User login
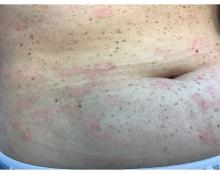
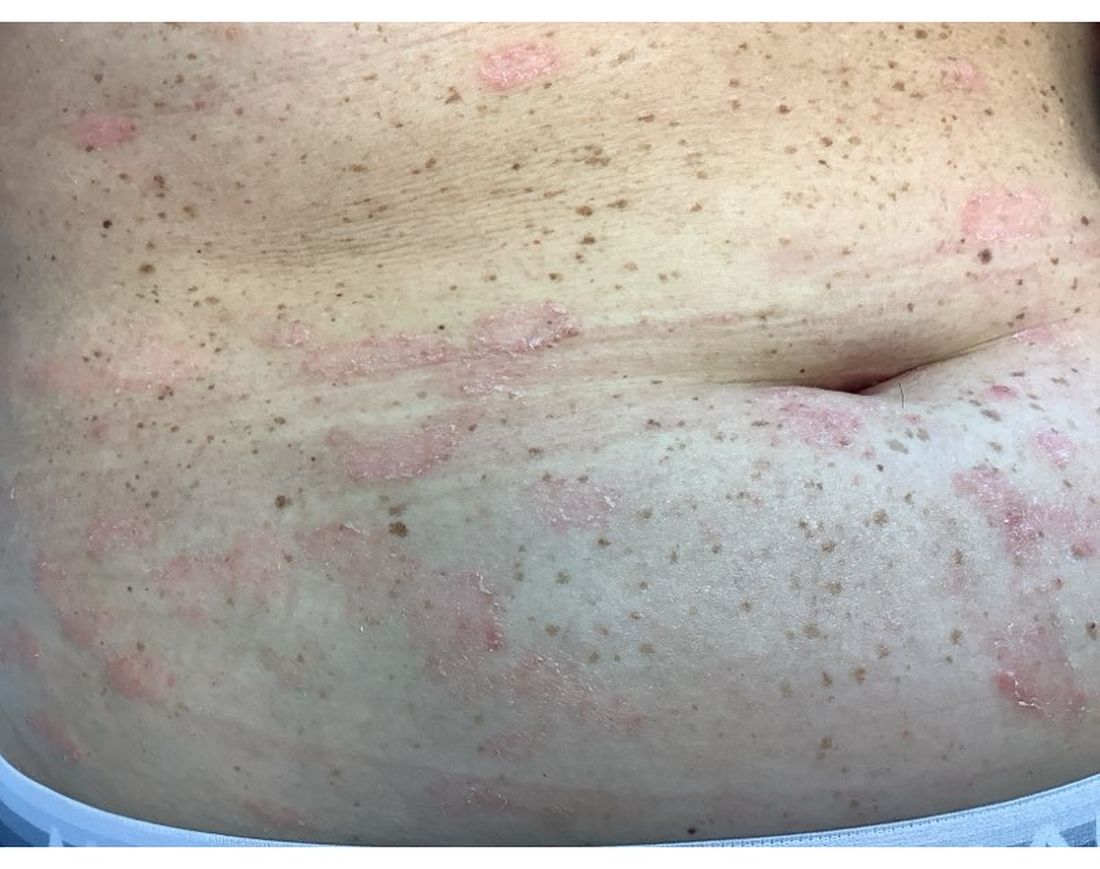
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Mild Hidradenitis Suppurativa: Positive Results Reported for Topical Therapy
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
FROM AAD 2024
Study Identifies Several Factors That Influence Longterm Antibiotic Prescribing for Acne
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
FROM JAMA DERMATOLOGY
Androgenetic Alopecia: Study Finds Efficacy of Topical and Oral Minoxidil Similar
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
FROM JAMA DERMATOLOGY
Bimekizumab Under FDA Review for Hidradenitis Suppurativa
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
Childhood Atopic Dermatitis Linked to IBD Risk
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
New Tool Helps Clinicians Detect Zoom Dysmorphia in Virtual Settings
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
FROM AAD 2024
Analysis Finds Low Malignancy Rate in Pediatric Longitudinal Melanonychia
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
Tooth Enamel Disorder Is a Feature of Kindler EB
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Dx Across the Skin Color Spectrum: Longitudinal Melanonychia
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009