-002 approval letter. Jan 30 2014. Available at http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/202155Orig1s002ltr.pdf
84. Hinojar R, Jimenez-Natcher JJ, Fernandez-Golfin C, Zamorano JL. New oral anticoagulants: a practical guide for physicians. Eur Heart J Cardiovasc Pharmacother 2015;1:134-45.
85. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patiets with atrial fibrillation. N Engl J Med 2011;364;806–17.
1. Fanikos J, Stapinski C, Koo S, et al. Medication errors associated with anticoagulant therapy in the hospital. Am J Cardiol 2004;94:532–5.
2. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med2011;365:2002–12.
3. Institute for Safe Medication Practices. QuarterWatch. 9 January 2013. Available at http://www.ismp.org/quarterwatch/pdfs/2012Q2.pdf.
4. Hajjar ER, Hanlon JT, Artz MB, Let al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother 2003;1:82–9.
5. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107–16.
6. Singh S. Defining ‘elderly’ in clinical practice guidelines for pharmacotherapy. Pharm Pract 2014;12:489.
7. Singh S, Bajorek B. Pharmacotherapy in the aging patient: The impact of age per se (a review). Ageing Res Rev 2015 Jul 28. pii: S1568-1637(15)30008-8.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA 2001;285:2370–5.
9. Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe Chest 2012;142:1489–98.
10. Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation-developed with the special contribution of the European Heart Rhythm Association Europace 2012;14:1385–413.
11. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17–40.
12. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden 2010 study. Circulation 2014;129:837-47.
13. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25.
14. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51.
15. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91.
16. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92.
17. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED Bleeding Risk–Prediction Scores in Patients With Atrial Fibrillation Undergoing Anticoagulation: The AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol 2012;60:861–7.
18. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
19. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138:1093–100.
20. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507.
21. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173–80.
22. Roldán V, Marín F, Fernández H, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a "real-world" population with atrial fibrillation receiving anticoagulant therapy. Chest 2013;43:179–84.
23. Robert-Ebadi H, Le Gal G, Righini M. Use of anticoagulants in elderly patients: practical recommendations. Clin Interv Aging 2009;4:165–77.
24. Barcellona D, Contu P, Sorano GG, et al. The management of oral anticoagulant therapy: the patient's point of view. Thromb Haemost 2000;83:49–53.
25. Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991;151:1944–9.
26. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22.
27. Giugliano RP, Ruff CT, Braunwald E, Murphy SA. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104.
28. Barco S, Cheung YW, Eikelboom JW, Coppens M. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol 2013;26:215–24
29. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–72.
30. Coppens M, Eikelboom JW, Ezekowitz M, et al. Dabigatran versus warfarin in very elderly patients with atrial fibrillation: results from the RE-LY trial. Abstract. Circulation 2012;126:A15l537.
31. Halperin JL, Wojdyla D, Piccini JP, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the ROCKET-AF trial. Abstract. Stroke 2012;43:A148.
32. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62
33. Clemens A, Strack A, Noack H, et al. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014;46:672–8.
34. Petty GW, Khandheria BK, Whisnant JP, et al. Predictors of cerebrovascular events and death among patients with valvular heart disease: A population-based study. Stroke 2000;31:2628–35.
35. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14.
36. Schomburg JL, Medina EM, Lahti MT, Bianco RW. Dabigatran versus warfarin after mechanical mitral valve replacement in the swine model. J Invest Surg 2012;25:150–5.
37. Douketis J, Bell AD, Eikelboom J, Liew A. Approach to the new oral anticoagulants in family practice: part 2: addressing frequently asked questions. Can Fam Physician 2014;60:997–1001.
38. Frost CE, Nepal S, Barrett YC, LaCreta F. Effects of age and gender on the singledose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban. Abstract. J Thromb Haemost 2009;7(Suppl 2):PP-MO-407..
39. Stangier J, Stahle H, Rathgen K et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47–59.
40. Kubitza D, Becka M, Mueck W. The effect of extreme age, and gender on the pharmacology and tolerability of rivaroxaban, an oral direct factor Xa inhibitor. Blood 2006;108: Abstract 905.
41. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J 2013;34:489–98.
42. Evans A, Kalra L. Are the results of randomized controlled trials on anticoagulation in patients with atrial fibrillation generalizable to clinical practice? Arch Intern Med 2001;161:1443–7.
43. Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012;366:864–6.
44. Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64.
45. Avgil-Tsadok M, Jackevicius CA, Essebag V, et al. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost 2015;115(1).
46. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012;172:397–402.
47. Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–72.
48. Lega JC, Bertoletti L, Gremillet C, et al. Consistency of safety profile of new oral anticoagulants in patients with renal failure. J Thromb Haemost 2014;12:337–43.
49. Ng KH, Hart RG, Eikelboom JW. Anticoagulation in patients aged ≥ 75 years with atrial fibrillation: role of novel oral anticoagulants. Cardiol Ther 2013;2:135–49.
50. Kato ET, Guigliano RP, Ruff CT, et al. Efficacy and safety of edoxaban for the management of elderly patients with atrial fibrillation: Engage-AF TIMI 48. Circulation 2014;130:A16612.
51. Tripodi A. The laboratory and the new oral anticoagulants. Clin Chem 2013;59:353–62.
52. Tripodi A, Di Iorio G, Lippi G, et al. Position paper on laboratory testing for patients taking new oral anticoagulants. Consensus document of FCSA, SIMeL, SIBioC and CISMEL. Clin Chem Lab Med 2012;50:2137-40.
53. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51.
54. Chin PK, Wright DF, Patterson DM, et al. A proposal for dose-adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol 2014;78:599–609.
55. Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012;69:1473–84.
56. Xarelto [package insert]. Titusville, NJ. Janssen Pharmaceuticals. September 2014.
57. Eliquis [package insert]. Princeton, NJ: Bristol-Meyers Squibb. June 2015.
58. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals. October 2010.
59. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo. September 2015.
60. Michels WM, Grootendorst DC, Verduijn M, et al. Performance of the Cockcroft-Gault, MDRD, and new CKDEPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5: 1003–9.
61. Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs 2012;72:1739–53.
62. Hariharan S, Madabushi R. Clinical pharmacology basis of deriving dosing recommendations for dabigatran in patients with severe renal impairment. J Clin Pharmacol 2012;52:119S–25S.
63. Lehr T, Haertter S, Liesenfeld KH, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. J Clin Pharmacol 2012;52:1373–8.
64. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387–94.
65. Pengo V, Crippa L, Falanga A et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost 2011;106:868–76.
66. Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999;14:141–52.
67. Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867–78.
68. Skov J, Bladbjerg EM, Sidelmann J, et al. Plenty of pills: polypharmacy prevails in patients of a Danish anticoagulant clinic. Eur J Clin Pharmacol 2011;67:1169–74.
69. Ukena C, Bohm M, Schirmer SH. Hot topics in cardiology: data from IABP-SHOCK II, TRILOGY-ACS, WOEST, ALTIDUDE, FAME II and more. Clin Res Cardiol 2012;101):861–74.
70. Dewilde, Willem JM, Oirbans T, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet;381:1107–15.
71. Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:
2562–70.
72. Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908–19.
73. Adcock AK, Lee-Iannotti JK, Aguilar MI, et al. Is dabigatran cost effective compared with warfarin for stroke prevention in atrial fibrillation?: a critically appraised topic. Neurologist 2012;18:102–7.
74. Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 2012;43:881–3.
75. Langkilde LK, Bergholdt AM, Overgaard M. Cost-effectiveness of dabigatran etexilate for stroke prevention in non-valvular atrial fibrillation. J Med Econ 2012;15:695-703.
76. Kansal AR, Sorensen SV, Gani R, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012; 98:573–8.
77. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1–11.
78. Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343:d6333.
79. Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation-cost analysis. Age Ageing 2012;41:681–4.
80. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676–81.
81. Amin A, Lingohr-Smith M, Bruno A, et al. Economic evaluations of medical cost differences: use of targeted-specific oral anticoagulants vs. warfarin among patients with nonvalvular atrial fibrillation and venous thromboembolism in the US. J Hematol Thrombo Dis 2015;3:209.
82. Lexicomp, Lexi-Drugs. Hudson, OH: Lexi-Comp.
83. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Apixaban NDA 202155/S-002 approval letter. Jan 30 2014. Available at http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/202155Orig1s002ltr.pdf
84. Hinojar R, Jimenez-Natcher JJ, Fernandez-Golfin C, Zamorano JL. New oral anticoagulants: a practical guide for physicians. Eur Heart J Cardiovasc Pharmacother 2015;1:134-45.
85. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patiets with atrial fibrillation. N Engl J Med 2011;364;806–17.
The new oral anticoagulants: Reasonable alternatives to warfarin
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
For decades, vitamin K antagonists such as warfarin, acenocoumarol, phenindione, and phenprocoumon have been the only available oral anticoagulants. These drugs have similar pharmacologic profiles and share significant drawbacks in clinical use: a narrow therapeutic window, food and drug interactions, and the need for repeated blood testing to ensure the desired international normalized ratio.
Such problems have fostered research in the field of coagulation, and new oral agents that selectively target coagulation factors have become available. At least three such products are already available in most countries: dabigatran (a thrombin or factor IIa inhibitor) and rivaroxaban and apixaban (factor Xa inhibitors).1,2 Other factor Xa inhibitors, including edoxaban3 (available in the United States and Japan) and betrixaban,4 may also soon become available worldwide.
The new oral anticoagulants are more effective than vitamin K antagonists in preventing several thromboembolic conditions, have fewer drug interactions, and likely have fewer side effects.5 Indications for these new agents are expected to expand as new clinical trial results become available.6,7
This review summarizes the clinically relevant characteristics of the new oral anticoagulants (Table 1) and provides guidance on their usage (Table 2).
THROMBIN (FACTOR IIa) INHIBITORS
Dabigatran
Dabigatran etexilate is a prodrug that is rapidly and completely converted by esterases in the plasma and liver into its active metabolite, dabigatran. It competitively and reversibly binds to freely circulating and clot-bound thrombin, thereby blocking thrombin’s procoagulant properties (Figure 1).
Clinical trials have shown dabigatran to be similar to warfarin and enoxaparin in efficacy and safety in preventing and treating thromboembolic disease.8–10
Indications. Dabigatran is approved by the US Food and Drug Administration (FDA) for:
- Preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days
- Preventing recurrence of deep vein thrombosis and pulmonary embolism in patients who have previously been treated with other medications.
Precautions. Dabigatran should not be used, or should be used only in a reduced dosage, in patients with renal failure. It can be used in patients with moderate liver impairment but should be avoided in patients with advanced liver disease (cirrhosis), especially if they have coagulopathy. Its use in pregnant and nursing women is not recommended.
