User login
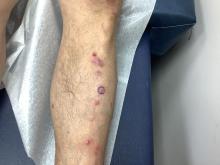
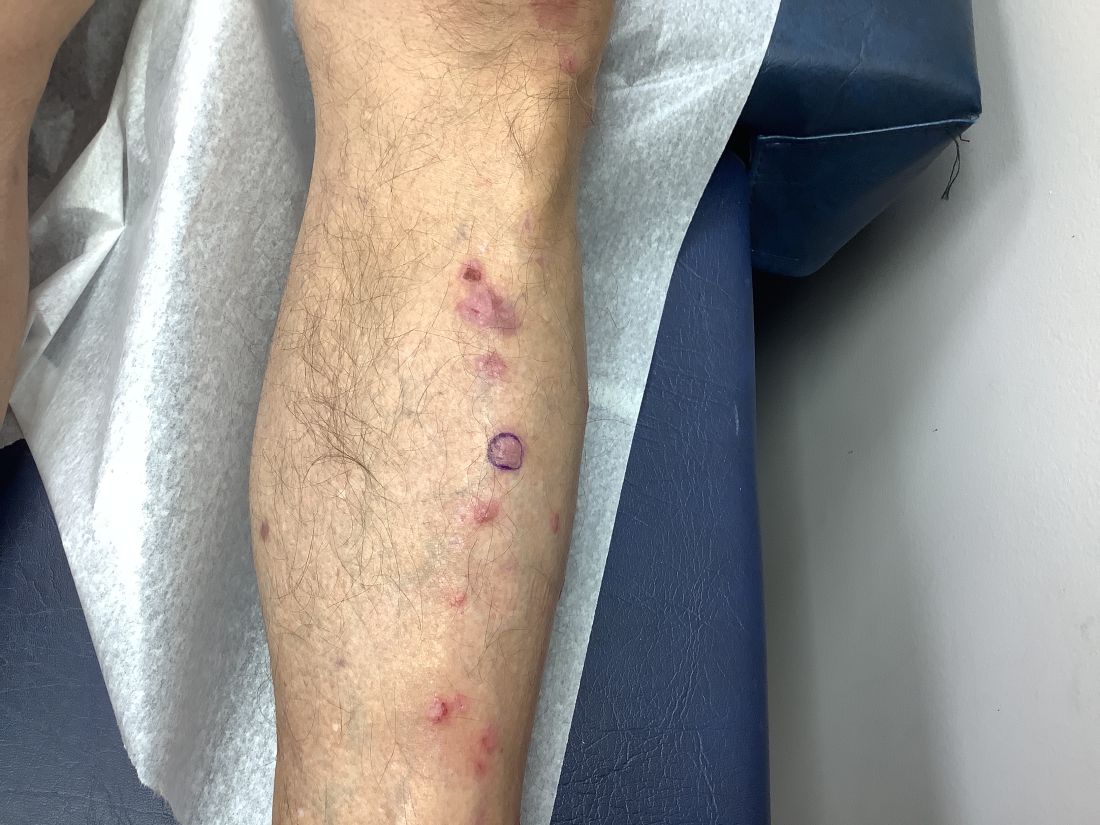
Pruritic, violaceous papules in a patient with renal cell carcinoma
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Photoprotection: Benefits of Sunscreens With Iron Oxide
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CHICAGO — One of the more recent developments in sunscreen technology is the addition of iron oxide to mineral sunscreens.
Iron oxide is “an excellent pigment” that absorbs and blocks visible light, which is particularly important in individuals with Fitzpatrick skin types III-VI, Zoe D. Draelos, MD, consulting professor of dermatology at Duke University, Durham, North Carolina, said at the Pigmentation Disorders Exchange symposium.
Susan C. Taylor, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, who spoke at the conference, also recommended tinted sunscreen with iron oxide for patients with skin of color. “It still needs to be broad spectrum,” she said, “and at least an SPF [Sun Protection Factor] 30.”
