User login
Is Laundry Detergent a Common Cause of Allergic Contact Dermatitis?
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13
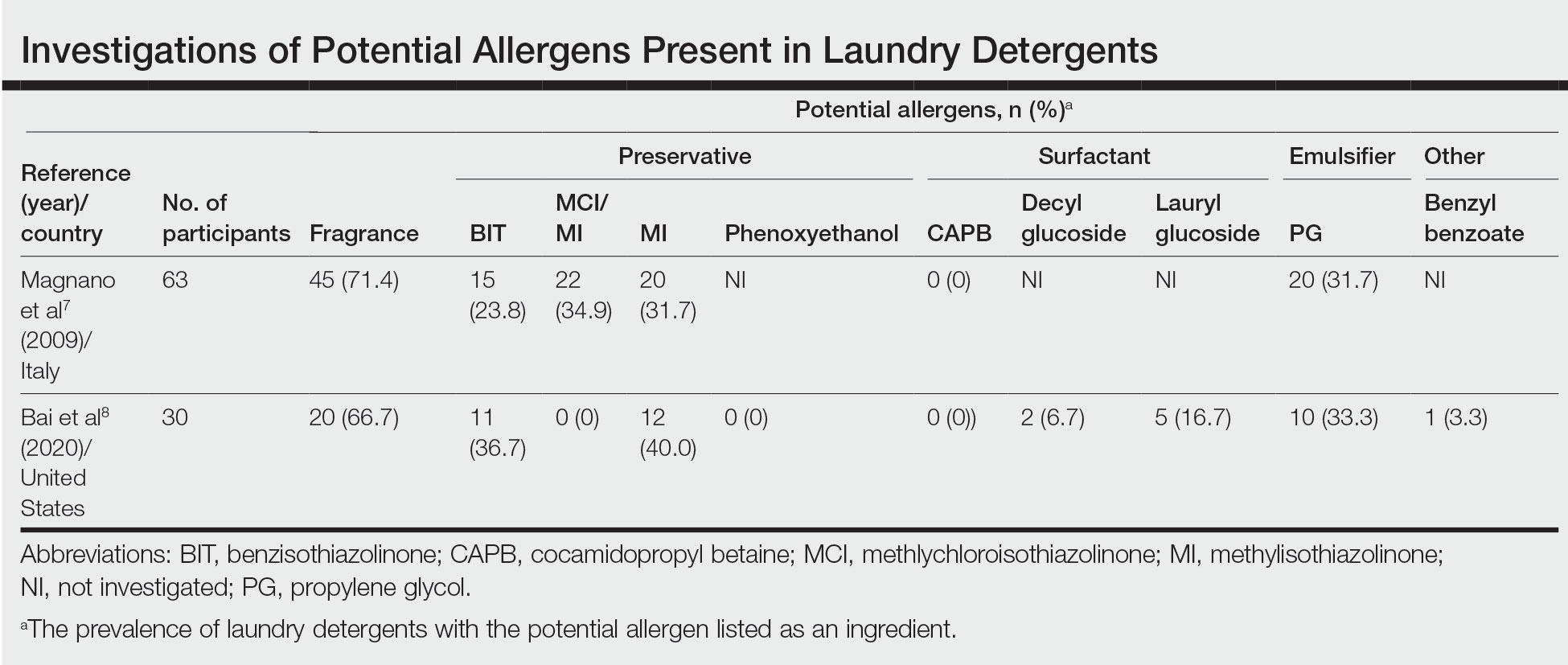
Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Practice Points
- Although laundry detergent commonly is believed to be a cause of allergic contact dermatitis (ACD), the actual prevalence is quite low (<1%).
- Common allergens present in laundry detergent such as fragrances and isothiazolinone preservatives likely are reduced to clinically irrelevant levels during routine machine washing.
- Other diagnoses to consider when laundry detergent–associated ACD is suspected include textile ACD, atopic dermatitis, and cutaneous T-cell lymphoma.
Pilot study evaluates sensitive skin burden in persons of color
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
AT AAD 2023
Papular Rash in a New Tattoo
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
A healthy 21-year-old woman presented with a pruritic papulovesicular rash on the left arm of 2 days’ duration. The day before rash onset, she received a black ink tattoo on the left arm to complete the second half of a monochromatic sleevestyle design. She previously underwent initial tattooing of the left arm by the same artist 2 weeks prior and experienced a similar but less extensive rash that self-resolved after 1 week. She had 8 older tattoos on various other body parts and denied any reactions. Physical examination showed numerous scattered papules and papulovesicles confined to areas of newly tattooed skin throughout the left arm. In the larger swaths of the tattoo, the papules coalesced into well-defined plaques. There was a discrete rim of faint erythema bordering the newly tattooed skin. No erosions, ulcerations, or purulent areas were observed, and there was no tenderness or excess warmth of the affected skin. Adjacent previously tattooed areas of the left arm were unaffected.