Adverse effects. Bleeding, including gastrointestinal and intracranial hemorrhage, is the most important adverse effect,11 but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,12 Dyspepsia is common and may be severe enough to require stopping treatment.13 Other possible effects are pain or burning in the throat, skin rash, and syncope. The risk of acute coronary syndrome is slightly increased but is outweighed by the benefit of ischemic stroke prevention.14,15
Drug interactions. Normally, permeability (P)-glycoprotein intestinal transporter extrudes substrate drugs back into the gut lumen after initial absorption, thereby interfering with drug bioavailability. Strong P-glycoprotein inhibitors (eg, ketoconazole, cyclosporine, tacrolimus, dronedarone, amiodarone, verapamil, clarithromycin) increase the plasma concentration of dabigatran. Despite that, giving these drugs with dabigatran is generally safe except in patients with renal failure (and especially with ketoconazole and dronedarone). To reduce interaction with verapamil, dabigatran should be taken at least 2 hours before this drug.
Potent P-glycoprotein transporter inducers such as rifampicin, carbamazepine, and phenytoin reduce the plasma concentration of dabigatran, and concomitant use of dabigatran with these drugs should be avoided.1
Another selective thrombin inhibitor
Ximelagatran was extensively investigated and approved in several countries in 2006. However, it was withdrawn after reports of severe hepatotoxicity.16 No other selective thrombin inhibitors are currently in an advanced stage of development.
FACTOR Xa INHIBITORS
Factor Xa is an ideal target for anticoagulants because of its important role in thrombin formation (Figure 1). Selective or direct factor Xa inhibitors significantly reduce the number of strokes and systemic embolic events compared with warfarin in patients with atrial fibrillation. They also may cause fewer major bleeding events than warfarin, although evidence supporting this is less robust.17 These agents have shown an advantage over enoxaparin for thromboprophylaxis after elective hip or knee replacement surgery and after hip fracture surgery without increasing the rate of bleeding events.18
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor. It reversibly binds to factor Xa with high specificity and inhibits free and clot-bound factor Xa as well as factor Xa in the prothrombinase complex (which catalyzes the conversion of prothrombin to thrombin).19
Indications. Clinical trials have shown rivaroxaban to have suitable efficacy and safety in several clinical situations.20–23 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation
- Preventing deep vein thrombosis after hip or knee replacement surgery
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrence of deep vein thrombosis and pulmonary embolism.
In addition, the European Medicines Agency has approved the use of rivaroxaban together with antiplatelet medications to prevent atherothrombotic events after an acute coronary syndrome with elevated cardiac biomarkers.
Precautions. Rivaroxaban should be taken with food to maximize its absorption. Like dabigatran, it should be avoided or used cautiously in patients with renal failure and liver disease, and it is not recommended for pregnant and nursing women.
Adverse effects. The most common adverse event is bleeding, although the incidence of major hemorrhage is similar to that with vitamin K antagonists and low-molecular-weight heparins.1 Other effects include osteoarticular pain, weakness, wound secretion, skin rash, pruritus, abdominal pain, and syncope.
Drug interactions. Inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes can alter the metabolism of rivaroxaban, making its levels too high. Rivaroxaban is not recommended for patients receiving systemic treatment with azole-antimycotics (eg, ketoconazole) or protease inhibitors to treat human immunodeficiency virus (HIV) infection (eg, ritonavir), as these drugs are strong inhibitors of both systems and may considerably increase plasma rivaroxaban concentrations.24 Interactions of rivaroxaban with most other inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes are considered clinically inconsequential, but caution is still recommended, especially in patients already at risk of bleeding (eg, those taking antiplatelet agents).25
Strong inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes (eg, rifampicin, phenytoin) can reduce plasma rivaroxaban concentrations and thus decrease its efficacy. Caution is needed if rivaroxaban is taken with these drugs.
Apixaban
Apixaban also selectively and reversibly inhibits free and clot-bound factor Xa, as well as factor Xa in the prothrombinase complex.
Indications. Apixaban has a suitable efficacy and safety profile, and in clinical trials fewer patients died while taking it than those taking warfarin.26–28 It is FDA-approved for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Prophylaxis of deep vein thrombosis and pulmonary embolism in patients who have undergone hip or knee replacement
- Treating deep vein thrombosis and pulmonary embolism
- Reducing the risk of recurrent deep vein thrombosis and pulmonary embolism after initial therapy.
Precautions. Apixaban can be used in most patients with renal failure, but at a lower dosage in some circumstances (Table 2). It can be used without dosage adjustment for patients with mild hepatic impairment but should be avoided in those with moderate or advanced liver failure. It is contraindicated in pregnant and nursing women.
Adverse effects. As with other anticoagulants, the most common adverse effect is bleeding, but the incidence is similar to that with vitamin K antagonists and low-molecular-weight heparins.1,26–28 Other adverse reactions, such as nausea, skin rash, and liver enzyme elevation, are uncommon.
Drug interactions are similar to those of rivaroxaban but are generally less intense. Concomitant use with strong dual inhibitors of the P-glycoprotein transporter or the cytochrome P450 enzymes, especially azole-antimycotics and HIV protease inhibitors, should be avoided, but if used, the apixaban dosage may be halved. Caution is also recommended if using apixaban with dual inducers of the P-glycoprotein transporter and the cytochrome P450 enzymes.29
Edoxaban
Edoxaban, another direct factor Xa inhibitor, has a rapid onset of action. It is taken orally once daily and has antithrombotic efficacy similar to other agents in this group.1,30
Indications. Edoxaban has been approved by the Japanese Pharmaceuticals and Medical Devices Agency and the FDA for:
- Reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
- Treating deep vein thrombosis and pulmonary embolism after 10 days of initial therapy with a parenteral anticoagulant.
Precautions. Edoxaban should not be used in patients with creatinine clearance above 95 mL/min because patients with this excellent level of renal function may clear the drug too well and therefore have a higher risk of ischemic stroke than those receiving warfarin.31
Adverse effects and drug interactions are similar to those of other factor Xa inhibitors.
Other factor Xa inhibitors
Betrixaban is similar to other factor Xa inhibitors but has some unique pharmacokinetic characteristics, including minimal metabolism through the cytochrome P450 system, limited renal excretion, and a long half-life. This profile may have the advantages of fewer drug interactions and greater flexibility for use in patients with poor renal function, as well as the convenience of once-daily dosing.4,32 The drug has not yet been approved for clinical use by the FDA or the European Medicines Agency.
Additional oral factor Xa inhibitors, including letaxaban, darexaban, and eribaxaban, are being developed with the aim of overcoming the limitations of available drugs in the group.33
COAGULATION MONITORING
Given their rapid onset of action, stable pharmacokinetic properties, and few significant drug interactions, the new oral anticoagulants do not generally require coagulation monitoring. However, these drugs may produce alterations in coagulation tests: thrombin inhibitors tend to prolong the activated partial thromboplastin time, and factor Xa inhibitors tend to prolong the prothrombin time. These alterations vary from laboratory to laboratory, depending on the reagents used.34,35
The new agents have also been reported to cause false-positive results on lupus anticoagulant assays and falsely elevated activated protein C ratio assays, misclassifying patients with the factor V Leiden mutation as normal.36,37
Anticoagulation from dabigatran therapy can be monitored with the ecarin clotting time test, which yields a dose-dependent prolongation of clotting time.38 Rivaroxaban, apixaban, and edoxaban can be monitored using modified chromogenic anti-Xa assays.25 These tests may help manage overdoses, bleeding events, and emergency perioperative situations, but their usefulness in clinical practice is limited at this time because they are not widely available and they are not validated for this use.
SWITCHING FROM VITAMIN K ANTAGONISTS TO THE NEW AGENTS
Important issues to consider when switching anticoagulant agents are the delayed onset of action after initiating treatment and the persistent anticoagulant effect after stopping it. In both cases, the international normalized ratio can be used to monitor the anticoagulant effect of the drugs. Renal failure should also be considered, as it can prolong the plasma half-life of the agents.1,39
MANAGING BLEEDING
Dabigatran is the only new anticoagulant with an antidote commercially available: idarucizumab can completely reverse the anticoagulant effect of dabigatran within minutes.
The other new oral anticoagulants lack antidotes, which can present a major problem if a patient has a major bleed or needs emergency surgery. Giving vitamin K is probably useless in this situation. In general, patients taking one of the new oral anticoagulants who present with bleeding should be treated with traditional measures—eg, oral activated charcoal to retard absorption of recently ingested drugs and cauterization and packing of localized bleeding sites. Dialysis may be useful for patients taking dabigatran40 but probably not the other drugs, because they are more highly protein-bound.
Other measures to consider include giving:
- Fresh frozen plasma, which may have some potential for reversing the action of thrombin inhibitors and factor Xa inhibitors but lacks data in humans41
- Activated prothrombin complex concentrate for reversing thrombin inhibitors
- Nonactivated prothrombin complex concentrates and factor Xa analogues for reversing anti-factor Xa agents42–44
- Recombinant factor VIIa, but serious adverse effects—disseminated intravascular coagulation and systemic thrombosis— limit its usefulness.45
More research is needed to assess the efficacy and safety of these measures.46,47
STOPPING THERAPY BEFORE SURGERY
How long to withhold a new oral anticoagulant before patients undergo surgery depends on the type and urgency of the procedure, the indication for anticoagulation, the patient’s renal function, and the drug used.
For procedures with a low risk of bleeding (eg, laparoscopy, colonoscopy), dabigatran should be stopped at least 48 hours before the procedure, and factor Xa inhibitors at least 24 hours before. More time should be allowed for patients with renal failure to clear the drug, according to creatinine clearance.
For procedures entailing a high bleeding risk (eg, major surgery, insertion of pacemaker or defibrillator, neurosurgery, spinal puncture), any new oral anticoagulant should be stopped at least 48 hours before the procedure, with a longer time needed for patients with renal failure.
If urgent surgery is needed and performed within a few hours after the last dose of a drug, bleeding complications should be anticipated.
Resuming anticoagulation therapy after surgery should also be individualized depending on the procedure, the indication for anticoagulation, and renal function. In most patients, if good hemostasis is achieved, the drug may be resumed 4 to 6 hours after surgery. Generally, the first dose should be reduced by 50%, after which the usual maintenance dose can be resumed.39
OTHER POSSIBLE USES
Cardioversion. Anticoagulation with dabigatran before and after cardioversion in patients with atrial fibrillation48 appears as effective and safe as anticoagulation with warfarin.49 There are insufficient data for the other new oral anticoagulants.
