User login
Use of Fecal Immunochemical Testing in Acute Patient Care in a Safety Net Hospital System
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
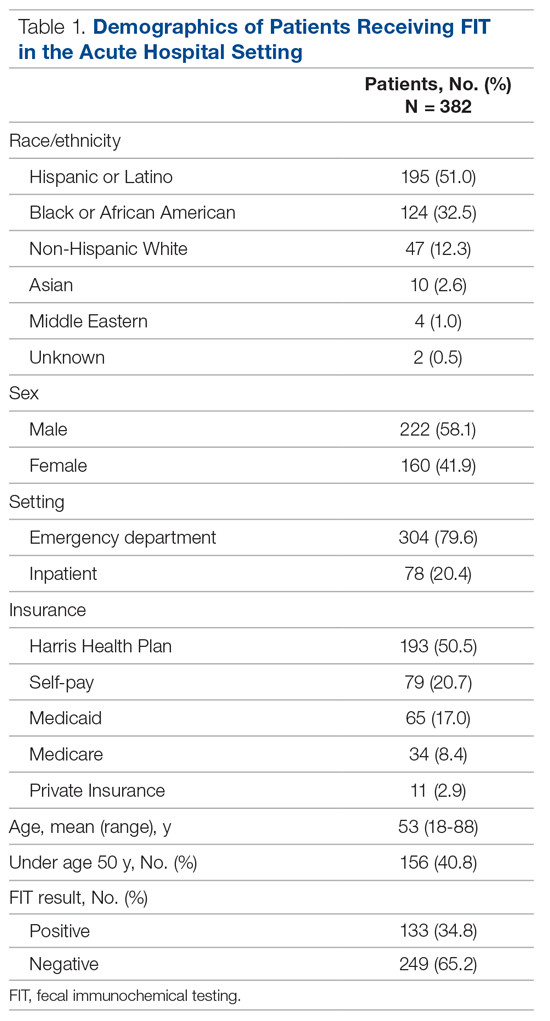
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
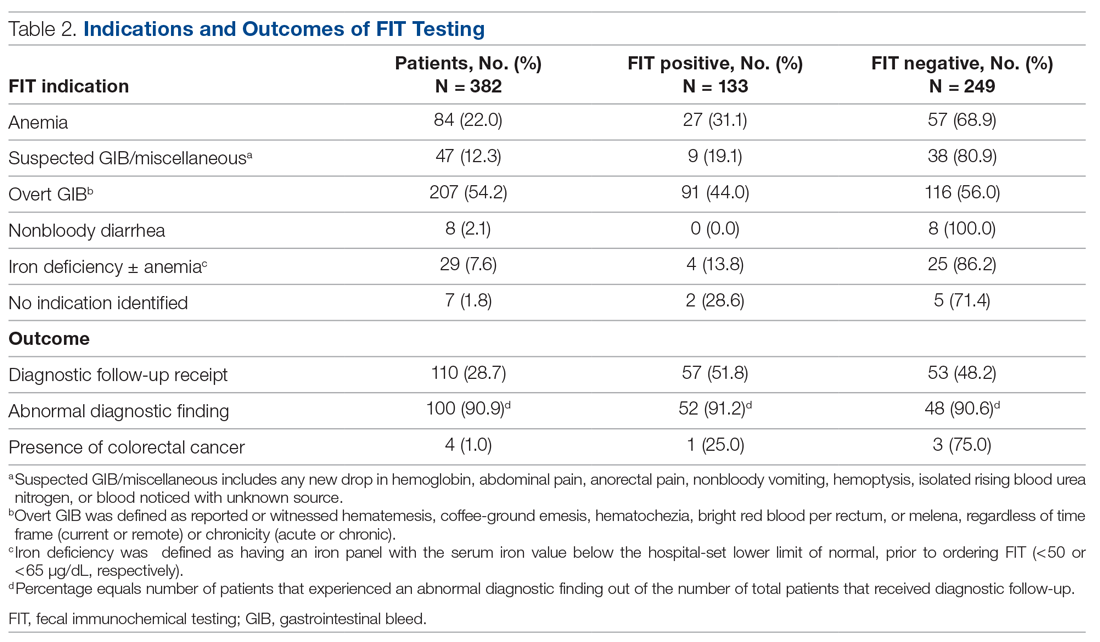
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
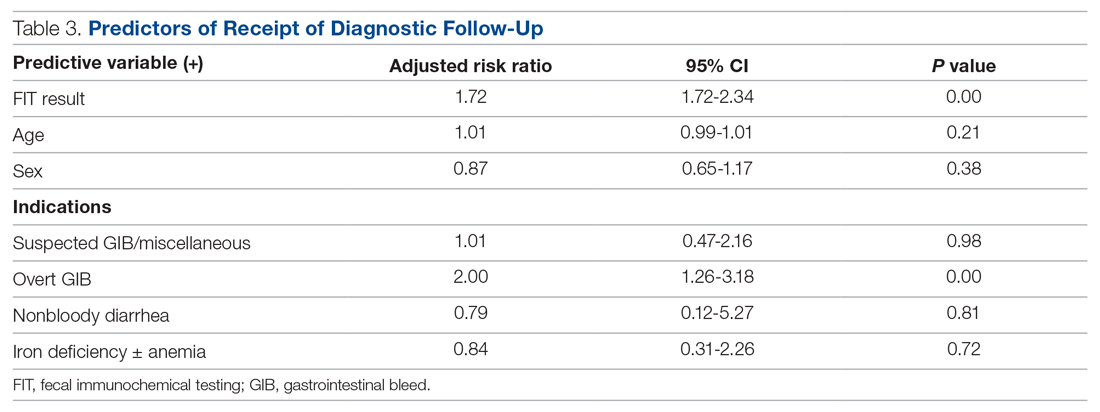
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
Postintubation tracheal injury in the COVID-19 era
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
The revenge of the ‘late COVID adopters’
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
Late-window stroke thrombolysis not linked to clot migration
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier antibiotic initiation for sepsis did not lead to overuse
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
FROM CCC50
Pulmonary and critical care session highlights new advances and research
An overview of five important advances in pulmonary and critical care medicine are on the agenda for the “Update in Pulmonary and Critical Care” session on Tuesday, May 4, at the virtual 2021 SHM Converge conference.
“I hope this session gives attendees a nice, broad look at advances both in the intensive care unit and in general pulmonary medicine,” said James Walter, MD, of Northwestern Medicine in Chicago, who serves as director of the session.
On the critical care medicine side, Dr. Walter will review the latest research on the efficacy of ascorbic acid in treating patients with severe sepsis and septic shock. “There was a lot of excitement and some skepticism about early results promising a really large treatment effect in giving critically ill patients with sepsis large doses of vitamin C,” Dr. Walter said. The last year has produced some high-quality randomized trials that have contributed to a better understanding of the potential effects ascorbic acid in sepsis can have, he noted.
Dr. Walter, who is also medical director of the Northwestern Lung Rescue Program, intends to discuss what he believes is a definitive trial regarding the benefit of preemptively starting critically ill patients with acute kidney injury on renal replacement therapy instead of waiting until there are specific clinical signs. “This has been another area of uncertainty in critical care and I think we finally have a very definitive answer with this high quality, randomized, controlled trial that I plan to review,” he said.