Lanolin gets nod for Allergen of the Year
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
FROM ACDS 2023
Are you misdiagnosing IBS? Watch out for this mimic
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
Josh struggled for more than a decade with what his doctors had told him was irritable bowel syndrome (IBS). But curiously, the 39-year-old’s flare-ups were caused by some foods that aren’t typical IBS triggers.
So, Josh (not his real name) sought the care of New York gastroenterologist Yevgenia Pashinsky, MD. She conducted a comprehensive nutritional assessment and sent him for allergy testing. The results: Josh had a little-known condition called systemic nickel allergy syndrome (SNAS), which can mimic some of the symptoms of IBS.
Dr. Pashinsky, of the department of medicine at Icahn School of Medicine at Mount Sinai, New York, and a partner with New York Gastroenterology Associates, presented Josh’s case as part of a seminar on SNAS and IBS “mimickers” at the Food and Nutrition Conference and Expo in Orlando last October, sponsored by the Academy of Nutrition and Dietetics.
She and two registered dietitians in her practice, Suzie Finkel, MS, RD, CDN, and Tamara Duker Freuman, MS, RD, CDN, told seminar attendees that SNAS is rarely diagnosed and can be mistaken for IBS. They noted that it probably strikes more people than doctors suspect.
“Systemic nickel allergy is present in at least 10% of the U.S. population (and much higher in some subgroups),” Dr. Pashinsky told this news organization. “But its connection to GI symptoms and functional GI disorders is still being learned about.
“I think of nickel allergy and other allergic disorders when, in addition to GI symptoms, the patient reports skin and mucous membrane involvement along with their abdominal reactions,” she said.
For patients like Josh with SNAS, the diagnosis and treatment of this condition are surprisingly simple and effective.
“Josh had these really [unusual] symptoms and nontraditional IBS food triggers,” Ms. Finkel said in an interview. “So, that’s a situation where, as dietitians we say, ‘Hmm, that’s weird; if you have IBS, then peanuts and shrimp shouldn’t really cause an issue here.’ But this might be something physicians might not be attuned to because it’s not part of their training.”
Ms. Finkel said that Josh was referred to an allergist. Josh tested positive for skin sensitization to nickel, and he was started on a low-nickel diet, which improved his symptoms.
“So, that was this happy ending,” she added.
The upshot?
“Doctors who treat IBS patients [who are not responding to treatment] need to consider the possibility that they have SNAS and send them for allergy testing,” Ms. Finkel said. “If they come back positive, simple dietary changes can address it.”
An underrecognized condition
There has been very little research regarding SNAS in patients with IBS, and there are no standard guidelines for diagnosing and treating it.
What’s more, many gastroenterologists aren’t familiar with it. More than a dozen gastroenterologists who were contacted for comment declined to be interviewed because they didn’t know about SNAS – or enough about it to provide useful information for the story.
Ms. Finkel said she’s not surprised that many gastroenterologists don’t know much about how SNAS can mimic IBS, which is why she and her colleagues presented the seminar last October in Orlando. “It’s really an allergy and not a GI disease. It manifests with GI symptoms, but the root is not in the digestive tract; the root is in a true allergy – a clinical allergy – to nickel.”
Complicating the issue is that people who have IBS and those with SNAS typically share some common symptoms.
Like IBS, SNAS can cause GI symptoms – such as cramping, abdominal pain, heartburn, constipation, gaseous distension, and mucus in the stool. It can be triggered by certain fresh, cooked, and canned foods.
But the food triggers that cause SNAS are not usually those that cause IBS symptoms. Rather, SNAS flare-ups are nearly always triggered by foods with high levels of nickel. Examples include apricots, artichokes, asparagus, beans, cauliflower, chickpeas, cocoa/chocolate, figs, lentils, licorice, oats, onions, peas, peanuts, potatoes, spinach, tomatoes, and tea.
According to the American Academy of Allergy, Asthma & Immunology, a distinguishing feature of SNAS is that it can cause allergic contact dermatitis when a person touches something made with nickel. Coins, jewelry, eyeglasses, home fixtures, keys, zippers, dental devices, and even stainless-steel cookware can contain allergy-triggering nickel.
What Ms. Finkel sees the most are skin reactions from touching a surface containing nickel or from ingesting it, she said.
The other immediate symptom is abdominal pain or changes in bowel movements, such as diarrhea, she added.