Heparin-induced thrombocytopenia. The new oral anticoagulants do not affect the interaction of platelets with platelet factor 4 or antibodies to the platelet factor 4-heparin complex, indicating that they may be an appropriate option for anticoagulation in patients with heparin-induced thrombocytopenia.50–53
Other conditions. The new oral anticoagulants have demonstrated efficacy in preventing or treating thromboembolic disease in patients with cancer54 and critical illnesses,55 and in treating acute coronary syndrome56–58 and other conditions.59 However, their role in these settings is not well established.60,61
SITUATIONS TO AVOID
Valvular heart disease. The new oral anticoagulants should not be prescribed for patients with a prosthetic heart valve or other significant valvular heart disease because of an increased risk of thrombotic complications with dabigatran and the lack of evidence of efficacy and safety of factor Xa inhibitors.62–64
Concurrent thrombolytic therapy along with any of the new oral anticoagulants poses a very high risk of bleeding. Some cases in which dabigatran was used successfully in this situation have been reported, but definitive recommendations are lacking.65
Elderly patients. The safety of the new oral anticoagulants in the elderly is of concern because of the high prevalence of renal failure and other comorbidities and the underrepresentation of this population in many clinical trials assessing these drugs. Data on interactions with foods or other drugs in this population are also scant.66
CHOOSING AN ORAL ANTICOAGULANT
New oral anticoagulants are now a viable alternative to vitamin K antagonists for preventing and treating thromboembolic disease.67,68
When oral anticoagulation is indicated, the choice of drug should be individualized. Cost is an important consideration: direct costs of the new drugs are substantially higher than those of vitamin K antagonists and heparin, but their cost-effectiveness may be comparable or superior to that of warfarin or enoxaparin when clinical efficacy and savings in avoiding coagulation tests are considered.18
Many experts estimate that the new oral anticoagulants are not remarkably superior to vitamin K antagonists, and thus patients whose coagulation is well controlled and stable on a traditional drug would probably not benefit much from changing.1,18
There is currently no conclusive evidence to determine which new oral anticoagulant drug is more effective and safe for long-term treatment, as head-to-head studies of the different medications have not yet been performed.17,69,70 However, there are factors to consider when choosing a drug:
- Rivaroxaban and edoxaban can be taken once daily and so may be better choices for patients who may have difficulties with compliance.
- Dabigatran should be avoided in patients with dyspepsia because of gastrointestinal adverse effects.13
- Dabigatran should be avoided in patients at risk of myocardial infarction because of a possible additional increase in risk.1,71
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc 2013; 88:495–511.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014; 34:9–17.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs 2013; 22:1465–1472.
- Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013; 52:69–82.
- Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther 2013; 11:1297–1299.
- Biondi-Zoccai G, Malavasi V, D’Ascenzo F, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair-wise and warfarin-controlled network meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth 2013; 5:40–54.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105:721–729.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Donaldson M, Norbeck AO. Adverse events in patients initiated on dabigatran etexilate therapy in a pharmacist-managed anticoagulation clinic. Pharm Pract (Granada) 2013; 11:90–95.
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013; 368:1272–1274.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol 2013; 11:246–252.
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012; 172:397–402.
- Artang R, Rome E, Nielsen JD, Vidaillet HJ. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol 2013; 112:1973–1979.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol 2010; 196:407–418.
- Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; 8:CD008980.
- Capranzano P, Miccichè E, D’Urso L, Privitera F, Tamburino C. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther 2013; 11:959-973.
- Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol 2012; 26:27-32.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Cohen AT, Spiro TE, Büller HR, et al; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013; 368:513–523.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; 76:455–466.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Agnelli G, Buller HR, Cohen A, et al; PLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368:699–708.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363:2487–2498.
- Keating GM. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs 2013; 73:825–843.
- Giugliano RP, Ruff CT, Braunwald E, et al; NGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093–2104.
- Traynor K. Edoxaban approved for embolism prevention. Am J Health Syst Pharm 2015; 72:258.
- Connolly SJ, Eikelboom J, Dorian P, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013; 34:1498–1505.
- Bondarenko M, Curti C, Montana M, Rathelot P, Vanelle P. Efficacy and toxicity of factor Xa inhibitors. J Pharm Pharm Sci 2013; 16:74–88.
- Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program 2012; 2012:460–465.
- Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111:240–248.
- Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 2012; 50:1601–1615.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost 2011; 105:385–386.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Akwaa F, Spyropoulos AC. Treatment of bleeding complications when using oral anticoagulants for prevention of strokes. Curr Treat Options Cardiovasc Med 2013; 15:288–298.
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol 2013; 26:191–202.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446–451.
- Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost 2014; 111:189–198.
- Holster IL, Hunfeld NG, Kuipers EJ, Kruip MJ, Tjwa ET. On the treatment of new oral anticoagulant-associated gastrointestinal hemorrhage. J Gastrointestin Liver Dis 2013; 22:229–231.
- Nitzki-George D, Wozniak I, Caprini JA. Current state of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother 2013; 47:841–855.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1935–1944.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 2011; 123:131–136.
- Warkentin TE. HIT: treatment easier, prevention harder. Blood 2012; 119:1099–1100.
- Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler 2013; 9:112–114.
- Walenga JM, Prechel M, Hoppensteadt D, et, al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013; 19:482–487.
- Bakchoul T, Greinacher A. Recent advances in the diagnosis and treatment of heparin-induced thrombocytopenia. Ther Adv Hematol 2012; 3:237–251.
- Den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2013; 26:163–169.
- Adriance SM, Murphy CV. Prophylaxis and treatment of venous thromboembolism in the critically ill. Int J Crit Illn Inj Sci 2013; 3:143–151.
- Mega JL, Braunwald E, Wiviott SD, et al; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9–19.
- Chatterjee S, Sharma A, Uchino K, Biondi-Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta-analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis 2013; 24:628–635.
- Liew A, Darvish-Kazem S, Douketis JD. Is there a role for the novel oral anticoagulants in patients with an acute coronary syndrome? A review of the clinical trials. Pol Arch Med Wewn 2013; 123:617–622.
- Säily VM, Pétas A, Joutsi-Korhonen L, Taari K, Lassila R, Rannikko AS. Dabigatran for thromboprophylaxis after robotic assisted laparoscopic prostatectomy: retrospective analysis of safety profile and effect on blood coagulation. Scand J Urol 2014; 48:153–159.
- Kearon C, Akl EA, Comerota AJ, et al; ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. J Am Heart Assoc 2013; 2:e000136.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol 2013; 69:1617–1633.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013; 34:2094–2106.
- Matute MC, Guillan M, Garcia-Caldentey J, et al. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011; 106:178–179.
- Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging 2013; 30:949–958.
- Mantha S. Target-specific oral anticoagulants in atrial fibrillation: results of phase III trials and comments on sub-analyses. J Thromb Thrombolysis 2013; 36:155–162.
- Prandoni P, Dalla Valle F, Piovella C, Tormene D, Pesavento R. New anticoagulants for the treatment of venous thromboembolism. Minerva Med 2013; 104:131–139.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Weitz JI. Anticoagulation therapy in 2015: where we are and where we are going. J Thromb Thrombolysis 2015; 39:264–272.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program 2012; 2012:536–540.
KEY POINTS
- The new oral anticoagulants have favorable pharmacologic properties and similar efficacy and safety as vitamin K antagonists.
- The new agents are indicated for preventing stroke and systemic embolism in patients with nonvalvular atrial fibrillation and preventing and treating deep vein thrombosis and pulmonary embolism (the indications regarding venous thromboembolism differ somewhat among agents).
- Except for dabigatran, lack of an antidote in case of bleeding or emergency surgery is a major drawback.
- Be cautious when using these drugs in patients with renal or liver disease and in those taking an inhibitor or inducer of the P-glycoprotein transporter or the cytochrome P450 enzymes.
Insulin pumps: Beyond basal-bolus
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
The advent of the insulin pump in the late 1970s was a step forward in diabetes treatment,1 and recent improvements make these devices easier to use in intensive insulin management. Today, more than 400,000 people in the United States are thought to be using an insulin pump.2
With a pump, patients can adjust the dosage and discreetly give themselves boluses by simply pushing a button instead of giving themselves multiple daily injections. Also, pump therapy can be tailored to correct for hepatic glucose production in a way that injections cannot.
This article reviews the clinical application of continuous subcutaneous insulin therapy—ie, the insulin pump—and provides recommendations for patient selection and management.
INDICATIONS FOR AN INSULIN PUMP
The American Association of Clinical Endocrinologists3 recommends considering an insulin pump for patients with type 1 or 2 diabetes mellitus who have a clear indication:
- Suboptimal control on basal-bolus injections, ie, not achieving glycemic goals despite maximal adherence to multiple daily injections
- Wide and erratic glycemic excursions
- Frequent severe hypoglycemia, or hypoglycemia unawareness
- A marked “dawn phenomenon” (spike in blood glucose level early in the morning)
- Pregnancy or planning for pregnancy
- Erratic lifestyle
- Personal preference.
WHO IS A GOOD CANDIDATE FOR AN INSULIN PUMP?
Good candidates for a pump are patients with type 1 diabetes (and some with type 2) who are well versed in taking multiple daily injections, are already checking their glucose four or more times daily, “counting carbs” (estimating or, preferably, measuring how much carbohydrate they are eating, and limiting their intake accordingly), and demonstrate the ability to adjust their dosing appropriately (Table 1).
A pump is not a shortcut to checking glucose less frequently or making fewer decisions. However, for those who actively manage their diabetes, it provides more real-time flexibility and some important safety features, as discussed below.
IS A PUMP BETTER THAN INJECTIONS?
Several studies have compared insulin pump therapy and multiple daily injections.4–7 While some found no difference in glucose control in terms of hemoglobin A1c or hypoglycemia, others showed improved glucose control with pumps in patients who had higher baseline hemoglobin A1c levels (> 10%).6 In this subgroup, a pump lowered hemoglobin A1c an additional estimated 0.65% compared with multiple daily injections.6 Fructosamine levels also improved in pump users.6
Using continuous glucose monitoring for 3 days in a study in children with type 1 diabetes, Schreiver et al8 found lower insulin requirements and less-severe glycemic excursions with a pump than with multiple daily injections.
A 2013 study9 of 57 patients ages 13 to 71 with type 2 diabetes who were struggling to control their blood sugar with multiple daily injections found that they achieved better control with less insulin using a pump.
A meta-analysis found pump therapy to be more effective than multiple daily injections for those who used it more than 1 year.10
ADVANTAGES AND DISADVANTAGES OF INSULIN PUMP THERAPY
Intensive glucose control reduces microvascular complications in type 1 diabetes.11–14 The advantages of using a pump include better adherence, more accurate dosing, greater lifestyle flexibility, control of the dawn phenomenon without induction of nocturnal hypoglycemia, and the ability to suspend or temporarily reduce basal insulin to compensate for increased physical activity.15
Disadvantages include the high degree of technical aptitude required, the need for high-level engagement, skin reactions to tape, a higher risk of diabetic ketoacidosis from pump malfunction, infusion-site problems such as “tunneling” of insulin (leakage of insulin along the outside of the cannula and back to the skin surface) and clogging of the infusion set, and a risk of inactivation of insulin from exposure to heat, which can lead to ketoacidosis in a few hours if not addressed promptly.15
IS IT COST-EFFECTIVE?
