User login
Device positioning may be culprit behind post-LVAD pump thrombosis
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
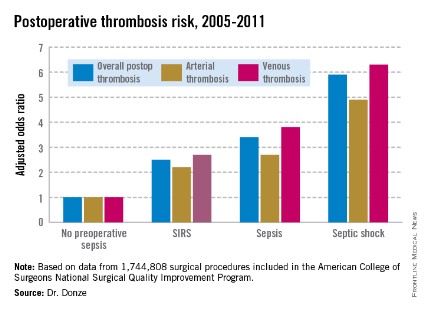
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Cannula malpositioning may be related to pump thrombosis after LVAD placement.
Major finding: The rate of pump thrombosis after LVAD implantation has rapidly increased since 2010, increasing steadily over the past 3 years. Excluding patients with device positioning concerns eliminated the significant difference seen in pump thrombosis across time.
Data source: Single-center, retrospective study of 63 LVAD implant patient records.
Disclosures: Dr. Bhama reported no relevant disclosures.
Upcoming ESC revascularization guidelines cement heart team’s role
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
PARIS – A joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery task force that will publish revised revascularization guidelines in late August gave a sneak peak of some important elements of the revision, including renewed endorsement of and a refinement to the heart team concept that was first introduced in the prior, 2010 version of the guidelines.
"One of the most important aspects of the 2010 guidelines was the introduction of the heart team (Eur. Heart J. 2010;31:2501-55) said Dr. Philippe H. Kolh. "In 2010, the heart team concept was still controversial, but I think now it is well accepted. We are further supporting and emphasizing the importance of the heart team," he said of the revised guidelines that will be released in August, during a session that previewed selected parts of the new guidelines at the annual congress of the European Association of Percutaneous Cardiovascular Interventions, an organization that also collaborated on the guidelines.
The revision also calls on each institution where operators perform revascularization to establish local protocols to guide the choice in routine cases between percutaneous coronary interventions (PCIs) or coronary artery bypass grafting (CABG), said Dr. Kolh, a cardiac surgeon at University Hospital in Liège, Belgium, and cochairman of the guideline-writing panel.
"The 2010 guidelines produced a misconception that every patient needs to be discussed by a heart team; the 2014 revision makes it clear that the heart team should develop institutional protocols for appropriate revascularization strategies for different types of patients. So if a patient has single-vessel disease, you can go ahead and do PCI and not wait for a heart-team decision," said Dr. Ulf Landmesser, professor and head of the acute cardiology clinic at University Hospital, Zurich, and a member of the 2014 panel. "Hopefully, it will now be clear that the heart team only needs to discuss complex patients that involve difficult decisions, and that institutional protocols can handle routine cases," Dr. Landmesser said.
The revision comes at a time when "the competition today is not so much between CABG and PCI; the more burning question is who should have revascularization, and how do patients get to the cath lab," noted Dr. Spencer B. King III, an interventional cardiologist at St. Joseph’s Medical Group in Atlanta who was invited to the session to comment on the new revision.
Results from a new meta-analysis highlight the critical role of revascularization relative to medical therapy alone in improving outcomes of patients with coronary artery disease. This finding is especially relevant in 2014, because it marks the 50th anniversary of the launch of revascularization with the first successful CABG performed, observed Dr. Stephan Windecker, professor and chief of cardiology at University Hospital in Bern, Switzerland, and cochairman of the guidelines-writing panel.
He presented an analysis of results from 100 randomized, controlled trials that compared some form of revascularization against medical therapy in 93,553 randomized patients followed for more than 260,000 patient-years. The results showed that CABG cut the rate of all-cause mortality by 20%, compared with medical therapy, a statistically significant difference, and that treatment with new-generation drug-eluting stents produced a significant reduction of more than 25%, according to an as-yet unpublished report by members of the European Myocardial Revascularization Collaborative. Dr. Windecker also noted that all the recommendations in the new revision were approved with 100% consensus by the panel, which included cardiac surgeons, interventional cardiologists, and noninterventional cardiologists in equal numbers.