Christopher Randolph, MD, an allergist based in Connecticut, told this news organization that it’s important for doctors to realize that patients who have a skin reaction to nickel may also have inflammatory GI symptoms.
“We definitely need more controlled studies,” said Dr. Randolph, of the department of allergy and immunology at Yale University, New Haven, Conn. “But the takeaway here is for patients and certainly providers to be mindful that you can have systemic reactions to nickel, even though you implicate only the contact dermatitis.”
Diagnosis and treatment recommendations
Skin patch allergy testing – in which a person’s skin is exposed to nickel – can quickly determine whether a patient with IBS is actually experiencing inflammatory reactions to dietary nickel and would benefit from a low-nickel or no-nickel diet, research shows.
For these patients, Dr. Pashinsky recommends the following:
- Avoiding high-nickel foods.
- Limiting canned foods.
- Using nonstainless cookware, especially for acidic foods.
- Boiling foods for potential nickel reduction, especially grains and vegetables.
- Running the tap before using water to drink or cook with first thing in the morning.
Dr. Pashisky and her team also recommend the following guidelines for doctors:
- Ask patients if symptoms occur immediately after eating certain high-nickel foods or worsen with a low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet.
- Determine whether a patient is not responding to typical medical and dietary interventions used to treat IBS.
- Conduct a food/symptom history to identify potential nickel allergy triggers.
- Try a low-nickel dietary intervention to see whether a patient’s symptoms improve in a week or two.
- Refer the patient for additional diagnostic skin-patch testing or treatment.
A multidisciplinary approach
Ms. Finkel said it’s important for doctors, particularly gastroenterologists who treat patients for suspected GI disorders to consider nickel allergy as a cause.
“SNAS is this overlooked condition ... and the research is really in its nascency here,” Ms. Finkel said.
“I would say only give [a low- or no-nickel diet] consideration if the high-nickel foods are a possible trigger,” she said. “It is very specific, looking at their diet history, to have a clear hypothesis based on what their triggers are. It’s not something to try out lightly because it’s a very restrictive diet, so I would never put a patient on a diet that I didn’t think was necessary.”
Ms. Finkel added that treatment of SNAS requires a multidisciplinary approach with a gastroenterologist, an allergist, and a dietitian.
Doctors and dietitians have distinct roles in identifying and treating these patients, Ms. Finkel said.
“If there is a suspicion of IBS symptoms and the patient is not responding to first-line treatments, then it is worth having the input of a dietitian and an allergist,” she said.
A version of this article first appeared on Medscape.com.
Silicone-based film for radiation dermatitis: It works, so why isn’t it used?
Radiation dermatitis is one of the most common side effects of radiotherapy for women with breast cancer. Results from a phase 3 trial add to previous evidence from smaller trials that show that a silicone-based film can protect skin from this side effect.
But it is not being used much in clinical practice. Instead, radiation dermatitis is usually treated after the fact, most often with aqueous creams.
said Edward Chow, MBBS, PhD, of the department of radiation oncology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, who was the senior author of the phase 3 study published recently in the Journal of Clinical Oncology.
“Other doctors think that because radiation dermatitis isn’t life-threatening it isn’t as important, but the condition does affect the quality of life for patients,” Dr. Chow said. “If we can lessen the pain and discomfort, why wouldn’t we as physicians?”
Dr. Chow’s open-label, multicenter trial was conducted in 376 women with large breasts (bra cup size C or larger) who were undergoing radiotherapy after lumpectomy or mastectomy. The primary endpoint was grade 2 or 3 radiation dermatitis using the Common Terminology Criteria for Adverse Events. (Grade 2 is described as moderate, whereas grade 3 is severe.)
The film significantly reduced the incidence of grade 2 or 3 radiation dermatitis, down to 15.5% compared with 45.6% in patients receiving standard care (odds ratio, 0.20, 95% confidence interval, 0.12-0.34, P < .0001).
There was also a significant reduction in grade 3 radiation dermatitis (2.8% vs. 13.6%; OR, 0.19; P < .0002) and moist desquamation (8% vs. 19.2%; OR, 0.36; P = .002).
“The film was remarkably effective and helped protect patients from potentially debilitating side effects,” commented Corey Speers, MD, PhD, a radiation oncologist with University Hospitals, Cleveland, who saw the study data presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
He believes that preventing radiation dermatitis before it develops is the best way to care for patients.
“[Radiation dermatitis] is usually associated with pain and discomfort and can lead to more serious issues like infection or delayed wound healing, and unfortunately, there aren’t effective treatments for it once it’s developed, so preventing it is our most effective strategy,” Dr. Speers said.
