User login
B6 a new approach for depression, anxiety?
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pharmacogenomic testing may curb drug interactions in severe depression
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
FROM JAMA
Reversing depression: A plethora of therapeutic strategies and mechanisms
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7
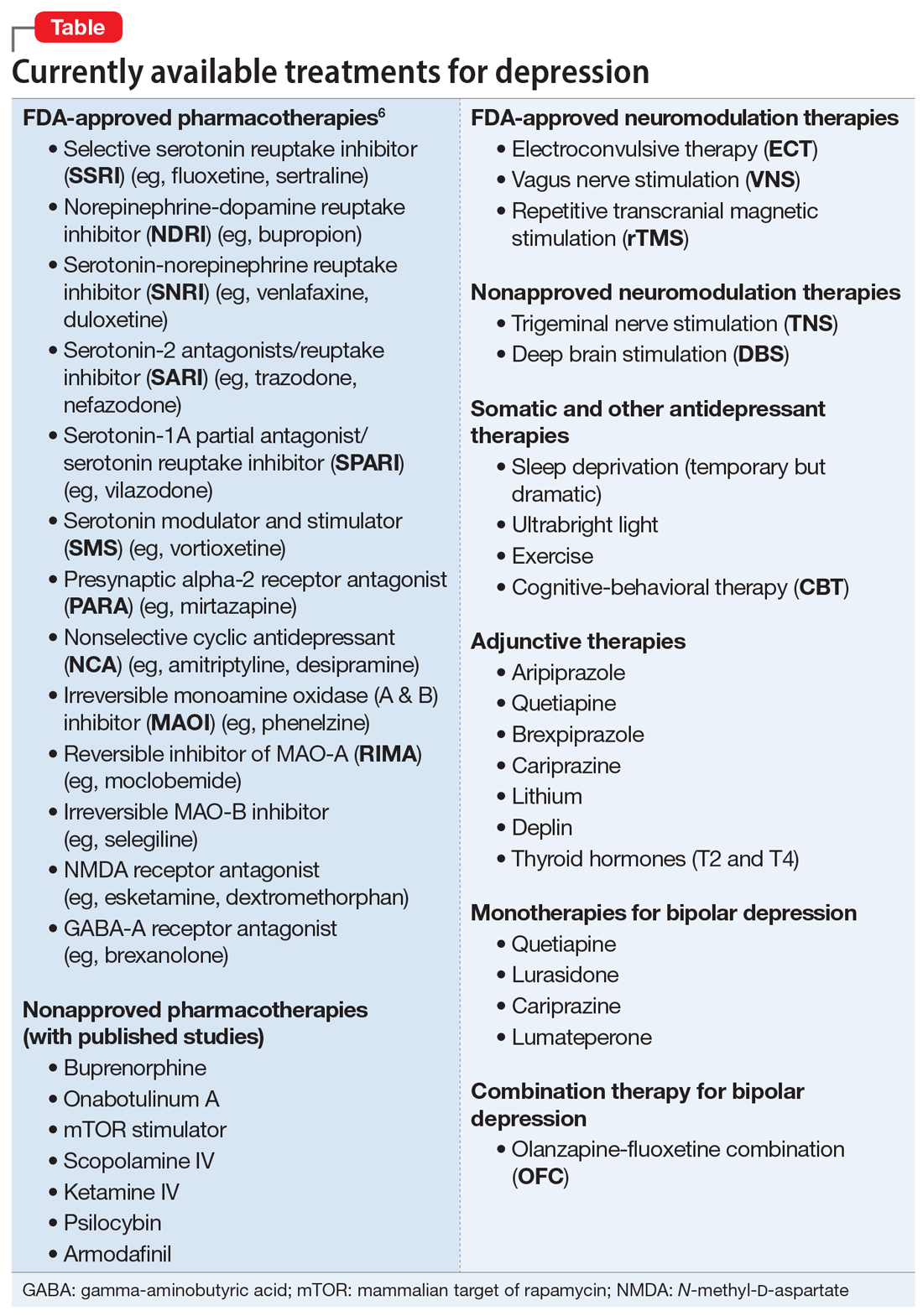
These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
High rate of mental health problems in transgender children
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Guideline advises against depression screening in pregnancy
The Canadian Task Force on Preventive Health Care recommends against the routine screening of all pregnant and postpartum women for depression using a standard questionnaire, according to its new guideline.
The basis for its position is the lack of evidence that such screening “adds value beyond discussions about overall wellbeing, depression, anxiety, and mood that are currently a part of established perinatal clinical care.
“We should not be using a one-size-fits all approach,” lead author Eddy Lang, MD, professor and head of emergency medicine at the Cumming School of Medicine, University of Calgary (Alta.), told this news organization.
Instead, the task force emphasizes regular clinical care, including asking patients about their wellbeing and support systems. The task force categorizes the recommendation as conditional and as having very low-certainty evidence.
The recommendation was published in CMAJ.
One randomized study
The task force is an independent panel of clinicians and scientists that makes recommendations on primary and secondary prevention in primary care. A working group of five members of the task force developed this recommendation with scientific support from Public Health Agency of Canada staff.
In its research, the task force found only one study that showed a benefit of routine depression screening in this population. This study was a randomized controlled trial conducted in Hong Kong. Researchers evaluated 462 postpartum women who were randomly assigned to receive screening with the Edinburgh Postnatal Depression Scale (EPDS) or no screening 2 months post partum.
“We found the effect of screening in this study to be very uncertain for the important outcomes of interest,” said Dr. Lang.
“These included parent-child stress, marital stress, and the number of infant hospital admissions. The effects of screening on all of these outcomes were very uncertain, mainly because it was such a small trial,” he said.
The task force also assessed how pregnant and postpartum women feel about being screened. What these women most wanted was a good relationship with a trusted primary care provider who would initiate discussions about their mental health in a caring atmosphere.
“Although they told us they liked the idea of universal screening, they admitted to their family doctors that they actually preferred to be asked about their wellbeing, [to be asked] how things were going at home, and [to have] a discussion about their mental health and wellbeing, rather than a formal screening process. They felt a discussion about depression with a primary health care provider during the pregnancy and postpartum period is critical,” said Dr. Lang.
Thus, the task force recommends “against instrument-based depression screening using a questionnaire with cutoff score to distinguish ‘screen positive’ and ‘screen negative’ administered to all individuals during pregnancy and the postpartum period (up to 1 year after childbirth).”
Screening remains common
“There’s a lot of uncertainty in the scientific community about whether it’s a good idea to administer a screening test to all pregnant and postpartum women to determine in a systematic way if they might be suffering from depression,” said Dr. Lang.
The task force recommended against screening for depression among perinatal or postpartum women in 2013, but screening is still performed in many provinces, said Dr. Lang.
Dr. Lang emphasized that the recommendation does not apply to usual care, in which the provider asks questions about and discusses a patient’s mental health and proceeds on the basis of their clinical judgment; nor does it apply to diagnostic pathways in which the clinician suspects that the individual may have depression and tests her accordingly.
“What we are saying in our recommendation is that all clinicians should ask about a patient’s wellbeing, about their mood, their anxiety, and these questions are an important part of the clinical assessment of pregnant and postpartum women. But we’re also saying the usefulness of doing so with a questionnaire and using a cutoff score on the questionnaire to decide who needs further assessment or possibly treatment is unproven by the research,” Dr. Lang said.
A growing problem
For Diane Francoeur, MD, CEO of the Society of Obstetricians and Gynecologists of Canada, this is all well and good, but the reality is that such screening is better than nothing.
Quebec is the only Canadian province that conducts universal screening for all pregnant and postpartum women, Dr. Francoeur said in an interview. She was not part of the task force.
“I agree that it should be more than one approach, but the problem is that there is such a shortage of resources. There are many issues that can arise when you follow a woman during her pregnancy,” she said.
Dr. Francoeur said that COVID-19 has been particularly tough on women, including pregnant and postpartum women, who are the most vulnerable.
“Especially during the COVID era, it was astonishing how women were not doing well. Their stress level was so high. We need to have a specific approach dedicated to prenatal mental health, because it’s a problem that is bigger than it used to be,” she said.
Violence against women has increased considerably since the beginning of the COVID-19 pandemic, said Dr. Francoeur. “Many more women have been killed by their partners. We have never seen anything like this before, and I hope we will never see this again,” she said.
“Help was more available a few years ago, but now, it’s really hard if and when you need to have a quick consultation with a specialist and the woman is really depressed. It can take forever. So, it’s okay to screen, but then, what’s next? Who is going to be there to take these women and help them? And we don’t have the answer,” Dr. Francoeur said.
Pregnant and postpartum women who suffer from depression need more than pills, she added. “We reassure them and treat their depression pharmacologically, but it’s also a time to give appropriate support and help them through the pregnancy and get well prepared to receive their newborn, because, as we now know, that first year of life is really important for the child, and the mom needs to be supported.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. Dr. Lang and Dr. Francoeur reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Canadian Task Force on Preventive Health Care recommends against the routine screening of all pregnant and postpartum women for depression using a standard questionnaire, according to its new guideline.
The basis for its position is the lack of evidence that such screening “adds value beyond discussions about overall wellbeing, depression, anxiety, and mood that are currently a part of established perinatal clinical care.
“We should not be using a one-size-fits all approach,” lead author Eddy Lang, MD, professor and head of emergency medicine at the Cumming School of Medicine, University of Calgary (Alta.), told this news organization.
Instead, the task force emphasizes regular clinical care, including asking patients about their wellbeing and support systems. The task force categorizes the recommendation as conditional and as having very low-certainty evidence.
The recommendation was published in CMAJ.
One randomized study
The task force is an independent panel of clinicians and scientists that makes recommendations on primary and secondary prevention in primary care. A working group of five members of the task force developed this recommendation with scientific support from Public Health Agency of Canada staff.
In its research, the task force found only one study that showed a benefit of routine depression screening in this population. This study was a randomized controlled trial conducted in Hong Kong. Researchers evaluated 462 postpartum women who were randomly assigned to receive screening with the Edinburgh Postnatal Depression Scale (EPDS) or no screening 2 months post partum.