Though he said there have been a number of important advances in pulmonary medicine over the past year, Dr. Walter will highlight just two.
Up until recently, the antifibrotics nintedanib and pirfenidone have mostly been used in patients with idiopathic pulmonary fibrosis. However, recent research suggests there may be a potential benefit to using these drugs in patients with fibrotic lung disease outside of idiopathic pulmonary fibrosis. “I think this is an important advance for hospital medicine providers to be aware of,” said Dr. Walter.
He will also go over some large randomized controlled trials of the use of triple therapy – a combination of a long-acting beta agonist (LABA), a long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid in one inhaler – in chronic obstructive pulmonary disease. The trials looked at whether triple inhaler therapy was beneficial compared to the typical therapies used for COPD.
The session wouldn’t be complete without a nod to COVID-19, which Dr. Walter said has significantly changed the landscape for hospital medicine providers. He plans to discuss what he considers the most impactful study – the RECOVERY trial. This study looked at the role of dexamethasone in patients with more severe manifestations of SARS-CoV-2.
“From the incredible amount of data that’s come out in the last year about COVID, I think this is probably the trial that’s changed practice the most and shown the largest therapeutic benefit of all the pharmacotherapies,” Dr. Walter said. “It’s an important one for providers to be aware of in terms of what the trial shows and how it informs which patients are most likely to benefit from dexamethasone therapy.”
Dr. Walter hopes clinicians who participate in the session will leave with these takeaways:
- Be able to summarize recent trials of ascorbic acid in sepsis and think about how to incorporate – or not – the use of vitamin C in critically ill sepsis patients.
- A thorough understanding of when renal replacement therapy should be offered to critically ill patients with acute kidney dysfunction.
- Be able to discuss the impact of antifibrotic therapy in interstitial lung diseases outside of idiopathic pulmonary fibrosis.