One reason for the film not being used much could be that it takes time apply the film, suggested Patries Herst, PhD, department of radiation therapy, University of Otago, Wellington, New Zealand. She was the lead author of a study published in 2014 that also analyzed the effectiveness of the film in preventing radiation dermatitis.
In their trial, a research radiation therapist applied the film to women when they were starting their radiotherapy. The film is applied to a portion of the breast or chest wall, and Dr. Herst emphasized the importance of applying the film correctly, making sure the film is not stretched during application and not overlapping other pieces of the film, while also making sure that it conforms to the breast shape. The film was replaced when it would curl too much around the sides, approximately every 1 or 2 weeks.
“Radiation therapy itself is very short. And so you have about 10 minutes for every patient,” she explained.
“But applying the film adds 20-30 minutes and it’s really awkward to apply properly,” Dr. Herst said. “You have to tap it in and then have to maybe cut it so that it fits better. And hospitals say, ‘We don’t have the time’ and that is still the biggest issue that we’re seeing right now.”
In Dr. Chow’s study, the average time spent applying the film on lumpectomy patients was 55 minutes and was slightly shorter at 45 minutes for mastectomy patients. He acknowledged that it does take time that staff at most hospitals and clinics simply don’t have.
Dr. Chow suggested that perhaps a family member or other caregiver could apply the film, and he referenced an educational video from the manufacturer that provides in-depth instructions on the correct way to apply the film for radiotherapy patients. However, this could lead to errors and a waste of product if not the film was not applied properly.
The cost of Mepitel film may also be a deterrent. Dr. Chow’s study noted that, during the entire course of radiotherapy, the cost for the film was about $80-$100 per patient. However, he believes the benefits outweigh the cost.
In addition, there have been issues with supplies, and it has been difficult for people to get their hands on the actual product.
Currently, the Mayo Clinic is also conducting a study testing Mepitel Film for radiation dermatitis in breast cancer patients following mastectomy. Mayo Clinic principal investigator Kimberly Corbin, MD, could not go into great detail about the ongoing trial, but she said it has been difficult to get the product.
“We have been using the film at Mayo for a number of years,” Dr. Corbin said, but we “have found that it is challenging to get supplies.”
“While we have generally been able to have some supply established through our store here, we know that is not typical and it is difficult for patients to access,” she said. In addition, “there are not a ton of centers with experience in application.”
A representative with Mölnlycke Health Care, Allyson Bower-Willner, could not comment on the distribution of Mepitel film in the United States or if the company plans to increase the amount of product shipped. The film is available “to a limited set of customers,” she said.
A version of this article first appeared on Medscape.com.
Radiation dermatitis is one of the most common side effects of radiotherapy for women with breast cancer. Results from a phase 3 trial add to previous evidence from smaller trials that show that a silicone-based film can protect skin from this side effect.
But it is not being used much in clinical practice. Instead, radiation dermatitis is usually treated after the fact, most often with aqueous creams.
said Edward Chow, MBBS, PhD, of the department of radiation oncology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, who was the senior author of the phase 3 study published recently in the Journal of Clinical Oncology.
“Other doctors think that because radiation dermatitis isn’t life-threatening it isn’t as important, but the condition does affect the quality of life for patients,” Dr. Chow said. “If we can lessen the pain and discomfort, why wouldn’t we as physicians?”
Dr. Chow’s open-label, multicenter trial was conducted in 376 women with large breasts (bra cup size C or larger) who were undergoing radiotherapy after lumpectomy or mastectomy. The primary endpoint was grade 2 or 3 radiation dermatitis using the Common Terminology Criteria for Adverse Events. (Grade 2 is described as moderate, whereas grade 3 is severe.)
The film significantly reduced the incidence of grade 2 or 3 radiation dermatitis, down to 15.5% compared with 45.6% in patients receiving standard care (odds ratio, 0.20, 95% confidence interval, 0.12-0.34, P < .0001).
There was also a significant reduction in grade 3 radiation dermatitis (2.8% vs. 13.6%; OR, 0.19; P < .0002) and moist desquamation (8% vs. 19.2%; OR, 0.36; P = .002).
“The film was remarkably effective and helped protect patients from potentially debilitating side effects,” commented Corey Speers, MD, PhD, a radiation oncologist with University Hospitals, Cleveland, who saw the study data presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
He believes that preventing radiation dermatitis before it develops is the best way to care for patients.
“[Radiation dermatitis] is usually associated with pain and discomfort and can lead to more serious issues like infection or delayed wound healing, and unfortunately, there aren’t effective treatments for it once it’s developed, so preventing it is our most effective strategy,” Dr. Speers said.
