User login
Pruritus: Diagnosing and Treating Older Adults
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
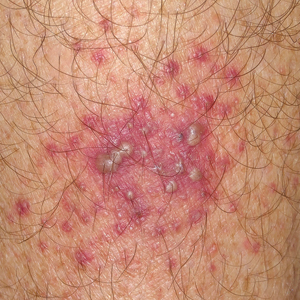
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Central Line Skin Reactions in Children: Survey Addresses Treatment Protocols in Use
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Allergic Contact Dermatitis: New Culprits
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Ob.Gyn. Says Collaboration with Dermatologists Essential for Managing Vulvar Dermatoses
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Sulfites: The 2024 American Contact Dermatitis Society Allergen of the Year
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
The American Contact Dermatitis Society (ACDS) selected sulfites as the 2024 Allergen of the Year.1 Due to their preservative and antioxidant properties, sulfites are prevalent in a variety of foods, beverages, medications, and personal care products; however, sulfites also have been implicated as a potential contact allergen. In this article, we review common sources of sulfite exposure, clinical manifestations of allergic contact dermatitis (ACD) to sulfites, and patch testing considerations for this emerging allergen.
What Are Sulfites?
Sulfiting agents are compounds that contain the sulfite ion SO32-, including sulfur dioxide, sodium disulfite (sodium metabisulfite), and potassium metabisulfite.2 Sulfites occur naturally in the environment and commonly are used as preservatives, antibrowning agents, and antioxidants in various foods, beverages, medications, cosmetics, and skin care products. As antibrowning agents and antioxidants, sulfites help maintain the natural appearance of foods and other products and prevent premature spoiling by inactivating oxidative enzymes.3 It should be noted that sulfites and sulfates are distinct and unrelated compounds that do not cross-react.1
Common Sources of Sulfite Exposure
From a morning glass of juice to an evening shower, in the pharmacy and at the hair salon, sulfite exposure is ubiquitous in most daily routines. Sulfites are present in many foods and beverages, either as a byproduct of natural fermentation or as an additive to prevent spoiling and color change. The Table provides examples of foods with high sulfite content.1,4-6 In particular, dried fruit, bottled lemon juice, wine, grape juice, sauerkraut juice, and pickled onions have high sulfite content.

Topical medications and personal care products represent other potential sources of sulfite exposure. A number of reports have shown that sulfites may be included in topical steroids,7 antibiotics,8 antifungals,9 hemorrhoidal preparations,10 local anesthetics,11 and urinary catheterization gel,12 highlighting their many potential applications. In addition, a comprehensive ingredient analysis of 264 ophthalmic medications found that 3.8% of the products contained sodium disulfite.13 Sulfites may be found in personal care products, including facial and hand cleansers, shampoos, moisturizers, and toothpastes. Hair dyes also commonly contain sulfites,7 which are listed in as many as 90% of hair dye kits in the ACDS Contact Allergen Management Program database.1
Occupational exposures also are widespread, as sulfites are extensively utilized across diverse industries such as pharmaceuticals, health care, leather manufacturing, mineral extraction, food preparation, chemical manufacturing, textiles, alcohol brewing, and wine production.1
Sulfites also are used in the rubber industry—particularly in gloves—due to their anticoagulant and preservative properties.4 This is relevant to health care providers, who may use dozens of disposable gloves in a single day. In an experimental pilot study, researchers detected sulfites in 83% (5/6) of natural rubber latex gloves, 96% (23/24) of synthetic (nitrile) gloves, and 0% (0/5) of polyvinyl chloride gloves.14 While this study was limited to a small sample size, it demonstrates the common use of sulfites in certain rubber gloves and encourages future studies to determine whether there is a quantitative threshold to elicit allergic reactions.
Sulfite Allergy
In 1968, an early case report of ACD to sulfites was published involving a pharmaceutical worker who developed hand eczema after working at a factory for 3 months and had a positive patch test to potassium metabisulfite.15 There have been other cases published in the literature since then, including localized ACD as well as less common cases of systemic contact dermatitis following oral, injectable, and rectal sulfite exposures.16
The North American Contact Dermatitis Group found that, among 132 (2.7%) of 4885 patients with positive patch tests to sodium disulfite from 2017 to 2018, the most commonly involved body sites were the face (28.8%) and hands (20.5%) followed by a scattered/generalized distribution (13.6%). Involvement of the face and hands may correlate with the most frequent sources of exposure that were identified, including personal care products (particularly hair dyes)(18.9%), medications (9.1%), and foods (7.6%).17 A multicenter analysis of patch test results from Germany, Austria, and Switzerland from 1999 to 2013 showed that 357 (2.9%) of 12,156 patients had positive reactions to sodium disulfite, with the most commonly identified exposure sources being topical pharmaceutical agents (59.3%); cosmetics, creams, and sunscreens (13.6%); and systemic drugs (6.8%).18 However, it is not always possible to determine the clinical relevance of a positive patch test to sulfites.1
Other than the face and hands, there have been other unexpected anatomic locations for sulfite ACD (eg, the lower back), and systemic contact dermatitis has manifested with widespread rashes due to oral, rectal, and parenteral exposure.4,16,19 There is no definitive link between sulfite contact allergy and patient sex, but there seems to be a higher prevalence in patients older than 40 years, perhaps related to overall lifetime exposure.1
Immediate hypersensitivity reactions to sulfites also have been reported, including urticaria, angioedema, and anaphylaxis.4 Due to multiple cases of severe dermatologic and respiratory reactions to food products containing sulfites,20 the US Food and Drug Administration prohibited their use in fresh fruit and vegetables as antibrowning agents in 1986 and required labels on packaged foods that contained sulfites at more than 10 parts per million.21 However, food and drinks produced in restaurants, bakeries, and cafes as well as those that are distributed directly to consumers from the preparation site are exempt from these rules.17
In addition, consuming high amounts of dietary sulfites has been linked to headaches through unclear (ie, not necessarily allergic) mechanisms.4,22 One study found that wine with a higher sulfite concentration was associated with increased risk for headaches in participants who had a history of headaches related to wine consumption.22
Patch Testing to Sulfites
The North American Contact Dermatitis Group has tested sodium disulfite since 2017 and found an increased frequency of positive patch tests from 2.7% (N=4885) in 2017 and 201817 to 3.3% (N=4115) in 2019 and 202023 among patients referred for testing. Similarly, patch testing to sodium disulfite in nearly 40,000 patients in 9 European countries showed a pooled prevalence of reactions of 3.1%.17 However, this contact allergy may go unrecognized, as sulfites are not included in common patch test series, including the thin-layer rapid use epicutaneous test and the ACDS Core Allergen Series.24,25 The relatively high patch test positivity to sulfites along with the prevalence of daily exposures supports the addition of sulfites to more patch test screening series.