- An understanding of the role of triple inhaler combinations in COPD.
- Be able to explain when dexamethasone is most likely to benefit hypoxemic patients with COVID-19.
An overview of five important advances in pulmonary and critical care medicine are on the agenda for the “Update in Pulmonary and Critical Care” session on Tuesday, May 4, at the virtual 2021 SHM Converge conference.
“I hope this session gives attendees a nice, broad look at advances both in the intensive care unit and in general pulmonary medicine,” said James Walter, MD, of Northwestern Medicine in Chicago, who serves as director of the session.
On the critical care medicine side, Dr. Walter will review the latest research on the efficacy of ascorbic acid in treating patients with severe sepsis and septic shock. “There was a lot of excitement and some skepticism about early results promising a really large treatment effect in giving critically ill patients with sepsis large doses of vitamin C,” Dr. Walter said. The last year has produced some high-quality randomized trials that have contributed to a better understanding of the potential effects ascorbic acid in sepsis can have, he noted.
Dr. Walter, who is also medical director of the Northwestern Lung Rescue Program, intends to discuss what he believes is a definitive trial regarding the benefit of preemptively starting critically ill patients with acute kidney injury on renal replacement therapy instead of waiting until there are specific clinical signs. “This has been another area of uncertainty in critical care and I think we finally have a very definitive answer with this high quality, randomized, controlled trial that I plan to review,” he said.
Though he said there have been a number of important advances in pulmonary medicine over the past year, Dr. Walter will highlight just two.
Up until recently, the antifibrotics nintedanib and pirfenidone have mostly been used in patients with idiopathic pulmonary fibrosis. However, recent research suggests there may be a potential benefit to using these drugs in patients with fibrotic lung disease outside of idiopathic pulmonary fibrosis. “I think this is an important advance for hospital medicine providers to be aware of,” said Dr. Walter.
He will also go over some large randomized controlled trials of the use of triple therapy – a combination of a long-acting beta agonist (LABA), a long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid in one inhaler – in chronic obstructive pulmonary disease. The trials looked at whether triple inhaler therapy was beneficial compared to the typical therapies used for COPD.
The session wouldn’t be complete without a nod to COVID-19, which Dr. Walter said has significantly changed the landscape for hospital medicine providers. He plans to discuss what he considers the most impactful study – the RECOVERY trial. This study looked at the role of dexamethasone in patients with more severe manifestations of SARS-CoV-2.
“From the incredible amount of data that’s come out in the last year about COVID, I think this is probably the trial that’s changed practice the most and shown the largest therapeutic benefit of all the pharmacotherapies,” Dr. Walter said. “It’s an important one for providers to be aware of in terms of what the trial shows and how it informs which patients are most likely to benefit from dexamethasone therapy.”
Dr. Walter hopes clinicians who participate in the session will leave with these takeaways:
- Be able to summarize recent trials of ascorbic acid in sepsis and think about how to incorporate – or not – the use of vitamin C in critically ill sepsis patients.
- A thorough understanding of when renal replacement therapy should be offered to critically ill patients with acute kidney dysfunction.
- Be able to discuss the impact of antifibrotic therapy in interstitial lung diseases outside of idiopathic pulmonary fibrosis.
- An understanding of the role of triple inhaler combinations in COPD.
- Be able to explain when dexamethasone is most likely to benefit hypoxemic patients with COVID-19.
An overview of five important advances in pulmonary and critical care medicine are on the agenda for the “Update in Pulmonary and Critical Care” session on Tuesday, May 4, at the virtual 2021 SHM Converge conference.
“I hope this session gives attendees a nice, broad look at advances both in the intensive care unit and in general pulmonary medicine,” said James Walter, MD, of Northwestern Medicine in Chicago, who serves as director of the session.
On the critical care medicine side, Dr. Walter will review the latest research on the efficacy of ascorbic acid in treating patients with severe sepsis and septic shock. “There was a lot of excitement and some skepticism about early results promising a really large treatment effect in giving critically ill patients with sepsis large doses of vitamin C,” Dr. Walter said. The last year has produced some high-quality randomized trials that have contributed to a better understanding of the potential effects ascorbic acid in sepsis can have, he noted.
Dr. Walter, who is also medical director of the Northwestern Lung Rescue Program, intends to discuss what he believes is a definitive trial regarding the benefit of preemptively starting critically ill patients with acute kidney injury on renal replacement therapy instead of waiting until there are specific clinical signs. “This has been another area of uncertainty in critical care and I think we finally have a very definitive answer with this high quality, randomized, controlled trial that I plan to review,” he said.