One reason for the film not being used much could be that it takes time apply the film, suggested Patries Herst, PhD, department of radiation therapy, University of Otago, Wellington, New Zealand. She was the lead author of a study published in 2014 that also analyzed the effectiveness of the film in preventing radiation dermatitis.
In their trial, a research radiation therapist applied the film to women when they were starting their radiotherapy. The film is applied to a portion of the breast or chest wall, and Dr. Herst emphasized the importance of applying the film correctly, making sure the film is not stretched during application and not overlapping other pieces of the film, while also making sure that it conforms to the breast shape. The film was replaced when it would curl too much around the sides, approximately every 1 or 2 weeks.
“Radiation therapy itself is very short. And so you have about 10 minutes for every patient,” she explained.
“But applying the film adds 20-30 minutes and it’s really awkward to apply properly,” Dr. Herst said. “You have to tap it in and then have to maybe cut it so that it fits better. And hospitals say, ‘We don’t have the time’ and that is still the biggest issue that we’re seeing right now.”
In Dr. Chow’s study, the average time spent applying the film on lumpectomy patients was 55 minutes and was slightly shorter at 45 minutes for mastectomy patients. He acknowledged that it does take time that staff at most hospitals and clinics simply don’t have.
Dr. Chow suggested that perhaps a family member or other caregiver could apply the film, and he referenced an educational video from the manufacturer that provides in-depth instructions on the correct way to apply the film for radiotherapy patients. However, this could lead to errors and a waste of product if not the film was not applied properly.
The cost of Mepitel film may also be a deterrent. Dr. Chow’s study noted that, during the entire course of radiotherapy, the cost for the film was about $80-$100 per patient. However, he believes the benefits outweigh the cost.
In addition, there have been issues with supplies, and it has been difficult for people to get their hands on the actual product.
Currently, the Mayo Clinic is also conducting a study testing Mepitel Film for radiation dermatitis in breast cancer patients following mastectomy. Mayo Clinic principal investigator Kimberly Corbin, MD, could not go into great detail about the ongoing trial, but she said it has been difficult to get the product.
“We have been using the film at Mayo for a number of years,” Dr. Corbin said, but we “have found that it is challenging to get supplies.”
“While we have generally been able to have some supply established through our store here, we know that is not typical and it is difficult for patients to access,” she said. In addition, “there are not a ton of centers with experience in application.”
A representative with Mölnlycke Health Care, Allyson Bower-Willner, could not comment on the distribution of Mepitel film in the United States or if the company plans to increase the amount of product shipped. The film is available “to a limited set of customers,” she said.
A version of this article first appeared on Medscape.com.
Radiation dermatitis is one of the most common side effects of radiotherapy for women with breast cancer. Results from a phase 3 trial add to previous evidence from smaller trials that show that a silicone-based film can protect skin from this side effect.
But it is not being used much in clinical practice. Instead, radiation dermatitis is usually treated after the fact, most often with aqueous creams.
said Edward Chow, MBBS, PhD, of the department of radiation oncology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, who was the senior author of the phase 3 study published recently in the Journal of Clinical Oncology.
“Other doctors think that because radiation dermatitis isn’t life-threatening it isn’t as important, but the condition does affect the quality of life for patients,” Dr. Chow said. “If we can lessen the pain and discomfort, why wouldn’t we as physicians?”
Dr. Chow’s open-label, multicenter trial was conducted in 376 women with large breasts (bra cup size C or larger) who were undergoing radiotherapy after lumpectomy or mastectomy. The primary endpoint was grade 2 or 3 radiation dermatitis using the Common Terminology Criteria for Adverse Events. (Grade 2 is described as moderate, whereas grade 3 is severe.)
The film significantly reduced the incidence of grade 2 or 3 radiation dermatitis, down to 15.5% compared with 45.6% in patients receiving standard care (odds ratio, 0.20, 95% confidence interval, 0.12-0.34, P < .0001).
There was also a significant reduction in grade 3 radiation dermatitis (2.8% vs. 13.6%; OR, 0.19; P < .0002) and moist desquamation (8% vs. 19.2%; OR, 0.36; P = .002).
“The film was remarkably effective and helped protect patients from potentially debilitating side effects,” commented Corey Speers, MD, PhD, a radiation oncologist with University Hospitals, Cleveland, who saw the study data presented during a plenary session at the annual meeting of the American Society of Clinical Oncology.
He believes that preventing radiation dermatitis before it develops is the best way to care for patients.
“[Radiation dermatitis] is usually associated with pain and discomfort and can lead to more serious issues like infection or delayed wound healing, and unfortunately, there aren’t effective treatments for it once it’s developed, so preventing it is our most effective strategy,” Dr. Speers said.