“We found the effect of screening in this study to be very uncertain for the important outcomes of interest,” said Dr. Lang.
“These included parent-child stress, marital stress, and the number of infant hospital admissions. The effects of screening on all of these outcomes were very uncertain, mainly because it was such a small trial,” he said.
The task force also assessed how pregnant and postpartum women feel about being screened. What these women most wanted was a good relationship with a trusted primary care provider who would initiate discussions about their mental health in a caring atmosphere.
“Although they told us they liked the idea of universal screening, they admitted to their family doctors that they actually preferred to be asked about their wellbeing, [to be asked] how things were going at home, and [to have] a discussion about their mental health and wellbeing, rather than a formal screening process. They felt a discussion about depression with a primary health care provider during the pregnancy and postpartum period is critical,” said Dr. Lang.
Thus, the task force recommends “against instrument-based depression screening using a questionnaire with cutoff score to distinguish ‘screen positive’ and ‘screen negative’ administered to all individuals during pregnancy and the postpartum period (up to 1 year after childbirth).”
Screening remains common
“There’s a lot of uncertainty in the scientific community about whether it’s a good idea to administer a screening test to all pregnant and postpartum women to determine in a systematic way if they might be suffering from depression,” said Dr. Lang.
The task force recommended against screening for depression among perinatal or postpartum women in 2013, but screening is still performed in many provinces, said Dr. Lang.
Dr. Lang emphasized that the recommendation does not apply to usual care, in which the provider asks questions about and discusses a patient’s mental health and proceeds on the basis of their clinical judgment; nor does it apply to diagnostic pathways in which the clinician suspects that the individual may have depression and tests her accordingly.
“What we are saying in our recommendation is that all clinicians should ask about a patient’s wellbeing, about their mood, their anxiety, and these questions are an important part of the clinical assessment of pregnant and postpartum women. But we’re also saying the usefulness of doing so with a questionnaire and using a cutoff score on the questionnaire to decide who needs further assessment or possibly treatment is unproven by the research,” Dr. Lang said.
A growing problem
For Diane Francoeur, MD, CEO of the Society of Obstetricians and Gynecologists of Canada, this is all well and good, but the reality is that such screening is better than nothing.
Quebec is the only Canadian province that conducts universal screening for all pregnant and postpartum women, Dr. Francoeur said in an interview. She was not part of the task force.
“I agree that it should be more than one approach, but the problem is that there is such a shortage of resources. There are many issues that can arise when you follow a woman during her pregnancy,” she said.
Dr. Francoeur said that COVID-19 has been particularly tough on women, including pregnant and postpartum women, who are the most vulnerable.
“Especially during the COVID era, it was astonishing how women were not doing well. Their stress level was so high. We need to have a specific approach dedicated to prenatal mental health, because it’s a problem that is bigger than it used to be,” she said.
Violence against women has increased considerably since the beginning of the COVID-19 pandemic, said Dr. Francoeur. “Many more women have been killed by their partners. We have never seen anything like this before, and I hope we will never see this again,” she said.
“Help was more available a few years ago, but now, it’s really hard if and when you need to have a quick consultation with a specialist and the woman is really depressed. It can take forever. So, it’s okay to screen, but then, what’s next? Who is going to be there to take these women and help them? And we don’t have the answer,” Dr. Francoeur said.
Pregnant and postpartum women who suffer from depression need more than pills, she added. “We reassure them and treat their depression pharmacologically, but it’s also a time to give appropriate support and help them through the pregnancy and get well prepared to receive their newborn, because, as we now know, that first year of life is really important for the child, and the mom needs to be supported.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. Dr. Lang and Dr. Francoeur reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Canadian Task Force on Preventive Health Care recommends against the routine screening of all pregnant and postpartum women for depression using a standard questionnaire, according to its new guideline.
The basis for its position is the lack of evidence that such screening “adds value beyond discussions about overall wellbeing, depression, anxiety, and mood that are currently a part of established perinatal clinical care.
“We should not be using a one-size-fits all approach,” lead author Eddy Lang, MD, professor and head of emergency medicine at the Cumming School of Medicine, University of Calgary (Alta.), told this news organization.
Instead, the task force emphasizes regular clinical care, including asking patients about their wellbeing and support systems. The task force categorizes the recommendation as conditional and as having very low-certainty evidence.
The recommendation was published in CMAJ.
One randomized study
The task force is an independent panel of clinicians and scientists that makes recommendations on primary and secondary prevention in primary care. A working group of five members of the task force developed this recommendation with scientific support from Public Health Agency of Canada staff.
In its research, the task force found only one study that showed a benefit of routine depression screening in this population. This study was a randomized controlled trial conducted in Hong Kong. Researchers evaluated 462 postpartum women who were randomly assigned to receive screening with the Edinburgh Postnatal Depression Scale (EPDS) or no screening 2 months post partum.
“We found the effect of screening in this study to be very uncertain for the important outcomes of interest,” said Dr. Lang.
“These included parent-child stress, marital stress, and the number of infant hospital admissions. The effects of screening on all of these outcomes were very uncertain, mainly because it was such a small trial,” he said.
The task force also assessed how pregnant and postpartum women feel about being screened. What these women most wanted was a good relationship with a trusted primary care provider who would initiate discussions about their mental health in a caring atmosphere.
“Although they told us they liked the idea of universal screening, they admitted to their family doctors that they actually preferred to be asked about their wellbeing, [to be asked] how things were going at home, and [to have] a discussion about their mental health and wellbeing, rather than a formal screening process. They felt a discussion about depression with a primary health care provider during the pregnancy and postpartum period is critical,” said Dr. Lang.
Thus, the task force recommends “against instrument-based depression screening using a questionnaire with cutoff score to distinguish ‘screen positive’ and ‘screen negative’ administered to all individuals during pregnancy and the postpartum period (up to 1 year after childbirth).”
Screening remains common
“There’s a lot of uncertainty in the scientific community about whether it’s a good idea to administer a screening test to all pregnant and postpartum women to determine in a systematic way if they might be suffering from depression,” said Dr. Lang.
The task force recommended against screening for depression among perinatal or postpartum women in 2013, but screening is still performed in many provinces, said Dr. Lang.
Dr. Lang emphasized that the recommendation does not apply to usual care, in which the provider asks questions about and discusses a patient’s mental health and proceeds on the basis of their clinical judgment; nor does it apply to diagnostic pathways in which the clinician suspects that the individual may have depression and tests her accordingly.
“What we are saying in our recommendation is that all clinicians should ask about a patient’s wellbeing, about their mood, their anxiety, and these questions are an important part of the clinical assessment of pregnant and postpartum women. But we’re also saying the usefulness of doing so with a questionnaire and using a cutoff score on the questionnaire to decide who needs further assessment or possibly treatment is unproven by the research,” Dr. Lang said.
A growing problem
For Diane Francoeur, MD, CEO of the Society of Obstetricians and Gynecologists of Canada, this is all well and good, but the reality is that such screening is better than nothing.
Quebec is the only Canadian province that conducts universal screening for all pregnant and postpartum women, Dr. Francoeur said in an interview. She was not part of the task force.
“I agree that it should be more than one approach, but the problem is that there is such a shortage of resources. There are many issues that can arise when you follow a woman during her pregnancy,” she said.
Dr. Francoeur said that COVID-19 has been particularly tough on women, including pregnant and postpartum women, who are the most vulnerable.
“Especially during the COVID era, it was astonishing how women were not doing well. Their stress level was so high. We need to have a specific approach dedicated to prenatal mental health, because it’s a problem that is bigger than it used to be,” she said.
Violence against women has increased considerably since the beginning of the COVID-19 pandemic, said Dr. Francoeur. “Many more women have been killed by their partners. We have never seen anything like this before, and I hope we will never see this again,” she said.
“Help was more available a few years ago, but now, it’s really hard if and when you need to have a quick consultation with a specialist and the woman is really depressed. It can take forever. So, it’s okay to screen, but then, what’s next? Who is going to be there to take these women and help them? And we don’t have the answer,” Dr. Francoeur said.
Pregnant and postpartum women who suffer from depression need more than pills, she added. “We reassure them and treat their depression pharmacologically, but it’s also a time to give appropriate support and help them through the pregnancy and get well prepared to receive their newborn, because, as we now know, that first year of life is really important for the child, and the mom needs to be supported.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. Dr. Lang and Dr. Francoeur reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMAJ
Hospital programs tackle mental health effects of long COVID
There’s little doubt that long COVID is real. Even as doctors and federal agencies struggle to define the syndrome, hospitals and health care systems are opening long COVID specialty treatment programs. As of July 25, there’s at least one long COVID center in almost every state – 48 out of 50, according to the patient advocacy group Survivor Corps.
Among the biggest challenges will be treating the mental health effects of long COVID.
Specialized centers will be tackling these problems even as the United States struggles to deal with mental health needs.
One study of COVID patients found more than one-third of them had symptoms of depression, anxiety, or PTSD 3-6 months after their initial infection. Another analysis of 30 previous studies of long COVID patients found roughly one in eight of them had severe depression – and that the risk was similar regardless of whether people were hospitalized for COVID-19.
“Many of these symptoms can emerge months into the course of long COVID illness,” said Jordan Anderson, DO, a neuropsychiatrist who sees patients at the Long COVID-19 Program at Oregon Health & Science University, Portland. Psychological symptoms are often made worse by physical setbacks like extreme fatigue and by challenges of working, caring for children, and keeping up with daily routines, he said.
“This impact is not only severe, but also chronic for many,” he said.
Like dozens of hospitals around the country, Oregon Health & Science opened its center for long COVID as it became clear that more patients would need help for ongoing physical and mental health symptoms. Today, there’s at least one long COVID center – sometimes called post-COVID care centers or clinics – in every state but Kansas and South Dakota, Survivor Corps said.
Many long COVID care centers aim to tackle both physical and mental health symptoms, said Tracy Vannorsdall, PhD, a neuropsychologist with the Johns Hopkins Post-Acute COVID-19 Team program. One goal at Hopkins is to identify patients with psychological issues that might otherwise get overlooked.