There is evidence that continuous subcutaneous insulin infusion is cost-effective, both in general and compared with multiple daily injections for children and adults with type 1 diabetes mellitus. Cohen and Shaw16 found that life expectancy and quality-adjusted life-years increased in pump users, although the price per life-year gained varied greatly depending on the model used.
And this therapy is expensive. Most pumps cost more than $6,000, and supplies cost about $300 per month. Most insurance providers cover this therapy for patients with type 1 diabetes (Table 2) but less often for those with type 2. Further, many insurance policies have copayments, and patients may find a 20% co-payment a significant financial burden. Physicians need to obtain preapproval for insulin pumps from the insurance company. Typically, prescriptions for supplies are written annually. Despite these significant costs, most patients with type 1 diabetes who use an insulin pump find that the benefits of improved control and greater independence justify the cost.
An annual review of currently available insulin pumps and other diabetes-related equipment is published in Diabetes Forecast.17
PATIENT PERSPECTIVE ON INSULIN PUMP USE
Many patients who use a pump find that it gives them greater flexibility to adjust to day-to-day changes in schedules and routines. For example, consuming an extra serving at a meal could necessitate another injection for a patient on multiple daily injections, but a pump user would need only to push a few buttons. With cell phone apps available to control some pumps, many people find that an insulin pump is more discreet and easier to manage than carrying around injection supplies. Further, the complex calculations of carbohydrate ratios and correction factors are easier and more accurate with a pump.
In an open-label randomized study,18 29 of 41 patients with type 1 diabetes said they preferred a pump to multiple daily injections.
Conversely, some people do not want a pump because it is attached all the time and identifies them to others as having an illness. Other patients do not trust a machine and want control in their own hands. (Actually, machines typically are much more reliable and less mistake-prone than humans.)
HOW DOES A PUMP WORK COMPARED WITH MULTIPLE DAILY INJECTIONS?
Patients taking multiple daily injections must use two types of insulin: a long-acting one that reaches a steady level in the blood without a peak and lasts from 12 to 24 hours, and a rapid-acting one taken with meals, usually having a peak of action and an effect lasting 3 to 5 hours. The idea is to approximate normal insulin patterns, with a basal level in the background and peaks (boluses) of insulin with carbohydrate intake.
Insulin pumps use only one kind of insulin—a rapid-acting one, ie, lispro, aspart, or glulisine. They preserve the basal-bolus concept, but with many refinements (discussed below).15
Most pumps are attached to the patient by plastic tubing that connects the reservoir to a subcutaneous cannula or steel needle. However, some pumps have a reservoir directly attached to a subcutaneous cannula without the tubing. This type of pump is controlled with a remote device.
The infusion set (cannula or needle and tubing) and the site should be changed every third day to minimize the risk of infection and abnormal delivery due to protein buildup on the cannula os, epithelial healing, and irritation around the site. Failure to do so often results in higher blood glucose concentrations.19
The patient and healthcare team work together to calculate the patient’s daily insulin needs, and the pump is programmed based on the patient’s requirements, lifestyle, and sensitivity to insulin. Once the pump is started, the patient operates it to deliver the insulin dose according to carbohydrate intake and blood glucose level.
PUMP SETTINGS
Basal rate
The basal rate is programmed by the physician and is intended to mimic physiologic insulin release. The pump can be set to a number of basal rates within any 24-hour period. This provides more physiologic matching of insulin delivery to hourly insulin needs based on the patient’s daily schedule.
If the patient has been taking multiple daily injections, the hourly basal rate can be calculated by dividing the daily basal dose by 24. However, lower rates are usually used after midnight, and rates are increased early in the morning to counteract the dawn phenomenon.
The rates can also be adjusted temporarily (for up to 24 hours), with a feature called the temporary basal rate. People tend to have higher blood glucose levels when they have a respiratory illness, are under significant stress, or are menstruating. Thus, a person with influenza could increase the basal rate by 25%, or a student could run a temporary basal rate of 150% for 4 hours before taking a final exam.
Conversely, exercising increases insulin’s effectiveness at the muscle level, and insulin requirements drop. To counteract this, one would temporarily decrease the basal rate in the pump before exercising.
Many factors affect the bolus dose
A bolus of insulin is given for meals and to correct hyperglycemia, as with multiple daily injections. A pump calculates the bolus based on the carbohydrate ratio, correction factor, or both. These ratios are programmed into the pump by the physician. A benefit of the insulin pump is that the patient just has to input the amount of carbohydrates to be eaten or record a blood glucose level and the pump will calculate the bolus dose of insulin to be given.
The carbohydrate ratio is the amount of insulin that should be taken per amount of carbohydrate. A typical ratio is 1:15, meaning that the patient should take 1 unit of insulin for every 15 g of carbohydrates to be eaten. This varies by patient depending on insulin sensitivity.
The correction factor describes how much the glucose level is expected to drop per unit of insulin given. For example, if the target glucose level is 100 mg/dL and the correction factor is 25, then the patient will get 1 unit of correction of insulin if his or her glucose level is 125 mg/dL, 2 units if it is 150 mg/dL, and so on. A pump can dispense fractions of a unit.
The target glucose level or range is set by the physician and patient and is one of the factors the pump uses in calculating a bolus dose. Insulin pumps allow for multiple target glucose levels. Commonly, to minimize the risk of hypoglycemia, a higher (less strict) target is set for bedtime and overnight than for daytime.
Active insulin time defines how soon the patient can take another bolus.
Often, people eat more than they thought they would. They may also find that the glucose level did not increase or decrease as much as expected. Many patients who actively manage their glucose take additional boluses of insulin after a meal if their glucose is higher than they thought it would be. A patient taking injections cannot know how much of the insulin from the before-meal bolus is still working and has to guess.
Insulin pumps use a logarithmic formula to calculate this and prevent the user from “stacking” insulin boluses and lowering the glucose level too much. For example, if the active insulin time is 4 hours and the patient took a bolus for lunch at noon, he or she would be unable to take a full insulin correction dose until 4:00 pm. The patient can override this feature. Although the active insulin time varies from patient to patient, it is rarely more than 4 hours.
Additional safety features
Suspend. When a person who is taking insulin injections starts to experience hypoglycemia, he or she has one option—to eat something to treat the low blood glucose. The insulin injection has already been taken and cannot be reversed. However, with an insulin pump the patient can first suspend the pump so that no additional insulin is infused until it is safe again, and then eat to treat the low sugar level. This allows the patient to eat less, prevent overtreating, and, hopefully, prevent rebound hyperglycemia.
Reverse correction. When patients take insulin for an upcoming meal, they estimate the amount needed for the carbohydrates that they are about to eat as well as how much correction is needed. If their glucose level is below the target range, they may or may not subtract insulin from the dose to achieve the glucose target. The pump does this automatically, resulting in a lower dose of insulin for that bolus. This allows the patient to take a bolus for a meal even if he or she is below the target, and thus prevent hyperglycemia.
CAN INSULIN PUMPS BE USED IN THE HOSPITAL?
Patients can keep using their insulin pump in the hospital under the right conditions.
Inpatient hypoglycemia increases the risk of death, and although not all patients require tight glycemic control, there is still benefit in avoiding extremes in blood sugar levels,20 including at night.20–22 Insulin pump therapy, when used in the hospital, results in fewer episodes of severe hyperglycemia (glucose levels > 300 mg/dL) and hypoglycemia (levels < 40 mg/dL) than multiple daily injections.22 Moreover, most pump users feel more comfortable when they can manage their own therapy. Using the pump in the hospital has the additional benefit that patients can treat themselves before and after meals easily with less staff time and effort.
Bailon et al23 retrospectively studied 35 patients with insulin pumps in 50 hospitalizations. More than half of the patients were allowed to continue using their pump in the hospital. Reasons for discontinuing the pump included lack of access to supplies, unfamiliarity with the pump, attempted suicide, malfunctioning hardware, diabetic ketoacidosis, and altered mental status. Patients using their pump had fewer episodes of hypoglycemia (glucose levels < 70 mg/dL) than patients who removed their pump. In patients who continued using the pump throughout their hospitalization, no adverse events (eg, site infection or mechanical failure) were noted.
Leonhardi et al24 reviewed 25 hospital admissions, and the outcomes were similar to those reported by Bailon et al,23 with no adverse outcomes related to the pumps.
When using an insulin pump in the hospital
When a physician wants a patient to continue using an insulin pump in the hospital, a number of things must happen. The nursing staff must be informed that the patient is wearing a pump and can self-administer insulin. Most facilities will still follow routine protocols for checking blood glucose but will document that the patient is administering his or her own insulin. The patient must be well enough to manage the pump. If the infusion site needs to be changed, the patient would be expected to do so with his or her own supplies.
Imaging and insulin pumps
Advice differs on what to do if a patient with an insulin pump needs to undergo radiographic imaging. For example, the University of Wisconsin radiology department says it is safe to keep an insulin pump in place if the x-ray beam will be on for less than 3 seconds at a time and if the device is covered by a lead apron.25 However, radiation can induce electrical currents in the circuitry, which can alter the function of the pump. For this reason, some manufacturers recommend removing the device before the patient enters any room in which radiation or magnetic resonance imaging will be used.26–31
Insulin pumps and surgery
Insulin pumps have been used in the perioperative and intraoperative periods, with positive outcomes.32 An analysis of 20 patients on pumps undergoing a total of 23 surgeries (mostly orthopedic procedures) found that 13 of the 20 patients wore their pump during surgery. No adverse events were noted in any of these cases, although the sample size was small.33
Corney et al34 retrospectively compared insulin pumps with alternative methods of perioperative glucose management. Multiple surgical specialties were included. No significant difference in mean blood glucose levels was found between those who continued to use their pump and those who used other methods. In those who continued to use their pump, there were no episodes of intraoperative technical difficulties related to the pump.
Any patient who may be undergoing a procedure or surgery must let the surgeon and anesthesiologist know that he or she has a pump. If the infusion site is too close to the site of the surgery or procedure, it must be moved.
Concerns during surgery include catheter or site disconnection or loss, crystallization within the tubing (a potential problem not limited to surgery), and pump malfunction. If the procedure involves imaging, the pump should probably be disconnected or covered by lead shielding as directed in the pump manufacturer’s manual. The surgeon and anesthesiologist must decide whether to continue use of a pump during a surgical procedure. However, the study by Corney et al34 shows it is possible.
Most office-based procedures can be done with the insulin pump in place, as the patient is not under general anesthesia and so can adjust the insulin regimen as needed.
Abdelmalak et al,35 in a comprehensive review of insulin pump use in noncardiac surgery, commented that the type of surgery may play a role in determining the best approach to perioperative glucose management. Major surgery causes a large inflammatory response that makes it difficult to control blood sugar, especially when steroids or beta agonists are given, whereas minor surgery does not affect blood glucose nearly as much. The authors offered recommendations on pump use during various surgical procedures depending on the length of the procedure:
- If surgery is anticipated to last less than 1 hour, then keep the insulin pump on, and have the patient manage corrections preoperatively and postoperatively.