The recommended patch test concentration for sodium disulfite is 1% in petrolatum.5 Testing in aqueous solutions is not recommended because they can cause sulfites to break down, potentially producing false-positive or irritant patch test reactions.7,26,27
Recommendations for Patients With Sulfite Allergies
Individuals with contact allergies to sulfites should be counseled on exposure sources and should be given resources providing a list of safe products, such as the ACDS Contact Allergen Management Program (https://www.acdscamp.org/login) or SkinSAFE (https://www.skinsafeproducts.com/). Prescribers should be cognizant of sulfites that are present in prescription medications. Just because a patient has a positive patch test to sulfites does not automatically imply that they will need to modify their diet to avoid sulfite-containing foods; in the absence of cheilitis or a distribution suggestive of systemic contact dermatitis (eg, vesicular hand/foot dermatitis, intertriginous eruptions), this step may be unnecessary. On the other hand, individuals who have experienced immediate hypersensitivity reactions to sulfites should avoid sulfite-containing foods and carry an epinephrine autoinjector.
Final Interpretation
Sulfites are ubiquitous compounds found in various foods, beverages, medications, and personal care products in addition to a range of occupational exposures. The face and hands are the most common sites of sulfite ACD. Despite patch test positivity in as many as 3% of tested patients,17,23 sulfite allergy may be missed due to lack of routine testing on standard screening series.
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
- Ekstein SF, Warshaw EM. Sulfites: allergen of the year 2024. Dermatitis. 2024;35:6-12. doi:10.1089/derm.2023.0154
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. a critical review. CRC Crit Rev Toxicol. 1987;17:185-214. doi:10.3109/10408448709071208
- Clough SR. Sodium sulfite. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014: 341-343.
- Vally H, Misso NL, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643-1651. doi:10.1111/j.1365-2222.2009.03362.x
- Ralph N, Verma S, Merry S, et al. What is the relevance of contact allergy to sodium metabisulfite and which concentration of the allergen should we use? Dermatitis. 2015;26:162-165. doi:10.1097/der.0000000000000120
- Madan V, Walker SL, Beck MH. Sodium metabisulfite allergy is common but is it relevant? Contact Dermatitis. 2007;57:173-176. doi:10.1111/j.1600-0536.2007.01188.x
- García-Gavín J, Parente J, Goossens A. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen. a case series and literature review. Contact Dermatitis. 2012;67:260-269. doi:10.1111/j.1600-0536.2012.02135.x
- Milpied B, van Wassenhove L, Larousse C, et al. Contact dermatitis from rifamycin. Contact Dermatitis. 1986;14:252-253. doi:10.1111/j.1600-0536.1986.tb01240.x
- Lodi A, Chiarelli G, Mancini LL, et al. Contact allergy to sodium sulfite contained in an antifungal preparation. Contact Dermatitis. 1993;29:97. doi:10.1111/j.1600-0536.1993.tb03493.x
- Sánchez-Pérez J, Abajo P, Córdoba S, et al. Allergic contact dermatitis from sodium metabisulfite in an antihemorrhoidal cream. Contact Dermatitis. 2000;42:176-177.
- Boyd AH, Warshaw EM. Sulfites: no longer a zebra? Dermatitis. 2017;28:364-366. doi:10.1097/der.0000000000000312
- Grosch E, Mahler V. Allergic contact dermatitis caused by a catheter system containing sodium metabisulfite. Contact Dermatitis. 2017;76:186-187. doi:10.1111/cod.12675
- Shaver RL, Warshaw EM. Contact allergens in prescription topical ophthalmic medications. Dermatitis. 2022;33:135-143. doi:10.1097/der.0000000000000751
- Dendooven E, Darrigade AS, Foubert K, et al. The presence of sulfites in ‘natural rubber latex’ and ‘synthetic’ rubber gloves: an experimental pilot study. Br J Dermatol. 2020;182:1054-1055. doi:10.1111/bjd.18608
- Nater JP. Allergic contact dermatitis caused by potassium metabisulfite. Dermatologica. 1968;136:477-478. doi:10.1159/000254143
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430. doi:10.1111/cod.12971
- Warshaw EM, Buonomo M, DeKoven JG, et al. Patch testing with sodium disulfite: North American Contact Dermatitis Group experience, 2017 to 2018. Contact Dermatitis. 2021;85:285-296. doi:10.1111/cod.13860
- Häberle M, Geier J, Mahler V. Contact allergy to sulfites: clinical and occupational relevance—new data from the German Contact Dermatitis Research Group and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2016;14:938-941. doi:10.1111/ddg.13009
- Tan MG, Li HO, Pratt MD. Systemic allergic dermatitis to sodium metabisulfite in local anesthetic solution. Contact Dermatitis. 2022;86:120-121. doi:10.1111/cod.13978
- D’Amore T, Di Taranto A, Berardi G, et al. Sulfites in meat: occurrence, activity, toxicity, regulation, and detection. a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2701-2720. doi:10.1111/1541-4337.12607
- Grotheer P, Marshall M, Simonne A. Sulfites: separating fact from fiction. May 11, 2022. UF IFAS Extension. University of Florida. Accessed October 4, 2024. https://edis.ifas.ufl.edu/publication/FY731
- Silva M, Gama J, Pinto N, et al. Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr. 2019;73:1316-1322. doi:10.1038/s41430-019-0420-2
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- T.R.U.E. Test. Thin-layer rapid use epicutaneous patch test. SmartPractice Dermatology Allergy. Accessed October 4, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Schalock PC, Dunnick CA, Nedorost, et al; American Contact Dermatitis Society Core Allergen Series Committee. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282.