A sizable minority of patients at the Johns Hopkins center – up to about 35% – report mental health problems that they didn’t have until after they got COVID-19, Dr. Vannorsdall says. The most common mental health issues providers see are depression, anxiety, and trauma-related distress.
“Routine assessment is key,” Dr. Vannorsdall said. “If patients are not asked about their mental health symptoms, they may not spontaneously report them to their provider due to fear of stigma or simply not appreciating that there are effective treatments available for these issues.”
Fear that doctors won’t take symptoms seriously is common, says Heather Murray MD, a senior instructor in psychiatry at the University of Colorado at Denver, Aurora.
“Many patients worry their physicians, loved ones, and society will not believe them or will minimize their symptoms and suffering,” said Dr. Murray, who treats patients at the UCHealth Post-COVID Clinic.
Diagnostic tests in long COVID patients often don’t have conclusive results, which can lead doctors and patients themselves to question whether symptoms are truly “physical versus psychosomatic,” she said. “It is important that providers believe their patients and treat their symptoms, even when diagnostic tests are unrevealing.”
Growing mental health crisis
Patients often find their way to academic treatment centers after surviving severe COVID-19 infections. But a growing number of long COVID patients show up at these centers after milder cases. These patients were never hospitalized for COVID-19 but still have persistent symptoms like fatigue, thinking problems, and mood disorders.
Among the major challenges is a shortage of mental health care providers to meet the surging need for care since the start of the pandemic. Around the world, anxiety and depression surged 25% during the first year of the pandemic, according to the World Health Organization.
In the United States, 40% of adults report feelings of anxiety and depression, and one in three high school students have feelings of sadness and hopelessness, according to a March 2022 statement from the White House.
Despite this surging need for care, almost half of Americans live in areas with a severe shortage of mental health care providers, according to the Health Resources and Services Administration. As of 2019, the United States had a shortage of about 6,790 mental health providers. Since then, the shortage has worsened; it’s now about 7,500 providers.
“One of the biggest challenges for hospitals and clinics in treating mental health disorders in long COVID is the limited resources and long wait times to get in for evaluations and treatment,” said Nyaz Didehbani, PhD, a neuropsychologist who treats long COVID patients at the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
These delays can lead to worse outcomes, Dr. Didehbani said. “Additionally, patients do not feel that they are being heard, as many providers are not aware of the mental health impact and relationship with physical and cognitive symptoms.” .
Even when doctors recognize that psychological challenges are common with long COVID, they still have to think creatively to come up with treatments that meet the unique needs of these patients, said Thida Thant, MD, an assistant professor of psychiatry at the University of Colorado who treats patients at the UCHealth Post-COVID Clinic.
“There are at least two major factors that make treating psychological issues in long COVID more complex: The fact that the pandemic is still ongoing and still so divisive throughout society, and the fact that we don’t know a single best way to treat all symptoms of long COVID,” she said.
Some common treatments for anxiety and depression, like psychotherapy and medication, can be used for long COVID patients with these conditions. But another intervention that can work wonders for many people with mood disorders – exercise – doesn’t always work for long COVID patients. That’s because many of them struggle with physical challenges like chronic fatigue and what’s known as postexertional malaise, or a worsening of symptoms after even limited physical effort.
“While we normally encourage patients to be active, have a daily routine, and to engage in physical activity as part of their mental health treatment, some long COVID patients find that their symptoms worsen after increased activity,” Dr. Vannorsdall said.
Patients who are able to reach long COVID care centers are much more apt to get mental health problems diagnosed and treated, doctors at many programs around the country agree. But many patients hardest hit by the pandemic – the poor and racial and ethnic minorities – are also less likely to have ready access to hospitals that offer these programs, said Dr. Anderson.
“Affluent, predominantly White populations are showing up in these clinics, while we know that non-White populations have disproportionally high rates of acute infection, hospitalization, and death related to the virus,” he said.
Clinics are also concentrated in academic medical centers and in urban areas, limiting options for people in rural communities who may have to drive for hours to access care, Dr. Anderson said.
“Even before long COVID, we already knew that many people live in areas where there simply aren’t enough mental health services available,” said John Zulueta, MD, an assistant professor of clinical psychiatry at the University of Illinois at Chicago who provides mental health evaluations at the UI Health Post-COVID Clinic.
“As more patients develop mental health issues associated with long COVID, it’s going to put more stress on an already stressed system,” he said.
A version of this article first appeared on WebMD.com.
There’s little doubt that long COVID is real. Even as doctors and federal agencies struggle to define the syndrome, hospitals and health care systems are opening long COVID specialty treatment programs. As of July 25, there’s at least one long COVID center in almost every state – 48 out of 50, according to the patient advocacy group Survivor Corps.
Among the biggest challenges will be treating the mental health effects of long COVID.
Specialized centers will be tackling these problems even as the United States struggles to deal with mental health needs.
One study of COVID patients found more than one-third of them had symptoms of depression, anxiety, or PTSD 3-6 months after their initial infection. Another analysis of 30 previous studies of long COVID patients found roughly one in eight of them had severe depression – and that the risk was similar regardless of whether people were hospitalized for COVID-19.
“Many of these symptoms can emerge months into the course of long COVID illness,” said Jordan Anderson, DO, a neuropsychiatrist who sees patients at the Long COVID-19 Program at Oregon Health & Science University, Portland. Psychological symptoms are often made worse by physical setbacks like extreme fatigue and by challenges of working, caring for children, and keeping up with daily routines, he said.
“This impact is not only severe, but also chronic for many,” he said.
Like dozens of hospitals around the country, Oregon Health & Science opened its center for long COVID as it became clear that more patients would need help for ongoing physical and mental health symptoms. Today, there’s at least one long COVID center – sometimes called post-COVID care centers or clinics – in every state but Kansas and South Dakota, Survivor Corps said.
Many long COVID care centers aim to tackle both physical and mental health symptoms, said Tracy Vannorsdall, PhD, a neuropsychologist with the Johns Hopkins Post-Acute COVID-19 Team program. One goal at Hopkins is to identify patients with psychological issues that might otherwise get overlooked.
A sizable minority of patients at the Johns Hopkins center – up to about 35% – report mental health problems that they didn’t have until after they got COVID-19, Dr. Vannorsdall says. The most common mental health issues providers see are depression, anxiety, and trauma-related distress.
“Routine assessment is key,” Dr. Vannorsdall said. “If patients are not asked about their mental health symptoms, they may not spontaneously report them to their provider due to fear of stigma or simply not appreciating that there are effective treatments available for these issues.”
Fear that doctors won’t take symptoms seriously is common, says Heather Murray MD, a senior instructor in psychiatry at the University of Colorado at Denver, Aurora.
“Many patients worry their physicians, loved ones, and society will not believe them or will minimize their symptoms and suffering,” said Dr. Murray, who treats patients at the UCHealth Post-COVID Clinic.
Diagnostic tests in long COVID patients often don’t have conclusive results, which can lead doctors and patients themselves to question whether symptoms are truly “physical versus psychosomatic,” she said. “It is important that providers believe their patients and treat their symptoms, even when diagnostic tests are unrevealing.”
Growing mental health crisis
Patients often find their way to academic treatment centers after surviving severe COVID-19 infections. But a growing number of long COVID patients show up at these centers after milder cases. These patients were never hospitalized for COVID-19 but still have persistent symptoms like fatigue, thinking problems, and mood disorders.
Among the major challenges is a shortage of mental health care providers to meet the surging need for care since the start of the pandemic. Around the world, anxiety and depression surged 25% during the first year of the pandemic, according to the World Health Organization.
In the United States, 40% of adults report feelings of anxiety and depression, and one in three high school students have feelings of sadness and hopelessness, according to a March 2022 statement from the White House.
Despite this surging need for care, almost half of Americans live in areas with a severe shortage of mental health care providers, according to the Health Resources and Services Administration. As of 2019, the United States had a shortage of about 6,790 mental health providers. Since then, the shortage has worsened; it’s now about 7,500 providers.
“One of the biggest challenges for hospitals and clinics in treating mental health disorders in long COVID is the limited resources and long wait times to get in for evaluations and treatment,” said Nyaz Didehbani, PhD, a neuropsychologist who treats long COVID patients at the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
These delays can lead to worse outcomes, Dr. Didehbani said. “Additionally, patients do not feel that they are being heard, as many providers are not aware of the mental health impact and relationship with physical and cognitive symptoms.” .
Even when doctors recognize that psychological challenges are common with long COVID, they still have to think creatively to come up with treatments that meet the unique needs of these patients, said Thida Thant, MD, an assistant professor of psychiatry at the University of Colorado who treats patients at the UCHealth Post-COVID Clinic.
“There are at least two major factors that make treating psychological issues in long COVID more complex: The fact that the pandemic is still ongoing and still so divisive throughout society, and the fact that we don’t know a single best way to treat all symptoms of long COVID,” she said.
Some common treatments for anxiety and depression, like psychotherapy and medication, can be used for long COVID patients with these conditions. But another intervention that can work wonders for many people with mood disorders – exercise – doesn’t always work for long COVID patients. That’s because many of them struggle with physical challenges like chronic fatigue and what’s known as postexertional malaise, or a worsening of symptoms after even limited physical effort.
“While we normally encourage patients to be active, have a daily routine, and to engage in physical activity as part of their mental health treatment, some long COVID patients find that their symptoms worsen after increased activity,” Dr. Vannorsdall said.
Patients who are able to reach long COVID care centers are much more apt to get mental health problems diagnosed and treated, doctors at many programs around the country agree. But many patients hardest hit by the pandemic – the poor and racial and ethnic minorities – are also less likely to have ready access to hospitals that offer these programs, said Dr. Anderson.
“Affluent, predominantly White populations are showing up in these clinics, while we know that non-White populations have disproportionally high rates of acute infection, hospitalization, and death related to the virus,” he said.
Clinics are also concentrated in academic medical centers and in urban areas, limiting options for people in rural communities who may have to drive for hours to access care, Dr. Anderson said.
