User login
Military Medical Teams Deploy to Relieve COVID-Battered Hospitals
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Military Medical Teams Deploy to Relieve COVID-Battered Hospitals
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
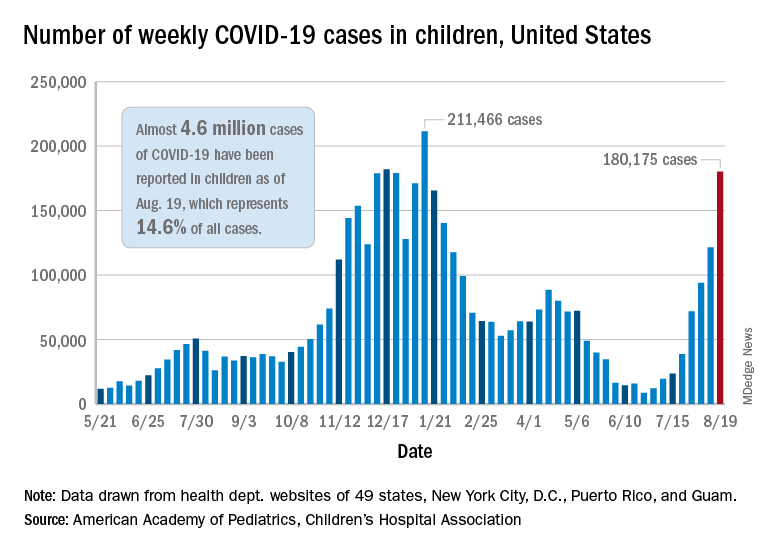
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.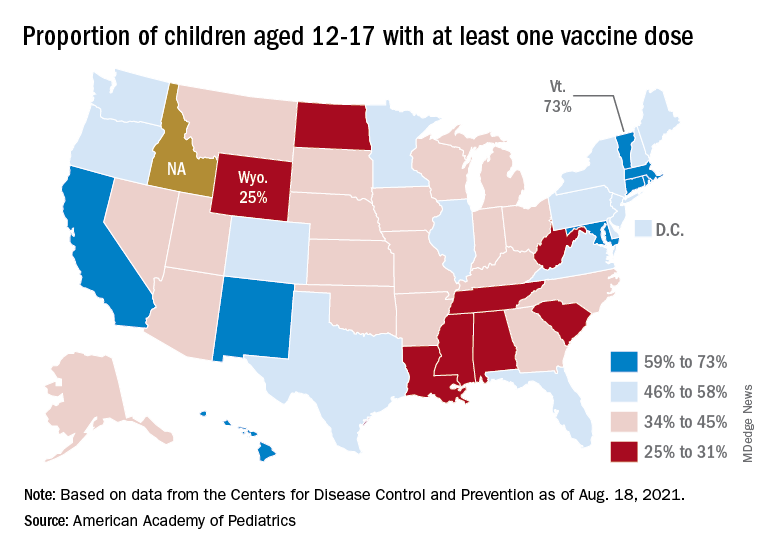
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Nearly 1 in 5 parents put off care for their kids in pandemic
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
Guidance on additional COVID-19 vaccine dose for MS patients
Patients aged 12 years and older with multiple sclerosis (MS) who are fully immunized against COVID-19 with either the Pfizer-BioNTech or Moderna mRNA vaccine may be eligible to receive an additional dose now, the National Multiple Sclerosis Society has announced.
New guidance, which is “based on available data from studies and expert consensus opinion” by a panel of MS neurologists and experts, was published Aug. 19 on the organization’s website.
The Food and Drug Administration has authorized an additional dose of the coronavirus vaccine for patients who are expected to not have a normal or adequate immune response to the first two doses. Patients with MS who use certain treatments have a reduced or absent antibody response to the vaccine, according to recent data.
“We want people living with MS to be aware of this additional dose and discuss when they need an additional dose or booster dose with their health care provider,” Julie Fiol, RN, MSW, associate vice president of health care access, National MS Society, said in an interview.