Though he said there have been a number of important advances in pulmonary medicine over the past year, Dr. Walter will highlight just two.
Up until recently, the antifibrotics nintedanib and pirfenidone have mostly been used in patients with idiopathic pulmonary fibrosis. However, recent research suggests there may be a potential benefit to using these drugs in patients with fibrotic lung disease outside of idiopathic pulmonary fibrosis. “I think this is an important advance for hospital medicine providers to be aware of,” said Dr. Walter.
He will also go over some large randomized controlled trials of the use of triple therapy – a combination of a long-acting beta agonist (LABA), a long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid in one inhaler – in chronic obstructive pulmonary disease. The trials looked at whether triple inhaler therapy was beneficial compared to the typical therapies used for COPD.
The session wouldn’t be complete without a nod to COVID-19, which Dr. Walter said has significantly changed the landscape for hospital medicine providers. He plans to discuss what he considers the most impactful study – the RECOVERY trial. This study looked at the role of dexamethasone in patients with more severe manifestations of SARS-CoV-2.
“From the incredible amount of data that’s come out in the last year about COVID, I think this is probably the trial that’s changed practice the most and shown the largest therapeutic benefit of all the pharmacotherapies,” Dr. Walter said. “It’s an important one for providers to be aware of in terms of what the trial shows and how it informs which patients are most likely to benefit from dexamethasone therapy.”
Dr. Walter hopes clinicians who participate in the session will leave with these takeaways:
- Be able to summarize recent trials of ascorbic acid in sepsis and think about how to incorporate – or not – the use of vitamin C in critically ill sepsis patients.
- A thorough understanding of when renal replacement therapy should be offered to critically ill patients with acute kidney dysfunction.
- Be able to discuss the impact of antifibrotic therapy in interstitial lung diseases outside of idiopathic pulmonary fibrosis.
- An understanding of the role of triple inhaler combinations in COPD.
- Be able to explain when dexamethasone is most likely to benefit hypoxemic patients with COVID-19.
Six-month follow-up shows continuing morbidity for COVID-19 survivors
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
FROM CCC50
PPE protected critical care staff from COVID-19 transmission
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
FROM CCC50
Burnout rates in ICU staff fueled by shortages, overtime
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
Health care professionals working in critical care settings have been overburdened because of the plethora of COVID-19 cases, which has led to symptoms of burnout in both physicians and nurses, findings from a new study show.
“Overburdening ICU professionals during an extended period of time leads to burnout,” said lead study author Niek Kok, MSc, of IQ healthcare, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands. “All ICU professionals are at the risk of this, and in our study, the incidence of physicians experiencing burnout was significantly higher than that of nurses in June 2020.”
This burnout can be explained by conditions caused by the pandemic, he noted, such as the scarcity of staff and resources and having to work with colleagues who were not qualified to work in critical care but who were there out of necessity.
Mr. Kok presented the findings of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Burnout highest among critical care physicians
The ICU can be a stressful environment for both patients and health care personnel, and burnout is not uncommon among ICU clinicians. However, COVID-19 has amplified the degree of burnout being experienced by clinicians working in this setting. Critical care physicians now top the list of physicians experiencing burnout, at 51%, up from 44% last year, according to the Medscape report ‘Death by 1000 Thousand Cuts’: Physician Burnout and Suicide Report 2021.
The Medscape Nurse Career Satisfaction Report 2020, while not restricted to those working in critical care, also reported higher rates of burnout, compared with the prepandemic period. The percentage of nurses reporting being “very burned out” prior to the pandemic was 4%. Six months into the pandemic, that percentage soared to 18%.
In this study, Mr. Kok and colleagues examined the prevalence and incidence of burnout symptoms and moral distress in health care professionals working in the ICU, both before and during the COVID-19 pandemic.
“When the COVID-19 pandemic surfaced in the Netherlands, the health care professionals in our hospitals were motivated to do everything they could to provide the best care possible,” said Mr. Kok. “Many of the ICU professionals immediately realized that they would have to work longer hours.”
However, the health care professionals that he spoke with did have mixed feelings. Some were afraid of being infected with the virus, while others said that “it was very interesting times for them and that gave them extra motivation to do the work.
“Some physicians [and] the WHO warned that COVID-19 is not going to weathered by a heroic sprint – it is an arduous marathon that is going to go hand in hand with burnout symptoms,” Mr. Kok added. “It will eat away at our qualified ICU staff.”
Before and after data on burnout
It was widely believed that the COVID-19 pandemic would increase burnout symptoms, as had been demonstrated in studies of previous pandemics. However, Mr. Kok emphasized that there are no before and after measurements that transcend cross-sectional designs.