When blended with mineral sunscreens, iron oxide can reduce transmission of visible light by 90% and can protect patients from hyperpigmentation. Iron oxide comes in different colors blended together for various degrees of tinting.
Dr. Taylor noted that iron oxide is listed under the inactive ingredients. “The literature indicates a 3% concentration to aim for, but we don’t know the concentration in most of the products,” she added.
During her presentation, Dr. Draelos noted that inorganic sunscreens, such as zinc oxide and titanium oxides, are highly effective but make the skin white and pasty. To address this issue, many companies are now grinding these materials into such small particles that they are transparent.
“That’s great, except the smaller the particle is, the less UV [ultraviolet] radiation it reflects and that lowers the [SPF],” she said.
In addition to providing photoprotection, sunscreens in general provide protection from nanoparticles in tobacco and combustion, such as traffic exhaust, which can harm skin over time. “Moisturizers and sunscreens are the best way to protect against pollution and tobacco nanoparticle damage, which can contribute to inflammation,” she noted. They create a film over the skin and trap the nanoparticles.
Start the Patient Visit With a Photoprotection Talk
At the meeting, Dr. Taylor recommended that for all patients with hypopigmentation and hyperpigmentation disorders, “treatment really begins with photoprotection.”
She acknowledged that photoprotection discussions, including the basics of seeking shade, wearing protective clothing, and avoiding midday sun, often come at the end of the patient visit but she urged dermatologists to make that the first topic instead.
Dr. Taylor said a question often asked of patients of color about prolonged sun exposure — whether their skin turns bright red after too much sun — may get a negative reply. The better question is whether the patient has experienced tender skin after too much sun — which can signify a sunburn, she said.
Dr. Draelos reported no relevant financial relationships. Dr. Taylor reported financial relationships and grant support from multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year
Diagnosis