One reason for the film not being used much could be that it takes time apply the film, suggested Patries Herst, PhD, department of radiation therapy, University of Otago, Wellington, New Zealand. She was the lead author of a study published in 2014 that also analyzed the effectiveness of the film in preventing radiation dermatitis.
In their trial, a research radiation therapist applied the film to women when they were starting their radiotherapy. The film is applied to a portion of the breast or chest wall, and Dr. Herst emphasized the importance of applying the film correctly, making sure the film is not stretched during application and not overlapping other pieces of the film, while also making sure that it conforms to the breast shape. The film was replaced when it would curl too much around the sides, approximately every 1 or 2 weeks.
“Radiation therapy itself is very short. And so you have about 10 minutes for every patient,” she explained.
“But applying the film adds 20-30 minutes and it’s really awkward to apply properly,” Dr. Herst said. “You have to tap it in and then have to maybe cut it so that it fits better. And hospitals say, ‘We don’t have the time’ and that is still the biggest issue that we’re seeing right now.”
In Dr. Chow’s study, the average time spent applying the film on lumpectomy patients was 55 minutes and was slightly shorter at 45 minutes for mastectomy patients. He acknowledged that it does take time that staff at most hospitals and clinics simply don’t have.
Dr. Chow suggested that perhaps a family member or other caregiver could apply the film, and he referenced an educational video from the manufacturer that provides in-depth instructions on the correct way to apply the film for radiotherapy patients. However, this could lead to errors and a waste of product if not the film was not applied properly.
The cost of Mepitel film may also be a deterrent. Dr. Chow’s study noted that, during the entire course of radiotherapy, the cost for the film was about $80-$100 per patient. However, he believes the benefits outweigh the cost.
In addition, there have been issues with supplies, and it has been difficult for people to get their hands on the actual product.
Currently, the Mayo Clinic is also conducting a study testing Mepitel Film for radiation dermatitis in breast cancer patients following mastectomy. Mayo Clinic principal investigator Kimberly Corbin, MD, could not go into great detail about the ongoing trial, but she said it has been difficult to get the product.
“We have been using the film at Mayo for a number of years,” Dr. Corbin said, but we “have found that it is challenging to get supplies.”
“While we have generally been able to have some supply established through our store here, we know that is not typical and it is difficult for patients to access,” she said. In addition, “there are not a ton of centers with experience in application.”
A representative with Mölnlycke Health Care, Allyson Bower-Willner, could not comment on the distribution of Mepitel film in the United States or if the company plans to increase the amount of product shipped. The film is available “to a limited set of customers,” she said.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Botanical Briefs: Primula obconica Dermatitis
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Practice Points
- Primula obconica is a household plant that can cause contact dermatitis (CD). Spent blossoms must be pinched off to keep the plant blooming, resulting in fingertip dermatitis.
- In the United States, P obconica is not a component of routine patch testing; therefore, it might be missed as the cause of an allergic reaction.
- Primin and miconidin are the principal allergens known to be responsible for causing P obconica dermatitis.
- Treatment of this condition is similar to the usual treatment of plant-induced CD: avoiding exposure to the plant and applying a topical steroid.
Can skin care aid use of diabetes devices?
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Technologies that allow people to monitor blood sugar and automate the administration of insulin have radically transformed the lives of patients – and children in particular – with type 1 diabetes. But the devices often come with a cost: Insulin pumps and continuous glucose monitors can irritate the skin at the points of contact, causing some people to stop using their pumps or monitors altogether.
Regular use of lipid-rich skin creams can reduce eczema in children who use insulin pumps and continuous glucose monitors to manage type 1 diabetes, Danish researchers reported last month. The article is currently undergoing peer review at The Lancet Diabetes and Endocrinology, and the authors said they hope their approach will deter more children from abandoning diabetes technology.
“A simple thing can actually change a lot,” said Anna Korsgaard Berg, MD, a pediatrician who specializes in diabetes care at Copenhagen University Hospital’s Steno Diabetes Center in Herlev, Denmark, and a coauthor of the new study. “Not all skin reactions can be solved by the skin care program, but it can help improve the issue.”
More than 1.5 million children and adolescents worldwide live with type 1 diabetes, a condition that requires continuous insulin infusion. Insulin pumps meet this need in many wealthier countries, and are often used in combination with sensors that measure a child’s glucose level. Both the American Diabetes Association and the International Society for Adolescent and Pediatric Diabetes recommend insulin pumps and continuous glucose monitors as core treatment tools.
Dr. Berg and colleagues, who have previously shown that as many as 90% of children who use these devices experience some kind of skin reaction, want to minimize the rate of such discomfort in hopes that fewer children stop using the devices. According to a 2014 study, 18% of people with type 1 diabetes who stopped using continuous glucose monitors did so because of skin irritation.