- For surgery of intermediate length (1–3 hours), have the patient take a bolus of 1 hour’s worth of insulin (based on the basal rate for that time period) before the procedure, then remove the insulin pump. Do this only if blood sugar is normal or close to normal. If the patient is severely hyperglycemic, remove the insulin pump and start an intravenous insulin infusion.
- If the procedure will take more than 3 hours, remove the pump and start an insulin infusion regardless of the blood sugar level.35
AIR TRAVEL AND INSULIN PUMPS
Insulin pumps can be easy to manage during airline travel if the user is prepared (Table 3).
First, it is important to have a letter from the treating physician stating that the pump is a necessary medical device. All supplies should be carried on and in a separate bag for easy inspection. The more forthcoming the user is at the security checkpoint, the easier the process.
According to the Transportation Security Administration, insulin pump users can keep their pump on during screening, and the metal detectors and full-body scanners will not harm the device.36
However, manufacturer recommendations differ. Medtronic recommends that patients not expose their insulin pump to x-rays, and that instead of going through a full-body scanner the patient should request a pat-down.37 Animas recommends the same.38 OmniPod states that their system can be worn through airport imaging, making it the only approved continuous insulin delivery system that can be taken through airport imaging.39
Another potential problem is the change in atmospheric pressure during takeoff and landing. Bubbles can form in the insulin reservoir as air pressure decreases with ascent, thereby displacing insulin from the pump to the patient. The opposite happens during descent. King et al40 corroborated this phenomenon with Animas and Medtronic pumps. Asante recommends removing their pump tubing during takeoff and landing.30
If PROBLEMS ARISE
Like any machine, an insulin pump can fail. Most failures result in lack of insulin delivery—the patient does not get excess insulin from insulin pump failure. Excess insulin delivery is most often due to operator error. All insulin is either preprogrammed (basal by provider or patient) or must be confirmed by the patient at the time of delivery (meal or correction boluses).
Pump manufacturers have 24-hour support programs and hotlines, with experts who will either walk the patient through the problem or send a replacement pump—often within 24 hours.
EVOLVING TECHNOLOGY
Pump technology is evolving quickly. On the way are “smart” pumps that interact with other systems, smaller pumps with advanced touch-screen features, and patch pumps that do not have tubing but operate similarly to pumps with tubing (ie, a cannula is still required for insulin delivery).
Some insulin pumps can be linked to an external glucose sensor. These systems provide a great amount of information to the patient and provider. Often, there is increased awareness of fluctuations in glucose, allowing earlier intervention to prevent high and low glucose excursions. Sensor-augmented pumps may further improve safety by suspending infusion during hypoglycemia.41,42
Researchers continue to strive for closed-loop systems that would allow the pump to automatically respond to circulating glucose and thus provide truly physiologic control.43 A recent study showed the effectiveness of the outpatient use of a bihormonal (insulin and glucagon) “bionic pancreas,” which provided improved glucose control and similar or less hypoglycemia in adults and adolescents who had been using a traditional insulin pump.44
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002; 25:593–598.
- JDRF and BD collaborate to improve insulin pump delivery. www.bd.com/_Images/BD_JDRF_press_release_2010_tcm49-19552.pdf. Accessed October 14, 2015.
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology insulin pump management task force. Endocr Pract 2014; 20:463–489.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001; 24:1722–1727.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005; 28:1568–1573.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27:2590–2596.
- Hirsch IB, Bode BW, Garg S, et al; Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005; 28:533–538.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (Oxf) 2013; 79:641–647.
- Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract 2013; 19:9–13.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 26:1079-1087.
- Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995; 18:361–376.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes 2007; 25:50–56.
- Cohen N, Shaw J. Cost effectiveness of insulin pump therapy. Infusystems Asia 2007; 2:25–28.
- Tucker ME. Insulin pumps: closer to a pancreas. Diabetes Forecast. www.diabetesforecast.org/2015/mar-apr/insulin-pumps-closer-to-pancreas.html. Accessed October 14, 2015.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000; 23:1232–1235.
- Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010; 4:976–982.
- Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Cook CB, Beer KA, Seifert KM, Boyle ME, Mackey PA, Castro JC. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol 2012; 6:995–1002.
- Bailon RM, Partlow BJ, Miller-Cage V, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract 2009; 15:24–29.
- Leonhardi BJ, Boyle ME, Beer KA, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol 2008; 2:948–962.
- Department of Radiology, University of Wisconsin School of Medicine and Public Health. Precautions with implanted devices. www.radiology.wisc.edu/fileShelf/forReferring/PrecautionsWithImplantedDevices_CTandXRAY.php. Accessed October 14, 2015.
- Indications, contraindications, warnings and precautions. Medtronicdiabetes.com/important-safety-information. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- T:slim user guide. www.tandemdiabetes.com/uploadedFiles/Content/_Configuration/Files/Manuals/tslim_User_Guide.pdf. Tandem Diabetes Care. Accessed October 14, 2015.
- OmniPod user guide. www.myomnipodtraining.com/pdf/OmniPod-User-Guide-UST400.pdf. Insulet Corporation. Accessed October 14, 2015.
- Important safety information.Animas Vibe Insulin Pump and CGM System. www.animas.com/safety. Animas Corporation. Accessed October 14, 2015.
- Snap insulin pump safety information. Snappump.com/safety-information. Asante Solutions, Inc. Accessed October 14, 2015.
- ACCU-CHEK Spirit insulin pump system. Pump user guide. www.accu-chekinsulinpumps.com/documents/PumpUserGuide.pdf. Disetronic Medical Systems, Inc. Accessed October 14, 2015.
- White WA Jr, Montalvo H, Monday JM. Continuous subcutaneous insulin infusion during general anesthesia: a case report. AANA J 2004; 72:353–357.
- Boyle ME, Seifert KM, Beer KA, et al. Insulin pump therapy in the perioperative period: a review of care after implementation of institutional guidelines. J Diabetes Sci Technol 2012; 6:1016–1021.
- Corney SM, Dukatz T, Rosenblatt S, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol 2012; 6:1003–1015.
- Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des 2012; 18:6204–6214.
- US Department of Homeland Security. Travelers with disabilities and medical conditions. www.tsa.gov/travel/special-procedures. Transportation Security Administration. Accessed October 14, 2015.
- Medical emergency card/airport information. www.medtronicdiabetes.com/sites/default/files/library/support/Airport%20Information%20Card.pdf. Medtronic MiniMed, Inc. Accessed October 14, 2015.
- Traveling with an insulin pump. www.animas.com/about-insulin-pump-therapy/traveling-with-diabetes. Animas Corporation. Accessed October 14, 2015.
- Tips for air travel with diabetes supplies. www.myomnipod.com/pdf/14986-AWAirTravelTipsFlyerR2-11-11.pdf. Insulet Corporation. Accessed October 14, 2015.
- King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34:1932–1933.
- Bergenstal RM, Tamborlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010; 363:311–320.
- Bergenstal RM, Klonoff DC, Garg SK, et al; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369:224–232.
- Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control 2012; 36:255–266.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371:313–325.
KEY POINTS
- Insulin pumps allow for more accurate insulin dosing than multiple daily injections, resulting in less drastic extremes in blood sugar.
- Insulin pumps allow for more individualized basal insulin coverage than long-acting injectable insulin.
- Both the patient and provider need a good understanding of insulin pump therapy for successful pump management.
Insulin pumps: Great devices, but you still have to press the button
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
In this issue of the Journal, Millstein et al provide an elegant, practical, and up-to-date review of insulin pump therapy (also known as continuous subcutaneous insulin infusion), emphasizing its benefits and comparing it with multiple daily insulin injections.1
NOT FOR EVERYONE
While insulin pumps make the lives of many patients much easier, we should be careful when generalizing their indications. These devices have been with us for 4 decades, during which they have progressively been made more precise and more intelligent—and smaller. The technology may be attractive to some patients but undesirable to others (Table 1).
Many healthcare providers are unfamiliar with pump technology, and some are intimidated by it because it involves a dynamic device-user interface that is more complex than that of other concealed programmed devices such as pacemakers. Inadequate glycemic management is complex and may result from factors such as fear of hypoglycemia, difficulty with insulin dose adjustment, and poor math skills.2
Unfortunately, some patients are given a pump without proper screening and education, and they tend to call the pump manufacturer’s help line or their provider often for help with technical problems. Selecting the right patient for this technology is more important than the converse.
Indications for an insulin pump vary by country. In some countries, a pump is started as soon as type 1 diabetes is diagnosed. In the United States, the indications are very rigorous and restrictive, especially for patients with type 2 diabetes, in whom a lack of endogenous insulin production must first be proved.
There is no question that a pump should be offered to every patient with type 1 diabetes who demonstrates good motivation to improve his or her glucose control, but only after a rigorous education program. This option is too costly to be tried just to see if the patient likes it.
ADVERSE EVENTS WITH INSULIN PUMPS: MORE DATA NEEDED
A worrisome aspect of continuous subcutaneous insulin infusion at a population level is a lack of information on the root causes of adverse events (diabetic ketoacidosis or severe hypoglycemia) in patients who use it. These events may be serious and sometimes even fatal.
Outside of a controlled environment, it is difficult to ascertain whether an adverse event represents device error or user error, since pumps contain different components (electronic, mechanical, and pharmacologic) that interface with the human user.3 How adverse events are tracked or categorized is unclear, and given the risks associated with this technology, better postmarketing evaluation is needed. Furthermore, we do not know if the precision of insulin delivery decreases over the life of a pump.
While most pump manufacturers have good customer service and make every effort to provide the patient with a replacement pump in case of failure, we do not know if anyone maintains a database of such failures or adverse events, and if those failures can be analyzed to improve safety.3
INTERFACES ARE NOT STANDARD
When one buys a new car, little time is needed to learn how to operate it because most cars use the same basic features.
The situation is different with insulin pumps. To compete with each other, pump manufacturers create different looks, different insulin delivery methods, and different ways of administering a bolus. Switching from one pump to another is difficult without detailed education on the “bells and whistles” of the new pump.
Most patients use just a few features of the pump. They look at it as more of a convenience. They sometimes forget they are wearing it, and even forget to take a bolus before a meal.