“Even before long COVID, we already knew that many people live in areas where there simply aren’t enough mental health services available,” said John Zulueta, MD, an assistant professor of clinical psychiatry at the University of Illinois at Chicago who provides mental health evaluations at the UI Health Post-COVID Clinic.
“As more patients develop mental health issues associated with long COVID, it’s going to put more stress on an already stressed system,” he said.
A version of this article first appeared on WebMD.com.
There’s little doubt that long COVID is real. Even as doctors and federal agencies struggle to define the syndrome, hospitals and health care systems are opening long COVID specialty treatment programs. As of July 25, there’s at least one long COVID center in almost every state – 48 out of 50, according to the patient advocacy group Survivor Corps.
Among the biggest challenges will be treating the mental health effects of long COVID.
Specialized centers will be tackling these problems even as the United States struggles to deal with mental health needs.
One study of COVID patients found more than one-third of them had symptoms of depression, anxiety, or PTSD 3-6 months after their initial infection. Another analysis of 30 previous studies of long COVID patients found roughly one in eight of them had severe depression – and that the risk was similar regardless of whether people were hospitalized for COVID-19.
“Many of these symptoms can emerge months into the course of long COVID illness,” said Jordan Anderson, DO, a neuropsychiatrist who sees patients at the Long COVID-19 Program at Oregon Health & Science University, Portland. Psychological symptoms are often made worse by physical setbacks like extreme fatigue and by challenges of working, caring for children, and keeping up with daily routines, he said.
“This impact is not only severe, but also chronic for many,” he said.
Like dozens of hospitals around the country, Oregon Health & Science opened its center for long COVID as it became clear that more patients would need help for ongoing physical and mental health symptoms. Today, there’s at least one long COVID center – sometimes called post-COVID care centers or clinics – in every state but Kansas and South Dakota, Survivor Corps said.
Many long COVID care centers aim to tackle both physical and mental health symptoms, said Tracy Vannorsdall, PhD, a neuropsychologist with the Johns Hopkins Post-Acute COVID-19 Team program. One goal at Hopkins is to identify patients with psychological issues that might otherwise get overlooked.
A sizable minority of patients at the Johns Hopkins center – up to about 35% – report mental health problems that they didn’t have until after they got COVID-19, Dr. Vannorsdall says. The most common mental health issues providers see are depression, anxiety, and trauma-related distress.
“Routine assessment is key,” Dr. Vannorsdall said. “If patients are not asked about their mental health symptoms, they may not spontaneously report them to their provider due to fear of stigma or simply not appreciating that there are effective treatments available for these issues.”
Fear that doctors won’t take symptoms seriously is common, says Heather Murray MD, a senior instructor in psychiatry at the University of Colorado at Denver, Aurora.
“Many patients worry their physicians, loved ones, and society will not believe them or will minimize their symptoms and suffering,” said Dr. Murray, who treats patients at the UCHealth Post-COVID Clinic.
Diagnostic tests in long COVID patients often don’t have conclusive results, which can lead doctors and patients themselves to question whether symptoms are truly “physical versus psychosomatic,” she said. “It is important that providers believe their patients and treat their symptoms, even when diagnostic tests are unrevealing.”
Growing mental health crisis
Patients often find their way to academic treatment centers after surviving severe COVID-19 infections. But a growing number of long COVID patients show up at these centers after milder cases. These patients were never hospitalized for COVID-19 but still have persistent symptoms like fatigue, thinking problems, and mood disorders.
Among the major challenges is a shortage of mental health care providers to meet the surging need for care since the start of the pandemic. Around the world, anxiety and depression surged 25% during the first year of the pandemic, according to the World Health Organization.
In the United States, 40% of adults report feelings of anxiety and depression, and one in three high school students have feelings of sadness and hopelessness, according to a March 2022 statement from the White House.
Despite this surging need for care, almost half of Americans live in areas with a severe shortage of mental health care providers, according to the Health Resources and Services Administration. As of 2019, the United States had a shortage of about 6,790 mental health providers. Since then, the shortage has worsened; it’s now about 7,500 providers.
“One of the biggest challenges for hospitals and clinics in treating mental health disorders in long COVID is the limited resources and long wait times to get in for evaluations and treatment,” said Nyaz Didehbani, PhD, a neuropsychologist who treats long COVID patients at the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
These delays can lead to worse outcomes, Dr. Didehbani said. “Additionally, patients do not feel that they are being heard, as many providers are not aware of the mental health impact and relationship with physical and cognitive symptoms.” .
Even when doctors recognize that psychological challenges are common with long COVID, they still have to think creatively to come up with treatments that meet the unique needs of these patients, said Thida Thant, MD, an assistant professor of psychiatry at the University of Colorado who treats patients at the UCHealth Post-COVID Clinic.
“There are at least two major factors that make treating psychological issues in long COVID more complex: The fact that the pandemic is still ongoing and still so divisive throughout society, and the fact that we don’t know a single best way to treat all symptoms of long COVID,” she said.
Some common treatments for anxiety and depression, like psychotherapy and medication, can be used for long COVID patients with these conditions. But another intervention that can work wonders for many people with mood disorders – exercise – doesn’t always work for long COVID patients. That’s because many of them struggle with physical challenges like chronic fatigue and what’s known as postexertional malaise, or a worsening of symptoms after even limited physical effort.
“While we normally encourage patients to be active, have a daily routine, and to engage in physical activity as part of their mental health treatment, some long COVID patients find that their symptoms worsen after increased activity,” Dr. Vannorsdall said.
Patients who are able to reach long COVID care centers are much more apt to get mental health problems diagnosed and treated, doctors at many programs around the country agree. But many patients hardest hit by the pandemic – the poor and racial and ethnic minorities – are also less likely to have ready access to hospitals that offer these programs, said Dr. Anderson.
“Affluent, predominantly White populations are showing up in these clinics, while we know that non-White populations have disproportionally high rates of acute infection, hospitalization, and death related to the virus,” he said.
Clinics are also concentrated in academic medical centers and in urban areas, limiting options for people in rural communities who may have to drive for hours to access care, Dr. Anderson said.
“Even before long COVID, we already knew that many people live in areas where there simply aren’t enough mental health services available,” said John Zulueta, MD, an assistant professor of clinical psychiatry at the University of Illinois at Chicago who provides mental health evaluations at the UI Health Post-COVID Clinic.
“As more patients develop mental health issues associated with long COVID, it’s going to put more stress on an already stressed system,” he said.
A version of this article first appeared on WebMD.com.
Mental health assessment for gender-diverse patients
Over the past several years, the number of patients seeking gender-affirming services has exponentially increased.1 Unfortunately, the number of patients presenting for treatment has exceeded evidence-based guidelines, research, and the number of providers familiar with gender-affirming care. Many institutions and associations such as the American College of Obstetricians and Gynecologists and the World Professional Association for Transgender Health (WPATH) advocate for training of providers; however, many patients will be seen by providers who are not qualified in diagnosing gender dysphoria. As a result, many practitioners rely on the mental health evaluation of gender-diverse individuals prior to prescribing hormonal therapy or before planning surgery.
Practitioners qualified to provide mental health services can include persons within in the field of psychology, psychiatry, social work, licensed professional counseling, nursing, or family medicine (with specific training in mental health).2 WPATH also defines specific criteria as part of the mental health assessment. For example, providers should have a master’s degree or higher in clinical behavioral science, competence in using the DSM/ICD, the ability to recognize and diagnose coexisting mental health concerns, and undergo continuing education in the treatment of gender dysphoria.2 Unfortunately, the demand for gender-competent mental health professionals exceeds the number available, and many patients are seen by therapists lacking experience within this field.3 This discrepancy can present an additional barrier to the health needs of transgender patients and sometimes inhibit access to hormone therapy, or even more catastrophically, compromise their presurgical assessment and surgical outcome.
For patients seeking chest surgery (mastectomy or breast augmentation), one letter from a mental health provider is necessary. If a patient is interested in pursuing genital surgery or the removal or reproductive organs, two letters from two separate mental health providers are required. Typically, one letter is from the patient’s primary therapist, and the other is often a second opinion. These letters must include a patient’s general characteristics, psychosocial assessment results, duration of the mental health professional’s relationship with the client, an explanation that the criteria for surgery have been met, a statement supporting the patient’s request for surgery and that informed consent was obtained, and a statement that the mental health professional is available for coordination of care.2 It is crucial to delineate that while a mental health evaluation is mandated, psychotherapy is not.
A therapist’s letter is not essential prior to initiating hormones; however, it is recommended if practitioners are unfamiliar with gender-diverse patients and current standards of care. If a provider such as a family physician, endocrinologist, or obstetrician/gynecologist is knowledgeable about the diagnostic criteria for gender dysphoria, they can prescribe hormones without a therapist’s letter. Additional considerations include establishing whether a patient has persistent gender dysphoria, has the capacity to give informed consent, and has “reasonably well-controlled” mental illness.3 The prevalence of both depression and anxiety is exceptionally high in this population, whereas rates of bipolar disorder and schizophrenia mirror that of the general population.3 Mental illness is not a contraindication to hormone therapy because there is sufficient evidence to support the benefits of gender-affirming hormones in reducing both anxiety and depression.
In contrast, concurrent severe psychiatric illness (i.e., bipolar disorder, schizophrenia, borderline personality disorder) that is not well controlled could prohibit patients from undergoing gender-affirming surgeries. Even the most well-educated patients do not truly understand the process of surgery and the rigorous postoperative care required, particularly after genital surgery. Many patients underestimate the need for a support system in the postoperative period and cannot predict their emotional response after undergoing such complex procedures. During a surgical consultation, the surgeon can help identify any mental, physical, monetary, or social constraints patients may have and work closely with other providers, including a well-trained mental health professional, to optimize a patient’s surgical recovery. Ideally, patients undergoing surgery are seen at multidisciplinary centers with the capabilities of addressing these concerns.