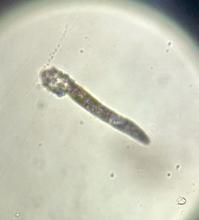
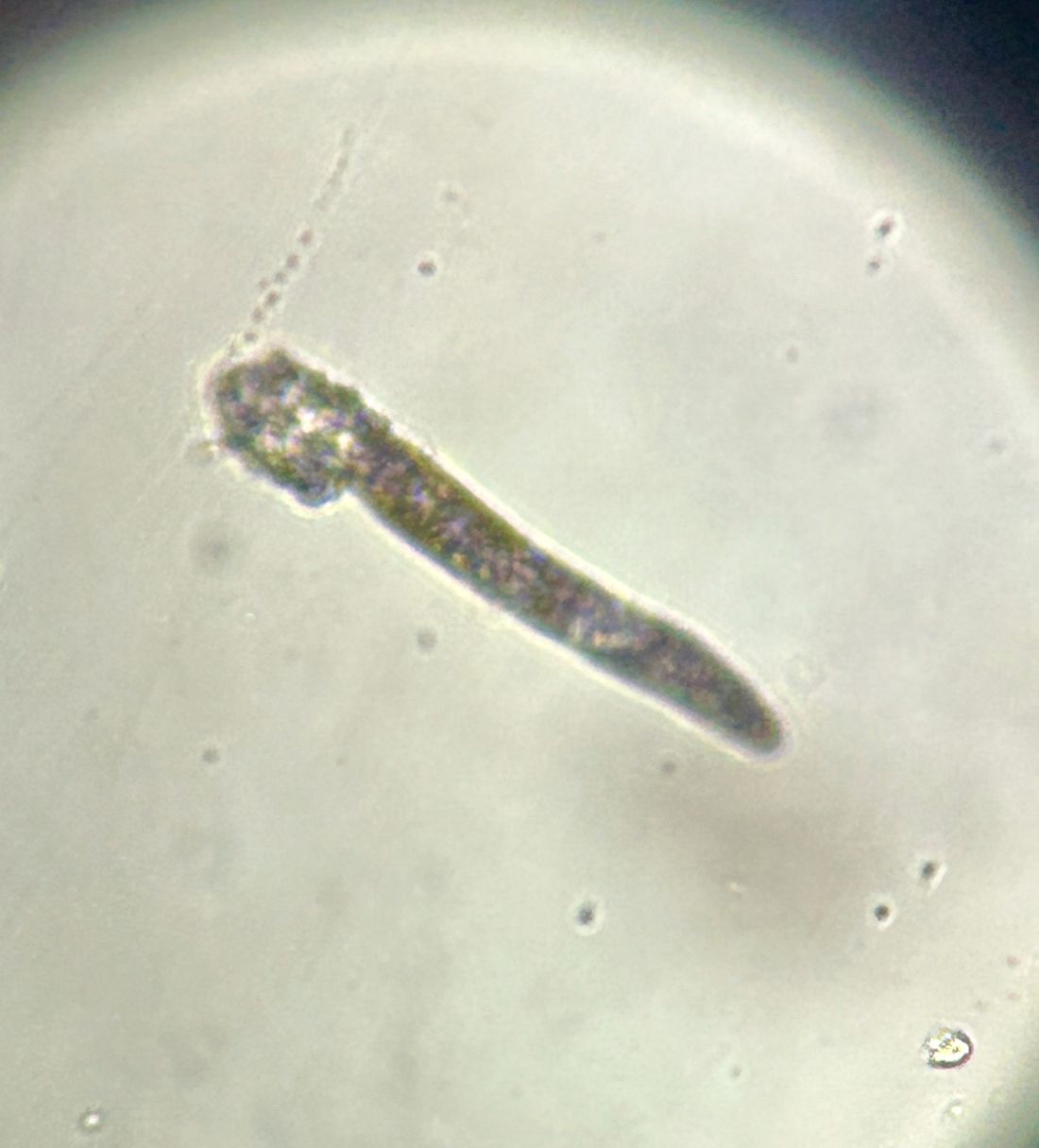
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year. She had been treated with several topical antibiotics and acne washes without resolution of the lesions. There were no signs of early puberty, and the child had no history of medical conditions. Her mother has a history of rosacea. Physical examination revealed erythematous papules on the nose and cheeks bilaterally.
Cosmetic Tattoo Ingredients Associated With Contact Dermatitis
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Study Highlights Melanoma Survival Disparities in Rural vs Urban Settings
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
, results from an analysis of data from the National Cancer Institute showed.
“Melanoma is currently the fifth most common malignancy in the United States, with approximately 106,000 new cases and 7180 reported deaths occurring in 2021,” the study’s first author, Mitchell Taylor, MD, a dermatology research fellow at the University of Nebraska, Omaha, and colleagues wrote in the abstract, which was presented during a poster session at the annual meeting of the Society for Investigative Dermatology. “Rural areas have been shown to bear a higher melanoma disease burden, yet there is a paucity of national-level studies examining these disparities.”
To characterize the rural population diagnosed with cutaneous melanoma and assess associated disparities in the United States, the researchers queried the NCI’s Surveillance, Epidemiology, and End Results database to identify individuals diagnosed with cutaneous melanoma from 2000 to 2020 (International Classification of Diseases, 3rd Edition, 8720/3 — 8780/3; Primary Site codes C44.0-C44.9). They drew from US Office of Management and Budget terminology to define and categorize rural and urban communities.
Among 391,047 patients included during the study period, binary logistic regression analysis revealed that patients in rural areas had a greater odds of being older, from ages 50 to 75 years (odds ratio [OR], 1.10; P < .001); had annual incomes < $70,000 (OR, 16.80; P < .001); had tumors located on the head and neck (OR, 1.24; P < .001); and presented with regional/distant disease (OR, 1.13; P < .001).