PATIENT SATISFACTION DEPENDS ON THE PATIENT
For years, we thought insulin pumps were better at improving hypoglycemia awareness. But in a prospective study, multiple daily injections with frequent self-monitoring of blood glucose provided identical outcomes without worsening hemoglobin A1c compared with continuous infusion with real-time continuous glucose monitoring, although satisfaction with treatment was better in the latter group.4
Patients’ satisfaction with continuous subcutaneous insulin infusion depends on their baseline hemoglobin A1c level. Patients with relatively low hemoglobin A1c tend to take an active approach to self-care, describe the pump as a tool for meeting glycemic goals, and say the pump makes them feel more normal. Patients with high hemoglobin A1c tend to have a more passive approach to their self-care and have more negative experiences with the pump. Women are more concerned than men with the effect of the pump on body image and social acceptance.5
DOLLARS AND CENTS
According to 2012 estimates, 29 million Americans had diabetes mellitus, of whom 1.25 million had type 1. The direct medical costs of diabetes are estimated at $176 billion, of which 12% covers overall pharmacy costs.6 About 31% of adults with diabetes use insulin.7
For a device that costs $6,000, has a life span of only 4 years, and requires supplies that cost $300 per month, rigorous interpretation of superiority data would be needed to confirm that this technology would have a positive impact on public health if every insulin-using patient with diabetes were to say yes to it. It is true that switching from multiple daily injections to a pump leads to a significant reduction in insulin expenditures in patients with type 2 diabetes, according to a retrospective analysis of claims data.8
However, not all studies comparing pumps and multiple daily injections in type 2 diabetes have shown an advantage of one over the other in terms of a reduction in fasting glucose, hemoglobin A1c, or incidence of hypoglycemia.9 A meta-analysis10 found that the two therapies had similar effects on glycemic control and hypoglycemia. Continuous infusion had a more favorable effect in adults with type 1 diabetes.10
Neither continuous infusion nor multiple daily injections can mimic physiologic endogenous insulin secretion. Endogenous insulin is secreted into the portal system, and its main site of action is the liver. As a result, there is more hepatic glucose uptake and thus a lower peripheral plasma insulin concentration with endogenous secretion than with systemic administration. Endogenous insulin secretion also suppresses hepatic glucose production and reduces the risk of hypoglycemia.11
PROGRESS, BUT NOT PERFECTION
Diabetes mellitus constitutes a big burden on patients and on society. The discovery of insulin was a giant leap forward; the insulin pump was another great advance. We are getting closer to an integrated bionic pancreas. We are far from achieving a perfect system, but we are much better off than we were 50 or 80 years ago. And although insulin pump technology is sophisticated and precise, it still interfaces with a human user, and the human user still must press its buttons.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
- Millstein R, Mora Becerra N, Shubrook JH. Insulin pumps: beyond basal-bolus. Cleve Clin J Med 2015; 82:835–842.
- Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [NCT01460446]. BMC Fam Pract 2012; 13:102.
- Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015; 38:716–722.
- Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37:2114–2122.
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007; 30:549–554.
- American Diabetes Association. Statistics about diabetes. Overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 4, 2015.
- Centers for Disease Control and Prevention (CDC). Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2011. www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed November 4, 2015.
- David G, Gill M, Gunnarsson C, Shafiroff J, Edelman S. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care; 20:e490–e497.
- Gao GQ, Heng XY, Wang YL, et al. Comparison of continuous subcutaneous insulin infusion and insulin glargine-based multiple daily insulin aspart injections with preferential adjustment of basal insulin in patients with type 2 diabetes. Exp Ther Med 2014; 8:1191–1196.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012; 157:336–347.
- Logtenberg SJ, van Ballegooie E, Israêl-Bultman H, van Linde A, Bilo HJ. Glycaemic control, health status and treatment satisfaction with continuous intraperitoneal insulin infusion. Neth J Med 2007; 65:65–70.
Does allergic conjunctivitis always require prescription eyedrops?
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
Noncosmetic uses of botulinum toxin in otolaryngology
Botulinum toxin is commonly used to treat movement disorders of the head and neck. It was first used to treat focal eye dystonia (blepharospasm) and laryngeal dystonia (spasmodic dysphonia) and is now also used for other head and neck dystonias, movement disorders, and muscle spasticity or contraction.
This article reviews the use of botulinum toxin for primary disorders of the laryngopharynx—adductor and abductor spasmodic dysphonias, laryngopharyngeal tremor, and cricopharyngeus muscle dysfunction—and its efficacy and side effects for the different conditions.
ABNORMAL MUSCLE MOVEMENT
Dystonia is abnormal muscle movement characterized by repetitive involuntary contractions. Dystonic contractions are described as either sustained (tonic) or spasmodic (clonic) and are typically induced by conscious action to move the muscle group.1,2 Dystonia can be categorized according to the amount of muscle involvement: generalized (widespread muscle activity), segmental (involving neighboring groups of muscles), or focal (involving only one or a few local small muscles).3 Activity may be associated with gross posturing and disfigurement, depending on the size and location of the muscle contractions, although the muscle action is usually normal during rest.
The cause of dystonia has been the focus of much debate and investigation. Some types of dystonia have strong family inheritance patterns, but most are sporadic, possibly brought on by trauma or infection. In most cases, dystonia is idiopathic, although it may be associated with other muscle group dystonias, tremor, neurologic injury or insults, other neurologic diseases and neurodegenerative disorders, or tardive syndromes.1 Because of the relationship with other neurologic diseases, consultation with a neurologist should be considered.
Treatment of the muscle contractions of the various dystonias includes drug therapy and physical, occupational, and voice therapy. Botulinum toxin is a principal treatment for head and neck dystonias and works by blocking muscular contractions.4 It has the advantages of having few side effects and predictable results for many conditions, although repeat injections are usually required to achieve a sustained effect.
LARYNGEAL DYSTONIAS CAUSE VOICE ABNORMALITIES
The most common laryngeal dystonia is spasmodic dysphonia, a focal dystonia of the larynx. It is subdivided into two types according to whether spasm of the vocal folds occurs during adduction or abduction.
Adductor spasmodic dysphonia accounts for 80% to 90% of cases. It is characterized by irregular speech with pitch breaks and a strained or strangulated voice. It was formerly treated by resection of the nerve to the vocal folds, but results were neither consistent nor persistent. Currently, the primary treatment is injection of botulinum toxin, which has a high success rate,5 with patients reporting about a 90% return of normal function.
Abductor spasmodic dysphonia accounts for 10% to 20% of cases.6 Patients have a breathy quality to the voice with a short duration of vocalization due to excessive loss of air on phonation. This is especially noticeable when the patient speaks words that begin with a voiceless consonant followed by a vowel (eg, pat, puppy). Response to botulinum toxin injection is more variable,6 possibly because of the pathophysiology of the disorder or because of the technical challenges of administering the injection.
Fewer than 1% of patients have both abductor and adductor components, and their treatment can be particularly challenging.
Adductor spasmodic dysphonia: Treatment usually successful
Botulinum toxin can be injected for adductor spasmodic dysphonia via a number of approaches, the most common being through the cricothyroid membrane (Figure 1). Injections can be made into one or both vocal folds and can be performed under guidance with laryngeal electromyography or with a flexible laryngoscope to visualize the larynx.
Patients typically experience breathiness beginning 1 or 2 days after the injection, and this effect may last for up to 2 weeks. During that time, the patient may be more susceptible to aspiration of thin liquids and so is instructed to drink cautiously. Treatment benefits typically last for 3 to 6 months. As the botulinum toxin wears off, the patient notices a gradual increase in vocal straining and effort.
Dosages of botulinum toxin for subsequent treatments are adjusted by balancing the period of benefit with postinjection breathiness. The desire of the patient should be paramount. Some are willing to tolerate more side effects to avoid frequent injections, so they can be given a larger dose. Others cannot tolerate the breathiness but are willing to accept more frequent injections, so they should be given a smaller dose. In rare cases, patients have significant breathiness from even small doses; they may be helped by injecting into only one vocal fold or, alternatively, into a false vocal fold, allowing diffusion of the toxin down to the muscle of the true vocal fold.
Abductor spasmodic dysphonia: Treatment more challenging
The success of botulinum toxin treatment for abductor spasmodic dysphonia is more variable than for the adductor type. The injections are made into the posterior cricoarytenoid muscle (Figure 2); because this muscle cannot be directly visualized, this procedure requires guidance with laryngeal electromyography. Most patients note improvement, and about 20% have a good response.6 Most require a second injection about 1 month later, often on the other side. Bilateral injections at one sitting may compromise the airway, and vocal fold motion should be evaluated at the time of the contralateral injection to assess airway patency. Interest has increased in simultaneous bilateral injections with lower doses of botulinum toxin, and this approach has been shown to be safe.7
ESSENTIAL TREMOR OF THE VOICE
Essential tremor is an action tremor that can occur with voluntary movement. It can occur anywhere in the body, often the head or hand, but the voice can also be affected. About half of cases are hereditary. Essential tremor of the voice causes a rhythmic oscillation of pitch and intensity.
Consultation with a neurologist is recommended to evaluate the cause, although voice tremor is often idiopathic and occurs in about 30% of patients with essential tremor in the arms or legs, as well as in about 30% of patients with spasmodic dysphonia. Extremity tremor can usually be successfully managed medically, but this is not true for voice tremor.
Botulinum toxin injection is the mainstay of treatment for essential tremor of the voice, although its success is marginal. About two-thirds of patients have some degree of improvement from traditional botulinum toxin injections in the true vocal fold.8
The results of treatment are likely to be inconsistent because tremor tends to involve several different muscles used in voice production, commonly in the soft palate, tongue base, pharyngeal walls, strap muscles, false vocal folds, and true vocal folds. A location-oriented tremor scoring system9 can help identify the involved muscles to guide injections. Treatment is less likely to be successful in patients with multiple sites of voice tremor. Injection into the false vocal fold, true vocal fold, and interarytenoid muscle10 can safely be performed; injections into the palate, tongue base, and strap muscles are to be avoided because of the high risk of postinjection aspiration.
Patients who have good results can have repeat treatments as needed. The dosage of botulinum toxin is adjusted according to response, side effects (eg, breathy voice, dysphagia), and patient preference.
CRICOPHARYNGEUS MUSCLE DYSFUNCTION: TROUBLE SWALLOWING
Dysfunction of the cricopharyngeus muscle causes difficulty swallowing, especially swallowing solid foods. It can be attributed to a mechanical stricture or to hyperfunction (spasm).
Mechanical stricture at the esophageal inlet frequently occurs in patients who have had a total laryngectomy for advanced laryngeal cancer. Fibrosis tends to be worse in patients who have also undergone radiation therapy.
Stricture can be treated with botulinum toxin injections and dilation. Conservative treatment is preferred to surgical myotomy for patients with complex postlaryngectomy anatomy and scarring from radiation therapy.
Cricopharyngeus muscle spasm or hyperfunction can be an important cause of dysphagia, especially in the elderly. Patients should be evaluated with barium esophagography or a modified barium swallow. The finding of a cricopharyngeal “bar” provides evidence of contraction of the muscle that impedes the passage of food.
Botulinum toxin injections for cricopharyngeus muscle dysfunction (Figure 2) can be effective in some cases, especially if the toxin is injected bilaterally. However, because the cricopharyngeus muscle plays an important role in preventing esophageal reflux into the laryngopharynx, botulinum toxin injection in patients with substantial hiatal hernia or laryngopharyngeal reflux disease should only be done with caution. In addition, treatment of reflux disease should be considered in any patient undergoing botulinum toxin injection for cricopharyngeus muscle dysfunction.