“The claim [has been] that it increases burnout – but there are no assessments of how it progresses in ICU professionals through time,” he said. “So what we really need is a comparison [of] before and after the pandemic.”
It is quite difficult to obtain this type of information because disruptive events like the COVID-19 pandemic cannot be predicted, he said. Thus, it is challenging to get a baseline measurement. But Mr. Kok pointed out that the study has both “before and after” measurements.
“By coincidence really, we had baseline data to measure the impact of the COVID-19 pandemic and had information that was collected before the pandemic,” he said.
In January 2020, a study began looking at the effects of ethics meetings on moral distress in ICU professionals. Data had been collected on moral distress and burnout on ICU professionals in December 2019. The first COVID-19 cases appeared in the Netherlands in February 2020.
A follow-up study was then conducted in May and June 2020, several months into the pandemic.
The longitudinal open cohort study included all ICU personnel who were working in five units within a single university medical center, plus another adult ICU that was based in a separate teaching hospital.
A total of 352 health care professionals responded to a baseline survey in October through December 2019, and then 233 responded to a follow-up survey sent in May and June 2020. The authors measured burnout symptoms and moral distress with the Maslach Burnout Inventory and the Moral Distress Scale, respectively.
Findings
The overall prevalence of burnout symptoms was 23.0% prior to the pandemic, and that jumped to 36.1% at post-peak time. Higher rates of burnout were reported by nurses (38.0%) than physicians (28.6%).
However, the incidence rate of new burnout cases was higher among physicians, compared with nurses (26.7% vs 21.9%). Not surprisingly, a higher prevalence of burnout symptoms was observed in the post-peak period for all clinicians (odds ratio, 1.83; 95% confidence interval, 1.32-2.53), and was higher for nurses (odds ratio, 1.77; 95% confidence interval, 1.03-3.04), for those working overtime (OR, 2.11; 95% CI, 1.48-3.02), and for personnel who directly engaged in patient care (OR, 1.87; 95% CI, 1.35-2.60).
Physicians in general were much more likely to develop burnout symptoms related to the pandemic, compared with nurses (OR, 3.56; 95% CI, 1.06-12.21).
When looking at findings on moral distress, Kok pointed out that it often arises in situations when the health care professional knows the right thing to do but is prevented from doing so. “Morally distressful situations all rose from December to June,” said Mr. Kok. “Scarcity was the most distressing. The other was where colleagues were perceived to be less skilled, and this had to do with the recruitment of people from outside of the ICU to provide care.”
Moral distress from scarcity and unskilled colleagues were both significantly related to burnout, he noted.
In the final model, working in a COVID-19 unit, stress from scarcity of resources and people, stress from unskilled colleagues, and stress from unsafe conditions were all related to burnout. “The stress of physicians was significantly higher,” said Kok. “Even though nurses had higher baseline burnout, it became less pronounced in June 2020. This indicates that burnout was significantly higher in physicians.”
Thus, Mr. Kok and colleagues concluded that overburdening ICU professionals during an extended period of time leads to burnout, and all ICU workers are at risk.
Burnout rates higher in physicians
Weighing in on the study, Greg S. Martin, MD, FCCP, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that the differences observed between physicians and nurses may have to do with the fact that “nurses have been smoldering all along and experiencing higher rates of burnout.
“They may have adapted better to the pandemic conditions, since they are more used to working overtime and short staffed, and spending far more time at the bedside,” he said. “Because of the volume of patients, physicians may be spending more hours doing patient care and are experiencing more burnout.”
For physicians, this may be a more significant change in the workload, as well as the complexity of the situation because of the pandemic. “Many things layer into it, such as [the fact] that there are no families present to give patients support, the complexity of care of these patients, and things like lack of PPE,” Dr. Martin said.
The study did not differentiate among physician groups, so it is unclear if the affected physicians were residents, fellows, or more senior staff. “Residents are often quite busy already, and don’t usually have the capacity to add more to their schedules, and maybe attendings were having to spend more time doing patient care,” Dr. Martin said. “In the United States, at least some personnel were restricted from working with COVID-19 patients. Medical students were removed in many places as well as nonessential staff, so that may have also added to their burnout.”
The study was conducted in the Netherlands, so there may be differences in the work environment, responsibilities of nurses vs. physicians, staffing, and so on. “But it still shows that burnout is very real among doctors and nurses working in the ICU in pandemic conditions,” he said.
FROM CCC50
COVID-19: Peginterferon lambda may prevent clinical deterioration, shorten viral shedding
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
FROM THE LANCET RESPIRATORY MEDICINE