- Kaaman AC, Boman A, Wrangsjö K, et al. Contact allergy to sodium metabisulfite: an occupational problem. Contact Dermatitis. 2010;63:110-112. doi:10.1111/j.1600-0536.2010.01756.x
- Vena GA, Foti C, Angelini G. Sulfite contact allergy. Contact Dermatitis. 1994;31:172-175. doi:10.1111/j.1600-0536.1994.tb01959.x
Practice Points
- Sulfites are ubiquitous compounds that serve as preservatives and antioxidants in various foods, beverages, medications, and personal care products.
- Allergic contact dermatitis to sulfites most commonly affects the face and hands.
- Because sulfites are not included in most patch test screening series, contact allergy to sulfites may be missed unless expanded testing is performed.
Cosmetic Dermatology Product Recalls Still Common, Analysis Finds
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Asteraceae Dermatitis: Everyday Plants With Allergenic Potential
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18
Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18

Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
The Asteraceae (formerly Compositae) family of plants is derived from the ancient Greek word aster, meaning “star,” referring to the starlike arrangement of flower petals around a central disc known as a capitulum. What initially appears as a single flower is actually a composite of several smaller flowers, hence the former name Compositae.1 Well-known members of the Asteraceae family include ornamental annuals (eg, sunflowers, marigolds, cosmos), herbaceous perennials (eg, chrysanthemums, dandelions), vegetables (eg, lettuce, chicory, artichokes), herbs (eg, chamomile, tarragon), and weeds (eg, ragweed, horseweed, capeweed)(Figure 1).2

There are more than 25,000 species of Asteraceae plants that thrive in a wide range of climates worldwide. Cases of Asteraceae-induced skin reactions have been reported in North America, Europe, Asia, and Australia.3 Members of the Asteraceae family are ubiquitous in gardens, along roadsides, and in the wilderness. Occupational exposure commonly affects gardeners, florists, farmers, and forestry workers through either direct contact with plants or via airborne pollen. Furthermore, plants of the Asteraceae family are used in various products, including pediculicides (eg, insect repellents), cosmetics (eg, eye creams, body washes), and food products (eg, cooking oils, sweetening agents, coffee substitutes, herbal teas).4-6 These plants have substantial allergic potential, resulting in numerous cutaneous reactions.
Allergic Potential
Asteraceae plants can elicit both immediate and delayed hypersensitivity reactions (HSRs); for instance, exposure to ragweed pollen may cause an IgE-mediated type 1 HSR manifesting as allergic rhinitis or a type IV HSR manifesting as airborne allergic contact dermatitis.7,8 The main contact allergens present in Asteraceae plants are sesquiterpene lactones, which are found in the leaves, stems, flowers, and pollen.9-11 Sesquiterpene lactones consist of an α-methyl group attached to a lactone ring combined with a sesquiterpene.12 Patch testing can be used to diagnose Asteraceae allergy; however, the results are not consistently reliable because there is no perfect screening allergen. Patch test preparations commonly used to detect Asteraceae allergy include Compositae mix (consisting of Anthemis nobilis extract, Chamomilla recutita extract, Achillea millefolium extract, Tanacetum vulgare extract, Arnica montana extract, and parthenolide) and sesquiterpene lactone mix (consisting of alantolactone, dehydrocostus lactone, and costunolide). In North America, the prevalence of positive patch tests to Compositae mix and sesquiterpene lactone mix is approximately 2% and 0.5%, respectively.13 When patch testing is performed, both Compositae mix and sesquiterpene lactone mix should be utilized to minimize the risk of missing Asteraceae allergy, as sesquiterpene lactone mix alone does not detect all Compositae-sensitized patients. Additionally, it may be necessary to test supplemental Asteraceae allergens, including preparations from specific plants to which the patient has been exposed. Exposure to Asteraceae-containing cosmetic products may lead to dermatitis, though this is highly dependent on the particular plant species involved. For instance, the prevalence of sensitization is high in arnica (tincture) and elecampane but low with more commonly used species such as German chamomile.14
Cutaneous Manifestations
Asteraceae dermatitis, which also is known as Australian bush dermatitis, weed dermatitis, and chrysanthemum dermatitis,2 can manifest on any area of the body that directly contacts the plant or is exposed to the pollen. Asteraceae dermatitis historically was reported in older adults with a recent history of plant exposure.6,15 However, recent data have shown a female preponderance and a younger mean age of onset (46–49 years).16
There are multiple distinct clinical manifestations of Asteraceae dermatitis. The most common cutaneous finding is localized vesicular or eczematous patches on the hands or wrists. Other variations include eczematous rashes on the exposed skin of the hands, arms, face, and neck; generalized eczema; and isolated facial eczema.16,17 These variations can be attributed to contact dermatitis caused by airborne pollen, which may mimic photodermatitis. However, airborne Asteraceae dermatitis can be distinguished clinically from photodermatitis by the involvement of sun-protected areas such as the skinfolds of the eyelids, retroauricular sulci, and nasolabial folds (Figure 2).2,9 In rare cases, systemic allergic contact dermatitis can occur if the Asteraceae allergen is ingested.2,18

Other diagnostic clues include dermatitis that flares during the summer, at the peak of the growing season, with remission in the cooler months. Potential risk factors include a childhood history of atopic dermatitis and allergic rhinitis.16 With prolonged exposure, patients may develop chronic actinic dermatitis, an immunologically mediated photodermatosis characterized by lichenified and pruritic eczematous plaques located predominantly on sun-exposed areas with notable sparing of the skin folds.19 The association between Asteraceae dermatitis and chronic actinic dermatitis is highly variable, with some studies reporting a 25% correlation and others finding a stronger association of up to 80%.2,15,20 Asteraceae allergy appears to be a relatively uncommon cause of photoallergy in North America. In one recent study, 16% (3/19) of patients with chronic actinic dermatitis had positive patch or photopatch tests to sesquiterpene lactone mix, but in another large study of photopatch testing it was reported to be a rare photoallergen.21,22
Parthenium dermatitis is an allergic contact dermatitis caused by exposure to Parthenium hysterophorus, a weed of the Asteraceae family that is responsible for 30% of cases of contact dermatitis in India.23,24 Unlike the more classic manifestation of Asteraceae dermatitis, which primarily affects the upper extremities in cases from North America and Europe, Parthenium dermatitis typically occurs in an airborne pattern distribution.24
Management
While complete avoidance of Asteraceae plants is ideal, it often is unrealistic due to their abundance in nature. Therefore, minimizing exposure to the causative plants is recommended. Primary preventive measures such as wearing protective gloves and clothing and applying bentonite clay prior to exposure should be taken when working outdoors. Promptly showering after contact with plants also can reduce the risk for Asteraceae dermatitis.