Most patients require repeat injections when the toxin wears off, although occasionally one or two injections provide long-term or permanent relief. Dosages are adjusted for the patient’s age, the presence of other swallowing problems, and reflux. Patients may experience increased difficulty swallowing for 1 or 2 weeks after the procedure and so should be counseled to eat slowly and carefully.
- Cultrara A, Chitkara A, Blitzer A. Botulinum toxin injections for the treatment of oromandibular dystonia. Oper Tech Otolaryngol Head Neck Surg 2004; 15:97–102.
- Fahn S. The varied clinical expressions of dystonia. Neurol Clin 1984; 2:541–554.
- Fahn S. Concept and classification of dystonia. Adv Neurol 1988; 50:1–8.
- Benninger MS, Knott PD. Techniques of botulinum toxin injections in the head and neck. San Diego, CA: Plural Publishing, Inc; 2012.
- Benninger MS, Gardner G, Grywalski C. Outcomes of botulinum toxin treatment for spasmodic dysphonia. Arch Otolaryngol Head Neck Surg 2001; 127:1083–1085.
- Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope 1998; 108:1435–1441.
- Klein AM, Stong BC, Wise J, DelGaudio JM, Hapner ER, Johns MM 3rd. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg 2008; 139:421–423.
- Hertegård S, Granqvist S, Lindestad PA. Botulinum toxin injections for essential voice tremor. Ann Otol Rhinol Laryngol 2000; 109:204–209.
- Bové M, Daamen N, Rosen C, Wang CC, Sulica L, Gartner-Schmidt J. Development and validation of the vocal tremor scoring system. Laryngoscope 2006; 116:1662–1667.
- Kendall KA, Leonard RJ. Interarytenoid muscle Botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J Voice 2001; 25:114–119.
Botulinum toxin is commonly used to treat movement disorders of the head and neck. It was first used to treat focal eye dystonia (blepharospasm) and laryngeal dystonia (spasmodic dysphonia) and is now also used for other head and neck dystonias, movement disorders, and muscle spasticity or contraction.
This article reviews the use of botulinum toxin for primary disorders of the laryngopharynx—adductor and abductor spasmodic dysphonias, laryngopharyngeal tremor, and cricopharyngeus muscle dysfunction—and its efficacy and side effects for the different conditions.
ABNORMAL MUSCLE MOVEMENT
Dystonia is abnormal muscle movement characterized by repetitive involuntary contractions. Dystonic contractions are described as either sustained (tonic) or spasmodic (clonic) and are typically induced by conscious action to move the muscle group.1,2 Dystonia can be categorized according to the amount of muscle involvement: generalized (widespread muscle activity), segmental (involving neighboring groups of muscles), or focal (involving only one or a few local small muscles).3 Activity may be associated with gross posturing and disfigurement, depending on the size and location of the muscle contractions, although the muscle action is usually normal during rest.
The cause of dystonia has been the focus of much debate and investigation. Some types of dystonia have strong family inheritance patterns, but most are sporadic, possibly brought on by trauma or infection. In most cases, dystonia is idiopathic, although it may be associated with other muscle group dystonias, tremor, neurologic injury or insults, other neurologic diseases and neurodegenerative disorders, or tardive syndromes.1 Because of the relationship with other neurologic diseases, consultation with a neurologist should be considered.
Treatment of the muscle contractions of the various dystonias includes drug therapy and physical, occupational, and voice therapy. Botulinum toxin is a principal treatment for head and neck dystonias and works by blocking muscular contractions.4 It has the advantages of having few side effects and predictable results for many conditions, although repeat injections are usually required to achieve a sustained effect.
LARYNGEAL DYSTONIAS CAUSE VOICE ABNORMALITIES
The most common laryngeal dystonia is spasmodic dysphonia, a focal dystonia of the larynx. It is subdivided into two types according to whether spasm of the vocal folds occurs during adduction or abduction.
Adductor spasmodic dysphonia accounts for 80% to 90% of cases. It is characterized by irregular speech with pitch breaks and a strained or strangulated voice. It was formerly treated by resection of the nerve to the vocal folds, but results were neither consistent nor persistent. Currently, the primary treatment is injection of botulinum toxin, which has a high success rate,5 with patients reporting about a 90% return of normal function.
Abductor spasmodic dysphonia accounts for 10% to 20% of cases.6 Patients have a breathy quality to the voice with a short duration of vocalization due to excessive loss of air on phonation. This is especially noticeable when the patient speaks words that begin with a voiceless consonant followed by a vowel (eg, pat, puppy). Response to botulinum toxin injection is more variable,6 possibly because of the pathophysiology of the disorder or because of the technical challenges of administering the injection.
Fewer than 1% of patients have both abductor and adductor components, and their treatment can be particularly challenging.
Adductor spasmodic dysphonia: Treatment usually successful
Botulinum toxin can be injected for adductor spasmodic dysphonia via a number of approaches, the most common being through the cricothyroid membrane (Figure 1). Injections can be made into one or both vocal folds and can be performed under guidance with laryngeal electromyography or with a flexible laryngoscope to visualize the larynx.
Patients typically experience breathiness beginning 1 or 2 days after the injection, and this effect may last for up to 2 weeks. During that time, the patient may be more susceptible to aspiration of thin liquids and so is instructed to drink cautiously. Treatment benefits typically last for 3 to 6 months. As the botulinum toxin wears off, the patient notices a gradual increase in vocal straining and effort.
Dosages of botulinum toxin for subsequent treatments are adjusted by balancing the period of benefit with postinjection breathiness. The desire of the patient should be paramount. Some are willing to tolerate more side effects to avoid frequent injections, so they can be given a larger dose. Others cannot tolerate the breathiness but are willing to accept more frequent injections, so they should be given a smaller dose. In rare cases, patients have significant breathiness from even small doses; they may be helped by injecting into only one vocal fold or, alternatively, into a false vocal fold, allowing diffusion of the toxin down to the muscle of the true vocal fold.
Abductor spasmodic dysphonia: Treatment more challenging
The success of botulinum toxin treatment for abductor spasmodic dysphonia is more variable than for the adductor type. The injections are made into the posterior cricoarytenoid muscle (Figure 2); because this muscle cannot be directly visualized, this procedure requires guidance with laryngeal electromyography. Most patients note improvement, and about 20% have a good response.6 Most require a second injection about 1 month later, often on the other side. Bilateral injections at one sitting may compromise the airway, and vocal fold motion should be evaluated at the time of the contralateral injection to assess airway patency. Interest has increased in simultaneous bilateral injections with lower doses of botulinum toxin, and this approach has been shown to be safe.7
ESSENTIAL TREMOR OF THE VOICE
Essential tremor is an action tremor that can occur with voluntary movement. It can occur anywhere in the body, often the head or hand, but the voice can also be affected. About half of cases are hereditary. Essential tremor of the voice causes a rhythmic oscillation of pitch and intensity.
Consultation with a neurologist is recommended to evaluate the cause, although voice tremor is often idiopathic and occurs in about 30% of patients with essential tremor in the arms or legs, as well as in about 30% of patients with spasmodic dysphonia. Extremity tremor can usually be successfully managed medically, but this is not true for voice tremor.
Botulinum toxin injection is the mainstay of treatment for essential tremor of the voice, although its success is marginal. About two-thirds of patients have some degree of improvement from traditional botulinum toxin injections in the true vocal fold.8
The results of treatment are likely to be inconsistent because tremor tends to involve several different muscles used in voice production, commonly in the soft palate, tongue base, pharyngeal walls, strap muscles, false vocal folds, and true vocal folds. A location-oriented tremor scoring system9 can help identify the involved muscles to guide injections. Treatment is less likely to be successful in patients with multiple sites of voice tremor. Injection into the false vocal fold, true vocal fold, and interarytenoid muscle10 can safely be performed; injections into the palate, tongue base, and strap muscles are to be avoided because of the high risk of postinjection aspiration.
Patients who have good results can have repeat treatments as needed. The dosage of botulinum toxin is adjusted according to response, side effects (eg, breathy voice, dysphagia), and patient preference.
CRICOPHARYNGEUS MUSCLE DYSFUNCTION: TROUBLE SWALLOWING
Dysfunction of the cricopharyngeus muscle causes difficulty swallowing, especially swallowing solid foods. It can be attributed to a mechanical stricture or to hyperfunction (spasm).
Mechanical stricture at the esophageal inlet frequently occurs in patients who have had a total laryngectomy for advanced laryngeal cancer. Fibrosis tends to be worse in patients who have also undergone radiation therapy.
Stricture can be treated with botulinum toxin injections and dilation. Conservative treatment is preferred to surgical myotomy for patients with complex postlaryngectomy anatomy and scarring from radiation therapy.
Cricopharyngeus muscle spasm or hyperfunction can be an important cause of dysphagia, especially in the elderly. Patients should be evaluated with barium esophagography or a modified barium swallow. The finding of a cricopharyngeal “bar” provides evidence of contraction of the muscle that impedes the passage of food.
Botulinum toxin injections for cricopharyngeus muscle dysfunction (Figure 2) can be effective in some cases, especially if the toxin is injected bilaterally. However, because the cricopharyngeus muscle plays an important role in preventing esophageal reflux into the laryngopharynx, botulinum toxin injection in patients with substantial hiatal hernia or laryngopharyngeal reflux disease should only be done with caution. In addition, treatment of reflux disease should be considered in any patient undergoing botulinum toxin injection for cricopharyngeus muscle dysfunction.
Most patients require repeat injections when the toxin wears off, although occasionally one or two injections provide long-term or permanent relief. Dosages are adjusted for the patient’s age, the presence of other swallowing problems, and reflux. Patients may experience increased difficulty swallowing for 1 or 2 weeks after the procedure and so should be counseled to eat slowly and carefully.
Botulinum toxin is commonly used to treat movement disorders of the head and neck. It was first used to treat focal eye dystonia (blepharospasm) and laryngeal dystonia (spasmodic dysphonia) and is now also used for other head and neck dystonias, movement disorders, and muscle spasticity or contraction.
This article reviews the use of botulinum toxin for primary disorders of the laryngopharynx—adductor and abductor spasmodic dysphonias, laryngopharyngeal tremor, and cricopharyngeus muscle dysfunction—and its efficacy and side effects for the different conditions.
ABNORMAL MUSCLE MOVEMENT
Dystonia is abnormal muscle movement characterized by repetitive involuntary contractions. Dystonic contractions are described as either sustained (tonic) or spasmodic (clonic) and are typically induced by conscious action to move the muscle group.1,2 Dystonia can be categorized according to the amount of muscle involvement: generalized (widespread muscle activity), segmental (involving neighboring groups of muscles), or focal (involving only one or a few local small muscles).3 Activity may be associated with gross posturing and disfigurement, depending on the size and location of the muscle contractions, although the muscle action is usually normal during rest.