As for disease-specific survival, patients living in rural areas had significantly reduced survival compared with those living in urban areas (a mean of 207.3 vs 216.3 months, respectively; P < .001). Multivariate Cox regression revealed that living in a rural setting was significantly associated with reduced disease-specific survival (hazard ratio [HR], 1.10; P < .001), as was having head and neck tumors (HR, 1.41; P < .001).“Overall, this study underscores a significant decrease in disease-specific survival among rural patients diagnosed with cutaneous melanoma and establishes a significant association between rural living and high-risk primary tumor locations, particularly the head and neck,” the authors concluded.
Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, Colorado, who was asked to comment on the results, said the findings echo the results of a recent study which characterized melanoma rates among non-Hispanic American Indian/Alaska Native individuals from 1999 to 2019.
“I suspect this decreased disease-specific survival highlights the issues our rural-residing patients face with access to dermatology care,” Dr. Kohn told this news organization. “Dermatologists are able to detect thinner melanomas than patients [and] are preferentially concentrated in metropolitan areas. Dermatologists are also the most skilled and knowledgeable to screen, diagnose, and manage melanomas. Having fewer dermatologists in rural areas impedes melanoma care for our rural-residing patients.”
Neither the researchers nor Dr. Kohn reported any relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SID 2024
Features of Merkel Cell in Hispanic Patients Explored
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
. In addition, the most affected site was the upper limb/shoulder, which differs from what has been reported in previous studies.
Those are key findings from a retrospective study of national cancer data that was presented during a poster session at the annual meeting of the Society for Investigative Dermatology.
“Merkel cell carcinoma is an infrequent and aggressive form of neuroendocrine skin cancer that mainly impacts individuals of White ethnicity, with a general occurrence rate of 0.7 instances per 100,000 person-years,” one of the study authors, Luis J. Borda, MD, chief dermatology resident at Eastern Virginia Medical School, Norfolk, Virginia, told this news organization. The incidence of MCC is increasing among all racial groups, especially in the Hispanic population, he added.
To determine how age, sex, and primary site of MCC differ in White vs non-White Hispanic patients, the researchers evaluated the 22 population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program from 2000 through 2020. They reported categorical variables as counts and percentages and used chi-square test with Yates’s correction to assess the association between categorical variables.
Of the 17,920 MCCs identified by the researchers, 40 (0.22%) were in non-White Hispanic patients. Compared with the White patients with MCC, significantly fewer non-White Hispanic patients were age 70 years or older (50% vs 72.1%, respectively; P < .001), and MCC was more common in female non-White Hispanic patients (23, or 57.5%), while White patients with MCC were predominantly male (11,309, or 63.2%; P < .05). “This suggests that MCC in non-White Hispanic patients may involve different risk factors related to age beyond just cumulative UV exposure and aging-related immunosenescence, which may additionally account for the higher prevalence of females in this cohort, as historically male outdoor occupation has resulted in increased lifetime cumulative UV exposure,” Dr. Borda said.
The head and neck were the most common sites of disease involvement in White patients (41.9% vs 27.5% in non-White Hispanic patients; P = .09), while the upper limb and shoulder were the most common sites of disease involvement in non-White Hispanic patients (37.5% vs 23.8% in White patients; P = .06). This finding “differs from previous studies showing head/neck being the most common site in Hispanics,” Dr. Borda said, adding that this could be a result of White patients not being included in the Hispanic cohort in this study. “Because non-White Hispanic patients have darker skin, they may have proportionally more cases on sun-protected skin, as is described by the present data, suggesting that they are less likely to have UV-driven MCC.”
The study “highlights distinct demographic and clinical characteristics of MCC among non-White Hispanic patients compared to their White counterparts, emphasizing the importance of considering race/ethnicity in understanding the epidemiology of this rare but increasingly prevalent cancer,” Dr. Borda said. He and his co-authors are planning to do further research on the increasing incidence of MCC in non-White Hispanic patients and on staging at diagnosis compared to White patients.
Dr. Borda acknowledged certain limitations of the analysis, including the small sample size in the non-White Hispanic group, the retrospective nature of SEER data, selection bias, and the potential for underreporting. He and his co-authors reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
FROM SID 2024
Study Finds Isotretinoin Effective for Acne in Transgender Patients on Hormone Rx
TOPLINE:
, but more information is needed on dosing and barriers to treatment.