Symptomatic treatment is appropriate for mild cases and includes topical corticosteroids and calcineurin inhibitors. For severe cases, systemic corticosteroids may be needed for acute flares, with azathioprine, mycophenolate, cyclosporine, or methotrexate available for recalcitrant disease. Verma et al25 found that treatment with azathioprine for 6 months resulted in greater than 60% clearance in all 12 patients, with a majority achieving 80% to 100% clearance. Methotrexate has been used at doses of 15 mg once weekly.26 Narrowband UVB and psoralen plus UVA have been effective in extensive cases; however, care should be exercised in patients with photosensitive dermatitis, who instead should practice strict photoprotection.27-29 Lakshmi et al30 reported the use of cyclosporine during the acute phase of Asteraceae dermatitis at a dose of 2.5 mg/kg daily for 4 to 8 weeks. There have been several case reports of dupilumab treating allergic contact dermatitis; however, there have been 3 cases of patients with atopic dermatitis developing Asteraceae dermatitis while taking dupilumab.31,32 Recently, oral Janus kinase inhibitors have shown success in treating refractory cases of airborne Asteraceae dermatitis.33,34 Further research is needed to determine the safety and efficacy of dupilumab and Janus kinase inhibitors for treatment of Asteraceae dermatitis.
Final Thoughts
The Asteraceae plant family is vast and diverse, with more than 200 species reported to cause allergic contact dermatitis.12 Common modes of contact include gardening, occupational exposure, airborne pollen, and use of pediculicides and cosmetics that contain components of Asteraceae plants. Educating patients on how to minimize contact with Asteraceae plants is the most effective management strategy; topical agents and oral immunosuppressives can be used for symptomatic treatment.
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
- Morhardt S, Morhardt E. California Desert Flowers: An Introduction to Families, Genera, and Species. University of California Press; 2004.
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123-130. doi:10.1046/j.1440-0960.1999.00341.x
- Denisow-Pietrzyk M, Pietrzyk Ł, Denisow B. Asteraceae species as potential environmental factors of allergy. Environ Sci Pollut Res Int. 2019;26:6290-6300. doi:10.1007/s11356-019-04146-w
- Paulsen E, Chistensen LP, Andersen KE. Cosmetics and herbal remedies with Compositae plant extracts—are they tolerated by Compositae-allergic patients? Contact Dermatitis. 2008;58:15-23. doi:10.1111/j.1600-0536.2007.01250.x
- Burry JN, Reid JG, Kirk J. Australian bush dermatitis. Contact Dermatitis. 1975;1:263-264. doi:10.1111/j.1600-0536.1975.tb05422.x
- Punchihewa N, Palmer A, Nixon R. Allergic contact dermatitis to Compositae: an Australian case series. Contact Dermatitis. 2022;87:356-362. doi:10.1111/cod.14162
- Chen KW, Marusciac L, Tamas PT, et al. Ragweed pollen allergy: burden, characteristics, and management of an imported allergen source in Europe. Int Arch Allergy Immunol. 2018;176:163-180. doi:10.1159/000487997
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274. doi:10.1111/ijd.12692
- Arlette J, Mitchell JC. Compositae dermatitis. current aspects. Contact Dermatitis. 1981;7:129-136. doi:10.1111/j.1600-0536.1981.tb04584.x
- Mitchell JC, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150. doi:10.1111/j.1365-2133.1971.tb06857.x
- Salapovic H, Geier J, Reznicek G. Quantification of Sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807-818. doi:10.3797/scipharm.1306-17
- Paulsen E. Compositae dermatitis: a survey. Contact Dermatitis. 1992;26:76-86. doi:10.1111/j.1600-0536.1992.tb00888.x. Published correction appears in Contact Dermatitis. 1992;27:208.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Paulsen E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermatitis. 2002;47:189-198. doi:10.1034/j.1600-0536.2002.470401.x
- Frain-Bell W, Johnson BE. Contact allergic sensitivity to plants and the photosensitivity dermatitis and actinic reticuloid syndrome. Br J Dermatol. 1979;101:503-512.
- Paulsen E, Andersen KE. Clinical patterns of Compositae dermatitis in Danish monosensitized patients. Contact Dermatitis. 2018;78:185-193. doi:10.1111/cod.12916
- Jovanovic´ M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43-49. doi:10.2298/mpns0302043j
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Cichorium (endive). simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27-36. doi:10.1111/j.1600-0536.1977.tb03583.x
- Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32:355-361, viii-ix. doi:10.1016/j.det.2014.03.007
- du P Menagé H, Hawk JL, White IR. Sesquiterpene lactone mix contact sensitivity and its relationship to chronic actinic dermatitis: a follow-up study. Contact Dermatitis. 1998;39:119-122. doi:10.1111/j.1600-0536.1998.tb05859.x
- Wang CX, Belsito DV. Chronic actinic dermatitis revisited. Dermatitis. 2020;31:68-74. doi:10.1097/DER.0000000000000531
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291. doi:10.1111/phpp.12742
- McGovern TW, LaWarre S. Botanical briefs: the scourge of India—Parthenium hysterophorus L. Cutis. 2001;67:27-34. Published correction appears in Cutis. 2001;67:154.