The session highlighted several other notable new elements in the revised guidelines, although Dr. Windecker stressed several times during the session that everything presented remained pending until the final version is released later this summer. The changes include:
• An "upgrade" of the recommendation for PCI use in patients with left main disease and a SYNTAX score of 23-32 to a IIa, "should be considered" class recommendation, boosted from class III "not recommended" status in 2010. Five-year outcomes from the SYNTAX trial showed "no difference in outcomes between PCI and CABG, a major reason to upgrade the recommendation for PCI," said Dr. Landmesser (Lancet 2013;381:629-38). "The guidelines put a lot of weight on SYNTAX score."
• When performing PCI in patients with non–ST-elevation myocardial infarction (NSTEMI), bivalirudin (Angiomax) is recommended exclusively as the anticoagulant to use during and immediately following PCI – with unfractionated heparin recommended only for patients who cannot receive bivalirudin – based on bivalirudin’s proven reduced risk for causing major bleeds, said Dr. Franz-Josef Neumann, professor and director of the University Heart Center in Bad Krozingen, Germany.
• But for patients with ST-elevation MI (STEMI) undergoing primary PCI, unfractionated heparin received the only unqualified, level I recommendation for anticoagulation, with bivalirudin receiving a level IIa, "should be considered" recommendation. This repositioning of the two options occurred, based to some extent on yet unpublished results from a very large, single-center study in Liverpool, HEAT-PPCI, reported at the annual meeting of the American College of Cardiology meeting in March that showed unfractionated heparin outperformed bivalirudin for 28-day outcomes, Dr. Neumann said. "I was very pleased and sort of amazed that results from HEAT-PPCI jumped into the guidelines, and it’s not even published yet. That [recommendation] will have an impact, I suspect," commented Dr. King.
• For patients with either STEMI or NSTEMI, the preferred antiplatelet P2Y12 inhibitors are prasugrel (Effient) and ticagrelor (Brilinta), with clopidogrel reduced to a back-up role "only when prasugrel or ticagrelor are not available," said Dr. Neumann. "I was a little surprised that clopidogrel has fallen off the charts. With the new stents having a low stent thrombosis rate, U.S. physicians tend to stick with clopidogrel; there has been more of a shift in Europe," commented Dr. King. "For elective cases, we still have a clear statement in favor of clopidogrel," countered Dr. Neumann. "It is only for higher risk, acute coronary syndrome and STEMI patients where the guidelines recommend the new agents."
Dr. Kolh said that he has received honoraria from Astra Zeneca and Braun, and research support from Edwards. Dr. Landmesser said that he had no disclosures. Dr. King said that he had no disclosures. Dr. Windecker said that he had received honoraria from, had been a consultant to, or had been a speaker for nine companies and had received research grants from seven companies. Dr. Neumann said that his institution had received research grants from 15 companies.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM EUROPCR 2014
Patient outcomes not affected by attendings running ‘simultaneous’ ORs
TORONTO – In academic medical centers, attending cardiothoracic surgeons often perform simultaneous procedures in different operating rooms as a means of increasing training opportunities for surgical fellows and to decrease hospital costs.
However, the practice of running simultaneous operating rooms did not appear to affect perioperative timing or negatively affect patient outcomes, according to the results of a single-institution review presented by Dr. Kenan W. Yount at the annual meeting of the American Association for Thoracic Surgery.
He and his colleagues at the University of Virginia, Charlottesville, wanted to examine their own data in guiding hospital policy as several major centers have recently proposed implementing a 1:1 ratio of attending surgeon to operating room.
In his presentation, Dr. Yount discussed the results of their review, which categorized 1,377 cardiac and 1,682 general thoracic operations performed from July 2011 to July 2013 by attending, case type, and whether the attending was simultaneously supervising two operations. "Our institution adheres to a strict policy of attending surgeon oversight of and involvement in the critical and key portions of all operations," said Dr. Yount.
They compared operative duration, starting and closing times, postoperative complications, and 30-day mortality in each category. They also compared rates of postoperative complications, hospital length of stay, and operative mortality in each category.