METHODOLOGY:
- Acne can be a side effect of masculinizing hormone therapy for transmasculine individuals. While isotretinoin is an effective treatment option for acne, its effectiveness and safety in transgender and gender-diverse individuals are not well understood.
- This retrospective case series included 55 patients (mean age, 25.4 years) undergoing masculinizing hormone therapy at four medical centers, who were prescribed isotretinoin for acne associated with treatment.
- Isotretinoin treatment was started a median of 22.1 months after hormone therapy was initiated and continued for a median of 6 months with a median cumulative dose of 132.7 mg/kg.
- Researchers assessed acne improvement, clearance, recurrence, adverse effects, and reasons for treatment discontinuation.
TAKEAWAY:
- Overall, 48 patients (87.3%) experienced improvement, and 26 (47.3%) achieved clearance during treatment. A higher proportion of patients experienced improvement (97% vs 72.7%) and achieved clearance (63.6% vs 22.7%) with cumulative doses of ≥ 120 mg/kg than those who received cumulative doses < 120 mg/kg.
- The risk for recurrence was 20% (in four patients) among 20 patients who achieved clearance and had any subsequent health care encounters, with a mean follow-up time of 734.3 days.
- Common adverse effects included dryness (80%), joint pain (14.5%), and headaches (10.9%). Other adverse effects included nose bleeds (9.1%) and depression (5.5%).
- Of the 22 patients with a cumulative dose < 120 mg/kg, 14 (63.6%) were lost to follow-up; among those not lost to follow-up, 2 patients discontinued treatment because of transfer of care, 1 because of adverse effects, and 1 because of gender-affirming surgery, with concerns about wound healing.
IN PRACTICE:
“Although isotretinoin appears to be an effective treatment option for acne among individuals undergoing masculinizing hormone therapy, further efforts are needed to understand optimal dosing and treatment barriers to improve outcomes in transgender and gender-diverse individuals receiving testosterone,” the authors concluded.
SOURCE:
The study, led by James Choe, BS, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, was published online in JAMA Dermatology.
LIMITATIONS:
The study population was limited to four centers, and variability in clinician- and patient-reported acne outcomes and missing information could affect the reliability of data. Because of the small sample size, the association of masculinizing hormone therapy regimens with outcomes could not be evaluated.
DISCLOSURES:
One author is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Three authors reported receiving grants or personal fees from various sources. The other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but more information is needed on dosing and barriers to treatment.
METHODOLOGY:
- Acne can be a side effect of masculinizing hormone therapy for transmasculine individuals. While isotretinoin is an effective treatment option for acne, its effectiveness and safety in transgender and gender-diverse individuals are not well understood.
- This retrospective case series included 55 patients (mean age, 25.4 years) undergoing masculinizing hormone therapy at four medical centers, who were prescribed isotretinoin for acne associated with treatment.
- Isotretinoin treatment was started a median of 22.1 months after hormone therapy was initiated and continued for a median of 6 months with a median cumulative dose of 132.7 mg/kg.
- Researchers assessed acne improvement, clearance, recurrence, adverse effects, and reasons for treatment discontinuation.
TAKEAWAY:
- Overall, 48 patients (87.3%) experienced improvement, and 26 (47.3%) achieved clearance during treatment. A higher proportion of patients experienced improvement (97% vs 72.7%) and achieved clearance (63.6% vs 22.7%) with cumulative doses of ≥ 120 mg/kg than those who received cumulative doses < 120 mg/kg.
- The risk for recurrence was 20% (in four patients) among 20 patients who achieved clearance and had any subsequent health care encounters, with a mean follow-up time of 734.3 days.
- Common adverse effects included dryness (80%), joint pain (14.5%), and headaches (10.9%). Other adverse effects included nose bleeds (9.1%) and depression (5.5%).