- Sharma VK, Verma P, Maharaja K. Parthenium dermatitis. Photochem Photobiol Sci. 2013;12:85-94. doi:10.1039/c2pp25186h
- Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-27. doi:10.4103/0378-6323.19713
- Sharma VK, Bhat R, Sethuraman G, et al. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis. 2007;57:118-119. doi:10.1111/j.1600-0536.2006.00950.x
- Burke DA, Corey G, Storrs FJ. Psoralen plus UVA protocol for Compositae photosensitivity. Am J Contact Dermat. 1996;7:171-176.
- Lovell CR. Allergic contact dermatitis due to plants. In: Plants and the Skin. Blackwell Scientific Publications; 1993:96-254.
- Dogra S, Parsad D, Handa S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br J Dermatol. 2004;150:373-374. doi:10.1111/j.1365-2133.2004.05724.x
- Lakshmi C, Srinivas CR, Jayaraman A. Ciclosporin in Parthenium dermatitis—a report of 2 cases. Contact Dermatitis. 2008;59:245-248. doi:10.1111/j.1600-0536.2007.01208.x
- Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis—a systematic review. J Dermatolog Treat. 2021;32:19-28. doi:10.1080/09546634.2019.1689227
- Napolitano M, Fabbrocini G, Patruno C. Allergic contact dermatitis to Compositae: a possible cause of dupilumab-associated facial and neck dermatitis in atopic dermatitis patients? Contact Dermatitis. 2021;85:473-474. doi:10.1111/cod.13898
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis. Contact Dermatitis. 2023;88:150-152. doi:10.1111/cod.14234
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544. doi:10.1111/cod.14204
Practice Points
- Asteraceae dermatitis can occur from direct contact with plants of the Asteraceae family; through airborne pollen; or from exposure to topical medications, cooking products, and cosmetics.
- Patient education on primary prevention, especially protective clothing, is crucial, as these plants are ubiquitous outdoors and have diverse phenotypes.
- Management of mild Asteraceae dermatitis consists primarily of topical corticosteroids and calcineurin inhibitors, while systemic corticosteroids and other immunosuppressive agents are utilized for severe or recalcitrant cases.
Beware the Manchineel: A Case of Irritant Contact Dermatitis
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.


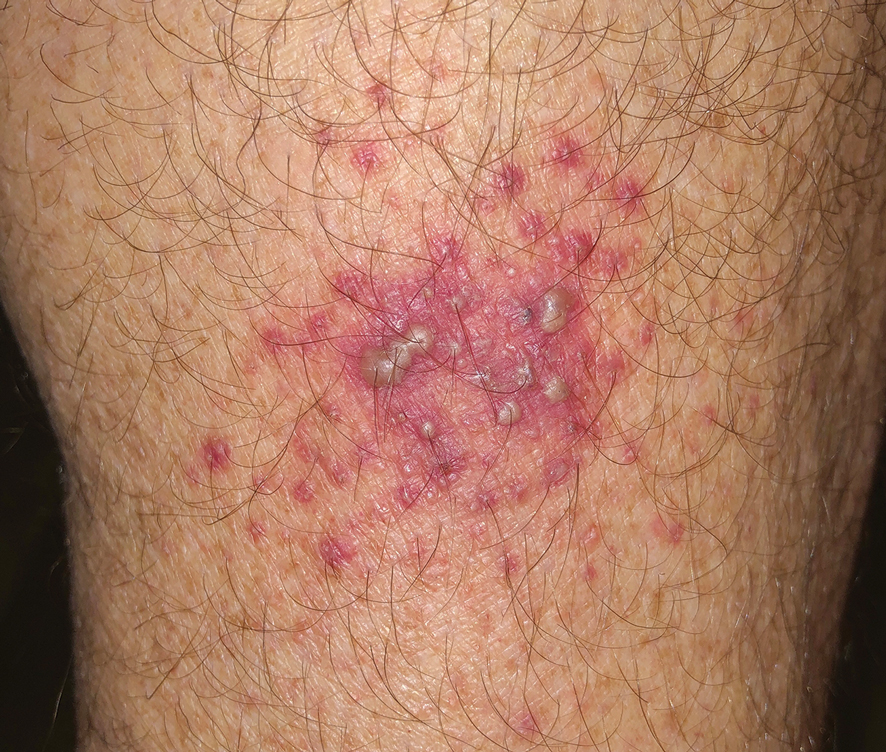
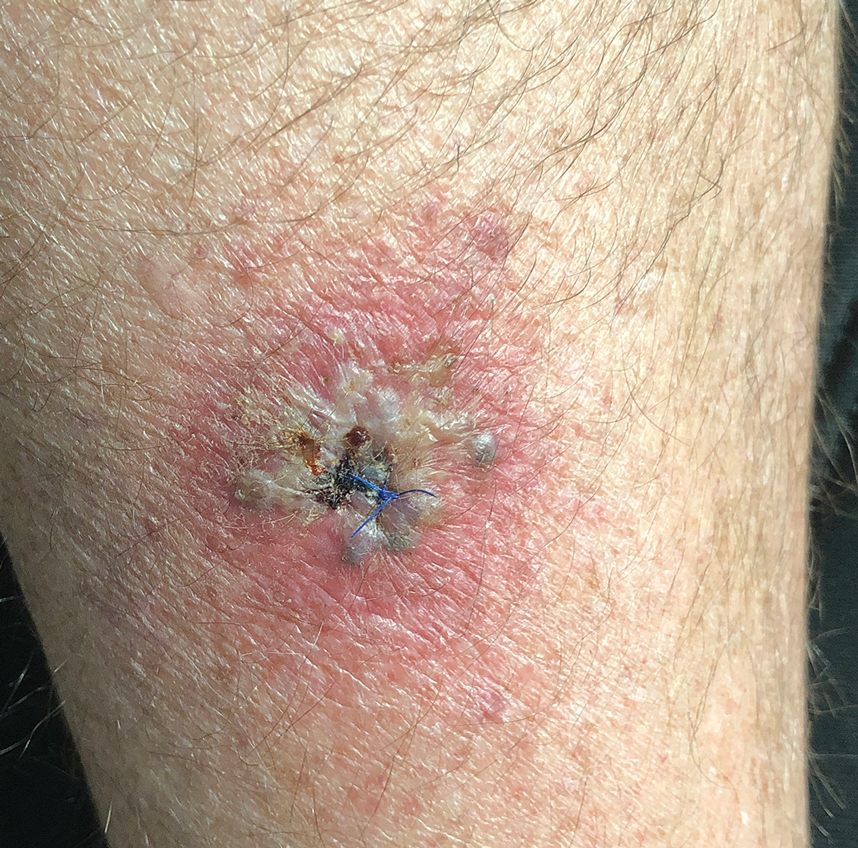
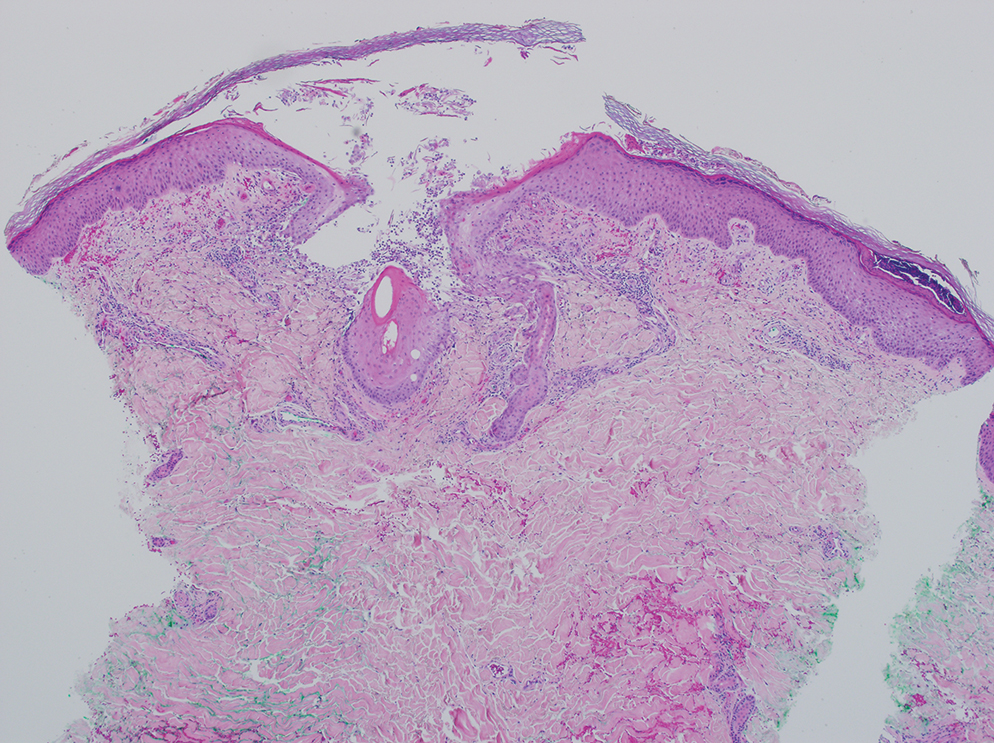
Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.





Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