Interestingly, timing effects varied between the two overall types of surgery. Running two rooms had no effect on room start times, but thoracic rooms finished 16 minutes later than scheduled. Across six surgeons and 15 types of surgery, however, there were no differences in operative times.
"Furthermore, running two rooms was not associated with any differences in operative duration, morbidity, or mortality in our multivariate regression analyses, and there were no statistically significant differences in observed outcomes in any category," Dr. Yount said.
"In academic cardiothoracic surgical centers that rely on surgical support from fellowship training, the practice of running simultaneous operating rooms can be efficient and does not appear to negatively impact patient outcomes," said Dr. Yount. "In addition, the practice did not significantly increase operative duration or dramatically impact operating room starting or closing times," he concluded.
In discussing the implications of these results, he said, "Obviously, there are caveats: Attendings must be intimately involved in operations and scrubbed for every key and critical portion of the operation; also, operations being scheduled in separate rooms must be done so with reasonable foresight." As long as institutions are following these practices, he concluded, "it would appear that lens of current policy efforts is too narrow by focusing on perception. The debate should be refocused by challenging training programs to strengthen attending involvement and ensure the requisite competence of their trainees."
Dr. Yount reported no relevant disclosures.
TORONTO – In academic medical centers, attending cardiothoracic surgeons often perform simultaneous procedures in different operating rooms as a means of increasing training opportunities for surgical fellows and to decrease hospital costs.
However, the practice of running simultaneous operating rooms did not appear to affect perioperative timing or negatively affect patient outcomes, according to the results of a single-institution review presented by Dr. Kenan W. Yount at the annual meeting of the American Association for Thoracic Surgery.
He and his colleagues at the University of Virginia, Charlottesville, wanted to examine their own data in guiding hospital policy as several major centers have recently proposed implementing a 1:1 ratio of attending surgeon to operating room.
In his presentation, Dr. Yount discussed the results of their review, which categorized 1,377 cardiac and 1,682 general thoracic operations performed from July 2011 to July 2013 by attending, case type, and whether the attending was simultaneously supervising two operations. "Our institution adheres to a strict policy of attending surgeon oversight of and involvement in the critical and key portions of all operations," said Dr. Yount.
They compared operative duration, starting and closing times, postoperative complications, and 30-day mortality in each category. They also compared rates of postoperative complications, hospital length of stay, and operative mortality in each category.
Interestingly, timing effects varied between the two overall types of surgery. Running two rooms had no effect on room start times, but thoracic rooms finished 16 minutes later than scheduled. Across six surgeons and 15 types of surgery, however, there were no differences in operative times.
"Furthermore, running two rooms was not associated with any differences in operative duration, morbidity, or mortality in our multivariate regression analyses, and there were no statistically significant differences in observed outcomes in any category," Dr. Yount said.
"In academic cardiothoracic surgical centers that rely on surgical support from fellowship training, the practice of running simultaneous operating rooms can be efficient and does not appear to negatively impact patient outcomes," said Dr. Yount. "In addition, the practice did not significantly increase operative duration or dramatically impact operating room starting or closing times," he concluded.
In discussing the implications of these results, he said, "Obviously, there are caveats: Attendings must be intimately involved in operations and scrubbed for every key and critical portion of the operation; also, operations being scheduled in separate rooms must be done so with reasonable foresight." As long as institutions are following these practices, he concluded, "it would appear that lens of current policy efforts is too narrow by focusing on perception. The debate should be refocused by challenging training programs to strengthen attending involvement and ensure the requisite competence of their trainees."
Dr. Yount reported no relevant disclosures.
TORONTO – In academic medical centers, attending cardiothoracic surgeons often perform simultaneous procedures in different operating rooms as a means of increasing training opportunities for surgical fellows and to decrease hospital costs.
However, the practice of running simultaneous operating rooms did not appear to affect perioperative timing or negatively affect patient outcomes, according to the results of a single-institution review presented by Dr. Kenan W. Yount at the annual meeting of the American Association for Thoracic Surgery.
He and his colleagues at the University of Virginia, Charlottesville, wanted to examine their own data in guiding hospital policy as several major centers have recently proposed implementing a 1:1 ratio of attending surgeon to operating room.
In his presentation, Dr. Yount discussed the results of their review, which categorized 1,377 cardiac and 1,682 general thoracic operations performed from July 2011 to July 2013 by attending, case type, and whether the attending was simultaneously supervising two operations. "Our institution adheres to a strict policy of attending surgeon oversight of and involvement in the critical and key portions of all operations," said Dr. Yount.
They compared operative duration, starting and closing times, postoperative complications, and 30-day mortality in each category. They also compared rates of postoperative complications, hospital length of stay, and operative mortality in each category.
Interestingly, timing effects varied between the two overall types of surgery. Running two rooms had no effect on room start times, but thoracic rooms finished 16 minutes later than scheduled. Across six surgeons and 15 types of surgery, however, there were no differences in operative times.
"Furthermore, running two rooms was not associated with any differences in operative duration, morbidity, or mortality in our multivariate regression analyses, and there were no statistically significant differences in observed outcomes in any category," Dr. Yount said.
"In academic cardiothoracic surgical centers that rely on surgical support from fellowship training, the practice of running simultaneous operating rooms can be efficient and does not appear to negatively impact patient outcomes," said Dr. Yount. "In addition, the practice did not significantly increase operative duration or dramatically impact operating room starting or closing times," he concluded.
In discussing the implications of these results, he said, "Obviously, there are caveats: Attendings must be intimately involved in operations and scrubbed for every key and critical portion of the operation; also, operations being scheduled in separate rooms must be done so with reasonable foresight." As long as institutions are following these practices, he concluded, "it would appear that lens of current policy efforts is too narrow by focusing on perception. The debate should be refocused by challenging training programs to strengthen attending involvement and ensure the requisite competence of their trainees."
Dr. Yount reported no relevant disclosures.
AT THE AATS ANNUAL MEETING
Major finding: Running two rooms was not associated with any differences in operative duration, morbidity, or mortality in multivariate regression analyses, and there were no statistically significant differences in observed outcomes in any category.
Data source: The study reviewed 1,377 cardiac and 1,682 general thoracic operations performed from July 2011 to July 2013 by attending, case type, and whether the attending was simultaneously supervising two operations.
Disclosures: Dr. Yount had no disclosures.
Delayed intervention can reduce TEVAR complications in acute type B dissections
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"We know that there has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [Food and Drug Administration] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery. Dr. Desai was the first recipient of the Randall B. Griepp Honorary Paper Presentation.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute early (TEVAR within 48 hours of symptom onset), n = 70; acute delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n=18.
Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, a cardiothoracic surgeon at the Hospital of the University of Pennsylvania, Philadelphia.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%, respectively) and stroke (5.6%, 4.6%, and 0%). Causes of early death included rupture of the descending aorta, retrograde type A rupture, or the consequences of severe malperfusion.
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category." He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
*Dr. Desai is a primary investigator for FDA clinical trials using TEVAR for W.L. Gore and Associates, Inc., Medtronic, and Cook Medical.
*Correction 5/22/14: An earlier version of this article did not state Dr. Desai's disclosure information.
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"We know that there has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [Food and Drug Administration] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery. Dr. Desai was the first recipient of the Randall B. Griepp Honorary Paper Presentation.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute early (TEVAR within 48 hours of symptom onset), n = 70; acute delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n=18.
Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, a cardiothoracic surgeon at the Hospital of the University of Pennsylvania, Philadelphia.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%, respectively) and stroke (5.6%, 4.6%, and 0%). Causes of early death included rupture of the descending aorta, retrograde type A rupture, or the consequences of severe malperfusion.
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category." He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
*Dr. Desai is a primary investigator for FDA clinical trials using TEVAR for W.L. Gore and Associates, Inc., Medtronic, and Cook Medical.
*Correction 5/22/14: An earlier version of this article did not state Dr. Desai's disclosure information.
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"We know that there has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [Food and Drug Administration] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery. Dr. Desai was the first recipient of the Randall B. Griepp Honorary Paper Presentation.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute early (TEVAR within 48 hours of symptom onset), n = 70; acute delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n=18.
Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, a cardiothoracic surgeon at the Hospital of the University of Pennsylvania, Philadelphia.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%, respectively) and stroke (5.6%, 4.6%, and 0%). Causes of early death included rupture of the descending aorta, retrograde type A rupture, or the consequences of severe malperfusion.
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category." He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
*Dr. Desai is a primary investigator for FDA clinical trials using TEVAR for W.L. Gore and Associates, Inc., Medtronic, and Cook Medical.
*Correction 5/22/14: An earlier version of this article did not state Dr. Desai's disclosure information.
AT AATS AORTIC SYMPOSIUM 2014
Key clinical point: Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait.
Major finding: The overall risk of severe complications was more than threefold higher in patients who underwent TEVAR for acute type B aortic dissections within 48 hours of initial presentation, compared with those whose treatment could be delayed until 2-6 weeks after symptom onset.
Data source: A single-center, retrospective registry analysis of 317 patients.
Disclosures: Dr. Desai said he had no relevant financial disclosures.
Open thoracoabdominal aortic aneurysm repair in octogenarians: Special considerations
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
AT AATS AORTIC SYMPOSIUM 2014
Key clinical point: Octogenarians with TAAAs present more challenges than younger individuals and their outcomes vary greatly according to the type of aneurysm repair.
Major finding: A study that compared octogenarians with thoracoabdominal aortic aneurysms (TAAAs) to a younger cohort found that octogenarians were more at risk for aneurysm rupture, were more likely to need visceral-branch endarterectomy/stenting, had more adverse postoperative outcomes, and higher rates of operative mortality and longer postoperative ICU and hospital stays. While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs can be performed with relatively low risk. Younger patients were more likely than octogenarians to present with aortic dissections.
Data source: Retrospective review.
Disclosures: Dr. Aftab had no relevant disclosures.
System overhaul eliminated CABG surgical site infections
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
ORLANDO – A system-wide quality improvement process that utilized define, measure, analyze, improve, and control and rapid adoption methodology eliminated surgical site infections among patients undergoing isolated coronary artery bypass with donor site surgery at a regional medical center.
Between March 2012 and March 2013 the overall decline in the rate of surgical site infections among 250 patients surpassed the center’s goal of a 40% decrease in the infection rate to a rate of 1.61; instead, the center achieved a rate of 0.7 during that time period. Since May, 2012, no surgical site infections have occurred among coronary artery bypass graft patients, Candis Kles, a critical care nurse at Athens (Ga.) Regional Medical Center, reported at the annual meeting of the Society of Thoracic Surgeons.
"We are now into our 23rd month without a deep/organ space infection with over 454 patients," Ms. Kles said in a May interview.
The quality improvement process was initiated after the infection rates at the center were found to be high (ranging up to 6.1), compared with National Healthcare Safety Network data. Using the define, measure, analyze, improve, and control methodology, a multidisciplinary team was convened, including cardiothoracic surgeons, nurses, and support personnel, and a 6-month process was undertaken to identify areas for improvement, said Ms. Kles who chaired the team.
"We also at this time partnered with the VHA National Hospital Engagement Network for some extra training and to help us along the process," she noted.
The team flow-charted processes – from scheduled surgery to discharge – and posted the flow charts in the operating room and cardiac intensive care unit to identify inconsistencies and potential problem areas. Frontline staff members were engaged and encouraged to participate in the improvement process by posting notes, suggestions, and questions on the flow charts.
A literature review was conducted to ensure that current practices mirrored best practices.