- Of the 22 patients with a cumulative dose < 120 mg/kg, 14 (63.6%) were lost to follow-up; among those not lost to follow-up, 2 patients discontinued treatment because of transfer of care, 1 because of adverse effects, and 1 because of gender-affirming surgery, with concerns about wound healing.
IN PRACTICE:
“Although isotretinoin appears to be an effective treatment option for acne among individuals undergoing masculinizing hormone therapy, further efforts are needed to understand optimal dosing and treatment barriers to improve outcomes in transgender and gender-diverse individuals receiving testosterone,” the authors concluded.
SOURCE:
The study, led by James Choe, BS, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, was published online in JAMA Dermatology.
LIMITATIONS:
The study population was limited to four centers, and variability in clinician- and patient-reported acne outcomes and missing information could affect the reliability of data. Because of the small sample size, the association of masculinizing hormone therapy regimens with outcomes could not be evaluated.
DISCLOSURES:
One author is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Three authors reported receiving grants or personal fees from various sources. The other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but more information is needed on dosing and barriers to treatment.
METHODOLOGY:
- Acne can be a side effect of masculinizing hormone therapy for transmasculine individuals. While isotretinoin is an effective treatment option for acne, its effectiveness and safety in transgender and gender-diverse individuals are not well understood.
- This retrospective case series included 55 patients (mean age, 25.4 years) undergoing masculinizing hormone therapy at four medical centers, who were prescribed isotretinoin for acne associated with treatment.
- Isotretinoin treatment was started a median of 22.1 months after hormone therapy was initiated and continued for a median of 6 months with a median cumulative dose of 132.7 mg/kg.
- Researchers assessed acne improvement, clearance, recurrence, adverse effects, and reasons for treatment discontinuation.
TAKEAWAY:
- Overall, 48 patients (87.3%) experienced improvement, and 26 (47.3%) achieved clearance during treatment. A higher proportion of patients experienced improvement (97% vs 72.7%) and achieved clearance (63.6% vs 22.7%) with cumulative doses of ≥ 120 mg/kg than those who received cumulative doses < 120 mg/kg.
- The risk for recurrence was 20% (in four patients) among 20 patients who achieved clearance and had any subsequent health care encounters, with a mean follow-up time of 734.3 days.
- Common adverse effects included dryness (80%), joint pain (14.5%), and headaches (10.9%). Other adverse effects included nose bleeds (9.1%) and depression (5.5%).
- Of the 22 patients with a cumulative dose < 120 mg/kg, 14 (63.6%) were lost to follow-up; among those not lost to follow-up, 2 patients discontinued treatment because of transfer of care, 1 because of adverse effects, and 1 because of gender-affirming surgery, with concerns about wound healing.
IN PRACTICE:
“Although isotretinoin appears to be an effective treatment option for acne among individuals undergoing masculinizing hormone therapy, further efforts are needed to understand optimal dosing and treatment barriers to improve outcomes in transgender and gender-diverse individuals receiving testosterone,” the authors concluded.
SOURCE:
The study, led by James Choe, BS, Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School, Boston, was published online in JAMA Dermatology.
LIMITATIONS:
The study population was limited to four centers, and variability in clinician- and patient-reported acne outcomes and missing information could affect the reliability of data. Because of the small sample size, the association of masculinizing hormone therapy regimens with outcomes could not be evaluated.
DISCLOSURES:
One author is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Three authors reported receiving grants or personal fees from various sources. The other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Hidradenitis Suppurativa: Clinical Outcomes for Bimekizumab Positive in Phase 3 Studies
TOPLINE:
, in two phase 3 studies.
METHODOLOGY:
- To assess the efficacy and safety of bimekizumab, an interleukin (IL)-17A and IL-17F antagonist, 320 mg for HS, researchers conducted two 48-week phase 3 trials BE HEARD I (n = 505) and II (n = 509), which enrolled patients with moderate to severe HS and a history of inadequate response to systemic antibiotics.