The patient’s perspective on the need for a therapist is often mixed. Historically, therapist letters have been viewed by patients as a form of “gatekeeping” and an additional barrier they are forced to overcome to receive treatment. However, the role of a mental health provider who specializes in gender-affirming care cannot be overstated. In the context of surgery, I often try to reframe the role the therapist as an integral part of the multidisciplinary team. Mental health assessments preoperatively can better prepare patients for their upcoming surgery. More importantly, this multidisciplinary approach can help identify potential issues with coping strategies or exacerbations of other mental health conditions that may arise in the immediate postoperative period.
There is no question that exceptional gender-affirming care requires a multidisciplinary approach. Establishing strong relationships between hormone prescribers, surgeons, and behavioral health specialists in an essential step toward providing competent patient-centered care.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Ettner R. Mental health evaluation for gender confirmation surgery. Clin Plastic Surg. 2018;45(3):307-11.
2. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier; 2020:8-11.
3. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th ed. Minneapolis: WPATH; 2012.
Over the past several years, the number of patients seeking gender-affirming services has exponentially increased.1 Unfortunately, the number of patients presenting for treatment has exceeded evidence-based guidelines, research, and the number of providers familiar with gender-affirming care. Many institutions and associations such as the American College of Obstetricians and Gynecologists and the World Professional Association for Transgender Health (WPATH) advocate for training of providers; however, many patients will be seen by providers who are not qualified in diagnosing gender dysphoria. As a result, many practitioners rely on the mental health evaluation of gender-diverse individuals prior to prescribing hormonal therapy or before planning surgery.
Practitioners qualified to provide mental health services can include persons within in the field of psychology, psychiatry, social work, licensed professional counseling, nursing, or family medicine (with specific training in mental health).2 WPATH also defines specific criteria as part of the mental health assessment. For example, providers should have a master’s degree or higher in clinical behavioral science, competence in using the DSM/ICD, the ability to recognize and diagnose coexisting mental health concerns, and undergo continuing education in the treatment of gender dysphoria.2 Unfortunately, the demand for gender-competent mental health professionals exceeds the number available, and many patients are seen by therapists lacking experience within this field.3 This discrepancy can present an additional barrier to the health needs of transgender patients and sometimes inhibit access to hormone therapy, or even more catastrophically, compromise their presurgical assessment and surgical outcome.
For patients seeking chest surgery (mastectomy or breast augmentation), one letter from a mental health provider is necessary. If a patient is interested in pursuing genital surgery or the removal or reproductive organs, two letters from two separate mental health providers are required. Typically, one letter is from the patient’s primary therapist, and the other is often a second opinion. These letters must include a patient’s general characteristics, psychosocial assessment results, duration of the mental health professional’s relationship with the client, an explanation that the criteria for surgery have been met, a statement supporting the patient’s request for surgery and that informed consent was obtained, and a statement that the mental health professional is available for coordination of care.2 It is crucial to delineate that while a mental health evaluation is mandated, psychotherapy is not.
A therapist’s letter is not essential prior to initiating hormones; however, it is recommended if practitioners are unfamiliar with gender-diverse patients and current standards of care. If a provider such as a family physician, endocrinologist, or obstetrician/gynecologist is knowledgeable about the diagnostic criteria for gender dysphoria, they can prescribe hormones without a therapist’s letter. Additional considerations include establishing whether a patient has persistent gender dysphoria, has the capacity to give informed consent, and has “reasonably well-controlled” mental illness.3 The prevalence of both depression and anxiety is exceptionally high in this population, whereas rates of bipolar disorder and schizophrenia mirror that of the general population.3 Mental illness is not a contraindication to hormone therapy because there is sufficient evidence to support the benefits of gender-affirming hormones in reducing both anxiety and depression.
In contrast, concurrent severe psychiatric illness (i.e., bipolar disorder, schizophrenia, borderline personality disorder) that is not well controlled could prohibit patients from undergoing gender-affirming surgeries. Even the most well-educated patients do not truly understand the process of surgery and the rigorous postoperative care required, particularly after genital surgery. Many patients underestimate the need for a support system in the postoperative period and cannot predict their emotional response after undergoing such complex procedures. During a surgical consultation, the surgeon can help identify any mental, physical, monetary, or social constraints patients may have and work closely with other providers, including a well-trained mental health professional, to optimize a patient’s surgical recovery. Ideally, patients undergoing surgery are seen at multidisciplinary centers with the capabilities of addressing these concerns.
The patient’s perspective on the need for a therapist is often mixed. Historically, therapist letters have been viewed by patients as a form of “gatekeeping” and an additional barrier they are forced to overcome to receive treatment. However, the role of a mental health provider who specializes in gender-affirming care cannot be overstated. In the context of surgery, I often try to reframe the role the therapist as an integral part of the multidisciplinary team. Mental health assessments preoperatively can better prepare patients for their upcoming surgery. More importantly, this multidisciplinary approach can help identify potential issues with coping strategies or exacerbations of other mental health conditions that may arise in the immediate postoperative period.
There is no question that exceptional gender-affirming care requires a multidisciplinary approach. Establishing strong relationships between hormone prescribers, surgeons, and behavioral health specialists in an essential step toward providing competent patient-centered care.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Ettner R. Mental health evaluation for gender confirmation surgery. Clin Plastic Surg. 2018;45(3):307-11.
2. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier; 2020:8-11.
3. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th ed. Minneapolis: WPATH; 2012.
Over the past several years, the number of patients seeking gender-affirming services has exponentially increased.1 Unfortunately, the number of patients presenting for treatment has exceeded evidence-based guidelines, research, and the number of providers familiar with gender-affirming care. Many institutions and associations such as the American College of Obstetricians and Gynecologists and the World Professional Association for Transgender Health (WPATH) advocate for training of providers; however, many patients will be seen by providers who are not qualified in diagnosing gender dysphoria. As a result, many practitioners rely on the mental health evaluation of gender-diverse individuals prior to prescribing hormonal therapy or before planning surgery.
Practitioners qualified to provide mental health services can include persons within in the field of psychology, psychiatry, social work, licensed professional counseling, nursing, or family medicine (with specific training in mental health).2 WPATH also defines specific criteria as part of the mental health assessment. For example, providers should have a master’s degree or higher in clinical behavioral science, competence in using the DSM/ICD, the ability to recognize and diagnose coexisting mental health concerns, and undergo continuing education in the treatment of gender dysphoria.2 Unfortunately, the demand for gender-competent mental health professionals exceeds the number available, and many patients are seen by therapists lacking experience within this field.3 This discrepancy can present an additional barrier to the health needs of transgender patients and sometimes inhibit access to hormone therapy, or even more catastrophically, compromise their presurgical assessment and surgical outcome.
For patients seeking chest surgery (mastectomy or breast augmentation), one letter from a mental health provider is necessary. If a patient is interested in pursuing genital surgery or the removal or reproductive organs, two letters from two separate mental health providers are required. Typically, one letter is from the patient’s primary therapist, and the other is often a second opinion. These letters must include a patient’s general characteristics, psychosocial assessment results, duration of the mental health professional’s relationship with the client, an explanation that the criteria for surgery have been met, a statement supporting the patient’s request for surgery and that informed consent was obtained, and a statement that the mental health professional is available for coordination of care.2 It is crucial to delineate that while a mental health evaluation is mandated, psychotherapy is not.
A therapist’s letter is not essential prior to initiating hormones; however, it is recommended if practitioners are unfamiliar with gender-diverse patients and current standards of care. If a provider such as a family physician, endocrinologist, or obstetrician/gynecologist is knowledgeable about the diagnostic criteria for gender dysphoria, they can prescribe hormones without a therapist’s letter. Additional considerations include establishing whether a patient has persistent gender dysphoria, has the capacity to give informed consent, and has “reasonably well-controlled” mental illness.3 The prevalence of both depression and anxiety is exceptionally high in this population, whereas rates of bipolar disorder and schizophrenia mirror that of the general population.3 Mental illness is not a contraindication to hormone therapy because there is sufficient evidence to support the benefits of gender-affirming hormones in reducing both anxiety and depression.
In contrast, concurrent severe psychiatric illness (i.e., bipolar disorder, schizophrenia, borderline personality disorder) that is not well controlled could prohibit patients from undergoing gender-affirming surgeries. Even the most well-educated patients do not truly understand the process of surgery and the rigorous postoperative care required, particularly after genital surgery. Many patients underestimate the need for a support system in the postoperative period and cannot predict their emotional response after undergoing such complex procedures. During a surgical consultation, the surgeon can help identify any mental, physical, monetary, or social constraints patients may have and work closely with other providers, including a well-trained mental health professional, to optimize a patient’s surgical recovery. Ideally, patients undergoing surgery are seen at multidisciplinary centers with the capabilities of addressing these concerns.
The patient’s perspective on the need for a therapist is often mixed. Historically, therapist letters have been viewed by patients as a form of “gatekeeping” and an additional barrier they are forced to overcome to receive treatment. However, the role of a mental health provider who specializes in gender-affirming care cannot be overstated. In the context of surgery, I often try to reframe the role the therapist as an integral part of the multidisciplinary team. Mental health assessments preoperatively can better prepare patients for their upcoming surgery. More importantly, this multidisciplinary approach can help identify potential issues with coping strategies or exacerbations of other mental health conditions that may arise in the immediate postoperative period.
There is no question that exceptional gender-affirming care requires a multidisciplinary approach. Establishing strong relationships between hormone prescribers, surgeons, and behavioral health specialists in an essential step toward providing competent patient-centered care.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Ettner R. Mental health evaluation for gender confirmation surgery. Clin Plastic Surg. 2018;45(3):307-11.
2. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier; 2020:8-11.
3. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th ed. Minneapolis: WPATH; 2012.
Clinical characteristics of recurrent RIME elucidated in chart review
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
‘Ecotrauma’: The effects of climate change on mental health
In June of this year, the World Health Organization launched a policy report to confront the increasingly strong and lasting impacts that climate change is having directly and indirectly on people’s mental health and psychosocial well-being.
In addition to the increasingly high incidence of mental disorders (for instance, emotional distress, stress, depression, and suicidal behavior) affecting people worldwide This term refers to anxiety in the face of the apocalyptic scenario predicted to result from the transformation of ecosystems by anthropogenic activity.