Lather on that lipid-rich lotion
Dr. Berg and colleagues studied 170 children and adolescents with type 1 diabetes (average age, 11 years) who use insulin pumps, continuous glucose monitors, or both. From March 2020 to July 2021, 112 children (55 girls) employed a skin care program developed for the study, while the other 58 (34 girls) did not receive any skin care advice.
The skin care group received instructions about how to gently insert and remove their insulin pumps or glucose monitors, to minimize skin damage. They also were told to avoid disinfectants such as alcohol, which can irritate skin. The children in this group used a cream containing 70% lipids to help rehydrate their skin, applying the salve each day a device was not inserted into their skin.
Eczema can be a real problem for kids who use insulin pumps and continuous glucose monitors to manage type 1 diabetes. Researchers found that regular use of lipid-rich skin creams can reduce its incidence.
Although insulin pumps and glucose monitors are kept in place for longer periods of time than they once were, Dr. Berg and colleagues noted, users do periodically remove them when bathing or when undergoing medical tests that involve x-rays. On days when the devices were not in place for a period of time, children in the skin care group were encouraged to follow the protocol.
Study results
One-third of children in the skin care group developed eczema or experienced a wound, compared with almost half of the children in the control group, according to the researchers. The absolute difference in developing eczema or wounds between the two groups was 12.9 % (95% confidence interval, –28.7% to 2.9%).
Children in the skin care group were much less likely to develop wounds, the researchers found, when they focused only on wounds and not eczema (odds ratio, 0.29, 95% CI, 0.12-0.68).
Dr. Berg said she would like to explore whether other techniques, such as a combination of patches, adhesives, or other lotions, yield even better results.
“Anything that can help people use technology more consistently is better for both quality of life and diabetes outcomes,” said Priya Prahalad, MD, a specialist in pediatric endocrinology and diabetes at Stanford Medicine Children’s Health in Palo Alto and Sunnyvale, Calif.
Dr. Prahalad, who was not involved in the Danish study, said that although the sample sizes in the trial were relatively small, the data are “headed in the right direction.”
Pediatricians already recommend using moisturizing creams at the sites where pumps or glucose monitors are inserted into the skin, she noted. But the new study simply employed an especially moisturizing cream to mitigate skin damage.
Although one reason for skin irritation may be the repeated insertion and removal of devices, Dr. Berg and Dr. Prahalad stressed that the medical devices themselves may contain allergy-causing components. Device makers are not required to disclose what’s inside the boxes.
“I do not understand why the full content of a device is not by law mandatory to declare, when declaration by law is mandatory for many other products and drugs but not for medical devices,” Dr. Berg said.
Dr. Berg reports receiving lipid cream from Teva Pharmaceuticals and research support from Medtronic. Dr. Prahalad reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Isothiazolinone contact allergy up in North America, down in Europe
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
, a trend that is likely driven by regulatory differences, a retrospective cohort study suggests.
“Between 2009 to 2018, the global burden of isothiazolinone allergy showed divergent trends between North American and European countries,” lead study author Margo J. Reeder, MD, of the University of Wisconsin in Madison and her colleagues write. The study was published online in JAMA Dermatology.
Isothiazolinone contact allergy peaked in Europe in 2013-2014 before gradually decreasing, they found. The prevalence of isothiazolinone allergy steadily increased in North America during the study period. “Earlier and more stringent regulation of MI [methylisothiazolinone] in Europe is associated with these divergent trends,” they write.
Common ingredients worldwide
Isothiazolinone preservatives, which are added to personal and industrial products, cause allergic contact dermatitis worldwide, the authors write. The preservatives are found in a wide range of leave-on and rinse-off water-based personal care products, such as shampoo and other hair products, dishwashing liquid, face cream, body lotion, shower gel, liquid soap, and wet wipes, as well as in water-based paint.
A mixture of methylchloroisothiazolinone (MCI) and MI has been used to prevent microbial growth in products since the 1980s. In 2005, U.S. and European regulators approved MI alone at higher concentrations as a preservative in personal care products. Coupled with consumer concerns about other preservatives, such as parabens (a rare allergen), use of MI in personal care products increased, the authors write.
Subsequently, researchers reported a global increase in the prevalence of contact allergy to isothiazolinones, the authors write. Regulatory restrictions on MI in personal care products were implemented in 2013 in Europe and in 2015 in Canada but not in the United States.
Patch test data reveal latest trends
To compare prevalence trends of allergic contact allergy to MI and sensitization to the MCI/MI mixture in North America and in Europe, Dr. Reeder and her colleagues compared the prevalence of positive patch test reactions to MCI/MI and to MI alone in North America and in Europe between 2009 and 2018.