- Patients were randomly assigned to one of four groups: Bimekizumab every 2 weeks, bimekizumab every 2 weeks for 16 weeks followed by every 4 weeks of dosing, bimekizumab every 4 weeks, or placebo for 16 weeks followed by bimekizumab every 2 weeks.
- The primary outcome was an HS clinical response of at least 50% (HiSCR50) at week 16, defined as at least a 50% reduction in total abscess and inflammatory nodule count.
TAKEAWAY:
- A higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved an HiSCR50 response at week 16 in BE HEARD I (48% vs 29%; odds ratio [OR], 2.23; P = .006) and II (52% vs 32%; OR, 2.29; P = .0032) trials.
- Patients receiving bimekizumab every 4 weeks also achieved a higher HiSCR50 response at week 16 vs placebo in the BE HEARD II trial (54% vs 32%; OR, 2.42; P = .0038).
- At week 16, a higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved at least a 75% HiSCR (HiSCR75) in both trials, and a higher proportion of those receiving bimekizumab every 4 weeks achieved HiSCR75 in the BE HEARD II trial.
- At week 48, 45%-68% of patients achieved HiSCR50 in both trials.
- Patients who received bimekizumab vs placebo for the initial 16 weeks had greater improvements in patient-reported outcomes, and bimekizumab was well tolerated with a low number of serious or severe treatment-emergent adverse events.
IN PRACTICE:
“Bimekizumab was well tolerated by patients with hidradenitis suppurativa and produced rapid and deep clinically meaningful responses that were maintained up to 48 weeks,” the authors wrote. “These data support the use of bimekizumab as a promising new therapeutic option for patients with moderate to severe hidradenitis suppurativa.”
SOURCE:
Alexa B. Kimball, MD, MPH, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, led this study, which was published online in The Lancet.
LIMITATIONS:
The placebo-controlled part of this trial was relatively short at 16 weeks and may affect the interpretation of later efficacy data, there was a lack of an active comparator group, and the efficacy of treatment was evaluated in the presence of rescue treatment with systemic antibiotics.
DISCLOSURES:
The studies were funded by bimekizumab manufacturer UCB Pharma. Seven authors disclosed being current or former employees of UCB Pharma. Other authors reported several ties with many companies, including UCB Pharma.
A version of this article first appeared on Medscape.com.
TOPLINE:
, in two phase 3 studies.
METHODOLOGY:
- To assess the efficacy and safety of bimekizumab, an interleukin (IL)-17A and IL-17F antagonist, 320 mg for HS, researchers conducted two 48-week phase 3 trials BE HEARD I (n = 505) and II (n = 509), which enrolled patients with moderate to severe HS and a history of inadequate response to systemic antibiotics.
- Patients were randomly assigned to one of four groups: Bimekizumab every 2 weeks, bimekizumab every 2 weeks for 16 weeks followed by every 4 weeks of dosing, bimekizumab every 4 weeks, or placebo for 16 weeks followed by bimekizumab every 2 weeks.
- The primary outcome was an HS clinical response of at least 50% (HiSCR50) at week 16, defined as at least a 50% reduction in total abscess and inflammatory nodule count.
TAKEAWAY:
- A higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved an HiSCR50 response at week 16 in BE HEARD I (48% vs 29%; odds ratio [OR], 2.23; P = .006) and II (52% vs 32%; OR, 2.29; P = .0032) trials.
- Patients receiving bimekizumab every 4 weeks also achieved a higher HiSCR50 response at week 16 vs placebo in the BE HEARD II trial (54% vs 32%; OR, 2.42; P = .0038).
- At week 16, a higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved at least a 75% HiSCR (HiSCR75) in both trials, and a higher proportion of those receiving bimekizumab every 4 weeks achieved HiSCR75 in the BE HEARD II trial.
- At week 48, 45%-68% of patients achieved HiSCR50 in both trials.