The cause of dystonia has been the focus of much debate and investigation. Some types of dystonia have strong family inheritance patterns, but most are sporadic, possibly brought on by trauma or infection. In most cases, dystonia is idiopathic, although it may be associated with other muscle group dystonias, tremor, neurologic injury or insults, other neurologic diseases and neurodegenerative disorders, or tardive syndromes.1 Because of the relationship with other neurologic diseases, consultation with a neurologist should be considered.
Treatment of the muscle contractions of the various dystonias includes drug therapy and physical, occupational, and voice therapy. Botulinum toxin is a principal treatment for head and neck dystonias and works by blocking muscular contractions.4 It has the advantages of having few side effects and predictable results for many conditions, although repeat injections are usually required to achieve a sustained effect.
LARYNGEAL DYSTONIAS CAUSE VOICE ABNORMALITIES
The most common laryngeal dystonia is spasmodic dysphonia, a focal dystonia of the larynx. It is subdivided into two types according to whether spasm of the vocal folds occurs during adduction or abduction.
Adductor spasmodic dysphonia accounts for 80% to 90% of cases. It is characterized by irregular speech with pitch breaks and a strained or strangulated voice. It was formerly treated by resection of the nerve to the vocal folds, but results were neither consistent nor persistent. Currently, the primary treatment is injection of botulinum toxin, which has a high success rate,5 with patients reporting about a 90% return of normal function.
Abductor spasmodic dysphonia accounts for 10% to 20% of cases.6 Patients have a breathy quality to the voice with a short duration of vocalization due to excessive loss of air on phonation. This is especially noticeable when the patient speaks words that begin with a voiceless consonant followed by a vowel (eg, pat, puppy). Response to botulinum toxin injection is more variable,6 possibly because of the pathophysiology of the disorder or because of the technical challenges of administering the injection.
Fewer than 1% of patients have both abductor and adductor components, and their treatment can be particularly challenging.
Adductor spasmodic dysphonia: Treatment usually successful
Botulinum toxin can be injected for adductor spasmodic dysphonia via a number of approaches, the most common being through the cricothyroid membrane (Figure 1). Injections can be made into one or both vocal folds and can be performed under guidance with laryngeal electromyography or with a flexible laryngoscope to visualize the larynx.
Patients typically experience breathiness beginning 1 or 2 days after the injection, and this effect may last for up to 2 weeks. During that time, the patient may be more susceptible to aspiration of thin liquids and so is instructed to drink cautiously. Treatment benefits typically last for 3 to 6 months. As the botulinum toxin wears off, the patient notices a gradual increase in vocal straining and effort.
Dosages of botulinum toxin for subsequent treatments are adjusted by balancing the period of benefit with postinjection breathiness. The desire of the patient should be paramount. Some are willing to tolerate more side effects to avoid frequent injections, so they can be given a larger dose. Others cannot tolerate the breathiness but are willing to accept more frequent injections, so they should be given a smaller dose. In rare cases, patients have significant breathiness from even small doses; they may be helped by injecting into only one vocal fold or, alternatively, into a false vocal fold, allowing diffusion of the toxin down to the muscle of the true vocal fold.
Abductor spasmodic dysphonia: Treatment more challenging
The success of botulinum toxin treatment for abductor spasmodic dysphonia is more variable than for the adductor type. The injections are made into the posterior cricoarytenoid muscle (Figure 2); because this muscle cannot be directly visualized, this procedure requires guidance with laryngeal electromyography. Most patients note improvement, and about 20% have a good response.6 Most require a second injection about 1 month later, often on the other side. Bilateral injections at one sitting may compromise the airway, and vocal fold motion should be evaluated at the time of the contralateral injection to assess airway patency. Interest has increased in simultaneous bilateral injections with lower doses of botulinum toxin, and this approach has been shown to be safe.7
ESSENTIAL TREMOR OF THE VOICE
Essential tremor is an action tremor that can occur with voluntary movement. It can occur anywhere in the body, often the head or hand, but the voice can also be affected. About half of cases are hereditary. Essential tremor of the voice causes a rhythmic oscillation of pitch and intensity.
Consultation with a neurologist is recommended to evaluate the cause, although voice tremor is often idiopathic and occurs in about 30% of patients with essential tremor in the arms or legs, as well as in about 30% of patients with spasmodic dysphonia. Extremity tremor can usually be successfully managed medically, but this is not true for voice tremor.
Botulinum toxin injection is the mainstay of treatment for essential tremor of the voice, although its success is marginal. About two-thirds of patients have some degree of improvement from traditional botulinum toxin injections in the true vocal fold.8
The results of treatment are likely to be inconsistent because tremor tends to involve several different muscles used in voice production, commonly in the soft palate, tongue base, pharyngeal walls, strap muscles, false vocal folds, and true vocal folds. A location-oriented tremor scoring system9 can help identify the involved muscles to guide injections. Treatment is less likely to be successful in patients with multiple sites of voice tremor. Injection into the false vocal fold, true vocal fold, and interarytenoid muscle10 can safely be performed; injections into the palate, tongue base, and strap muscles are to be avoided because of the high risk of postinjection aspiration.
Patients who have good results can have repeat treatments as needed. The dosage of botulinum toxin is adjusted according to response, side effects (eg, breathy voice, dysphagia), and patient preference.
CRICOPHARYNGEUS MUSCLE DYSFUNCTION: TROUBLE SWALLOWING
Dysfunction of the cricopharyngeus muscle causes difficulty swallowing, especially swallowing solid foods. It can be attributed to a mechanical stricture or to hyperfunction (spasm).
Mechanical stricture at the esophageal inlet frequently occurs in patients who have had a total laryngectomy for advanced laryngeal cancer. Fibrosis tends to be worse in patients who have also undergone radiation therapy.
Stricture can be treated with botulinum toxin injections and dilation. Conservative treatment is preferred to surgical myotomy for patients with complex postlaryngectomy anatomy and scarring from radiation therapy.
Cricopharyngeus muscle spasm or hyperfunction can be an important cause of dysphagia, especially in the elderly. Patients should be evaluated with barium esophagography or a modified barium swallow. The finding of a cricopharyngeal “bar” provides evidence of contraction of the muscle that impedes the passage of food.
Botulinum toxin injections for cricopharyngeus muscle dysfunction (Figure 2) can be effective in some cases, especially if the toxin is injected bilaterally. However, because the cricopharyngeus muscle plays an important role in preventing esophageal reflux into the laryngopharynx, botulinum toxin injection in patients with substantial hiatal hernia or laryngopharyngeal reflux disease should only be done with caution. In addition, treatment of reflux disease should be considered in any patient undergoing botulinum toxin injection for cricopharyngeus muscle dysfunction.
Most patients require repeat injections when the toxin wears off, although occasionally one or two injections provide long-term or permanent relief. Dosages are adjusted for the patient’s age, the presence of other swallowing problems, and reflux. Patients may experience increased difficulty swallowing for 1 or 2 weeks after the procedure and so should be counseled to eat slowly and carefully.
- Cultrara A, Chitkara A, Blitzer A. Botulinum toxin injections for the treatment of oromandibular dystonia. Oper Tech Otolaryngol Head Neck Surg 2004; 15:97–102.
- Fahn S. The varied clinical expressions of dystonia. Neurol Clin 1984; 2:541–554.
- Fahn S. Concept and classification of dystonia. Adv Neurol 1988; 50:1–8.
- Benninger MS, Knott PD. Techniques of botulinum toxin injections in the head and neck. San Diego, CA: Plural Publishing, Inc; 2012.
- Benninger MS, Gardner G, Grywalski C. Outcomes of botulinum toxin treatment for spasmodic dysphonia. Arch Otolaryngol Head Neck Surg 2001; 127:1083–1085.
- Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope 1998; 108:1435–1441.
- Klein AM, Stong BC, Wise J, DelGaudio JM, Hapner ER, Johns MM 3rd. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg 2008; 139:421–423.
- Hertegård S, Granqvist S, Lindestad PA. Botulinum toxin injections for essential voice tremor. Ann Otol Rhinol Laryngol 2000; 109:204–209.
- Bové M, Daamen N, Rosen C, Wang CC, Sulica L, Gartner-Schmidt J. Development and validation of the vocal tremor scoring system. Laryngoscope 2006; 116:1662–1667.
- Kendall KA, Leonard RJ. Interarytenoid muscle Botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J Voice 2001; 25:114–119.
- Cultrara A, Chitkara A, Blitzer A. Botulinum toxin injections for the treatment of oromandibular dystonia. Oper Tech Otolaryngol Head Neck Surg 2004; 15:97–102.
- Fahn S. The varied clinical expressions of dystonia. Neurol Clin 1984; 2:541–554.
- Fahn S. Concept and classification of dystonia. Adv Neurol 1988; 50:1–8.
- Benninger MS, Knott PD. Techniques of botulinum toxin injections in the head and neck. San Diego, CA: Plural Publishing, Inc; 2012.
- Benninger MS, Gardner G, Grywalski C. Outcomes of botulinum toxin treatment for spasmodic dysphonia. Arch Otolaryngol Head Neck Surg 2001; 127:1083–1085.
- Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope 1998; 108:1435–1441.
- Klein AM, Stong BC, Wise J, DelGaudio JM, Hapner ER, Johns MM 3rd. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg 2008; 139:421–423.
- Hertegård S, Granqvist S, Lindestad PA. Botulinum toxin injections for essential voice tremor. Ann Otol Rhinol Laryngol 2000; 109:204–209.
- Bové M, Daamen N, Rosen C, Wang CC, Sulica L, Gartner-Schmidt J. Development and validation of the vocal tremor scoring system. Laryngoscope 2006; 116:1662–1667.
- Kendall KA, Leonard RJ. Interarytenoid muscle Botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J Voice 2001; 25:114–119.
KEY POINTS
- Botulinum toxin can be injected with a variety of approaches directly into the affected muscle exhibiting abnormal contractions.
- Depending on the muscles involved, side effects may include breathiness or difficulty swallowing for a period soon after injection.
- Injections can be repeated as needed as the toxin wears off.
- Some conditions are more amenable to treatment than others. Benefit can be enhanced by altering the dosage or injection site.
Women’s health 2015: An update for the internist
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
KEY POINTS
- Earlier trials had raised concerns about possible teratogenic effects of selective serotonin reuptake inhibitors, but more recent trials have found no strong association between these drugs and congenital heart defects, and no association with miscarriage or autism spectrum disorder, though there may be a risk of attention deficit hyperactivity disorder in offspring.
- Paroxetine is approved for treating vasomotor symptoms of menopause, but in a lower dose (7.5 mg) than those used for depression and other psychiatric indications. Clinical trials have also shown good results with other antidepressants for treating hot flashes, but the drugs are not yet approved for this indication.
- Women with heart failure and left bundle-branch block can decrease their risk of death with cardiac resynchronization therapy more than men with the same condition. Moreover, women may benefit from this therapy even if their QRS duration is somewhat shorter than the established cutoff, ie, if it is in the range of 130 to 149 ms.