What is the world’s most dangerous tree? According to Guinness World Records1 (and one unlucky contestant on the wilderness survival reality show Naked and Afraid,2 who got its sap in his eyes and needed to be evacuated for treatment), the manchineel tree (Hippomane mancinella) has earned this designation.1-3 Manchineel trees are part of the strand vegetation of islands in the West Indies and along the Caribbean coasts of South and Central America, where their copious root systems help reduce coastal erosion. In the United States, this poisonous tree grows along the southern edge of Florida’s Everglades National Park; the Florida Keys; and the US Virgin Islands, especially Virgin Islands National Park. Although the manchineel tree appears on several endangered species lists,4-6 there are places within its distribution where it is locally abundant and thus poses a risk to residents and visitors.
The first European description of manchineel toxicity was by Peter Martyr d’Anghiera, a court historian and geographer of Christopher Columbus’s patroness, Isabella I, Queen of Castile and Léon. In the early 1500s, Peter Martyr wrote that on Columbus’s second New World voyage in 1493, the crew encountered a mysterious tree that burned the skin and eyes of anyone who had contact with it.7 Columbus called the tree’s fruit manzanilla de la muerte (“little apple of death”) after several sailors became severely ill from eating the fruit.8,9 Manchineel lore is rife with tales of agonizing death after eating the applelike fruit, and several contemporaneous accounts describe indigenous Caribbean islanders using manchineel’s toxic sap as an arrow poison.10
Eating manchineel fruit is known to cause abdominal pain, burning sensations in the oropharynx, and esophageal spasms.11 Several case reports mention that consuming the fruit can create an exaggerated
Case Report
A 64-year-old physician (S.A.N.) came across a stand of manchineel trees while camping in the Virgin Islands National Park on St. John in the US Virgin Islands (Figure 1). The patient—who was knowledgeable about tropical ecology and was familiar with the tree—was curious about its purported cutaneous toxicity and applied the viscous white sap of a broken branchlet (Figure 2) to a patch of skin measuring 4 cm in diameter on the medial left calf. He took serial photographs of the site on days 2, 4 (Figure 3), 6, and 10 (Figure 4), showing the onset of erythema and the subsequent development of follicular pustules. On day 6, a 4-mm punch biopsy specimen was taken of the most prominent pustule. Histopathology showed a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which was consistent with irritant contact dermatitis (Figure 5). On day 8, the region became indurated and tender to pressure; however, there was no warmth, edema, purulent drainage, lymphangitic streaks, or other signs of infection. The region was never itchy; it was uncomfortable only with firm direct pressure. The patient applied hot compresses to the site for 10 minutes 1 to 2 times daily for roughly 2 weeks, and the affected area healed fully (without any additional intervention) in approximately 6 weeks.





