User login
Damage control laparotomy an option for nontrauma secondary peritonitis
LAS VEGAS – Damage control laparotomy is a safe and reliable approach to the surgical management of patients with severe nontrauma secondary peritonitis who require bowel resection, according a review of 182 cases.
For example, the deferred ostomy rate was 16.7% among 72 patients who underwent damage control laparotomy (DCL), which was significantly lower than the primary ostomy rate of 53.6% in 110 patients who underwent a definitive surgical procedure (DSP), Dr. Maria P. Garcia-Garcia reported at the annual meeting of the American Association for the Surgery of Trauma.
Further, the fistula rate was lower among 60 DCL patients who underwent delayed anastomosis, compared with 51 DSP patients who underwent primary anastomosis (26.7% vs. 37.2%), and the mortality rate was lower in the DCL vs. DSP patients (16.7% vs. 24.5%). These differences did not meet statistical significance due to the sample size. Deaths in the DCL group all occurred in those who underwent delayed anastomosis; deaths in the DSP group occurred in 14 ostomy patients and 13 anastomosis patients, said Dr. Garcia-Garcia of Fundacion Valle del Lili, Cali, Colombia.
Disease severity, as measured by APACHE II scores, was similar in the two groups (mean of about 17 for each group). Septic shock was present in 37% at the time of admission. Mean hospital length of stay and mean intensive care unit length of stay did not differ significantly between the groups, nor did the systemic complication rate, or the rates of multiple organ failure and acute respiratory distress syndrome.
Small-bowel perforation occurred in 77 (42.3%), and colon perforation occurred in 105 (57.7%).
The patients included teens and adults aged 16 years or older (mean of 60.3 years) with severe nontrauma secondary peritonitis (NSPT) who were undergoing bowel resection after enteric perforations between 2003 and 2013. The DSP patients underwent either primary anastomosis or primary ostomy, and the DCL patients underwent segmental bowel resection, temporary abdominal closure, and subsequent delayed anastomosis or deferred ostomy.
DCL is a recognized strategy for managing bowel injuries in trauma patients. It was developed in response to the poor outcomes associated with attempting definitive repair, but evidence regarding the role and timing of anastomosis in DCL in NTSP – a condition associated with high morbidity and a 30% in-hospital mortality rate – is lacking, Dr. Garcia-Garcia said, noting that the current findings suggest it is the preferred approach.
“When a definite surgical repair is chosen, there is a 50/50 chance of performing anastomosis or ostomy. However, when a damage control abbreviated laparotomy is performed, there is a high bowel reconstruction success rate of about 80%. Therefore, damage control abbreviated laparotomy is a reliable and safe option in critically ill nontrauma secondary peritonitis patients. At the end of the day it’s your choice: Would you rather leave your patient with an ostomy or tube, or would you give your patient a chance of successful reconstruction without an ostomy?” she said.
Dr. Garcia-Garcia reported having no disclosures.
LAS VEGAS – Damage control laparotomy is a safe and reliable approach to the surgical management of patients with severe nontrauma secondary peritonitis who require bowel resection, according a review of 182 cases.
For example, the deferred ostomy rate was 16.7% among 72 patients who underwent damage control laparotomy (DCL), which was significantly lower than the primary ostomy rate of 53.6% in 110 patients who underwent a definitive surgical procedure (DSP), Dr. Maria P. Garcia-Garcia reported at the annual meeting of the American Association for the Surgery of Trauma.
Further, the fistula rate was lower among 60 DCL patients who underwent delayed anastomosis, compared with 51 DSP patients who underwent primary anastomosis (26.7% vs. 37.2%), and the mortality rate was lower in the DCL vs. DSP patients (16.7% vs. 24.5%). These differences did not meet statistical significance due to the sample size. Deaths in the DCL group all occurred in those who underwent delayed anastomosis; deaths in the DSP group occurred in 14 ostomy patients and 13 anastomosis patients, said Dr. Garcia-Garcia of Fundacion Valle del Lili, Cali, Colombia.
Disease severity, as measured by APACHE II scores, was similar in the two groups (mean of about 17 for each group). Septic shock was present in 37% at the time of admission. Mean hospital length of stay and mean intensive care unit length of stay did not differ significantly between the groups, nor did the systemic complication rate, or the rates of multiple organ failure and acute respiratory distress syndrome.
Small-bowel perforation occurred in 77 (42.3%), and colon perforation occurred in 105 (57.7%).
The patients included teens and adults aged 16 years or older (mean of 60.3 years) with severe nontrauma secondary peritonitis (NSPT) who were undergoing bowel resection after enteric perforations between 2003 and 2013. The DSP patients underwent either primary anastomosis or primary ostomy, and the DCL patients underwent segmental bowel resection, temporary abdominal closure, and subsequent delayed anastomosis or deferred ostomy.
DCL is a recognized strategy for managing bowel injuries in trauma patients. It was developed in response to the poor outcomes associated with attempting definitive repair, but evidence regarding the role and timing of anastomosis in DCL in NTSP – a condition associated with high morbidity and a 30% in-hospital mortality rate – is lacking, Dr. Garcia-Garcia said, noting that the current findings suggest it is the preferred approach.
“When a definite surgical repair is chosen, there is a 50/50 chance of performing anastomosis or ostomy. However, when a damage control abbreviated laparotomy is performed, there is a high bowel reconstruction success rate of about 80%. Therefore, damage control abbreviated laparotomy is a reliable and safe option in critically ill nontrauma secondary peritonitis patients. At the end of the day it’s your choice: Would you rather leave your patient with an ostomy or tube, or would you give your patient a chance of successful reconstruction without an ostomy?” she said.
Dr. Garcia-Garcia reported having no disclosures.
LAS VEGAS – Damage control laparotomy is a safe and reliable approach to the surgical management of patients with severe nontrauma secondary peritonitis who require bowel resection, according a review of 182 cases.
For example, the deferred ostomy rate was 16.7% among 72 patients who underwent damage control laparotomy (DCL), which was significantly lower than the primary ostomy rate of 53.6% in 110 patients who underwent a definitive surgical procedure (DSP), Dr. Maria P. Garcia-Garcia reported at the annual meeting of the American Association for the Surgery of Trauma.
Further, the fistula rate was lower among 60 DCL patients who underwent delayed anastomosis, compared with 51 DSP patients who underwent primary anastomosis (26.7% vs. 37.2%), and the mortality rate was lower in the DCL vs. DSP patients (16.7% vs. 24.5%). These differences did not meet statistical significance due to the sample size. Deaths in the DCL group all occurred in those who underwent delayed anastomosis; deaths in the DSP group occurred in 14 ostomy patients and 13 anastomosis patients, said Dr. Garcia-Garcia of Fundacion Valle del Lili, Cali, Colombia.
Disease severity, as measured by APACHE II scores, was similar in the two groups (mean of about 17 for each group). Septic shock was present in 37% at the time of admission. Mean hospital length of stay and mean intensive care unit length of stay did not differ significantly between the groups, nor did the systemic complication rate, or the rates of multiple organ failure and acute respiratory distress syndrome.
Small-bowel perforation occurred in 77 (42.3%), and colon perforation occurred in 105 (57.7%).
The patients included teens and adults aged 16 years or older (mean of 60.3 years) with severe nontrauma secondary peritonitis (NSPT) who were undergoing bowel resection after enteric perforations between 2003 and 2013. The DSP patients underwent either primary anastomosis or primary ostomy, and the DCL patients underwent segmental bowel resection, temporary abdominal closure, and subsequent delayed anastomosis or deferred ostomy.
DCL is a recognized strategy for managing bowel injuries in trauma patients. It was developed in response to the poor outcomes associated with attempting definitive repair, but evidence regarding the role and timing of anastomosis in DCL in NTSP – a condition associated with high morbidity and a 30% in-hospital mortality rate – is lacking, Dr. Garcia-Garcia said, noting that the current findings suggest it is the preferred approach.
“When a definite surgical repair is chosen, there is a 50/50 chance of performing anastomosis or ostomy. However, when a damage control abbreviated laparotomy is performed, there is a high bowel reconstruction success rate of about 80%. Therefore, damage control abbreviated laparotomy is a reliable and safe option in critically ill nontrauma secondary peritonitis patients. At the end of the day it’s your choice: Would you rather leave your patient with an ostomy or tube, or would you give your patient a chance of successful reconstruction without an ostomy?” she said.
Dr. Garcia-Garcia reported having no disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Damage control laparotomy is a safe and reliable approach to the surgical management of patients with severe nontrauma secondary peritonitis who require bowel resection, according a review of 182 cases.
Major finding: The ostomy rate was 16.7% vs. 53.6% with DCL vs. DSP.
Data source: A review of 182 cases.
Disclosures: Dr. Garcia-Garcia reported having no disclosures.
Utilization of Fusion PET/CT in Mapping Surgical/Medical Treatment Algorithms: Individualizing Patient Care for Suspicious Colorectal Masses
Purpose: Determine the utility of fusion positron emission tomography/computed tomography (PET/CT) in mapping surgical procedures for suspicious colorectal masses in the era of minimally invasive surgery—laparoscopy/robotics where haptic feedback is absent.
Background: The National Comprehensive Cancer Network (NCCN) guidelines recommend using CT of the chest, abdomen, and pelvis for colorectal cancer staging. This is largely because PET/CT is not widely available, thus limiting access. Colonoscopy is used to locate/diagnose colorectal masses. Gastroenterologists often “guestimate” the location of the lesion either by anatomical landmarks or by measurement on the colonoscope itself. These are often inaccurate. It is the standard of care to ink the location of the lesion as well. This is not always done or easy to identify. It is often necessary to perform an intraoperative colonoscopy to locate the lesion in question and then make incisions or dock the robot accordingly.
Methods: Retrospective data from a colorectal surgeon were reviewed. Surgeries performed at the Raymond G. Murphy VAMC from March 2012 to June 2015 were included. Data were reviewed for these patients to evaluate for the efficacy of fusion PET/CT studies in identifying the lesion in question regardless of benign or cancerous lesion, mapping of the planned procedure, and how it affected planned treatment algorithms.
Results: Fifty patients were referred for evaluation and treatment of a suspicious colorectal mass, and 45 patients underwent PET/CT for staging. The lesion was not PET avid in 9 patients, and 36 patients had positive findings on the study. Thirty-two of those patients had findings fairly consistent with the colonoscopy site identifiers. In 5 patients, the PET/ CT results changed the planned surgery or delayed surgery for neoadjuvant chemoradiotherapy. The nonvisualized patients were either mucinous or no residual tumor remained.
Conclusions: Although PET/CT is not the recommended staging study by NCCN guidelines for colorectal cancers, it is readily available at our VAMC and proves useful in differentiating scar from tumor when compared with CT alone. Our experience showed that PET/CT is often positive in suspicious colorectal masses, helps to map the surgery, and acts as a baseline for ongoing surveillance. It ultimately can change the entire treatment algorithm for our individual patients.
Purpose: Determine the utility of fusion positron emission tomography/computed tomography (PET/CT) in mapping surgical procedures for suspicious colorectal masses in the era of minimally invasive surgery—laparoscopy/robotics where haptic feedback is absent.
Background: The National Comprehensive Cancer Network (NCCN) guidelines recommend using CT of the chest, abdomen, and pelvis for colorectal cancer staging. This is largely because PET/CT is not widely available, thus limiting access. Colonoscopy is used to locate/diagnose colorectal masses. Gastroenterologists often “guestimate” the location of the lesion either by anatomical landmarks or by measurement on the colonoscope itself. These are often inaccurate. It is the standard of care to ink the location of the lesion as well. This is not always done or easy to identify. It is often necessary to perform an intraoperative colonoscopy to locate the lesion in question and then make incisions or dock the robot accordingly.
Methods: Retrospective data from a colorectal surgeon were reviewed. Surgeries performed at the Raymond G. Murphy VAMC from March 2012 to June 2015 were included. Data were reviewed for these patients to evaluate for the efficacy of fusion PET/CT studies in identifying the lesion in question regardless of benign or cancerous lesion, mapping of the planned procedure, and how it affected planned treatment algorithms.
Results: Fifty patients were referred for evaluation and treatment of a suspicious colorectal mass, and 45 patients underwent PET/CT for staging. The lesion was not PET avid in 9 patients, and 36 patients had positive findings on the study. Thirty-two of those patients had findings fairly consistent with the colonoscopy site identifiers. In 5 patients, the PET/ CT results changed the planned surgery or delayed surgery for neoadjuvant chemoradiotherapy. The nonvisualized patients were either mucinous or no residual tumor remained.