- Patients who received bimekizumab vs placebo for the initial 16 weeks had greater improvements in patient-reported outcomes, and bimekizumab was well tolerated with a low number of serious or severe treatment-emergent adverse events.
IN PRACTICE:
“Bimekizumab was well tolerated by patients with hidradenitis suppurativa and produced rapid and deep clinically meaningful responses that were maintained up to 48 weeks,” the authors wrote. “These data support the use of bimekizumab as a promising new therapeutic option for patients with moderate to severe hidradenitis suppurativa.”
SOURCE:
Alexa B. Kimball, MD, MPH, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, led this study, which was published online in The Lancet.
LIMITATIONS:
The placebo-controlled part of this trial was relatively short at 16 weeks and may affect the interpretation of later efficacy data, there was a lack of an active comparator group, and the efficacy of treatment was evaluated in the presence of rescue treatment with systemic antibiotics.
DISCLOSURES:
The studies were funded by bimekizumab manufacturer UCB Pharma. Seven authors disclosed being current or former employees of UCB Pharma. Other authors reported several ties with many companies, including UCB Pharma.
A version of this article first appeared on Medscape.com.
TOPLINE:
, in two phase 3 studies.
METHODOLOGY:
- To assess the efficacy and safety of bimekizumab, an interleukin (IL)-17A and IL-17F antagonist, 320 mg for HS, researchers conducted two 48-week phase 3 trials BE HEARD I (n = 505) and II (n = 509), which enrolled patients with moderate to severe HS and a history of inadequate response to systemic antibiotics.
- Patients were randomly assigned to one of four groups: Bimekizumab every 2 weeks, bimekizumab every 2 weeks for 16 weeks followed by every 4 weeks of dosing, bimekizumab every 4 weeks, or placebo for 16 weeks followed by bimekizumab every 2 weeks.
- The primary outcome was an HS clinical response of at least 50% (HiSCR50) at week 16, defined as at least a 50% reduction in total abscess and inflammatory nodule count.
TAKEAWAY:
- A higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved an HiSCR50 response at week 16 in BE HEARD I (48% vs 29%; odds ratio [OR], 2.23; P = .006) and II (52% vs 32%; OR, 2.29; P = .0032) trials.
- Patients receiving bimekizumab every 4 weeks also achieved a higher HiSCR50 response at week 16 vs placebo in the BE HEARD II trial (54% vs 32%; OR, 2.42; P = .0038).
- At week 16, a higher proportion of patients receiving bimekizumab every 2 weeks vs placebo achieved at least a 75% HiSCR (HiSCR75) in both trials, and a higher proportion of those receiving bimekizumab every 4 weeks achieved HiSCR75 in the BE HEARD II trial.
- At week 48, 45%-68% of patients achieved HiSCR50 in both trials.
- Patients who received bimekizumab vs placebo for the initial 16 weeks had greater improvements in patient-reported outcomes, and bimekizumab was well tolerated with a low number of serious or severe treatment-emergent adverse events.
IN PRACTICE:
“Bimekizumab was well tolerated by patients with hidradenitis suppurativa and produced rapid and deep clinically meaningful responses that were maintained up to 48 weeks,” the authors wrote. “These data support the use of bimekizumab as a promising new therapeutic option for patients with moderate to severe hidradenitis suppurativa.”
SOURCE:
Alexa B. Kimball, MD, MPH, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, led this study, which was published online in The Lancet.
LIMITATIONS:
The placebo-controlled part of this trial was relatively short at 16 weeks and may affect the interpretation of later efficacy data, there was a lack of an active comparator group, and the efficacy of treatment was evaluated in the presence of rescue treatment with systemic antibiotics.
DISCLOSURES:
The studies were funded by bimekizumab manufacturer UCB Pharma. Seven authors disclosed being current or former employees of UCB Pharma. Other authors reported several ties with many companies, including UCB Pharma.
A version of this article first appeared on Medscape.com.
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
FDA Grants New Pediatric Arthritis Indications for Upadacitinib
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.