Two weeks after the release of the policy report, which integrates key policies for countries to address one of the biggest challenges, the WHO published its largest review of global mental health since the turn of the century. The work provides a model for governments, academics, health professionals, and civil society to become key players when dealing with the mental health problems that our society is going through.
As the document highlights, almost 1 billion people, including 14% of the world’s adolescents, were living with a mental health disorder in 2019. Suicide accounted for more than 1 in 100 deaths, and 58% of cases occurred before age 50 years. Mental health disorders are already the leading cause of disability in the world, and people with serious but preventable diseases die on average 10-20 years earlier than the general population.
The COVID-19 crisis has significantly aggravated mental health disorders, especially in populations such as minors. Consequently, many experts refer to this public health phenomenon as the new major pandemic. “I’m not sure it’s correct to call a set of mental health problems a pandemic, but the reality is that many countries are ignoring or largely forgetting this crisis,” Sarah Sheppard, WHO communications officer, told this news organization. According to Ms. Sheppard, “stigma and lack of understanding are key drivers of these problems and have been one of the reasons for the lack of mental health funding for decades. Mental health receives less than 1% of international health aid.” We recently interviewed Ms. Sheppard about these challenges.
Univadis: As the data provided in the recently released Mental Health and Climate Change Policy Brief indicate, there are large gaps in many countries between mental health needs and the services and systems available to address them. Where can we start to change this reality?
Ms. Sheppard: The simplest answer to improve the situation we face begins with a change in people’s priorities when it comes to valuing mental health. This would lead to greater investment in human and financial resources for mental health services and systems. However, the challenge lies in the complexity of the problem. In the report we just published, we provide comprehensive recommendations on how to transform mental health systems for all, such as trying to integrate climate change considerations into policies and programs for mental health or building on existing global commitments, including the Sustainable Development Goals (SDGs) or the Paris Agreement.
Univadis: Is there evidence that mental illnesses and disorders affect some populations more than others, such as women, for example?
Ms. Sheppard: The prevalence of mental disorders varies according to conditions and according to sex and age. In general, I don’t think we can say that mental health conditions or disorders affect women more than men. There are groups at risk, but vulnerability depends on the context and varies a lot. Of course, social determinants such as poverty, unstable housing, and exposure to adversity can significantly increase risk.
Univadis: According to the statistics recently provided by the WHO, changes in the environment are directly and indirectly affecting people’s mental health and psychosocial well-being. The new report highlights the gap between countries when it comes to addressing this complex problem. Is there any country that is carrying out political or innovative initiatives in this regard?
Ms. Sheppard: Yes, there are many case studies in the policy brief that highlight important work in the area. There are strong examples that are highlighted in the summary. One of them is India and its resilient cities program. Focused on the reduction of disaster risk, climate resilience, and mental health and psychosocial support at city level, this project resulted from a collaboration between the United Nations Development Program and the Indian National Institute of Mental Health and Neurosciences, which began in 2017.
Univadis: In addition to its effects on mental health, we are seeing how climate change is causing the appearance and resurgence of zoonoses, such as the pandemic caused by coronavirus and now monkeypox.
Ms. Sheppard: Mike Ryan, head of emergency situations at WHO, stated at the beginning of June that the increase in zoonoses raises the risk of new pandemics. Infections transmitted from animals to humans, such as Ebola, COVID-19, or monkeypox, have multiplied in recent years. Climate change alters the conditions for pathogens and zoonotic disease vectors and their distribution. The intensification of travel, for example, allows them to spread more quickly and in a more uncontrolled way.
Human health, including mental health, is connected to animal health. As various materials available to us from our World Health Day 2022 campaign examine, the links between planetary health and human health are inextricable.
Univadis: How is it possible that while scientific progress advances and more powerful and efficient technologies are developed, we become increasingly vulnerable to environmental phenomena?
Ms. Sheppard: Scientific advancement improves our understanding of the quality and scale of the health impacts of climate change, including the identification of the most vulnerable groups, as well as the adaptation and mitigation measures that would work to reduce the consequences on health. At the same time, climate change is widespread, rapid, and intensifying. Technological advances have a role to play in mitigation, particularly those tools that reduce our dependence on burning fossil fuels, as well as adaptation to climate change. For example, early warning systems for extreme weather events could reduce those vulnerabilities your question mentioned.
On the other hand, the measures proposed by the latest report on mental health and climate change have multiple effects. Some are particularly powerful and are not overly dependent on new technology. These include changing our mode of transport to low-emission, physically active ways to get around (walking, cycling), the benefits of which are already more than proven for both the environment and human health.
This article was translated from Univadis Spain.
A version of this article first appeared on Medscape.com.
In June of this year, the World Health Organization launched a policy report to confront the increasingly strong and lasting impacts that climate change is having directly and indirectly on people’s mental health and psychosocial well-being.
In addition to the increasingly high incidence of mental disorders (for instance, emotional distress, stress, depression, and suicidal behavior) affecting people worldwide This term refers to anxiety in the face of the apocalyptic scenario predicted to result from the transformation of ecosystems by anthropogenic activity.
Two weeks after the release of the policy report, which integrates key policies for countries to address one of the biggest challenges, the WHO published its largest review of global mental health since the turn of the century. The work provides a model for governments, academics, health professionals, and civil society to become key players when dealing with the mental health problems that our society is going through.
As the document highlights, almost 1 billion people, including 14% of the world’s adolescents, were living with a mental health disorder in 2019. Suicide accounted for more than 1 in 100 deaths, and 58% of cases occurred before age 50 years. Mental health disorders are already the leading cause of disability in the world, and people with serious but preventable diseases die on average 10-20 years earlier than the general population.
The COVID-19 crisis has significantly aggravated mental health disorders, especially in populations such as minors. Consequently, many experts refer to this public health phenomenon as the new major pandemic. “I’m not sure it’s correct to call a set of mental health problems a pandemic, but the reality is that many countries are ignoring or largely forgetting this crisis,” Sarah Sheppard, WHO communications officer, told this news organization. According to Ms. Sheppard, “stigma and lack of understanding are key drivers of these problems and have been one of the reasons for the lack of mental health funding for decades. Mental health receives less than 1% of international health aid.” We recently interviewed Ms. Sheppard about these challenges.
Univadis: As the data provided in the recently released Mental Health and Climate Change Policy Brief indicate, there are large gaps in many countries between mental health needs and the services and systems available to address them. Where can we start to change this reality?
Ms. Sheppard: The simplest answer to improve the situation we face begins with a change in people’s priorities when it comes to valuing mental health. This would lead to greater investment in human and financial resources for mental health services and systems. However, the challenge lies in the complexity of the problem. In the report we just published, we provide comprehensive recommendations on how to transform mental health systems for all, such as trying to integrate climate change considerations into policies and programs for mental health or building on existing global commitments, including the Sustainable Development Goals (SDGs) or the Paris Agreement.
Univadis: Is there evidence that mental illnesses and disorders affect some populations more than others, such as women, for example?
Ms. Sheppard: The prevalence of mental disorders varies according to conditions and according to sex and age. In general, I don’t think we can say that mental health conditions or disorders affect women more than men. There are groups at risk, but vulnerability depends on the context and varies a lot. Of course, social determinants such as poverty, unstable housing, and exposure to adversity can significantly increase risk.
Univadis: According to the statistics recently provided by the WHO, changes in the environment are directly and indirectly affecting people’s mental health and psychosocial well-being. The new report highlights the gap between countries when it comes to addressing this complex problem. Is there any country that is carrying out political or innovative initiatives in this regard?
Ms. Sheppard: Yes, there are many case studies in the policy brief that highlight important work in the area. There are strong examples that are highlighted in the summary. One of them is India and its resilient cities program. Focused on the reduction of disaster risk, climate resilience, and mental health and psychosocial support at city level, this project resulted from a collaboration between the United Nations Development Program and the Indian National Institute of Mental Health and Neurosciences, which began in 2017.
Univadis: In addition to its effects on mental health, we are seeing how climate change is causing the appearance and resurgence of zoonoses, such as the pandemic caused by coronavirus and now monkeypox.
Ms. Sheppard: Mike Ryan, head of emergency situations at WHO, stated at the beginning of June that the increase in zoonoses raises the risk of new pandemics. Infections transmitted from animals to humans, such as Ebola, COVID-19, or monkeypox, have multiplied in recent years. Climate change alters the conditions for pathogens and zoonotic disease vectors and their distribution. The intensification of travel, for example, allows them to spread more quickly and in a more uncontrolled way.
Human health, including mental health, is connected to animal health. As various materials available to us from our World Health Day 2022 campaign examine, the links between planetary health and human health are inextricable.
Univadis: How is it possible that while scientific progress advances and more powerful and efficient technologies are developed, we become increasingly vulnerable to environmental phenomena?
Ms. Sheppard: Scientific advancement improves our understanding of the quality and scale of the health impacts of climate change, including the identification of the most vulnerable groups, as well as the adaptation and mitigation measures that would work to reduce the consequences on health. At the same time, climate change is widespread, rapid, and intensifying. Technological advances have a role to play in mitigation, particularly those tools that reduce our dependence on burning fossil fuels, as well as adaptation to climate change. For example, early warning systems for extreme weather events could reduce those vulnerabilities your question mentioned.
On the other hand, the measures proposed by the latest report on mental health and climate change have multiple effects. Some are particularly powerful and are not overly dependent on new technology. These include changing our mode of transport to low-emission, physically active ways to get around (walking, cycling), the benefits of which are already more than proven for both the environment and human health.
This article was translated from Univadis Spain.
A version of this article first appeared on Medscape.com.
In June of this year, the World Health Organization launched a policy report to confront the increasingly strong and lasting impacts that climate change is having directly and indirectly on people’s mental health and psychosocial well-being.
In addition to the increasingly high incidence of mental disorders (for instance, emotional distress, stress, depression, and suicidal behavior) affecting people worldwide This term refers to anxiety in the face of the apocalyptic scenario predicted to result from the transformation of ecosystems by anthropogenic activity.