Those who may benefit from an additional dose include patients with MS who use sphingosine 1-phosphate receptor modulators, anti-CD20 monoclonal antibodies, or alemtuzumab (Lemtrada), the National MS Society noted. These particular disease modifying therapies (DMTs) have a stronger effect on the immune system than do other treatments.
Protecting ‘the most vulnerable’
Sphingosine 1-phosphate receptor modulators include fingolimod (Gilenya), siponimod (Mayzent), ozanimod (Zeposia), and ponesimod (Ponvory).
Anti-CD20 monoclonal antibodies include ocrelizumab (Ocrevus), ofatumumab (Kesimpta), rituximab (Rituxan), and corresponding biosimilars.
Current data do not support an additional dose for immunocompromised patients who received the Johnson & Johnson vaccine. The FDA and the Centers for Disease Control and Prevention are developing recommendations for these patients, and the National MS Society will update its guidance as needed, the organization noted in its statement.
“Like other medical decisions, the decision to get an additional dose is best made in partnership with your health care provider,” said Ms. Fiol. “Talk to your MS health care provider to determine what is best for you.”
MS itself does not compromise the immune system, but some MS therapies alter the immune system and reduce the body’s response to vaccination. Patients with MS who use B cell-depleting therapies have a better antibody response when they receive the vaccine 3 months or more after the last dose of MS therapy, according to the National MS Society.
Data suggest that patients with MS are not more susceptible to COVID-19 infection, severe illness, or death than are patients without MS. However, certain groups of patients with MS, such as those who receive B cell-depleting treatments, are more susceptible to having a severe case of COVID-19.
That said, “everyone will need a booster at some point. Those who take DMTs that have greater impact on the immune system are the most urgent need now,” the organization noted.
“Vaccination against COVID-19 is critical for public safety and, especially, the safety of the most vulnerable among us,” said Ms. Fiol. “We encourage everyone with MS get vaccinated.”
A version of this article first appeared on Medscape.com.
Patients aged 12 years and older with multiple sclerosis (MS) who are fully immunized against COVID-19 with either the Pfizer-BioNTech or Moderna mRNA vaccine may be eligible to receive an additional dose now, the National Multiple Sclerosis Society has announced.
New guidance, which is “based on available data from studies and expert consensus opinion” by a panel of MS neurologists and experts, was published Aug. 19 on the organization’s website.
The Food and Drug Administration has authorized an additional dose of the coronavirus vaccine for patients who are expected to not have a normal or adequate immune response to the first two doses. Patients with MS who use certain treatments have a reduced or absent antibody response to the vaccine, according to recent data.
“We want people living with MS to be aware of this additional dose and discuss when they need an additional dose or booster dose with their health care provider,” Julie Fiol, RN, MSW, associate vice president of health care access, National MS Society, said in an interview.
Those who may benefit from an additional dose include patients with MS who use sphingosine 1-phosphate receptor modulators, anti-CD20 monoclonal antibodies, or alemtuzumab (Lemtrada), the National MS Society noted. These particular disease modifying therapies (DMTs) have a stronger effect on the immune system than do other treatments.
Protecting ‘the most vulnerable’
Sphingosine 1-phosphate receptor modulators include fingolimod (Gilenya), siponimod (Mayzent), ozanimod (Zeposia), and ponesimod (Ponvory).
Anti-CD20 monoclonal antibodies include ocrelizumab (Ocrevus), ofatumumab (Kesimpta), rituximab (Rituxan), and corresponding biosimilars.
Current data do not support an additional dose for immunocompromised patients who received the Johnson & Johnson vaccine. The FDA and the Centers for Disease Control and Prevention are developing recommendations for these patients, and the National MS Society will update its guidance as needed, the organization noted in its statement.
“Like other medical decisions, the decision to get an additional dose is best made in partnership with your health care provider,” said Ms. Fiol. “Talk to your MS health care provider to determine what is best for you.”