Conclusions: Although PET/CT is not the recommended staging study by NCCN guidelines for colorectal cancers, it is readily available at our VAMC and proves useful in differentiating scar from tumor when compared with CT alone. Our experience showed that PET/CT is often positive in suspicious colorectal masses, helps to map the surgery, and acts as a baseline for ongoing surveillance. It ultimately can change the entire treatment algorithm for our individual patients.
Purpose: Determine the utility of fusion positron emission tomography/computed tomography (PET/CT) in mapping surgical procedures for suspicious colorectal masses in the era of minimally invasive surgery—laparoscopy/robotics where haptic feedback is absent.
Background: The National Comprehensive Cancer Network (NCCN) guidelines recommend using CT of the chest, abdomen, and pelvis for colorectal cancer staging. This is largely because PET/CT is not widely available, thus limiting access. Colonoscopy is used to locate/diagnose colorectal masses. Gastroenterologists often “guestimate” the location of the lesion either by anatomical landmarks or by measurement on the colonoscope itself. These are often inaccurate. It is the standard of care to ink the location of the lesion as well. This is not always done or easy to identify. It is often necessary to perform an intraoperative colonoscopy to locate the lesion in question and then make incisions or dock the robot accordingly.
Methods: Retrospective data from a colorectal surgeon were reviewed. Surgeries performed at the Raymond G. Murphy VAMC from March 2012 to June 2015 were included. Data were reviewed for these patients to evaluate for the efficacy of fusion PET/CT studies in identifying the lesion in question regardless of benign or cancerous lesion, mapping of the planned procedure, and how it affected planned treatment algorithms.
Results: Fifty patients were referred for evaluation and treatment of a suspicious colorectal mass, and 45 patients underwent PET/CT for staging. The lesion was not PET avid in 9 patients, and 36 patients had positive findings on the study. Thirty-two of those patients had findings fairly consistent with the colonoscopy site identifiers. In 5 patients, the PET/ CT results changed the planned surgery or delayed surgery for neoadjuvant chemoradiotherapy. The nonvisualized patients were either mucinous or no residual tumor remained.
Conclusions: Although PET/CT is not the recommended staging study by NCCN guidelines for colorectal cancers, it is readily available at our VAMC and proves useful in differentiating scar from tumor when compared with CT alone. Our experience showed that PET/CT is often positive in suspicious colorectal masses, helps to map the surgery, and acts as a baseline for ongoing surveillance. It ultimately can change the entire treatment algorithm for our individual patients.
Colorectal Cancer Statistics Among Patients Reported in the Veterans Affairs Central Cancer Registry
Purpose: On average, VA patients are older and sicker than is the general population. Our objectives were to provide an overview of VA colorectal (CRC) incidence and make comparisons with the Surveillance, Epidemiology, and End Results (SEER) data, which provides U.S. cancer statistics.
Background: About 3,400 incidents of CRC are reported in the Veterans Affairs Central Cancer Registry (VACCR) annually. This equates to nearly 9% of VA cancers.
Methods/Data Analysis: Data were obtained from VACCR for incident CRC diagnosed/treated in VA from fiscal year (FY) 2009 to 2012. Using VHA Support Service Center information about the distribution of VA health care system enrollees for corresponding years, we made age and gender adjustments for the underlying VA population. Colorectal incidence among VA patients was descriptively compared with projected national 2014 CRC-specific SEER and supporting data sources.
Results: From FY 2009 to 2012, we identified 15,205 VA patients nationwide. For analysis, there were 12,551 patients (n = 322, 2.6% women; n = 12,229, 97.4% men). Among patients in the VACCR, the most common tumor location was proximal colon (n = 4,830, 38%), followed by rectum (n = 3,907, 31%), distal colon (n = 3,240, 26%), and other colon (n = 574, 5%). These percentages are comparable with those of SEER, in which proximal colon and rectum are most common. Among patients in the VACCR, SEER summary stage distribution was 44% (n = 5,517) local, 36% (n = 4,488) regional, 17% (n = 2,091) distant, and 4% (n = 455) unknown. These percentages also align with those of SEER, in which about 40% of CRC cases are diagnosed locally. Mirroring SEER, among the VACCR, overall CRC incidence rate decreased from 0.22 to 0.16 cases per 1,000 veterans in FYs 2009 and 2012, respectively.
Implications: VACCR data indicate that incident CRC in FY 2009 to 2012 approximated SEER projections during a similar time frame. National VA CRC incidence, location, and stage distribution are also similar. This suggests that despite VA patients being more complex than their general population counterparts, VA patients are generally diagnosed with comparable CRC locations and stages. This analysis also suggests that, like SEER, the VACCR may have utility for epidemiologic tracking and research.
Purpose: On average, VA patients are older and sicker than is the general population. Our objectives were to provide an overview of VA colorectal (CRC) incidence and make comparisons with the Surveillance, Epidemiology, and End Results (SEER) data, which provides U.S. cancer statistics.
Background: About 3,400 incidents of CRC are reported in the Veterans Affairs Central Cancer Registry (VACCR) annually. This equates to nearly 9% of VA cancers.
Methods/Data Analysis: Data were obtained from VACCR for incident CRC diagnosed/treated in VA from fiscal year (FY) 2009 to 2012. Using VHA Support Service Center information about the distribution of VA health care system enrollees for corresponding years, we made age and gender adjustments for the underlying VA population. Colorectal incidence among VA patients was descriptively compared with projected national 2014 CRC-specific SEER and supporting data sources.
Results: From FY 2009 to 2012, we identified 15,205 VA patients nationwide. For analysis, there were 12,551 patients (n = 322, 2.6% women; n = 12,229, 97.4% men). Among patients in the VACCR, the most common tumor location was proximal colon (n = 4,830, 38%), followed by rectum (n = 3,907, 31%), distal colon (n = 3,240, 26%), and other colon (n = 574, 5%). These percentages are comparable with those of SEER, in which proximal colon and rectum are most common. Among patients in the VACCR, SEER summary stage distribution was 44% (n = 5,517) local, 36% (n = 4,488) regional, 17% (n = 2,091) distant, and 4% (n = 455) unknown. These percentages also align with those of SEER, in which about 40% of CRC cases are diagnosed locally. Mirroring SEER, among the VACCR, overall CRC incidence rate decreased from 0.22 to 0.16 cases per 1,000 veterans in FYs 2009 and 2012, respectively.
Implications: VACCR data indicate that incident CRC in FY 2009 to 2012 approximated SEER projections during a similar time frame. National VA CRC incidence, location, and stage distribution are also similar. This suggests that despite VA patients being more complex than their general population counterparts, VA patients are generally diagnosed with comparable CRC locations and stages. This analysis also suggests that, like SEER, the VACCR may have utility for epidemiologic tracking and research.
Purpose: On average, VA patients are older and sicker than is the general population. Our objectives were to provide an overview of VA colorectal (CRC) incidence and make comparisons with the Surveillance, Epidemiology, and End Results (SEER) data, which provides U.S. cancer statistics.
Background: About 3,400 incidents of CRC are reported in the Veterans Affairs Central Cancer Registry (VACCR) annually. This equates to nearly 9% of VA cancers.
Methods/Data Analysis: Data were obtained from VACCR for incident CRC diagnosed/treated in VA from fiscal year (FY) 2009 to 2012. Using VHA Support Service Center information about the distribution of VA health care system enrollees for corresponding years, we made age and gender adjustments for the underlying VA population. Colorectal incidence among VA patients was descriptively compared with projected national 2014 CRC-specific SEER and supporting data sources.
Results: From FY 2009 to 2012, we identified 15,205 VA patients nationwide. For analysis, there were 12,551 patients (n = 322, 2.6% women; n = 12,229, 97.4% men). Among patients in the VACCR, the most common tumor location was proximal colon (n = 4,830, 38%), followed by rectum (n = 3,907, 31%), distal colon (n = 3,240, 26%), and other colon (n = 574, 5%). These percentages are comparable with those of SEER, in which proximal colon and rectum are most common. Among patients in the VACCR, SEER summary stage distribution was 44% (n = 5,517) local, 36% (n = 4,488) regional, 17% (n = 2,091) distant, and 4% (n = 455) unknown. These percentages also align with those of SEER, in which about 40% of CRC cases are diagnosed locally. Mirroring SEER, among the VACCR, overall CRC incidence rate decreased from 0.22 to 0.16 cases per 1,000 veterans in FYs 2009 and 2012, respectively.
Implications: VACCR data indicate that incident CRC in FY 2009 to 2012 approximated SEER projections during a similar time frame. National VA CRC incidence, location, and stage distribution are also similar. This suggests that despite VA patients being more complex than their general population counterparts, VA patients are generally diagnosed with comparable CRC locations and stages. This analysis also suggests that, like SEER, the VACCR may have utility for epidemiologic tracking and research.
The Importance of Lymph Node Retrieval and Lymph Node Ratio in Male Patients With Colorectal Cancer: A 5-Year Retrospective Single Institution Study
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
Colorectal Carcinoma and Emerging Targeted Therapies
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the U.S.1-3 Only 40% of cases are diagnosed in a localized stage with an estimated 5-year survival of 90%, whereas 20% of cases present with metastatic disease with a 5-year survival of about 12.5%.1 With recent advances in cancer genetics and immunology as well as approval of targeted agents by the FDA, different treatment options are now available, even for progressive disease.
This article presents a brief review of CRC with a special focus on targeted therapies in metastatic CRC (mCRC). Colorectal cancers exhibit certain mutations, which affect the tumor responsiveness to various treatment options. This article describes the role of targeted therapies in various well-established mutations.
Epidemiology
The most common tumor location is the proximal colon (42%), followed by the rectum (28%). More than 90% of patients with CRCs are aged > 50 years at diagnosis.
Women have a higher percentage of proximal tumors compared with men (46% vs 38%) and a lower percentage of rectal tumors (24% vs 31%).Among both sexes, incidence and mortality are highest in African Americans and lowest in Asian/Pacific Islanders. The estimated 5-year survival is slightly higher for rectal cancer (66.5%) than for colon cancer (64.2%), although the stage-specific survival is similar. The difference in 5-year overall survival (OS) is attributed to the higher percentage of rectal tumors diagnosed at a localized stage (44% vs 38%). Patients aged < 65 years have higher 5-year survival rates than do those aged 65 years (68.9% vs 62.0%).1
Risk Factors
Like most human cancers, multiple genetic and environmental factors are believed to play a role in the development of colorectal carcinomas, with environmental factors playing the dominant role.4
Environmental risk factors include a low-fiber diet,5 red and processed meat intake,6 a high-fat diet,7 smoking,8 heavy alcohol consumption,9 obesity,10 physical inactivity,11 alteration in intestinal flora,12 and chronic inflammation.13 Aspirin (at doses > 300 mg/d), nonsteroidal anti-inflammatory drugs, and folic acid are believed to protect against colon cancer.14
Some of the genetic factors involved in colorectal cancers include (1) the loss of tumor suppressor genes, such as APC (most common tumor suppressor mutation), p53, SMAD4 pathways, or TGF- ß pathways; (2) DNA mismatch repair defects: mutations in MLH1, MSH2 in hereditary nonpolyposis colon cancer or methylation of MLH1 in sporadic cases; and (3) CpG island methylation (CIMP pathway), methylation of MLH1, MINT1, MINT2, MINT3; and (4) activation of oncogenes such as RAS and BRAF.15
Pathogenesis
The colonic mucosa consists of epithelial cells arranged in cylindrical structures called crypts. The human colon contains about 10 millioncrypts. The cellular proliferation and migration in each of the crypts is believed to be tightly regulated, with the majority of cells arising from a small number of stem cells (around 4-6) at the bottom of the crypt, which migrate upward after division and are eventually shed into the lumen.16 Each crypt is renewed in 2 to 8 days, which makes colonic mucosa one of the organs with the most cell proliferation in the human body and, hence, a target for various genetic and epigenetic alterations as well as environmental mutagenesis.7
Traditionally, the majority of colorectal carcinomas were believed to evolve from adenomatous polyps, which transform into an advanced adenoma with high-grade
dysplasia and then progress to an invasive cancer often referred to as the adenoma-to-carcinoma sequence.15 However, 2 other pathways, alternative and serrated,
have been described, and CRCs are now regarded as complex malignancies with a wide array of genetic and epigenetic mutations.17
About 70% to 85% of CRCs generally develop from chromosomal instability resulting from inactivation of tumor suppressor genes (APC gene, p53, etc). About 15% of cases are attributed to the failure of the DNA mismatch repair system either by germline/somatic mutations or by epigenetic silencing of gene transcription by CpG island methylation.18 Mutation in the BRAF or K-ras oncogenes are also believed to promote carcinogenesis.15 All these changes are believed to give rise to a precursor microscopic mucosal lesion that precedes the development of macroscopic adenomas.17
Clinical Features
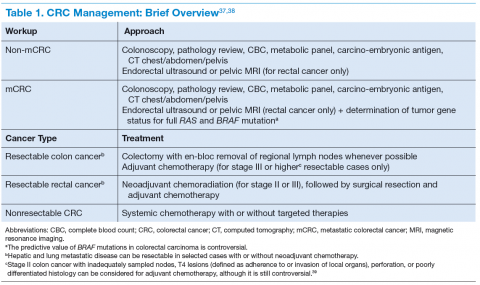
The clinical features of CRCs can be widely variable, from incidental findings during screening colonoscopy to intestinal obstruction. The most common clinical presentation is rectal bleeding, followed by weight loss, abdominal pain, constipation, or diarrhea.19 The likelihood of CRC is higher with the combination of rectal bleeding and weight loss as well as rectal bleeding and change in bowel habits. Other clinical features may include bloating, abdominal pain, or anemia.20 (See Table 1.)
Targeted Therapy in CRC
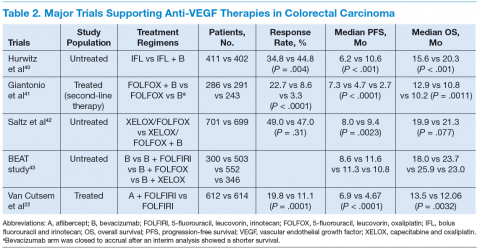
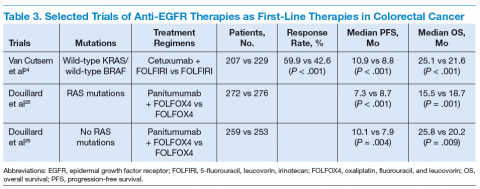
The targeted therapies in CRC include (1) the antivascular endothelial growth factor-A (anti–VEGF-A) antibody bevacizumab; (2) the VEGF-A, VEGF-B, and placental growth factor inhibitor aflibercept; (3) the multikinase inhibitor regorafenib; and (4) the anti-epidermal growth factor receptor (anti-EGFR) antibodies cetuximab and panitumumab.
Bevacizumab is an anti-VEGF monoclonal antibody. Vascular endothelial growth factor promotes angiogenesisnecessary for tumor growth. Bevacizumab was approved by the FDA in February 2004 as a first-line treatment in combination with IFL (irinotecan plus 5-fluorouracil [5-FU]/leucovorin) regimen and in June 2006 as a second-line treatment in combination with 5-FU–based chemotherapy for patients with mCRC. In January 2013, bevacizumab was approved for use in combination with fluoropyrimidine–irinotecan- or fluoropyrimidine–oxaliplatin-based chemotherapy for the treatment of patients with mCRC whose disease has progressed while on first-line treatment with a bevacizumab-containing regimen.21 (See Table 2.)
Aflibercept is a recombinant fusion protein, containing VEGF-binding portions from the extracellular domains of human VEGF receptors 1 and 2, fused to the Fc portion of human immunoglobulin IgG1 that blocks the activity of VEGF-A, VEGF-B, and placental growth factor by acting as a high-affinity ligand trap to prevent these ligands from binding to their endogenous receptors.22 It was approved by the FDA in August 2012 for use in combination with FOLFIRI (5-FU, leucovorin, irinotecan) for the treatment of patients with mCRC that is resistant to or has progressed following an oxaliplatin‑containing regimen.23
Cetuximab (a chimeric IgG1 anti-EGFR monoclonal antibody) and panitumumab (a human IgG2 anti-EGFR monoclonal antibody) have been shown to improve OS
and progression-free survival (PFS) in up to 20% of cases, either alone or in combination with chemotherapy.24,25 The FDA approved cetuximab in July 2012 for use in
combination with FOLFIRI for first-line treatment of patients with K-ras mutation-negative (wild-type), EGFRexpressing mCRC.26 The FDA approved panitumumab in
September 2006 for the treatment of patients with EGFRexpressing metastatic colorectal carcinoma with disease progression on or following FOLFOX (5-FU, leucovorin, oxaliplatin)/FOLFIRI.27 (See Table 3.)
KRAS, a member of the rat sarcoma virus (ras) gene family of oncogenes, encodes for a small G protein downstream of EGFR. KRAS is mutated in CRC in up to 37% cases, resulting in activation of the different downstream signaling pathways.15,28 Therefore, KRAS mutations predict resistance to anti-EGFR therapy.28,29 Testing for KRAS mutation prior to treatment with cetuximab or panitumumab leads to targeted us of the very costly monoclonal antibodies and hence is considered a cost-effective practice.30
Even in cases with wild-type KRAS mutation, response to anti-EGFR therapies is seen in only less than half of patients.31 Up to 17% of tumors with wild-type for KRAS exon 2 at codons 12 and 13 can have a mutation in another of the ras pathway genes (eg, KRAS exon 3, 4 and NRAS exon 2, 3, 4).25 Mutations in the BRAF oncogene have been described in up to 13% of colorectal carcinoma cases.15 The data to suggest a lack of antitumor activity from anti-EGFR therapies in the presence of BRAF V600E mutation are still limited, but BRAF mutation is considered a poor prognostic factor.
A recent trial involving 1,137 patients with KRAS exon 2 wild-type mCRC randomly assigned to cetuximab or bevacizumab with standard chemotherapy (FOLFOX or FOLFIRI) found OS of 29.9 vs 29.0 months and median PFS 10.4 vs 10.8 months for cetuximab and bevacizumab, respectively.32 The OS for 5-FU–based therapies was about 11 months.33
Regorafenib is an oral multikinase inhibitor of angiogenic, stromal, and oncogenic receptor protein kinases, including those involved in the regulation of tumor angiogenesis (eg, VEGFR1-3 and TIE2 [tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2]), tumor microenvironment (plateletderived growth factor receptor-β and fibroblast growth factor receptor 1), as well as tumor oncogenesis (KIT, RET, RAF1, BRAF, BRAF V600E).34 A randomized phase 3 study involving 760 patients with documented progressive mCRC found a higher median OS with regorafenib vs placebo (6.4 mo vs 5.0 mo, P = .0052) and a
higher PFS (2.0 mo vs 1.7 mo, P < .0001).35 The FDA approved regorafenib in September 2012 for the treatment of patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, with an anti-VEGF therapy and, if KRAS wild-type, with an anti-EGFR therapy.36
Conclusion
Targeted therapies in conjunction with newer chemotherapies have improved outcomes in metastatic colorectal carcinoma compared with those of conventional therapy (29 mo vs 11 mo).
Author disclosures
Peter T. Silberstein, MD, reports receiving payment for lectures from Bristol Myers and Celgene in the past. Drs. Khanal and Upadhyay report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
2. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
4. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78-85.
5. Bingham SA, Day NE, Luben R, et al; European Prospective Investigation into Cancer and Nutrition. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361(9368):1496-1501.
6. Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4(2):177-184.
7. Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis—update and perspectives. World J Gastroenterol. 2014;20(48):18151-18164.
8. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
9. Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958-1972.
10. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916.
11. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204-213.
12. Candela M, Turroni S, Biagi E, et al. Inflammation and colorectal cancer, when
microbiota-host mutualism breaks. World J Gastroenterol. 2014;20(4):908-922.
13. Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(4):405-410.
14. Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33-46.
15. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of
colorectal cancer. N Engl J Med. 2009;361(25):2449-2460.
16. Zhao R, Michor F. Patterns of proliferative activity in the colonic crypt determine
crypt stability and rates of somatic evolution. PLoS Comput Biol. 2013;9(6):e1003082.
17. Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple
pathways in colorectal cancer progression. Pathol Res Int. 2012;2012:509348.
18. Worthley DL, Whitehall VL, Spring KJ, Leggett BA. Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol. 2007;13(28):3784-3791.
19. Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399-405.
20. Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61(586):e231-e243.
21. National Cancer Institute. FDA approval for bevacizumab: first-line treatment of metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab#Anchor-Approva-23287. Updated December 4, 2014. Accessed July 6, 2015.
22. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatinbased regimen. J Clin Oncol. 2012;30(28):3499-3506.
23. U.S. Food and Drug Administration. Ziv-Aflibercept. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm314438.htm. Updated August 3, 2012. Accessed July 6, 2015.
24. Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019.
25. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
26. U.S. Food and Drug Administration. Cetuximab in combination with folfiri/therascreen. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm310933.htm. Updated July 9, 2012. Accessed July 6, 2015.
27. Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary:
panitumumab (Vectibix). Oncologist. 2007;12(5):577-583.
28. Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: antiepidermal
growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154(1):37-49.
29. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
30. Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40-49.
31. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncology. 2009;27(12):2091-2096.
32. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance); SWOG; ECOG. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(15)(suppl):Abstract LBA3.
33. Poon MA, O’Connell MJ, Moertel CG, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7(10):1407-1418.
34. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245-255.
35. Grothey A, Van Cutsem E, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312.
36. National Cancer Institute. FDA approval for regorafenib: previously treated metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-regorafenib#Anchor-MCRC. Updated July 3, 2013. Accessed July 6, 2015.
37. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Updated October 3, 2014. Accessed January 25, 2015.
38. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: rectal cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Updated December 9, 2014. Accessed January 25, 2015.
39. Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408-3419.
40. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342.
41. Giantonio BJ, Catalano PJ, Meropol NJ, et al; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544.
42. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013-2019.
43. Van Cutsem E, Rivera F, Berry S, et al; First BEAT investigators. Safety and efficacy of
first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842-1847.
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the U.S.1-3 Only 40% of cases are diagnosed in a localized stage with an estimated 5-year survival of 90%, whereas 20% of cases present with metastatic disease with a 5-year survival of about 12.5%.1 With recent advances in cancer genetics and immunology as well as approval of targeted agents by the FDA, different treatment options are now available, even for progressive disease.
This article presents a brief review of CRC with a special focus on targeted therapies in metastatic CRC (mCRC). Colorectal cancers exhibit certain mutations, which affect the tumor responsiveness to various treatment options. This article describes the role of targeted therapies in various well-established mutations.
Epidemiology
The most common tumor location is the proximal colon (42%), followed by the rectum (28%). More than 90% of patients with CRCs are aged > 50 years at diagnosis.
Women have a higher percentage of proximal tumors compared with men (46% vs 38%) and a lower percentage of rectal tumors (24% vs 31%).Among both sexes, incidence and mortality are highest in African Americans and lowest in Asian/Pacific Islanders. The estimated 5-year survival is slightly higher for rectal cancer (66.5%) than for colon cancer (64.2%), although the stage-specific survival is similar. The difference in 5-year overall survival (OS) is attributed to the higher percentage of rectal tumors diagnosed at a localized stage (44% vs 38%). Patients aged < 65 years have higher 5-year survival rates than do those aged 65 years (68.9% vs 62.0%).1
Risk Factors
Like most human cancers, multiple genetic and environmental factors are believed to play a role in the development of colorectal carcinomas, with environmental factors playing the dominant role.4
Environmental risk factors include a low-fiber diet,5 red and processed meat intake,6 a high-fat diet,7 smoking,8 heavy alcohol consumption,9 obesity,10 physical inactivity,11 alteration in intestinal flora,12 and chronic inflammation.13 Aspirin (at doses > 300 mg/d), nonsteroidal anti-inflammatory drugs, and folic acid are believed to protect against colon cancer.14
Some of the genetic factors involved in colorectal cancers include (1) the loss of tumor suppressor genes, such as APC (most common tumor suppressor mutation), p53, SMAD4 pathways, or TGF- ß pathways; (2) DNA mismatch repair defects: mutations in MLH1, MSH2 in hereditary nonpolyposis colon cancer or methylation of MLH1 in sporadic cases; and (3) CpG island methylation (CIMP pathway), methylation of MLH1, MINT1, MINT2, MINT3; and (4) activation of oncogenes such as RAS and BRAF.15
Pathogenesis
The colonic mucosa consists of epithelial cells arranged in cylindrical structures called crypts. The human colon contains about 10 millioncrypts. The cellular proliferation and migration in each of the crypts is believed to be tightly regulated, with the majority of cells arising from a small number of stem cells (around 4-6) at the bottom of the crypt, which migrate upward after division and are eventually shed into the lumen.16 Each crypt is renewed in 2 to 8 days, which makes colonic mucosa one of the organs with the most cell proliferation in the human body and, hence, a target for various genetic and epigenetic alterations as well as environmental mutagenesis.7
Traditionally, the majority of colorectal carcinomas were believed to evolve from adenomatous polyps, which transform into an advanced adenoma with high-grade
dysplasia and then progress to an invasive cancer often referred to as the adenoma-to-carcinoma sequence.15 However, 2 other pathways, alternative and serrated,
have been described, and CRCs are now regarded as complex malignancies with a wide array of genetic and epigenetic mutations.17
About 70% to 85% of CRCs generally develop from chromosomal instability resulting from inactivation of tumor suppressor genes (APC gene, p53, etc). About 15% of cases are attributed to the failure of the DNA mismatch repair system either by germline/somatic mutations or by epigenetic silencing of gene transcription by CpG island methylation.18 Mutation in the BRAF or K-ras oncogenes are also believed to promote carcinogenesis.15 All these changes are believed to give rise to a precursor microscopic mucosal lesion that precedes the development of macroscopic adenomas.17
Clinical Features
The clinical features of CRCs can be widely variable, from incidental findings during screening colonoscopy to intestinal obstruction. The most common clinical presentation is rectal bleeding, followed by weight loss, abdominal pain, constipation, or diarrhea.19 The likelihood of CRC is higher with the combination of rectal bleeding and weight loss as well as rectal bleeding and change in bowel habits. Other clinical features may include bloating, abdominal pain, or anemia.20 (See Table 1.)
Targeted Therapy in CRC
The targeted therapies in CRC include (1) the antivascular endothelial growth factor-A (anti–VEGF-A) antibody bevacizumab; (2) the VEGF-A, VEGF-B, and placental growth factor inhibitor aflibercept; (3) the multikinase inhibitor regorafenib; and (4) the anti-epidermal growth factor receptor (anti-EGFR) antibodies cetuximab and panitumumab.
Bevacizumab is an anti-VEGF monoclonal antibody. Vascular endothelial growth factor promotes angiogenesisnecessary for tumor growth. Bevacizumab was approved by the FDA in February 2004 as a first-line treatment in combination with IFL (irinotecan plus 5-fluorouracil [5-FU]/leucovorin) regimen and in June 2006 as a second-line treatment in combination with 5-FU–based chemotherapy for patients with mCRC. In January 2013, bevacizumab was approved for use in combination with fluoropyrimidine–irinotecan- or fluoropyrimidine–oxaliplatin-based chemotherapy for the treatment of patients with mCRC whose disease has progressed while on first-line treatment with a bevacizumab-containing regimen.21 (See Table 2.)
Aflibercept is a recombinant fusion protein, containing VEGF-binding portions from the extracellular domains of human VEGF receptors 1 and 2, fused to the Fc portion of human immunoglobulin IgG1 that blocks the activity of VEGF-A, VEGF-B, and placental growth factor by acting as a high-affinity ligand trap to prevent these ligands from binding to their endogenous receptors.22 It was approved by the FDA in August 2012 for use in combination with FOLFIRI (5-FU, leucovorin, irinotecan) for the treatment of patients with mCRC that is resistant to or has progressed following an oxaliplatin‑containing regimen.23
Cetuximab (a chimeric IgG1 anti-EGFR monoclonal antibody) and panitumumab (a human IgG2 anti-EGFR monoclonal antibody) have been shown to improve OS
and progression-free survival (PFS) in up to 20% of cases, either alone or in combination with chemotherapy.24,25 The FDA approved cetuximab in July 2012 for use in
combination with FOLFIRI for first-line treatment of patients with K-ras mutation-negative (wild-type), EGFRexpressing mCRC.26 The FDA approved panitumumab in
September 2006 for the treatment of patients with EGFRexpressing metastatic colorectal carcinoma with disease progression on or following FOLFOX (5-FU, leucovorin, oxaliplatin)/FOLFIRI.27 (See Table 3.)
KRAS, a member of the rat sarcoma virus (ras) gene family of oncogenes, encodes for a small G protein downstream of EGFR. KRAS is mutated in CRC in up to 37% cases, resulting in activation of the different downstream signaling pathways.15,28 Therefore, KRAS mutations predict resistance to anti-EGFR therapy.28,29 Testing for KRAS mutation prior to treatment with cetuximab or panitumumab leads to targeted us of the very costly monoclonal antibodies and hence is considered a cost-effective practice.30
Even in cases with wild-type KRAS mutation, response to anti-EGFR therapies is seen in only less than half of patients.31 Up to 17% of tumors with wild-type for KRAS exon 2 at codons 12 and 13 can have a mutation in another of the ras pathway genes (eg, KRAS exon 3, 4 and NRAS exon 2, 3, 4).25 Mutations in the BRAF oncogene have been described in up to 13% of colorectal carcinoma cases.15 The data to suggest a lack of antitumor activity from anti-EGFR therapies in the presence of BRAF V600E mutation are still limited, but BRAF mutation is considered a poor prognostic factor.
A recent trial involving 1,137 patients with KRAS exon 2 wild-type mCRC randomly assigned to cetuximab or bevacizumab with standard chemotherapy (FOLFOX or FOLFIRI) found OS of 29.9 vs 29.0 months and median PFS 10.4 vs 10.8 months for cetuximab and bevacizumab, respectively.32 The OS for 5-FU–based therapies was about 11 months.33
Regorafenib is an oral multikinase inhibitor of angiogenic, stromal, and oncogenic receptor protein kinases, including those involved in the regulation of tumor angiogenesis (eg, VEGFR1-3 and TIE2 [tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2]), tumor microenvironment (plateletderived growth factor receptor-β and fibroblast growth factor receptor 1), as well as tumor oncogenesis (KIT, RET, RAF1, BRAF, BRAF V600E).34 A randomized phase 3 study involving 760 patients with documented progressive mCRC found a higher median OS with regorafenib vs placebo (6.4 mo vs 5.0 mo, P = .0052) and a
higher PFS (2.0 mo vs 1.7 mo, P < .0001).35 The FDA approved regorafenib in September 2012 for the treatment of patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, with an anti-VEGF therapy and, if KRAS wild-type, with an anti-EGFR therapy.36
Conclusion
Targeted therapies in conjunction with newer chemotherapies have improved outcomes in metastatic colorectal carcinoma compared with those of conventional therapy (29 mo vs 11 mo).
Author disclosures
Peter T. Silberstein, MD, reports receiving payment for lectures from Bristol Myers and Celgene in the past. Drs. Khanal and Upadhyay report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the U.S.1-3 Only 40% of cases are diagnosed in a localized stage with an estimated 5-year survival of 90%, whereas 20% of cases present with metastatic disease with a 5-year survival of about 12.5%.1 With recent advances in cancer genetics and immunology as well as approval of targeted agents by the FDA, different treatment options are now available, even for progressive disease.
This article presents a brief review of CRC with a special focus on targeted therapies in metastatic CRC (mCRC). Colorectal cancers exhibit certain mutations, which affect the tumor responsiveness to various treatment options. This article describes the role of targeted therapies in various well-established mutations.
Epidemiology
The most common tumor location is the proximal colon (42%), followed by the rectum (28%). More than 90% of patients with CRCs are aged > 50 years at diagnosis.
Women have a higher percentage of proximal tumors compared with men (46% vs 38%) and a lower percentage of rectal tumors (24% vs 31%).Among both sexes, incidence and mortality are highest in African Americans and lowest in Asian/Pacific Islanders. The estimated 5-year survival is slightly higher for rectal cancer (66.5%) than for colon cancer (64.2%), although the stage-specific survival is similar. The difference in 5-year overall survival (OS) is attributed to the higher percentage of rectal tumors diagnosed at a localized stage (44% vs 38%). Patients aged < 65 years have higher 5-year survival rates than do those aged 65 years (68.9% vs 62.0%).1
Risk Factors
Like most human cancers, multiple genetic and environmental factors are believed to play a role in the development of colorectal carcinomas, with environmental factors playing the dominant role.4
Environmental risk factors include a low-fiber diet,5 red and processed meat intake,6 a high-fat diet,7 smoking,8 heavy alcohol consumption,9 obesity,10 physical inactivity,11 alteration in intestinal flora,12 and chronic inflammation.13 Aspirin (at doses > 300 mg/d), nonsteroidal anti-inflammatory drugs, and folic acid are believed to protect against colon cancer.14
Some of the genetic factors involved in colorectal cancers include (1) the loss of tumor suppressor genes, such as APC (most common tumor suppressor mutation), p53, SMAD4 pathways, or TGF- ß pathways; (2) DNA mismatch repair defects: mutations in MLH1, MSH2 in hereditary nonpolyposis colon cancer or methylation of MLH1 in sporadic cases; and (3) CpG island methylation (CIMP pathway), methylation of MLH1, MINT1, MINT2, MINT3; and (4) activation of oncogenes such as RAS and BRAF.15
Pathogenesis
The colonic mucosa consists of epithelial cells arranged in cylindrical structures called crypts. The human colon contains about 10 millioncrypts. The cellular proliferation and migration in each of the crypts is believed to be tightly regulated, with the majority of cells arising from a small number of stem cells (around 4-6) at the bottom of the crypt, which migrate upward after division and are eventually shed into the lumen.16 Each crypt is renewed in 2 to 8 days, which makes colonic mucosa one of the organs with the most cell proliferation in the human body and, hence, a target for various genetic and epigenetic alterations as well as environmental mutagenesis.7
Traditionally, the majority of colorectal carcinomas were believed to evolve from adenomatous polyps, which transform into an advanced adenoma with high-grade
dysplasia and then progress to an invasive cancer often referred to as the adenoma-to-carcinoma sequence.15 However, 2 other pathways, alternative and serrated,
have been described, and CRCs are now regarded as complex malignancies with a wide array of genetic and epigenetic mutations.17
About 70% to 85% of CRCs generally develop from chromosomal instability resulting from inactivation of tumor suppressor genes (APC gene, p53, etc). About 15% of cases are attributed to the failure of the DNA mismatch repair system either by germline/somatic mutations or by epigenetic silencing of gene transcription by CpG island methylation.18 Mutation in the BRAF or K-ras oncogenes are also believed to promote carcinogenesis.15 All these changes are believed to give rise to a precursor microscopic mucosal lesion that precedes the development of macroscopic adenomas.17
Clinical Features
The clinical features of CRCs can be widely variable, from incidental findings during screening colonoscopy to intestinal obstruction. The most common clinical presentation is rectal bleeding, followed by weight loss, abdominal pain, constipation, or diarrhea.19 The likelihood of CRC is higher with the combination of rectal bleeding and weight loss as well as rectal bleeding and change in bowel habits. Other clinical features may include bloating, abdominal pain, or anemia.20 (See Table 1.)
Targeted Therapy in CRC
The targeted therapies in CRC include (1) the antivascular endothelial growth factor-A (anti–VEGF-A) antibody bevacizumab; (2) the VEGF-A, VEGF-B, and placental growth factor inhibitor aflibercept; (3) the multikinase inhibitor regorafenib; and (4) the anti-epidermal growth factor receptor (anti-EGFR) antibodies cetuximab and panitumumab.
Bevacizumab is an anti-VEGF monoclonal antibody. Vascular endothelial growth factor promotes angiogenesisnecessary for tumor growth. Bevacizumab was approved by the FDA in February 2004 as a first-line treatment in combination with IFL (irinotecan plus 5-fluorouracil [5-FU]/leucovorin) regimen and in June 2006 as a second-line treatment in combination with 5-FU–based chemotherapy for patients with mCRC. In January 2013, bevacizumab was approved for use in combination with fluoropyrimidine–irinotecan- or fluoropyrimidine–oxaliplatin-based chemotherapy for the treatment of patients with mCRC whose disease has progressed while on first-line treatment with a bevacizumab-containing regimen.21 (See Table 2.)
Aflibercept is a recombinant fusion protein, containing VEGF-binding portions from the extracellular domains of human VEGF receptors 1 and 2, fused to the Fc portion of human immunoglobulin IgG1 that blocks the activity of VEGF-A, VEGF-B, and placental growth factor by acting as a high-affinity ligand trap to prevent these ligands from binding to their endogenous receptors.22 It was approved by the FDA in August 2012 for use in combination with FOLFIRI (5-FU, leucovorin, irinotecan) for the treatment of patients with mCRC that is resistant to or has progressed following an oxaliplatin‑containing regimen.23
Cetuximab (a chimeric IgG1 anti-EGFR monoclonal antibody) and panitumumab (a human IgG2 anti-EGFR monoclonal antibody) have been shown to improve OS
and progression-free survival (PFS) in up to 20% of cases, either alone or in combination with chemotherapy.24,25 The FDA approved cetuximab in July 2012 for use in
combination with FOLFIRI for first-line treatment of patients with K-ras mutation-negative (wild-type), EGFRexpressing mCRC.26 The FDA approved panitumumab in
September 2006 for the treatment of patients with EGFRexpressing metastatic colorectal carcinoma with disease progression on or following FOLFOX (5-FU, leucovorin, oxaliplatin)/FOLFIRI.27 (See Table 3.)
KRAS, a member of the rat sarcoma virus (ras) gene family of oncogenes, encodes for a small G protein downstream of EGFR. KRAS is mutated in CRC in up to 37% cases, resulting in activation of the different downstream signaling pathways.15,28 Therefore, KRAS mutations predict resistance to anti-EGFR therapy.28,29 Testing for KRAS mutation prior to treatment with cetuximab or panitumumab leads to targeted us of the very costly monoclonal antibodies and hence is considered a cost-effective practice.30
Even in cases with wild-type KRAS mutation, response to anti-EGFR therapies is seen in only less than half of patients.31 Up to 17% of tumors with wild-type for KRAS exon 2 at codons 12 and 13 can have a mutation in another of the ras pathway genes (eg, KRAS exon 3, 4 and NRAS exon 2, 3, 4).25 Mutations in the BRAF oncogene have been described in up to 13% of colorectal carcinoma cases.15 The data to suggest a lack of antitumor activity from anti-EGFR therapies in the presence of BRAF V600E mutation are still limited, but BRAF mutation is considered a poor prognostic factor.
A recent trial involving 1,137 patients with KRAS exon 2 wild-type mCRC randomly assigned to cetuximab or bevacizumab with standard chemotherapy (FOLFOX or FOLFIRI) found OS of 29.9 vs 29.0 months and median PFS 10.4 vs 10.8 months for cetuximab and bevacizumab, respectively.32 The OS for 5-FU–based therapies was about 11 months.33
Regorafenib is an oral multikinase inhibitor of angiogenic, stromal, and oncogenic receptor protein kinases, including those involved in the regulation of tumor angiogenesis (eg, VEGFR1-3 and TIE2 [tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2]), tumor microenvironment (plateletderived growth factor receptor-β and fibroblast growth factor receptor 1), as well as tumor oncogenesis (KIT, RET, RAF1, BRAF, BRAF V600E).34 A randomized phase 3 study involving 760 patients with documented progressive mCRC found a higher median OS with regorafenib vs placebo (6.4 mo vs 5.0 mo, P = .0052) and a
higher PFS (2.0 mo vs 1.7 mo, P < .0001).35 The FDA approved regorafenib in September 2012 for the treatment of patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, with an anti-VEGF therapy and, if KRAS wild-type, with an anti-EGFR therapy.36
Conclusion
Targeted therapies in conjunction with newer chemotherapies have improved outcomes in metastatic colorectal carcinoma compared with those of conventional therapy (29 mo vs 11 mo).
Author disclosures
Peter T. Silberstein, MD, reports receiving payment for lectures from Bristol Myers and Celgene in the past. Drs. Khanal and Upadhyay report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
2. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
4. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78-85.
5. Bingham SA, Day NE, Luben R, et al; European Prospective Investigation into Cancer and Nutrition. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361(9368):1496-1501.
6. Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4(2):177-184.
7. Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis—update and perspectives. World J Gastroenterol. 2014;20(48):18151-18164.
8. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
9. Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958-1972.
10. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916.
11. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204-213.
12. Candela M, Turroni S, Biagi E, et al. Inflammation and colorectal cancer, when
microbiota-host mutualism breaks. World J Gastroenterol. 2014;20(4):908-922.
13. Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(4):405-410.
14. Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33-46.
15. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of
colorectal cancer. N Engl J Med. 2009;361(25):2449-2460.
16. Zhao R, Michor F. Patterns of proliferative activity in the colonic crypt determine
crypt stability and rates of somatic evolution. PLoS Comput Biol. 2013;9(6):e1003082.
17. Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple
pathways in colorectal cancer progression. Pathol Res Int. 2012;2012:509348.
18. Worthley DL, Whitehall VL, Spring KJ, Leggett BA. Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol. 2007;13(28):3784-3791.
19. Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399-405.
20. Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61(586):e231-e243.
21. National Cancer Institute. FDA approval for bevacizumab: first-line treatment of metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab#Anchor-Approva-23287. Updated December 4, 2014. Accessed July 6, 2015.
22. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatinbased regimen. J Clin Oncol. 2012;30(28):3499-3506.
23. U.S. Food and Drug Administration. Ziv-Aflibercept. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm314438.htm. Updated August 3, 2012. Accessed July 6, 2015.
24. Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019.
25. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
26. U.S. Food and Drug Administration. Cetuximab in combination with folfiri/therascreen. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm310933.htm. Updated July 9, 2012. Accessed July 6, 2015.
27. Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary:
panitumumab (Vectibix). Oncologist. 2007;12(5):577-583.
28. Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: antiepidermal
growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154(1):37-49.
29. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
30. Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40-49.
31. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncology. 2009;27(12):2091-2096.
32. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance); SWOG; ECOG. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(15)(suppl):Abstract LBA3.
33. Poon MA, O’Connell MJ, Moertel CG, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7(10):1407-1418.
34. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245-255.
35. Grothey A, Van Cutsem E, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312.
36. National Cancer Institute. FDA approval for regorafenib: previously treated metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-regorafenib#Anchor-MCRC. Updated July 3, 2013. Accessed July 6, 2015.
37. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Updated October 3, 2014. Accessed January 25, 2015.
38. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: rectal cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Updated December 9, 2014. Accessed January 25, 2015.
39. Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408-3419.
40. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342.
41. Giantonio BJ, Catalano PJ, Meropol NJ, et al; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544.
42. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013-2019.
43. Van Cutsem E, Rivera F, Berry S, et al; First BEAT investigators. Safety and efficacy of
first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842-1847.
1. Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
2. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
4. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78-85.
5. Bingham SA, Day NE, Luben R, et al; European Prospective Investigation into Cancer and Nutrition. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361(9368):1496-1501.
6. Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4(2):177-184.
7. Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis—update and perspectives. World J Gastroenterol. 2014;20(48):18151-18164.
8. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
9. Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958-1972.
10. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916.
11. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204-213.
12. Candela M, Turroni S, Biagi E, et al. Inflammation and colorectal cancer, when
microbiota-host mutualism breaks. World J Gastroenterol. 2014;20(4):908-922.
13. Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(4):405-410.
14. Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33-46.
15. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of
colorectal cancer. N Engl J Med. 2009;361(25):2449-2460.
16. Zhao R, Michor F. Patterns of proliferative activity in the colonic crypt determine
crypt stability and rates of somatic evolution. PLoS Comput Biol. 2013;9(6):e1003082.
17. Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple
pathways in colorectal cancer progression. Pathol Res Int. 2012;2012:509348.
18. Worthley DL, Whitehall VL, Spring KJ, Leggett BA. Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol. 2007;13(28):3784-3791.
19. Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399-405.
20. Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61(586):e231-e243.
21. National Cancer Institute. FDA approval for bevacizumab: first-line treatment of metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab#Anchor-Approva-23287. Updated December 4, 2014. Accessed July 6, 2015.
22. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatinbased regimen. J Clin Oncol. 2012;30(28):3499-3506.
23. U.S. Food and Drug Administration. Ziv-Aflibercept. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm314438.htm. Updated August 3, 2012. Accessed July 6, 2015.
24. Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019.
25. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034.
26. U.S. Food and Drug Administration. Cetuximab in combination with folfiri/therascreen. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm310933.htm. Updated July 9, 2012. Accessed July 6, 2015.
27. Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary:
panitumumab (Vectibix). Oncologist. 2007;12(5):577-583.
28. Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: antiepidermal
growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154(1):37-49.
29. Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374-379.
30. Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40-49.
31. Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncology. 2009;27(12):2091-2096.
32. Venook AP, Niedzwiecki D, Lenz H-J, et al; Cancer and Leukemia Group B (Alliance); SWOG; ECOG. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(15)(suppl):Abstract LBA3.
33. Poon MA, O’Connell MJ, Moertel CG, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7(10):1407-1418.
34. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245-255.
35. Grothey A, Van Cutsem E, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312.
36. National Cancer Institute. FDA approval for regorafenib: previously treated metastatic colorectal cancer. National Cancer Institute Website. http://www.cancer.gov/cancertopics/druginfo/fda-regorafenib#Anchor-MCRC. Updated July 3, 2013. Accessed July 6, 2015.
37. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Updated October 3, 2014. Accessed January 25, 2015.
38. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: rectal cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Updated December 9, 2014. Accessed January 25, 2015.
39. Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408-3419.
40. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342.
41. Giantonio BJ, Catalano PJ, Meropol NJ, et al; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544.
42. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013-2019.
43. Van Cutsem E, Rivera F, Berry S, et al; First BEAT investigators. Safety and efficacy of
first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842-1847.
Modifiable risk factors foretell colonic anastomotic leak
CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.
CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.

CHICAGO – Several modifiable risk factors predicted the development of anastomotic leak following elective colon resection, a large national analysis found.
“Preoperative smoking cessation, preoperative administration of oral antibiotic bowel preparation, and laparoscopic approach are modifiable factors that could reduce the risk of anastomotic leak,” Dr. Cindy Wu said at the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) National Conference.
Anastomotic leakage results in increased morbidity and mortality, yet the current literature analyzing risk factors for this complication is generally limited to retrospective studies of single institutions, she said.
To examine data from a larger sample of colectomy patients from multiple centers, the investigators used the NSQIP Participant Use Data File specifically targeted to colectomy to identify 14,848 patients who underwent elective colon resection from 2012 to 2013. Chi-square, Wald chi-square, and logistic regression analyses were performed examining patient factors (sex, race, comorbidities, smoking status, American Society of Anesthesiologists class, functional status, steroid use, and preoperative albumin), oncologic factors (chemotherapy, tumor stage, and presence or absence of disseminated cancer), and operative factors (wound class, mechanical bowel preparation, oral antibiotic preparation, surgical approach, colectomy site, surgical indication, and operative time).
In all, 3.4%, or 498 patients, experienced an anastomotic leak, which is consistent with the literature, Dr. Wu of Temple University in Philadelphia said. Of these patients, 101 required no intervention, while 272 required surgery and 125 needed percutaneous drainage. The mean age of the patients was 60.7 years and 57% were male.
In a univariate analysis, male sex (chi-square = 17.4; P less than .01), diabetes controlled with either oral medication or insulin (X2 = 9.5; P less than .01), and smoking within the last year (X2 = 20.4; P less than .01) were associated with a greater incidence of anastomotic leak.
Other risk factors that were significant in additional univariate analysis were ASA class (X2 = 23.3; P = .0001), functional status (X2 = 9.15; P = .01), 10% weight loss over the last 6 months (X2 = 5.83; P = .02), wound class (X2 = 10.8; P = .01), mechanical bowel preparation (X2 = 5.89; P = .01), lack of oral antibiotic preparation (X2 = 17.5; P less than .0001), open vs. laparoscopic/minimally invasive surgery (X2 = 60.0; P less than .0001), chemotherapy in the last 90 days (X2 = 23.1; P less than .0001), and presence of disseminated cancer (X2 = 7.41; P = .01), Dr. Wu said.
With all of these factors taken into account in multivariate analysis, independent predictors of an increased risk of anastomotic leak were male sex (odds ratio, 1.74; P = .01), tobacco use (OR, 1.73; P = .03), and lack of a preoperative oral antibiotic bowel preparation (OR, 1.79; P less than .01).
Interestingly, use of a laparoscopic technique was protective against the development of anastomotic leakage (OR, 0.54; P less than .01), she said.
The authors reported having no relevant financial disclosures.

AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: Altering specific patient and operative factors can modify the risk of anastomotic leakage after colectomy.
Major finding: Male sex (OR, 1.74), tobacco use (OR, 1.73), and lack of an oral antibiotic bowel preparation (OR, 1.79) predicted anastomotic leak.
Data source: A retrospective study of 14,848 elective colectomies.
Disclosures: The authors reported having no relevant financial disclosures.
Intestinal obstruction risk increased in some childhood cancer survivors
Childhood cancer survivors are at an increased risk for developing an intestinal obstruction requiring surgery (IOS) 5 or more years after the initial cancer diagnosis, according to a study based on data from the Childhood Cancer Survivor Study.
The risk was greater among those who had a pelvic or abdominal tumor and had been exposed to pelvic or abdominal radiotherapy, reported the authors, who pointed out that no study has “rigorously” investigated the incidence of intestinal obstruction in childhood cancer survivors. The study appeared online in the Journal of Clinical Oncology (2015 Aug 10 doi: 10.1200/JCO.2015.61.5070).
The subjects in the study had been diagnosed with cancer before age 21 years between 1970 and 1986 and were followed longitudinally in the CCSS. The sample included 12,316 childhood cancer survivors who had survived at least 5 years from the time they were diagnosed with cancer and 4,023 of their siblings.
The cumulative incidence of “late” IOS (occurring at least 5 years after the cancer diagnosis) was 5.8% among those who had an abdominopelvic tumor and 1% among those who had other types of cancer, compared with 0.3% among siblings, who served as controls in the study. After adjusting for year of diagnosis, age at diagnosis, cancer type, radiotherapy, surgery, and other confounding factors, the risk of late IOS was significantly increased among those who had an abdominopelvic tumor (3.6 times greater) and those who had received abdominal/pelvic radiotherapy (2.4 times greater). Mortality was also almost twofold higher among those who developed late IOS when adjusted for the same factors, but there was no association with chemotherapy, cyclophosphamide equivalent dose, or platinum agent score and late IOS.
“The risk of IOS extends for decades beyond cancer diagnosis, implying the need for long-term vigilance, especially among survivors with abdominal or pelvic tumors and survivors who have undergone treatment with abdominal or pelvic surgery or radiotherapy,” concluded lead author Dr. Arin Madenci of Boston Children’s Hospital and his coauthors.
“Widespread awareness of the signs and symptoms of IOS will facilitate timely presentation and effective management of this complication. Although prevention of IOS is not currently possible, education of survivors of cancer, their families, and their health care providers is critical,” they added.
The study was supported by grants from the National Cancer Institute and Cancer Center Support (Centers of Research Excellence) and by the American Lebanese Syrian Associated Charities. Dr. Madenci and nine other authors had no disclosures. The remaining three authors had disclosures that included receiving honoraria, travel, and expenses from Sandoz, holding stock or other ownership in Pfizer and Novartis, serving as a consultant or advisor to United Therapeutics, and having an immediate family member with stock or other ownership in several pharmaceutical companies.
Childhood cancer survivors are at an increased risk for developing an intestinal obstruction requiring surgery (IOS) 5 or more years after the initial cancer diagnosis, according to a study based on data from the Childhood Cancer Survivor Study.
The risk was greater among those who had a pelvic or abdominal tumor and had been exposed to pelvic or abdominal radiotherapy, reported the authors, who pointed out that no study has “rigorously” investigated the incidence of intestinal obstruction in childhood cancer survivors. The study appeared online in the Journal of Clinical Oncology (2015 Aug 10 doi: 10.1200/JCO.2015.61.5070).
The subjects in the study had been diagnosed with cancer before age 21 years between 1970 and 1986 and were followed longitudinally in the CCSS. The sample included 12,316 childhood cancer survivors who had survived at least 5 years from the time they were diagnosed with cancer and 4,023 of their siblings.
The cumulative incidence of “late” IOS (occurring at least 5 years after the cancer diagnosis) was 5.8% among those who had an abdominopelvic tumor and 1% among those who had other types of cancer, compared with 0.3% among siblings, who served as controls in the study. After adjusting for year of diagnosis, age at diagnosis, cancer type, radiotherapy, surgery, and other confounding factors, the risk of late IOS was significantly increased among those who had an abdominopelvic tumor (3.6 times greater) and those who had received abdominal/pelvic radiotherapy (2.4 times greater). Mortality was also almost twofold higher among those who developed late IOS when adjusted for the same factors, but there was no association with chemotherapy, cyclophosphamide equivalent dose, or platinum agent score and late IOS.
“The risk of IOS extends for decades beyond cancer diagnosis, implying the need for long-term vigilance, especially among survivors with abdominal or pelvic tumors and survivors who have undergone treatment with abdominal or pelvic surgery or radiotherapy,” concluded lead author Dr. Arin Madenci of Boston Children’s Hospital and his coauthors.
“Widespread awareness of the signs and symptoms of IOS will facilitate timely presentation and effective management of this complication. Although prevention of IOS is not currently possible, education of survivors of cancer, their families, and their health care providers is critical,” they added.
The study was supported by grants from the National Cancer Institute and Cancer Center Support (Centers of Research Excellence) and by the American Lebanese Syrian Associated Charities. Dr. Madenci and nine other authors had no disclosures. The remaining three authors had disclosures that included receiving honoraria, travel, and expenses from Sandoz, holding stock or other ownership in Pfizer and Novartis, serving as a consultant or advisor to United Therapeutics, and having an immediate family member with stock or other ownership in several pharmaceutical companies.
Childhood cancer survivors are at an increased risk for developing an intestinal obstruction requiring surgery (IOS) 5 or more years after the initial cancer diagnosis, according to a study based on data from the Childhood Cancer Survivor Study.
The risk was greater among those who had a pelvic or abdominal tumor and had been exposed to pelvic or abdominal radiotherapy, reported the authors, who pointed out that no study has “rigorously” investigated the incidence of intestinal obstruction in childhood cancer survivors. The study appeared online in the Journal of Clinical Oncology (2015 Aug 10 doi: 10.1200/JCO.2015.61.5070).
The subjects in the study had been diagnosed with cancer before age 21 years between 1970 and 1986 and were followed longitudinally in the CCSS. The sample included 12,316 childhood cancer survivors who had survived at least 5 years from the time they were diagnosed with cancer and 4,023 of their siblings.
The cumulative incidence of “late” IOS (occurring at least 5 years after the cancer diagnosis) was 5.8% among those who had an abdominopelvic tumor and 1% among those who had other types of cancer, compared with 0.3% among siblings, who served as controls in the study. After adjusting for year of diagnosis, age at diagnosis, cancer type, radiotherapy, surgery, and other confounding factors, the risk of late IOS was significantly increased among those who had an abdominopelvic tumor (3.6 times greater) and those who had received abdominal/pelvic radiotherapy (2.4 times greater). Mortality was also almost twofold higher among those who developed late IOS when adjusted for the same factors, but there was no association with chemotherapy, cyclophosphamide equivalent dose, or platinum agent score and late IOS.
“The risk of IOS extends for decades beyond cancer diagnosis, implying the need for long-term vigilance, especially among survivors with abdominal or pelvic tumors and survivors who have undergone treatment with abdominal or pelvic surgery or radiotherapy,” concluded lead author Dr. Arin Madenci of Boston Children’s Hospital and his coauthors.
“Widespread awareness of the signs and symptoms of IOS will facilitate timely presentation and effective management of this complication. Although prevention of IOS is not currently possible, education of survivors of cancer, their families, and their health care providers is critical,” they added.
The study was supported by grants from the National Cancer Institute and Cancer Center Support (Centers of Research Excellence) and by the American Lebanese Syrian Associated Charities. Dr. Madenci and nine other authors had no disclosures. The remaining three authors had disclosures that included receiving honoraria, travel, and expenses from Sandoz, holding stock or other ownership in Pfizer and Novartis, serving as a consultant or advisor to United Therapeutics, and having an immediate family member with stock or other ownership in several pharmaceutical companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Some childhood cancer survivors are at a significantly increased risk of developing an intestinal obstruction requiring surgery (IOS), which should be considered in their long-term follow-up.
Major finding: The cumulative incidence of IOS occurring at least 5 years after the cancer diagnosis was 5.8% among those who had an abdominopelvic tumor and 1% among those who did not have an abdominopelvic tumor, compared with 0.3% among sibling controls in the study, and late IOS was associated with increased mortality.
Data source: A retrospective cohort study involving 12,316 childhood cancer survivors who had survived at least 5 years and 4,023 of their siblings, who served as controls, from the Childhood Cancer Survivor Study.
Disclosures: The study was supported by National Cancer Institute and Cancer Center Support (Centers of Research Excellence) grants, and by the American Lebanese Syrian Associated Charities. Dr. Madenci and nine other authors had no disclosures. The remaining three authors had disclosures that included receiving honoraria, travel, and expenses from Sandoz, holding stock or other ownership in Pfizer and Novartis, serving as a consultant or advisor to United Therapeutics, and having an immediate family member with stock or other ownership in several pharmaceutical companies.
Are oral antibiotics enough to reduce colorectal surgery infections?
CHICAGO – Oral antibiotics alone or in combination with mechanical bowel preparation were independently associated with reduced surgical site infections after elective colorectal resection in a large national patient sample.
Oral antibiotics (OA) alone significantly reduced the rate of any surgical site infection (SSI) by 44% (odds ratio, 0.56; 95% confidence interval, 0.36-0.87) and wound SSI by 59% (OR, 0.41; 95% CI, 0.23-0.72), compared with no bowel preparation in propensity-adjusted multivariate analysis.
OA combined with mechanical bowel preparation (MBP) was independently associated with significant reductions of 54%, 58%, and 41%, respectively, for any SSI (OR, 0.46; 95% CI, 0.38-0.55), wound SSI (OR, 0.42; 95% CI, 0.33-0.53), and organ space SSI (OR, 0.59; 95% CI, 0.44-0.78).
In contrast, MBP, which was used in 40.8% of cases, was not independently associated with reduced rates of any SSI (OR, 0.95; 95% CI, 0.82-1.10), wound SSI (OR, 0.91; 95% CI, 0.76-1.09), or organ space SSI (OR, 1.0; 95% CI, 0.79-1.27), according to Dr. Sarah Koller of Temple University Hospital in Philadelphia and her associates.
A limitation of the study was the lack of information on type of OA or MBP used, patient compliance, and use of parenteral antibiotic prophylaxis.
“Randomized clinical trials are needed to determine the true benefits of oral antibiotics alone versus combined oral antibiotics and mechanical bowel prep prior to elective colorectal resection,” Dr. Koller said at the American College of Surgeons/National Surgery Quality Improvement Program National Conference.
Session comoderator Dr. E. Patchen Dellinger, of the University of Washington in Seattle, commented, “Logically, it’s hard for me to believe that oral antibiotics would affect a couple of kilograms of stool in the colon and yet here are these tantalizing data. So we do need the prospective trial you mention.
“But, the other thing that blows my mind every time I see these data is 49% of people getting a mechanical bowel prep without oral antibiotics, which has conclusively been shown to be useless for anything but torture of the patient.”
Significant variability in the use of bowel preparation exists within the surgical community, with a recent survey of colorectal surgeons revealing that 76% routinely used MBP and only 36% routinely used oral antibiotics, Dr. Koller observed.
In the current analysis, just 3.3% of patients received OA, 30.4% OA plus MBP, 40.8% MBP, and 25.5% no bowel preparation.
Physicians have been slow to abandon MBP, despite multiple studies showing that MBP alone does not reduce SSIs in elective colon and rectal surgery. There also have been reports of higher rates of anastomotic leak, increased cardiac or metabolic complications, and a slower return of bowel function with MBP.
Several studies, including a recent Cochrane Database Review, have shown that oral or intravenous antibiotic prophylaxis reduces surgical wound infection after colorectal surgery. The comparison groups are not uniform across the studies, however, and the controversy persists as to which type of bowel prep best reduces SSI after colorectal surgery, she said.
To explore this issue, the investigators identified all patients who underwent elective, nonemergent colorectal resections in both the ACS NSQIP Participant Use Data File (PUF) and the Procedure Targeted PUF for colectomy from 2012 to 2013. The cohort included 19,372 patients with complete preoperative bowel preparation data. Patients who were ventilator dependent or had infections or open wounds at the time of surgery were excluded.
The overall rates of any SSI, wound SSI (superficial and/or deep), and organ space SSI were 9.5%, 6.4%, and 3.5%, respectively.
With regard to adverse outcomes cited in previous studies, only OA plus MBP was shown to independently reduce anastomotic leak (OR, 0.57; 95% CI, 0.42-0.78) and postoperative ileus (OR, 0.79; 95% CI, 0.68-0.92), compared with no bowel prep, Dr. Koller reported.
Both OA and OA plus MBP, however, decreased length of stay by 0.83 days.
None of the bowel preparations were independently associated with increased rates of cardiac or renal complications, she said.
The investigators were not able to track rates of Clostridium difficile colitis after administration of the oral antibiotics. Dr. Koller acknowledged this is a concern when using oral antibiotics and may contribute to why they aren’t used frequently.
Dr. Koller reported having no conflicts of interest. A coauthor disclosed consulting for Intuitive Surgical.
CHICAGO – Oral antibiotics alone or in combination with mechanical bowel preparation were independently associated with reduced surgical site infections after elective colorectal resection in a large national patient sample.
Oral antibiotics (OA) alone significantly reduced the rate of any surgical site infection (SSI) by 44% (odds ratio, 0.56; 95% confidence interval, 0.36-0.87) and wound SSI by 59% (OR, 0.41; 95% CI, 0.23-0.72), compared with no bowel preparation in propensity-adjusted multivariate analysis.
OA combined with mechanical bowel preparation (MBP) was independently associated with significant reductions of 54%, 58%, and 41%, respectively, for any SSI (OR, 0.46; 95% CI, 0.38-0.55), wound SSI (OR, 0.42; 95% CI, 0.33-0.53), and organ space SSI (OR, 0.59; 95% CI, 0.44-0.78).
In contrast, MBP, which was used in 40.8% of cases, was not independently associated with reduced rates of any SSI (OR, 0.95; 95% CI, 0.82-1.10), wound SSI (OR, 0.91; 95% CI, 0.76-1.09), or organ space SSI (OR, 1.0; 95% CI, 0.79-1.27), according to Dr. Sarah Koller of Temple University Hospital in Philadelphia and her associates.
A limitation of the study was the lack of information on type of OA or MBP used, patient compliance, and use of parenteral antibiotic prophylaxis.
“Randomized clinical trials are needed to determine the true benefits of oral antibiotics alone versus combined oral antibiotics and mechanical bowel prep prior to elective colorectal resection,” Dr. Koller said at the American College of Surgeons/National Surgery Quality Improvement Program National Conference.
Session comoderator Dr. E. Patchen Dellinger, of the University of Washington in Seattle, commented, “Logically, it’s hard for me to believe that oral antibiotics would affect a couple of kilograms of stool in the colon and yet here are these tantalizing data. So we do need the prospective trial you mention.
“But, the other thing that blows my mind every time I see these data is 49% of people getting a mechanical bowel prep without oral antibiotics, which has conclusively been shown to be useless for anything but torture of the patient.”
Significant variability in the use of bowel preparation exists within the surgical community, with a recent survey of colorectal surgeons revealing that 76% routinely used MBP and only 36% routinely used oral antibiotics, Dr. Koller observed.
In the current analysis, just 3.3% of patients received OA, 30.4% OA plus MBP, 40.8% MBP, and 25.5% no bowel preparation.
Physicians have been slow to abandon MBP, despite multiple studies showing that MBP alone does not reduce SSIs in elective colon and rectal surgery. There also have been reports of higher rates of anastomotic leak, increased cardiac or metabolic complications, and a slower return of bowel function with MBP.
Several studies, including a recent Cochrane Database Review, have shown that oral or intravenous antibiotic prophylaxis reduces surgical wound infection after colorectal surgery. The comparison groups are not uniform across the studies, however, and the controversy persists as to which type of bowel prep best reduces SSI after colorectal surgery, she said.
To explore this issue, the investigators identified all patients who underwent elective, nonemergent colorectal resections in both the ACS NSQIP Participant Use Data File (PUF) and the Procedure Targeted PUF for colectomy from 2012 to 2013. The cohort included 19,372 patients with complete preoperative bowel preparation data. Patients who were ventilator dependent or had infections or open wounds at the time of surgery were excluded.
The overall rates of any SSI, wound SSI (superficial and/or deep), and organ space SSI were 9.5%, 6.4%, and 3.5%, respectively.
With regard to adverse outcomes cited in previous studies, only OA plus MBP was shown to independently reduce anastomotic leak (OR, 0.57; 95% CI, 0.42-0.78) and postoperative ileus (OR, 0.79; 95% CI, 0.68-0.92), compared with no bowel prep, Dr. Koller reported.
Both OA and OA plus MBP, however, decreased length of stay by 0.83 days.
None of the bowel preparations were independently associated with increased rates of cardiac or renal complications, she said.
The investigators were not able to track rates of Clostridium difficile colitis after administration of the oral antibiotics. Dr. Koller acknowledged this is a concern when using oral antibiotics and may contribute to why they aren’t used frequently.
Dr. Koller reported having no conflicts of interest. A coauthor disclosed consulting for Intuitive Surgical.
CHICAGO – Oral antibiotics alone or in combination with mechanical bowel preparation were independently associated with reduced surgical site infections after elective colorectal resection in a large national patient sample.
Oral antibiotics (OA) alone significantly reduced the rate of any surgical site infection (SSI) by 44% (odds ratio, 0.56; 95% confidence interval, 0.36-0.87) and wound SSI by 59% (OR, 0.41; 95% CI, 0.23-0.72), compared with no bowel preparation in propensity-adjusted multivariate analysis.
OA combined with mechanical bowel preparation (MBP) was independently associated with significant reductions of 54%, 58%, and 41%, respectively, for any SSI (OR, 0.46; 95% CI, 0.38-0.55), wound SSI (OR, 0.42; 95% CI, 0.33-0.53), and organ space SSI (OR, 0.59; 95% CI, 0.44-0.78).
In contrast, MBP, which was used in 40.8% of cases, was not independently associated with reduced rates of any SSI (OR, 0.95; 95% CI, 0.82-1.10), wound SSI (OR, 0.91; 95% CI, 0.76-1.09), or organ space SSI (OR, 1.0; 95% CI, 0.79-1.27), according to Dr. Sarah Koller of Temple University Hospital in Philadelphia and her associates.
A limitation of the study was the lack of information on type of OA or MBP used, patient compliance, and use of parenteral antibiotic prophylaxis.
“Randomized clinical trials are needed to determine the true benefits of oral antibiotics alone versus combined oral antibiotics and mechanical bowel prep prior to elective colorectal resection,” Dr. Koller said at the American College of Surgeons/National Surgery Quality Improvement Program National Conference.
Session comoderator Dr. E. Patchen Dellinger, of the University of Washington in Seattle, commented, “Logically, it’s hard for me to believe that oral antibiotics would affect a couple of kilograms of stool in the colon and yet here are these tantalizing data. So we do need the prospective trial you mention.
“But, the other thing that blows my mind every time I see these data is 49% of people getting a mechanical bowel prep without oral antibiotics, which has conclusively been shown to be useless for anything but torture of the patient.”
Significant variability in the use of bowel preparation exists within the surgical community, with a recent survey of colorectal surgeons revealing that 76% routinely used MBP and only 36% routinely used oral antibiotics, Dr. Koller observed.
In the current analysis, just 3.3% of patients received OA, 30.4% OA plus MBP, 40.8% MBP, and 25.5% no bowel preparation.
Physicians have been slow to abandon MBP, despite multiple studies showing that MBP alone does not reduce SSIs in elective colon and rectal surgery. There also have been reports of higher rates of anastomotic leak, increased cardiac or metabolic complications, and a slower return of bowel function with MBP.
Several studies, including a recent Cochrane Database Review, have shown that oral or intravenous antibiotic prophylaxis reduces surgical wound infection after colorectal surgery. The comparison groups are not uniform across the studies, however, and the controversy persists as to which type of bowel prep best reduces SSI after colorectal surgery, she said.
To explore this issue, the investigators identified all patients who underwent elective, nonemergent colorectal resections in both the ACS NSQIP Participant Use Data File (PUF) and the Procedure Targeted PUF for colectomy from 2012 to 2013. The cohort included 19,372 patients with complete preoperative bowel preparation data. Patients who were ventilator dependent or had infections or open wounds at the time of surgery were excluded.
The overall rates of any SSI, wound SSI (superficial and/or deep), and organ space SSI were 9.5%, 6.4%, and 3.5%, respectively.
With regard to adverse outcomes cited in previous studies, only OA plus MBP was shown to independently reduce anastomotic leak (OR, 0.57; 95% CI, 0.42-0.78) and postoperative ileus (OR, 0.79; 95% CI, 0.68-0.92), compared with no bowel prep, Dr. Koller reported.
Both OA and OA plus MBP, however, decreased length of stay by 0.83 days.
None of the bowel preparations were independently associated with increased rates of cardiac or renal complications, she said.
The investigators were not able to track rates of Clostridium difficile colitis after administration of the oral antibiotics. Dr. Koller acknowledged this is a concern when using oral antibiotics and may contribute to why they aren’t used frequently.
Dr. Koller reported having no conflicts of interest. A coauthor disclosed consulting for Intuitive Surgical.
AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: Oral antibiotics alone or in combination with mechanical bowel preparation were independently associated with reduced surgical site infections after elective colorectal resection.
Major finding: Oral antibiotics alone significantly reduced the rate of any surgical site infection by 44% and wound SSI by 59%.
Data source: Data from 19,372 patients who underwent elective, nonemergent colorectal resections in both the ACS NSQIP Participant Use Data File (PUF) and the Procedure Targeted PUF for colectomy from 2012 to 2013.
Disclosures: Dr. Koller reported having no conflicts of interest. A coauthor disclosed consulting for Intuitive Surgical.
Elderly LARC patients had similar nCRT outcomes to younger patients
Clinical outcomes of elderly patients treated with neoadjuvant chemoradiation (nCRT) for locally advanced rectal cancer (LARC) were similar enough to outcomes of younger patients that doctors may want to reconsider using age alone for determining eligibility for nCRT and surgery, investigators reported online in the Annals of Oncology.
Dr. D. M. Jiang of the University of Ottawa (Ont.) and associates collected data from 1,172 patients with LARC who received nCRT and curative intent surgery, 25% (n = 295) of whom were 70 years old or older, from five major Canadian cancer centers between 2005 and 2012. When compared with younger patients, elderly patients were less likely to receive adjuvant chemotherapy (ACT) (60% vs. 79%; P less than .0001), oxaliplatin-based ACT (12% vs. 31%; P less than .0001), less likely to complete nCT (76% vs. 86%; P less than .001), and more likely to be anemic at initiation of nCRT (42% vs. 30%; P = .0004).
The investigators found that increasing age was not predictive of disease-free survival (hazard ratio, 1.00; 95% confidence interval, 0.99-1.02; P = .49) or cancer-specific survival (HR, 1.002; 95% CI, 0.98-1.02; P = .88) among LARC patients; however, advanced age correlated with an inferior overall survival (HR, 1.02; 95% CI, 1.00-1.03; P = .04).
Read the full article here.
Clinical outcomes of elderly patients treated with neoadjuvant chemoradiation (nCRT) for locally advanced rectal cancer (LARC) were similar enough to outcomes of younger patients that doctors may want to reconsider using age alone for determining eligibility for nCRT and surgery, investigators reported online in the Annals of Oncology.
Dr. D. M. Jiang of the University of Ottawa (Ont.) and associates collected data from 1,172 patients with LARC who received nCRT and curative intent surgery, 25% (n = 295) of whom were 70 years old or older, from five major Canadian cancer centers between 2005 and 2012. When compared with younger patients, elderly patients were less likely to receive adjuvant chemotherapy (ACT) (60% vs. 79%; P less than .0001), oxaliplatin-based ACT (12% vs. 31%; P less than .0001), less likely to complete nCT (76% vs. 86%; P less than .001), and more likely to be anemic at initiation of nCRT (42% vs. 30%; P = .0004).
The investigators found that increasing age was not predictive of disease-free survival (hazard ratio, 1.00; 95% confidence interval, 0.99-1.02; P = .49) or cancer-specific survival (HR, 1.002; 95% CI, 0.98-1.02; P = .88) among LARC patients; however, advanced age correlated with an inferior overall survival (HR, 1.02; 95% CI, 1.00-1.03; P = .04).
Read the full article here.
Clinical outcomes of elderly patients treated with neoadjuvant chemoradiation (nCRT) for locally advanced rectal cancer (LARC) were similar enough to outcomes of younger patients that doctors may want to reconsider using age alone for determining eligibility for nCRT and surgery, investigators reported online in the Annals of Oncology.
Dr. D. M. Jiang of the University of Ottawa (Ont.) and associates collected data from 1,172 patients with LARC who received nCRT and curative intent surgery, 25% (n = 295) of whom were 70 years old or older, from five major Canadian cancer centers between 2005 and 2012. When compared with younger patients, elderly patients were less likely to receive adjuvant chemotherapy (ACT) (60% vs. 79%; P less than .0001), oxaliplatin-based ACT (12% vs. 31%; P less than .0001), less likely to complete nCT (76% vs. 86%; P less than .001), and more likely to be anemic at initiation of nCRT (42% vs. 30%; P = .0004).
The investigators found that increasing age was not predictive of disease-free survival (hazard ratio, 1.00; 95% confidence interval, 0.99-1.02; P = .49) or cancer-specific survival (HR, 1.002; 95% CI, 0.98-1.02; P = .88) among LARC patients; however, advanced age correlated with an inferior overall survival (HR, 1.02; 95% CI, 1.00-1.03; P = .04).