Two weeks after the release of the policy report, which integrates key policies for countries to address one of the biggest challenges, the WHO published its largest review of global mental health since the turn of the century. The work provides a model for governments, academics, health professionals, and civil society to become key players when dealing with the mental health problems that our society is going through.
As the document highlights, almost 1 billion people, including 14% of the world’s adolescents, were living with a mental health disorder in 2019. Suicide accounted for more than 1 in 100 deaths, and 58% of cases occurred before age 50 years. Mental health disorders are already the leading cause of disability in the world, and people with serious but preventable diseases die on average 10-20 years earlier than the general population.
The COVID-19 crisis has significantly aggravated mental health disorders, especially in populations such as minors. Consequently, many experts refer to this public health phenomenon as the new major pandemic. “I’m not sure it’s correct to call a set of mental health problems a pandemic, but the reality is that many countries are ignoring or largely forgetting this crisis,” Sarah Sheppard, WHO communications officer, told this news organization. According to Ms. Sheppard, “stigma and lack of understanding are key drivers of these problems and have been one of the reasons for the lack of mental health funding for decades. Mental health receives less than 1% of international health aid.” We recently interviewed Ms. Sheppard about these challenges.
Univadis: As the data provided in the recently released Mental Health and Climate Change Policy Brief indicate, there are large gaps in many countries between mental health needs and the services and systems available to address them. Where can we start to change this reality?
Ms. Sheppard: The simplest answer to improve the situation we face begins with a change in people’s priorities when it comes to valuing mental health. This would lead to greater investment in human and financial resources for mental health services and systems. However, the challenge lies in the complexity of the problem. In the report we just published, we provide comprehensive recommendations on how to transform mental health systems for all, such as trying to integrate climate change considerations into policies and programs for mental health or building on existing global commitments, including the Sustainable Development Goals (SDGs) or the Paris Agreement.
Univadis: Is there evidence that mental illnesses and disorders affect some populations more than others, such as women, for example?
Ms. Sheppard: The prevalence of mental disorders varies according to conditions and according to sex and age. In general, I don’t think we can say that mental health conditions or disorders affect women more than men. There are groups at risk, but vulnerability depends on the context and varies a lot. Of course, social determinants such as poverty, unstable housing, and exposure to adversity can significantly increase risk.
Univadis: According to the statistics recently provided by the WHO, changes in the environment are directly and indirectly affecting people’s mental health and psychosocial well-being. The new report highlights the gap between countries when it comes to addressing this complex problem. Is there any country that is carrying out political or innovative initiatives in this regard?
Ms. Sheppard: Yes, there are many case studies in the policy brief that highlight important work in the area. There are strong examples that are highlighted in the summary. One of them is India and its resilient cities program. Focused on the reduction of disaster risk, climate resilience, and mental health and psychosocial support at city level, this project resulted from a collaboration between the United Nations Development Program and the Indian National Institute of Mental Health and Neurosciences, which began in 2017.
Univadis: In addition to its effects on mental health, we are seeing how climate change is causing the appearance and resurgence of zoonoses, such as the pandemic caused by coronavirus and now monkeypox.
Ms. Sheppard: Mike Ryan, head of emergency situations at WHO, stated at the beginning of June that the increase in zoonoses raises the risk of new pandemics. Infections transmitted from animals to humans, such as Ebola, COVID-19, or monkeypox, have multiplied in recent years. Climate change alters the conditions for pathogens and zoonotic disease vectors and their distribution. The intensification of travel, for example, allows them to spread more quickly and in a more uncontrolled way.
Human health, including mental health, is connected to animal health. As various materials available to us from our World Health Day 2022 campaign examine, the links between planetary health and human health are inextricable.
Univadis: How is it possible that while scientific progress advances and more powerful and efficient technologies are developed, we become increasingly vulnerable to environmental phenomena?
Ms. Sheppard: Scientific advancement improves our understanding of the quality and scale of the health impacts of climate change, including the identification of the most vulnerable groups, as well as the adaptation and mitigation measures that would work to reduce the consequences on health. At the same time, climate change is widespread, rapid, and intensifying. Technological advances have a role to play in mitigation, particularly those tools that reduce our dependence on burning fossil fuels, as well as adaptation to climate change. For example, early warning systems for extreme weather events could reduce those vulnerabilities your question mentioned.
On the other hand, the measures proposed by the latest report on mental health and climate change have multiple effects. Some are particularly powerful and are not overly dependent on new technology. These include changing our mode of transport to low-emission, physically active ways to get around (walking, cycling), the benefits of which are already more than proven for both the environment and human health.
This article was translated from Univadis Spain.
A version of this article first appeared on Medscape.com.
Growing evidence gardening cultivates mental health
The results of the small pilot study add to the growing body of evidence supporting the therapeutic value of gardening, study investigator Charles Guy, PhD, professor emeritus, University of Florida Institute of Food and Agricultural Sciences, Gainesville, told this news organization.
“If we can see therapeutic benefits among healthy individuals in a rigorously designed study, where variability was as controlled as you will see in this field, then now is the time to invest in some large-scale multi-institutional studies,” Dr. Guy added.
The study was published online in PLOS ONE.
Horticulture as therapy
Horticulture therapy involves engaging in gardening and plant-based activities facilitated by a trained therapist. Previous studies found that this intervention reduces apathy and improves cognitive function in some populations.
The current study included healthy, nonsmoking, and non–drug-using women, whose average age was about 32.5 years and whose body mass index was less than 32. The participants had no chronic conditions and were not allergic to pollen or plants.
Virtually all previous studies of therapeutic gardening included participants who had been diagnosed with conditions such as depression, chronic pain, or PTSD. “If we can see a therapeutic benefit with perfectly healthy people, then this is likely to have a therapeutic effect with whatever clinical population you might be interested in looking at,” said Dr. Guy.
In addition, including only women reduced variability, which is important in a small study, he said.
The researchers randomly assigned 20 participants to the gardening intervention and 20 to an art intervention. Each intervention consisted of twice-weekly 60-minute sessions for 4 weeks and a single follow-up session.
The art group was asked not to visit art galleries, museums, arts and crafts events, or art-related websites. Those in the gardening group were told not to visit parks or botanical gardens, not to engage in gardening activities, and not to visit gardening websites.
Activities in both groups involved a similar level of physical, cognitive, and social engagement. Gardeners were taught how to plant seeds and transplant and harvest edible crops, such as tomatoes, beans, and basil. Those in the art group learned papermaking and storytelling through drawing, printmaking, and mixed media collage.
At the beginning and end of the study, participants completed six questionnaires: the Profile of Mood States 2-A (POMS) short form, the Perceived Stress Scale (PSS), the Beck Depression Inventory II (BDI-II), the State-Trait Anxiety Inventory for Adults, the Satisfaction With Participation in Discretionary Social Activities, and the 36-item Short-Form Survey.
Participants wore wrist cuff blood pressure and heart rate monitors.
The analysis included 15 persons in the gardening group and 17 in the art group.
Participants in both interventions improved on several scales. For example, the mean preintervention POMS TMD (T score) for gardeners was 53.1, which was reduced to a mean of 46.9 post intervention (P = .018). In the art group, the means score was 53.5 before the intervention and 47.0 after the intervention (P = .009).
For the PSS, mean scores went from 14.9 to 9.4 (P = .002) for gardening and from 15.8 to 10.0 (P = .001) for artmaking.
For the BDI-II, mean scores dropped from 8.2 to 2.8 (P = .001) for gardening and from 9.0 to 5.1 (P = .009) for art.
However, gardening was associated with less trait anxiety than artmaking. “We concluded that both interventions were roughly equally therapeutic, with one glaring exception, and that was with trait anxiety, where the gardening resulted in statistical separation from the art group,” said Dr. Guy.
There appeared to be dose responses for total mood disturbance, perceived stress, and depression symptomatology for both gardening and artmaking.
Neither intervention affected heart rate or blood pressure. A larger sample might be needed to detect treatment differences in healthy women, the investigators noted.
The therapeutic benefit of gardening may lie in the role of plants in human evolution, during which “we relied on plants for shelter; we relied on them for protection; we relied on them obviously for nutrition,” said Dr. Guy.
The study results support carrying out large, well-designed, rigorously designed trials “that will definitively and conclusively demonstrate treatment effects with quantitative descriptions of those treatment effects with respect to dosage,” he said.
Good for the mind
Commenting on the study, Sir Richard Thompson, MD, past president, Royal College of Physicians, London, who has written about the health benefits of gardening, said this new study provides “more evidence that both gardening and art therapy are good for the mind” with mostly equal benefits for the two interventions.
“A much larger study would be needed to strengthen their case, but it fits in with much of the literature,” said Dr. Thompson.
However, he acknowledged the difficulty of carrying out scientifically robust studies in the field of alternative medicine, which “tends to be frowned upon” by some scientists.
Dr. Thompson identified some drawbacks of the study. In trying to measure so many parameters, the authors “may have had to resort to complex statistical analyses,” which may have led to some outcome changes being statistically positive by chance.
He noted that the study was small and that the gardening arm was “artificial” in that it was carried out in a greenhouse. “Maybe being outside would have been more beneficial; it would be interesting to test that hypothesis.”
As well, he pointed out initial differences between the two groups, including income and initial blood pressure, but he doubts these were significant.
He agreed that changes in cardiovascular parameters wouldn’t be expected in healthy young women, “as there’s little room for improvement.
“I wonder whether more improvement might have been seen in participants who were already suffering from anxiety, depression, etc.”
The study was supported by the Horticulture Research Institute, the Gene and Barbara Batson Endowed Nursery Fund, Florida Nursery Growers and Landscape Association, the Institute of Food and Agricultural Sciences, Wilmot Botanical Gardens, the Center for Arts in Medicine, Health Shands Arts in Medicine, and the department of environmental horticulture at the University of Florida. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results of the small pilot study add to the growing body of evidence supporting the therapeutic value of gardening, study investigator Charles Guy, PhD, professor emeritus, University of Florida Institute of Food and Agricultural Sciences, Gainesville, told this news organization.
“If we can see therapeutic benefits among healthy individuals in a rigorously designed study, where variability was as controlled as you will see in this field, then now is the time to invest in some large-scale multi-institutional studies,” Dr. Guy added.