Comment
Manchineel is a member of the Euphorbiaceae (also known as the euphorb or spurge) family, a mainly tropical or subtropical plant family that includes many useful as well as many toxic species. Examples of useful plants include cassava (Manihot esculenta) and the rubber tree (Hevea brasiliensis). Many euphorbs have well-described toxicities, and many (eg, castor bean, Ricinus communis) are useful in some circumstances and toxic in others.6,12-14 Many euphorbs are known to cause skin reactions, usually due to toxins in the milky sap that directly irritate the skin or to latex compounds that can induce IgE-mediated contact dermatitis.9,14
Manchineel contains a complex mix of toxins, though no specific one has been identified as the main cause of the associated irritant contact dermatitis. Manchineel sap (and sap of many other euphorbs) contains phorbol esters that may cause direct pH-induced cytotoxicity leading to keratinocyte necrosis. Diterpenes may augment this cytotoxic effect via induction of proinflammatory cytokines.12 Pitts et al5 pointed to a mixture of oxygenated diterpene esters as the primary cause of toxicity and suggested that their water solubility explained occurrences of keratoconjunctivitis after contact with rainwater or dew from the manchineel tree.
All parts of the manchineel tree—fruit, leaves, wood, and sap—are poisonous. In a retrospective series of 97 cases of manchineel fruit ingestion, the most common symptoms were oropharyngeal pain (68% [66/97]), abdominal pain (42% [41/97]), and diarrhea (37% [36/97]). The same series identified 1 (1%) case of bradycardia and hypotension.3 Contact with the wood, exposure to sawdust, and inhalation of smoke from burning the wood can irritate the skin, conjunctivae, or nasopharynx. Rainwater or dew dripping from the leaves onto the skin can cause dermatitis and ophthalmitis, even without direct contact with the tree.4,5
Management—There is no specific treatment for manchineel dermatitis. Because it is an irritant reaction and not a type IV hypersensitivity reaction, topical corticosteroids have minimal benefit. A regimen consisting of a thorough cleansing, wet compresses, and observation, as most symptoms resolve spontaneously within a few days, has been recommended.4 Our patient used hot compresses, which he believes helped heal the site, although his symptoms lasted for several weeks.
Given that there is no specific treatment for manchineel dermatitis, the wisest approach is strict avoidance. On many Caribbean islands, visitors are warned about the manchineel tree, advised to avoid direct contact, and reminded to avoid standing beneath it during a rainstorm (Figure 6).

Conclusion
This article begins with a question: “What is the world’s most dangerous tree?” Many sources from the indexed medical literature as well as the popular press and social media state that it is the manchineel. Although all parts of the manchineel tree are highly toxic, human exposures are uncommon, and deaths are more apocryphal than actual.
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
- Most dangerous tree. Guinness World Records. Accessed October 14, 2024. https://www.guinnessworldrecords.com/world-records/most-dangerous-tree
- Naked and Afraid: Garden of Evil (S4E9). Discovery Channel. June 21, 2015. Accessed October 14, 2024. https://go.discovery.com/video/naked-and-afraid-discovery/garden-of-evil
- Boucaud-Maitre D, Cachet X, Bouzidi C, et al. Severity of manchineel fruit (Hippomane mancinella) poisoning: a retrospective case series of 97 patients from French Poison Control Centers. Toxicon. 2019;161:28-32. doi:10.1016/j.toxicon.2019.02.014
- Blue LM, Sailing C, Denapoles C, et al. Manchineel dermatitis in North American students in the Caribbean. J Travel Medicine. 2011;18:422-424. doi:10.1111/j.1708-8305.2011.00568.x
- Pitts JF, Barker NH, Gibbons DC, et al. Manchineel keratoconjunctivitis. Br J Ophthalmol. 1993;77:284-288. doi:10.1136/bjo.77.5.284
- Lauter WM, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella, L. I. historical review. J Pharm Sci. 1952;41:199-201. https://doi.org/10.1002/jps.3030410412
- Martyr P. De Orbe Novo: the Eight Decades of Peter Martyr d’Anghera. Vol 1. FA MacNutt (translator). GP Putnam’s Sons; 1912. Accessed October 14, 2024. https://gutenberg.org/cache/epub/12425/pg12425.txt
- Fernandez de Ybarra AM. A forgotten medical worthy, Dr. Diego Alvarex Chanca, of Seville, Spain, and his letter describing the second voyage of Christopher Columbus to America. Med Library Hist J. 1906;4:246-263.
- Muscat MK. Manchineel apple of death. EJIFCC. 2019;30:346-348.
- Handler JS. Aspects of Amerindian ethnography in 17th century Barbados. Caribbean Studies. 1970;9:50-72.
- Howard RA. Three experiences with the manchineel (Hippomane spp., Euphorbiaceae). Biotropica. 1981;13:224-227. https://doi.org/10.2307/2388129
- Rao KV. Toxic principles of Hippomane mancinella. Planta Med. 1974;25:166-171. doi:10.1055/s-0028-1097927
- Lauter WM, Foote PA. Investigation of the toxic principles of Hippomane mancinella L. II. Preliminary isolation of a toxic principle of the fruit. J Am Pharm Assoc. 1955;44:361-363. doi:10.1002/jps.3030440616
- Carroll MN Jr, Fox LE, Ariail WT. Investigation of the toxic principles of Hippomane mancinella L. III. Toxic actions of extracts of Hippomane mancinella L. J Am Pharm Assoc. 1957;46:93-97. doi:10.1002/jps.3030460206
PRACTICE POINTS
- Sap from the manchineel tree—found on the coasts of Caribbean islands, the Atlantic coastline of Central and northern South America, and parts of southernmost Florida—can cause severe dermatologic and ophthalmologic injuries. Eating its fruit can lead to oropharyngeal pain and diarrhea.
- Histopathology of manchineel dermatitis reveals a subcorneal acantholytic blister and epidermal spongiosis overlying a mixed perivascular infiltrate and follicular necrosis, which is consistent with irritant contact dermatitis.
- There is no specific treatment for manchineel dermatitis. Case reports advocate a thorough cleansing, application of wet compresses, and observation.
Eyelid Dermatitis: Common Patterns and Contact Allergens
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.