They analyzed data from the North American Contact Dermatitis Group (NACDG), the European Surveillance System on Contact Allergies (ESSCA), and the Information Network of Departments of Dermatology (IVDK) in 2-year intervals. The data came from patients who had been patch tested at referral patch test clinics in North America and Europe.
Over the decade, the study sites conducted patch testing for 226,161 patients for MCI/MI and 118,779 for MI. Most data came from Europe. The researchers found the following:
- In Europe, isothiazolinone allergy peaked in 2013 and 2014; MCI/MI positivity reached 7.6% (ESSCA) and 5.4% (IVDK) before decreasing to 4.4% (ESSCA) and 3.2% (IVDK) in 2017-2018.
- In North America, MCI/MI positivity rose steadily from 2.5% in 2009-2010 to 10.8% in 2017-2018.
- In Europe, there were 5.5% (ESSCA) and 3.4% (IVDK) positive reactions to MI, compared with 15% (NACDG) in North America in 2017-2018.
Divergent contact allergy trends linked to regulatory approaches
The downward trend of isothiazolinone allergy in Europe after its peak in 2013 and 2014 may have been due in part, the authors explain, to a memo released in 2013 by Cosmetics Europe after it and the European Society of Contact Dermatitis reviewed reports of increased contact allergy to MI. The memo urged companies to remove MI from leave-on products.
Later that year, the European Union’s Scientific Committee on Consumer Safety advised omitting MI from leave-on consumer personal care products and moved to restrict the ingredient in rinse-off products to less than 15 ppm. The recommendation took effect in 2015.
That year, Canada banned the use of MCI/MI in leave-on products but allowed MI alone in leave-on products until 2018. The total concentration of MI and MCI in wash-off products was limited to less than 15 ppm.
The authors add that, to their knowledge, the U.S. government does not restrict the use of MCI/MI or MI.
Policy implications for contact allergy
MI is still widely used in “countless products,” including shampoos, skin cleansers, dishwashing and laundry detergents, paints, and adhesives, Daniel W. Shaw, MD, associate professor of dermatology at the University of California, San Diego, told this news organization by email.
“Exact figures between the U.S. and Europe are difficult to compare due to differing patch test concentrations, but the overall trends strongly suggest that stricter and earlier regulation in Europe resulted in lower MI allergy prevalence there than in the U.S.,” added Dr. Shaw, who was not involved in the study.
Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University in Winston-Salem, N.C., said by email that accurate information on allergic reaction prevalence is difficult to find.
“The NACDG, ESSCA, and IVDK databases may contain the best data available, but the data depend on people who get patch tested and are not directly informative of the allergy rates in the general population,” added Dr. Feldman, who was not involved in the study.
“The great majority of people in the population may not be allergic,” he said. “For those with itchy rashes, getting patch tested or avoiding products with preservatives may be prudent. Broad regulations, however, should consider the overall risks and benefits in the population, and this particular study does not fully capture those issues.”
“This study shows that government regulations are important to limit consumer exposure to common allergens, especially to the concentrations used in personal care products,” Kelly Tyler, MD, associate professor of dermatology at the Ohio State University Wexner Medical Center in Columbus, noted by email. She was not involved in the study.
She advised clinicians to ask their patients who may have allergic contact dermatitis whether they have been exposed to products containing these compounds.
“All personal care products in the store contain preservatives, and their maximum concentrations should be limited,” she advised. “The Expert Panel for Cosmetic Ingredient Safety should establish stricter guidelines for MI use in personal care products, especially given the findings of this study.”
Has MI contact allergy in North America peaked?
“In the U.S., MI has not been banned from leave-on skin-care products, but recently, its use has markedly decreased,” Dr. Shaw commented. “Hopefully, the prevalence of MI contact allergy will also begin to decrease.”
New evidence is promising. In a related study published online in Dermatology, Joel G. DeKoven, MD, MHSc, FRCPC, of the University of Toronto and his colleagues reported the NACDG 2019-2020 patch test results for MI in North America. They found that 13.8% of patients tested positive for MI.
“For the first time, MI positivity did not increase between reporting periods,” they conclude. “The epidemic of MI contact allergy in North America may have reached a plateau.”
Information regarding funding for the study was not provided. Dr. Reeder has financial relationships with the American Contact Dermatitis Society and a publishing company. Several coauthors have financial relationships with the pharmaceutical industry. Dr. Tyler, Dr. Shaw, and Dr. Feldman report no relevant financial relationship.
A version of this article first appeared on Medscape.com.
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.