The study was published online in PLOS ONE.
Horticulture as therapy
Horticulture therapy involves engaging in gardening and plant-based activities facilitated by a trained therapist. Previous studies found that this intervention reduces apathy and improves cognitive function in some populations.
The current study included healthy, nonsmoking, and non–drug-using women, whose average age was about 32.5 years and whose body mass index was less than 32. The participants had no chronic conditions and were not allergic to pollen or plants.
Virtually all previous studies of therapeutic gardening included participants who had been diagnosed with conditions such as depression, chronic pain, or PTSD. “If we can see a therapeutic benefit with perfectly healthy people, then this is likely to have a therapeutic effect with whatever clinical population you might be interested in looking at,” said Dr. Guy.
In addition, including only women reduced variability, which is important in a small study, he said.
The researchers randomly assigned 20 participants to the gardening intervention and 20 to an art intervention. Each intervention consisted of twice-weekly 60-minute sessions for 4 weeks and a single follow-up session.
The art group was asked not to visit art galleries, museums, arts and crafts events, or art-related websites. Those in the gardening group were told not to visit parks or botanical gardens, not to engage in gardening activities, and not to visit gardening websites.
Activities in both groups involved a similar level of physical, cognitive, and social engagement. Gardeners were taught how to plant seeds and transplant and harvest edible crops, such as tomatoes, beans, and basil. Those in the art group learned papermaking and storytelling through drawing, printmaking, and mixed media collage.
At the beginning and end of the study, participants completed six questionnaires: the Profile of Mood States 2-A (POMS) short form, the Perceived Stress Scale (PSS), the Beck Depression Inventory II (BDI-II), the State-Trait Anxiety Inventory for Adults, the Satisfaction With Participation in Discretionary Social Activities, and the 36-item Short-Form Survey.
Participants wore wrist cuff blood pressure and heart rate monitors.
The analysis included 15 persons in the gardening group and 17 in the art group.
Participants in both interventions improved on several scales. For example, the mean preintervention POMS TMD (T score) for gardeners was 53.1, which was reduced to a mean of 46.9 post intervention (P = .018). In the art group, the means score was 53.5 before the intervention and 47.0 after the intervention (P = .009).
For the PSS, mean scores went from 14.9 to 9.4 (P = .002) for gardening and from 15.8 to 10.0 (P = .001) for artmaking.
For the BDI-II, mean scores dropped from 8.2 to 2.8 (P = .001) for gardening and from 9.0 to 5.1 (P = .009) for art.
However, gardening was associated with less trait anxiety than artmaking. “We concluded that both interventions were roughly equally therapeutic, with one glaring exception, and that was with trait anxiety, where the gardening resulted in statistical separation from the art group,” said Dr. Guy.
There appeared to be dose responses for total mood disturbance, perceived stress, and depression symptomatology for both gardening and artmaking.
Neither intervention affected heart rate or blood pressure. A larger sample might be needed to detect treatment differences in healthy women, the investigators noted.
The therapeutic benefit of gardening may lie in the role of plants in human evolution, during which “we relied on plants for shelter; we relied on them for protection; we relied on them obviously for nutrition,” said Dr. Guy.
The study results support carrying out large, well-designed, rigorously designed trials “that will definitively and conclusively demonstrate treatment effects with quantitative descriptions of those treatment effects with respect to dosage,” he said.
Good for the mind
Commenting on the study, Sir Richard Thompson, MD, past president, Royal College of Physicians, London, who has written about the health benefits of gardening, said this new study provides “more evidence that both gardening and art therapy are good for the mind” with mostly equal benefits for the two interventions.
“A much larger study would be needed to strengthen their case, but it fits in with much of the literature,” said Dr. Thompson.
However, he acknowledged the difficulty of carrying out scientifically robust studies in the field of alternative medicine, which “tends to be frowned upon” by some scientists.
Dr. Thompson identified some drawbacks of the study. In trying to measure so many parameters, the authors “may have had to resort to complex statistical analyses,” which may have led to some outcome changes being statistically positive by chance.
He noted that the study was small and that the gardening arm was “artificial” in that it was carried out in a greenhouse. “Maybe being outside would have been more beneficial; it would be interesting to test that hypothesis.”
As well, he pointed out initial differences between the two groups, including income and initial blood pressure, but he doubts these were significant.
He agreed that changes in cardiovascular parameters wouldn’t be expected in healthy young women, “as there’s little room for improvement.
“I wonder whether more improvement might have been seen in participants who were already suffering from anxiety, depression, etc.”
The study was supported by the Horticulture Research Institute, the Gene and Barbara Batson Endowed Nursery Fund, Florida Nursery Growers and Landscape Association, the Institute of Food and Agricultural Sciences, Wilmot Botanical Gardens, the Center for Arts in Medicine, Health Shands Arts in Medicine, and the department of environmental horticulture at the University of Florida. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results of the small pilot study add to the growing body of evidence supporting the therapeutic value of gardening, study investigator Charles Guy, PhD, professor emeritus, University of Florida Institute of Food and Agricultural Sciences, Gainesville, told this news organization.
“If we can see therapeutic benefits among healthy individuals in a rigorously designed study, where variability was as controlled as you will see in this field, then now is the time to invest in some large-scale multi-institutional studies,” Dr. Guy added.
The study was published online in PLOS ONE.
Horticulture as therapy
Horticulture therapy involves engaging in gardening and plant-based activities facilitated by a trained therapist. Previous studies found that this intervention reduces apathy and improves cognitive function in some populations.
The current study included healthy, nonsmoking, and non–drug-using women, whose average age was about 32.5 years and whose body mass index was less than 32. The participants had no chronic conditions and were not allergic to pollen or plants.
Virtually all previous studies of therapeutic gardening included participants who had been diagnosed with conditions such as depression, chronic pain, or PTSD. “If we can see a therapeutic benefit with perfectly healthy people, then this is likely to have a therapeutic effect with whatever clinical population you might be interested in looking at,” said Dr. Guy.
In addition, including only women reduced variability, which is important in a small study, he said.
The researchers randomly assigned 20 participants to the gardening intervention and 20 to an art intervention. Each intervention consisted of twice-weekly 60-minute sessions for 4 weeks and a single follow-up session.
The art group was asked not to visit art galleries, museums, arts and crafts events, or art-related websites. Those in the gardening group were told not to visit parks or botanical gardens, not to engage in gardening activities, and not to visit gardening websites.
Activities in both groups involved a similar level of physical, cognitive, and social engagement. Gardeners were taught how to plant seeds and transplant and harvest edible crops, such as tomatoes, beans, and basil. Those in the art group learned papermaking and storytelling through drawing, printmaking, and mixed media collage.
At the beginning and end of the study, participants completed six questionnaires: the Profile of Mood States 2-A (POMS) short form, the Perceived Stress Scale (PSS), the Beck Depression Inventory II (BDI-II), the State-Trait Anxiety Inventory for Adults, the Satisfaction With Participation in Discretionary Social Activities, and the 36-item Short-Form Survey.
Participants wore wrist cuff blood pressure and heart rate monitors.
The analysis included 15 persons in the gardening group and 17 in the art group.
Participants in both interventions improved on several scales. For example, the mean preintervention POMS TMD (T score) for gardeners was 53.1, which was reduced to a mean of 46.9 post intervention (P = .018). In the art group, the means score was 53.5 before the intervention and 47.0 after the intervention (P = .009).
For the PSS, mean scores went from 14.9 to 9.4 (P = .002) for gardening and from 15.8 to 10.0 (P = .001) for artmaking.
For the BDI-II, mean scores dropped from 8.2 to 2.8 (P = .001) for gardening and from 9.0 to 5.1 (P = .009) for art.
However, gardening was associated with less trait anxiety than artmaking. “We concluded that both interventions were roughly equally therapeutic, with one glaring exception, and that was with trait anxiety, where the gardening resulted in statistical separation from the art group,” said Dr. Guy.
There appeared to be dose responses for total mood disturbance, perceived stress, and depression symptomatology for both gardening and artmaking.
Neither intervention affected heart rate or blood pressure. A larger sample might be needed to detect treatment differences in healthy women, the investigators noted.
The therapeutic benefit of gardening may lie in the role of plants in human evolution, during which “we relied on plants for shelter; we relied on them for protection; we relied on them obviously for nutrition,” said Dr. Guy.
The study results support carrying out large, well-designed, rigorously designed trials “that will definitively and conclusively demonstrate treatment effects with quantitative descriptions of those treatment effects with respect to dosage,” he said.
Good for the mind
Commenting on the study, Sir Richard Thompson, MD, past president, Royal College of Physicians, London, who has written about the health benefits of gardening, said this new study provides “more evidence that both gardening and art therapy are good for the mind” with mostly equal benefits for the two interventions.
“A much larger study would be needed to strengthen their case, but it fits in with much of the literature,” said Dr. Thompson.
However, he acknowledged the difficulty of carrying out scientifically robust studies in the field of alternative medicine, which “tends to be frowned upon” by some scientists.
Dr. Thompson identified some drawbacks of the study. In trying to measure so many parameters, the authors “may have had to resort to complex statistical analyses,” which may have led to some outcome changes being statistically positive by chance.
He noted that the study was small and that the gardening arm was “artificial” in that it was carried out in a greenhouse. “Maybe being outside would have been more beneficial; it would be interesting to test that hypothesis.”
As well, he pointed out initial differences between the two groups, including income and initial blood pressure, but he doubts these were significant.
He agreed that changes in cardiovascular parameters wouldn’t be expected in healthy young women, “as there’s little room for improvement.
“I wonder whether more improvement might have been seen in participants who were already suffering from anxiety, depression, etc.”
The study was supported by the Horticulture Research Institute, the Gene and Barbara Batson Endowed Nursery Fund, Florida Nursery Growers and Landscape Association, the Institute of Food and Agricultural Sciences, Wilmot Botanical Gardens, the Center for Arts in Medicine, Health Shands Arts in Medicine, and the department of environmental horticulture at the University of Florida. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE