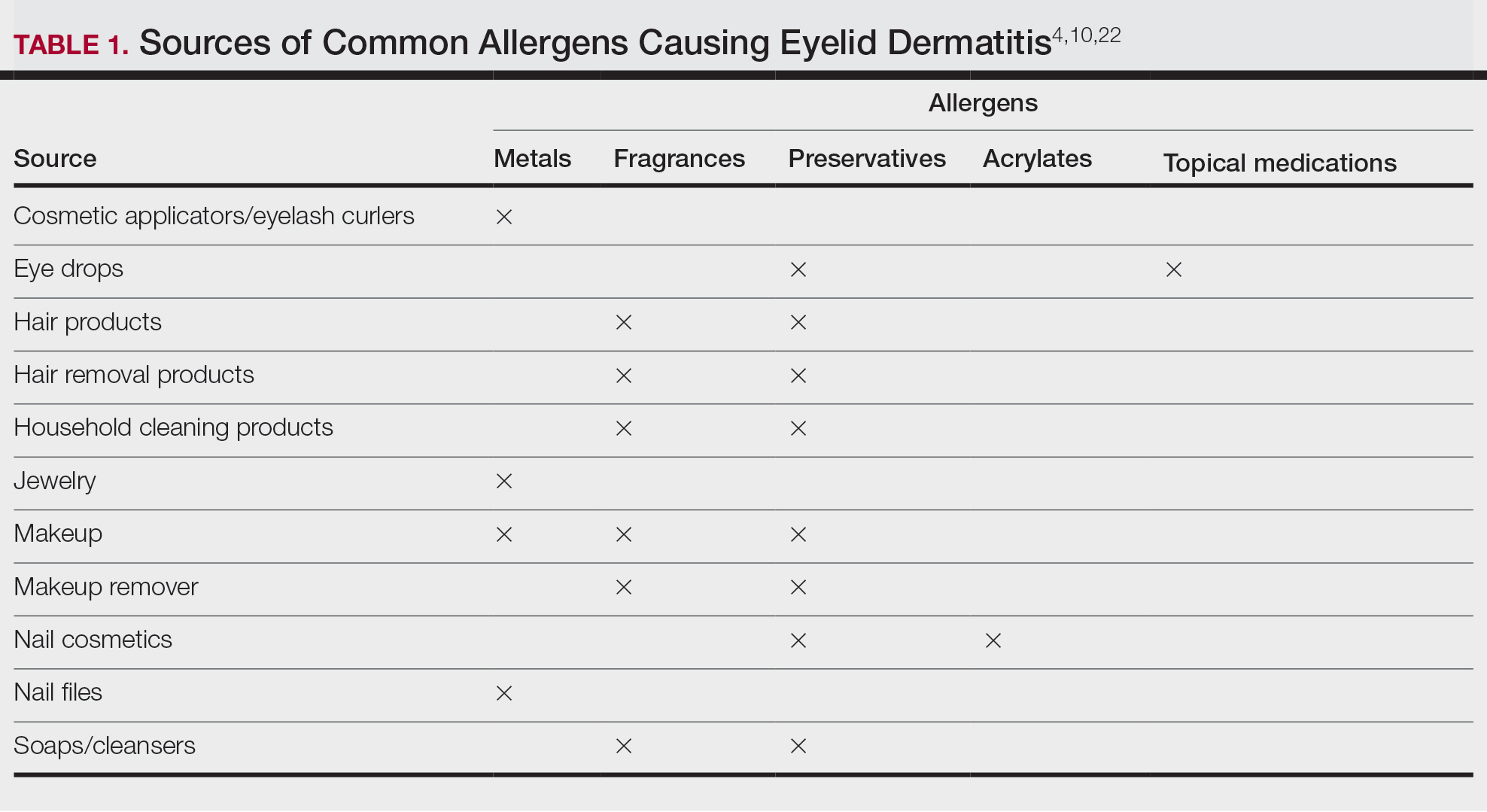
Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46
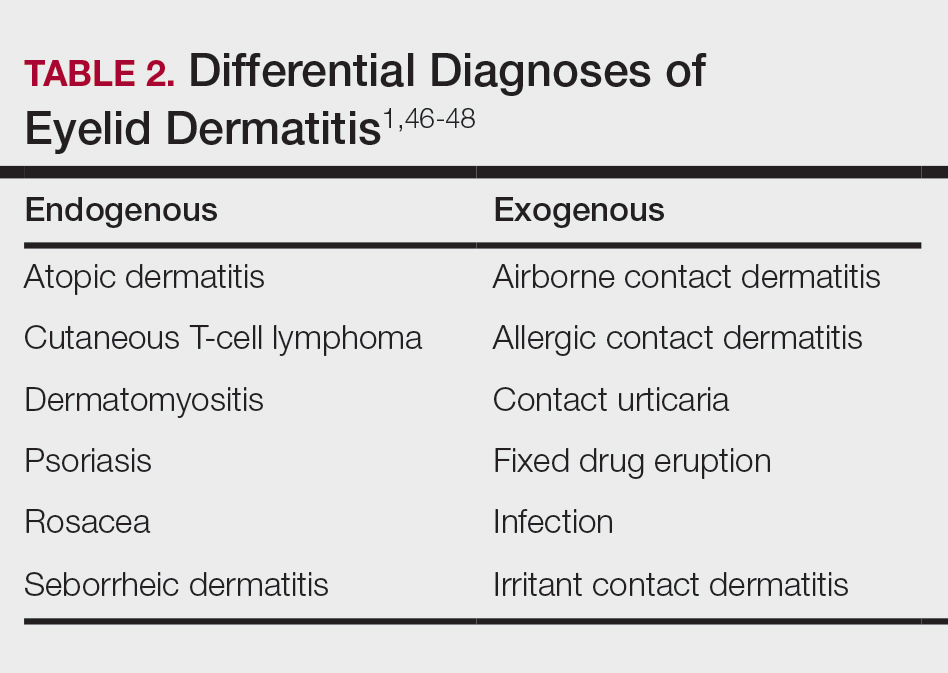
Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Practice Points
- Eyelid dermatitis is a common dermatologic concern representing a broad range of inflammatory dermatoses, most often caused by allergic contact dermatitis (ACD).
- The most common contact allergens associated with eyelid dermatitis are metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications, which may be found in a variety of sources, including cosmetics, ophthalmic medications, nail lacquers, and jewelry.
- Eyelid ACD is diagnosed via patch testing, and management involves strict allergen avoidance.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.