Read the full article here.
ERAS protocol cuts colorectal surgery morbidity, SSIs
CHICAGO – Implementing an enhanced recovery after surgery (ERAS) protocol at Canada’s second largest hospital significantly reduced morbidity and surgical site infections after elective colorectal surgery.
Rates of postoperative morbidity declined 48.7% from 27.3% before implementation to 14% after full ERAS implementation (P less than .05), while total surgical site infections fell 45% (20.2% vs. 11%; P less than .05).
Nonsignificant reductions were also seen in superficial surgical site infections (11.1% vs. 7.3%), deep SSIs (2% vs. 0.6%), and organ space SSIs (7.1% vs. 3.4%).
“Our results illustrate that using a multidisciplinary team, with attention to details and small multiple changes, aggregation of marginal gains can result in dramatic improvements in patient outcomes,” primary author Tracey Hong, R.N., said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
The ERAS protocol was implemented at Vancouver General Hospital, after ACS NSQIP risk-adjusted reports showed the 743-bed hospital had a high odds ratio of 1.50 for colorectal operative mortality.
“We had a problem,” Ms. Hong, the hospital’s quality and patient safety coordinator, said.
ERAS documents were developed, staff were educated on the protocol, intraoperative components were implemented and audited, and the full protocol was initiated in November 2013.
To explore the effects of ERAS implementation, chart reviews were conducted on 278 general surgery patients undergoing elective colorectal surgery: 99 patients before ERAS implementation (July 2011 through June 2013) and 179 patients in the first 10 months after full implementation (November 2013 through August 2014).
Laparoscopic colon resections were performed in 53% of the pre-ERAS group and 62% of the post-ERAS group, laparoscopic anterior and abdominoperineal resections in 10% and 23%, and open anterior and abdominoperineal resections in 23% and 18%, respectively. The median American Society of Anesthesiologists classification in both groups was 2.
After ERAS implementation, there was a trend for less postoperative pneumonia, unplanned intubation, ventilator use greater than 48 hours, and urinary tract infections (data not presented).
The median length of stay was reduced from 7 to 5 days, while readmissions increased from 7.1% to 11.7% (both changes were nonsignificant), according to Ms. Hong, who won the conference’s 2015 Surgical Clinical Reviewers Abstract Competition.
The reason for the increased readmissions is unclear, but opportunities to avoid preventable readmissions have been identified and are currently being worked on, she said.
Process measures showed that the goal of achieving a minimum 80% compliance from August 2014 to March 2015 was met within 4 months and sustained for the preoperative and intraoperative ERAS components, in aggregate. The aggregate postoperative components, which include early oral nutrition, early ambulation, early catheter removal, use of chewing gum, and defined discharge criteria, were the slowest to change, but are trending in the right direction, Ms. Hong said.
The key to achieving better outcomes with ERAS lies in involving a multidisciplinary team in all stages of planning and implementation, ongoing communication and sharing of results with stakeholders to foster commitment and ownership, and real-time auditing and use of plan-do-study-act cycles to enhance the rate of improvement, she said.
“It takes time to change culture; tenacity is important,” Ms. Hong added.
In a separate poster presentation, Ms. Hong and her colleagues reported compliance of ERAS components under the control of the anesthesiologist. The highest rate of compliance was seen in practices with few barriers to implementation such as active pre- and intraoperative warming (96%) and appropriate admission of antibiotics (92%) and antiemetics (86%). Conversely, rates were lower for multimodal analgesia (72%) and goal-directed fluid therapy (50%), which can be more labor intensive. Also, there is controversy around goal-directed fluid therapy’s benefit in low-risk patients, which may contribute to the lower compliance rates, the study authors noted. Overall, just under three-quarters of patients received at least four out of five components in their care.
On the basis of the success of the protocol, ERAS is now used for patients undergoing radical cystectomy, with plans to expand its use to all emergent and urgent cases within general surgery at Vancouver General as well as bariatric surgery at Richmond Hospital, also a member of Vancouver Coastal Health, Andrea Bisaillon, operations director of surgical services at Vancouver General Hospital, said in an interview.
“We’re rolling out ERAS to all the surgical patients because it’s best practice for all of surgery, not just colorectal surgery anymore,” she said.
CHICAGO – Implementing an enhanced recovery after surgery (ERAS) protocol at Canada’s second largest hospital significantly reduced morbidity and surgical site infections after elective colorectal surgery.
Rates of postoperative morbidity declined 48.7% from 27.3% before implementation to 14% after full ERAS implementation (P less than .05), while total surgical site infections fell 45% (20.2% vs. 11%; P less than .05).
Nonsignificant reductions were also seen in superficial surgical site infections (11.1% vs. 7.3%), deep SSIs (2% vs. 0.6%), and organ space SSIs (7.1% vs. 3.4%).
“Our results illustrate that using a multidisciplinary team, with attention to details and small multiple changes, aggregation of marginal gains can result in dramatic improvements in patient outcomes,” primary author Tracey Hong, R.N., said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
The ERAS protocol was implemented at Vancouver General Hospital, after ACS NSQIP risk-adjusted reports showed the 743-bed hospital had a high odds ratio of 1.50 for colorectal operative mortality.
“We had a problem,” Ms. Hong, the hospital’s quality and patient safety coordinator, said.
ERAS documents were developed, staff were educated on the protocol, intraoperative components were implemented and audited, and the full protocol was initiated in November 2013.
To explore the effects of ERAS implementation, chart reviews were conducted on 278 general surgery patients undergoing elective colorectal surgery: 99 patients before ERAS implementation (July 2011 through June 2013) and 179 patients in the first 10 months after full implementation (November 2013 through August 2014).
Laparoscopic colon resections were performed in 53% of the pre-ERAS group and 62% of the post-ERAS group, laparoscopic anterior and abdominoperineal resections in 10% and 23%, and open anterior and abdominoperineal resections in 23% and 18%, respectively. The median American Society of Anesthesiologists classification in both groups was 2.
After ERAS implementation, there was a trend for less postoperative pneumonia, unplanned intubation, ventilator use greater than 48 hours, and urinary tract infections (data not presented).
The median length of stay was reduced from 7 to 5 days, while readmissions increased from 7.1% to 11.7% (both changes were nonsignificant), according to Ms. Hong, who won the conference’s 2015 Surgical Clinical Reviewers Abstract Competition.
The reason for the increased readmissions is unclear, but opportunities to avoid preventable readmissions have been identified and are currently being worked on, she said.
Process measures showed that the goal of achieving a minimum 80% compliance from August 2014 to March 2015 was met within 4 months and sustained for the preoperative and intraoperative ERAS components, in aggregate. The aggregate postoperative components, which include early oral nutrition, early ambulation, early catheter removal, use of chewing gum, and defined discharge criteria, were the slowest to change, but are trending in the right direction, Ms. Hong said.
The key to achieving better outcomes with ERAS lies in involving a multidisciplinary team in all stages of planning and implementation, ongoing communication and sharing of results with stakeholders to foster commitment and ownership, and real-time auditing and use of plan-do-study-act cycles to enhance the rate of improvement, she said.
“It takes time to change culture; tenacity is important,” Ms. Hong added.
In a separate poster presentation, Ms. Hong and her colleagues reported compliance of ERAS components under the control of the anesthesiologist. The highest rate of compliance was seen in practices with few barriers to implementation such as active pre- and intraoperative warming (96%) and appropriate admission of antibiotics (92%) and antiemetics (86%). Conversely, rates were lower for multimodal analgesia (72%) and goal-directed fluid therapy (50%), which can be more labor intensive. Also, there is controversy around goal-directed fluid therapy’s benefit in low-risk patients, which may contribute to the lower compliance rates, the study authors noted. Overall, just under three-quarters of patients received at least four out of five components in their care.
On the basis of the success of the protocol, ERAS is now used for patients undergoing radical cystectomy, with plans to expand its use to all emergent and urgent cases within general surgery at Vancouver General as well as bariatric surgery at Richmond Hospital, also a member of Vancouver Coastal Health, Andrea Bisaillon, operations director of surgical services at Vancouver General Hospital, said in an interview.
“We’re rolling out ERAS to all the surgical patients because it’s best practice for all of surgery, not just colorectal surgery anymore,” she said.
CHICAGO – Implementing an enhanced recovery after surgery (ERAS) protocol at Canada’s second largest hospital significantly reduced morbidity and surgical site infections after elective colorectal surgery.
Rates of postoperative morbidity declined 48.7% from 27.3% before implementation to 14% after full ERAS implementation (P less than .05), while total surgical site infections fell 45% (20.2% vs. 11%; P less than .05).
Nonsignificant reductions were also seen in superficial surgical site infections (11.1% vs. 7.3%), deep SSIs (2% vs. 0.6%), and organ space SSIs (7.1% vs. 3.4%).
“Our results illustrate that using a multidisciplinary team, with attention to details and small multiple changes, aggregation of marginal gains can result in dramatic improvements in patient outcomes,” primary author Tracey Hong, R.N., said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
The ERAS protocol was implemented at Vancouver General Hospital, after ACS NSQIP risk-adjusted reports showed the 743-bed hospital had a high odds ratio of 1.50 for colorectal operative mortality.
“We had a problem,” Ms. Hong, the hospital’s quality and patient safety coordinator, said.
ERAS documents were developed, staff were educated on the protocol, intraoperative components were implemented and audited, and the full protocol was initiated in November 2013.
To explore the effects of ERAS implementation, chart reviews were conducted on 278 general surgery patients undergoing elective colorectal surgery: 99 patients before ERAS implementation (July 2011 through June 2013) and 179 patients in the first 10 months after full implementation (November 2013 through August 2014).
Laparoscopic colon resections were performed in 53% of the pre-ERAS group and 62% of the post-ERAS group, laparoscopic anterior and abdominoperineal resections in 10% and 23%, and open anterior and abdominoperineal resections in 23% and 18%, respectively. The median American Society of Anesthesiologists classification in both groups was 2.
After ERAS implementation, there was a trend for less postoperative pneumonia, unplanned intubation, ventilator use greater than 48 hours, and urinary tract infections (data not presented).
The median length of stay was reduced from 7 to 5 days, while readmissions increased from 7.1% to 11.7% (both changes were nonsignificant), according to Ms. Hong, who won the conference’s 2015 Surgical Clinical Reviewers Abstract Competition.
The reason for the increased readmissions is unclear, but opportunities to avoid preventable readmissions have been identified and are currently being worked on, she said.
Process measures showed that the goal of achieving a minimum 80% compliance from August 2014 to March 2015 was met within 4 months and sustained for the preoperative and intraoperative ERAS components, in aggregate. The aggregate postoperative components, which include early oral nutrition, early ambulation, early catheter removal, use of chewing gum, and defined discharge criteria, were the slowest to change, but are trending in the right direction, Ms. Hong said.
The key to achieving better outcomes with ERAS lies in involving a multidisciplinary team in all stages of planning and implementation, ongoing communication and sharing of results with stakeholders to foster commitment and ownership, and real-time auditing and use of plan-do-study-act cycles to enhance the rate of improvement, she said.
“It takes time to change culture; tenacity is important,” Ms. Hong added.
In a separate poster presentation, Ms. Hong and her colleagues reported compliance of ERAS components under the control of the anesthesiologist. The highest rate of compliance was seen in practices with few barriers to implementation such as active pre- and intraoperative warming (96%) and appropriate admission of antibiotics (92%) and antiemetics (86%). Conversely, rates were lower for multimodal analgesia (72%) and goal-directed fluid therapy (50%), which can be more labor intensive. Also, there is controversy around goal-directed fluid therapy’s benefit in low-risk patients, which may contribute to the lower compliance rates, the study authors noted. Overall, just under three-quarters of patients received at least four out of five components in their care.
On the basis of the success of the protocol, ERAS is now used for patients undergoing radical cystectomy, with plans to expand its use to all emergent and urgent cases within general surgery at Vancouver General as well as bariatric surgery at Richmond Hospital, also a member of Vancouver Coastal Health, Andrea Bisaillon, operations director of surgical services at Vancouver General Hospital, said in an interview.
“We’re rolling out ERAS to all the surgical patients because it’s best practice for all of surgery, not just colorectal surgery anymore,” she said.
AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: Through an ERAS protocol, attention to details and small multiple changes can result in dramatic improvements in patient outcomes.
Major finding: After full ERAS implementation, rates of postoperative morbidity and total surgical site infection were reduced 48.7% and 45%, respectively.
Data source: A retrospective analysis of 278 patients undergoing elective colorectal surgery.
Disclosures: The study authors reported having no relevant financial conflicts.