MS itself does not compromise the immune system, but some MS therapies alter the immune system and reduce the body’s response to vaccination. Patients with MS who use B cell-depleting therapies have a better antibody response when they receive the vaccine 3 months or more after the last dose of MS therapy, according to the National MS Society.
Data suggest that patients with MS are not more susceptible to COVID-19 infection, severe illness, or death than are patients without MS. However, certain groups of patients with MS, such as those who receive B cell-depleting treatments, are more susceptible to having a severe case of COVID-19.
That said, “everyone will need a booster at some point. Those who take DMTs that have greater impact on the immune system are the most urgent need now,” the organization noted.
“Vaccination against COVID-19 is critical for public safety and, especially, the safety of the most vulnerable among us,” said Ms. Fiol. “We encourage everyone with MS get vaccinated.”
A version of this article first appeared on Medscape.com.
Patients aged 12 years and older with multiple sclerosis (MS) who are fully immunized against COVID-19 with either the Pfizer-BioNTech or Moderna mRNA vaccine may be eligible to receive an additional dose now, the National Multiple Sclerosis Society has announced.
New guidance, which is “based on available data from studies and expert consensus opinion” by a panel of MS neurologists and experts, was published Aug. 19 on the organization’s website.
The Food and Drug Administration has authorized an additional dose of the coronavirus vaccine for patients who are expected to not have a normal or adequate immune response to the first two doses. Patients with MS who use certain treatments have a reduced or absent antibody response to the vaccine, according to recent data.
“We want people living with MS to be aware of this additional dose and discuss when they need an additional dose or booster dose with their health care provider,” Julie Fiol, RN, MSW, associate vice president of health care access, National MS Society, said in an interview.
Those who may benefit from an additional dose include patients with MS who use sphingosine 1-phosphate receptor modulators, anti-CD20 monoclonal antibodies, or alemtuzumab (Lemtrada), the National MS Society noted. These particular disease modifying therapies (DMTs) have a stronger effect on the immune system than do other treatments.
Protecting ‘the most vulnerable’
Sphingosine 1-phosphate receptor modulators include fingolimod (Gilenya), siponimod (Mayzent), ozanimod (Zeposia), and ponesimod (Ponvory).
Anti-CD20 monoclonal antibodies include ocrelizumab (Ocrevus), ofatumumab (Kesimpta), rituximab (Rituxan), and corresponding biosimilars.
Current data do not support an additional dose for immunocompromised patients who received the Johnson & Johnson vaccine. The FDA and the Centers for Disease Control and Prevention are developing recommendations for these patients, and the National MS Society will update its guidance as needed, the organization noted in its statement.
“Like other medical decisions, the decision to get an additional dose is best made in partnership with your health care provider,” said Ms. Fiol. “Talk to your MS health care provider to determine what is best for you.”
MS itself does not compromise the immune system, but some MS therapies alter the immune system and reduce the body’s response to vaccination. Patients with MS who use B cell-depleting therapies have a better antibody response when they receive the vaccine 3 months or more after the last dose of MS therapy, according to the National MS Society.
Data suggest that patients with MS are not more susceptible to COVID-19 infection, severe illness, or death than are patients without MS. However, certain groups of patients with MS, such as those who receive B cell-depleting treatments, are more susceptible to having a severe case of COVID-19.
That said, “everyone will need a booster at some point. Those who take DMTs that have greater impact on the immune system are the most urgent need now,” the organization noted.
“Vaccination against COVID-19 is critical for public safety and, especially, the safety of the most vulnerable among us,” said Ms. Fiol. “We encourage everyone with MS get vaccinated.”
A version of this article first appeared on Medscape.com.
Q&A: Get flu shot early this year? Same time as COVID vaccine?
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
Plastic barriers may not stop COVID-19 spread, experts say
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
Shouldn’t docs who spread false COVID-19 info lose their licenses?
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.