Despite an extensive evaluation of possible patient-, procedure-, and hospital-related factors that might be associated with the infection rate, no single cause of the high rate was identified, although a number of areas for improvement were recognized. Among the changes that were implemented were use of chlorhexidine oral rinse, which was administered until discharge; use of disposable EKG leads (after high bacterial counts were found on the leads in use prior to program initiation); use of silver-impregnated midsternal dressings; a change in surgical and suture techniques; development of patient educational materials, and standardization of all processes, Ms. Kles said, noting that the changes were implemented rapidly.
The success of the program is largely attributable to input from all levels and disciplines within the organization – including physicians who championed the process, and to staff engagement and buy in, she said.
The improvement in the infection rate resulted in cost avoidance of $212,355 (compared with a total excess cost of surgical site infections of $250,965 in 2011), and in a reduction from 156 to 24 excess hospital days.
Ms. Kles reported that as of February 2014 she is a member of the speakers bureau for Mölnlycke Health Care, a maker of surgical and wound care products.
AT THE STS ANNUAL MEETING
Key clinical point: Cross-discipline buy in obliterated CABG surgical site infections.
Major finding: Since May, 2012, no surgical site infections have occurred among CABG patients.
Data source: An overview of a quality improvement process at a regional medical center.
Disclosures: Ms. Kles reported that as of February, 2014 she is a member of the speakers bureau for Mölnlycke Health Care.
Six factors predict 1-year survival after TAVR
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
AT ACC 2014
Key clinical point: One-year outcome data from almost 6,000 U.S. TAVR patients give potential insight into the procedure’s best targets.
Major finding: Six factors significantly influenced 1-year survival following transcatheter aortic valve replacement.
Data source: The STS/ACC Transcatheter Valve Therapy Registry, which included 1-year outcomes data for the first 5,980 U.S. patients who underwent TAVR since November 2011.
Disclosures: The registry does not receive commercial support. Dr. Holmes had no relevant disclosures.
Even mild preop sepsis boosts postop clot risk
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.
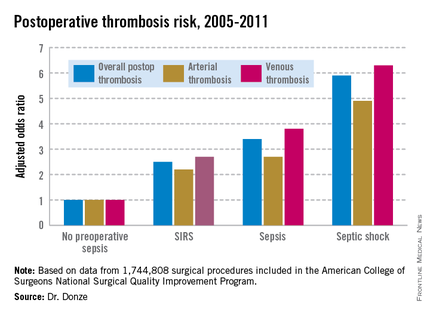
Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
Major finding: Preoperative sepsis is a strong independent risk factor for postoperative arterial and venous thrombosis; the more severe the sepsis, the greater the thrombosis risk.
Data source: This was an analysis of nearly 1.75 million surgical procedures at 314 U.S. hospitals detailed in the American College of Surgeons National Quality Improvement Program registry.
Disclosures: The presenter reported having no financial conflicts.
Hold the ACE inhibitors during surgery?
SCOTTSDALE, ARIZ. – When it comes to holding or continuing with ACE inhibitors before surgery, all bets are off, a perioperative medicine consultant suggested.
Patients on angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) have about a 50% risk of developing hypotension during surgery, and a significant proportion of those episodes could be severe, said Dr. Paul Grant, of the University of Michigan Health System in Ann Arbor.
"I recommend having some sort of standard approach [to perioperative ACE inhibitor use] at your institution if that’s at all possible, either for a certain surgery type or across the board," he said at a meeting on perioperative medicine sponsored by the University of Miami.
Evidence from a small number of randomized trials and observational studies suggests that continuing ACE inhibitors during cardiac surgery may result in less cardiac enzyme release, less kidney injury, and a lower incidence of atrial fibrillation. In vascular surgery, evidence suggests that patients on ACE inhibitors who are undergoing surgery have less of a drop in cardiac output and may have improved creatinine clearance.
On the other hand, patients who remain on ACE inhibitors during surgery can experience a "profound" drop in blood pressure requiring immediate intervention, he said.
Data to support the continue vs. hold debate are sparse, but include a trial of 51 patients randomized to continue ACE inhibitors on the day of surgery or to have the drugs held for 12-24 hours before surgery. In all, 33 of the patients were on captopril (Capoten), and 18 were on enalapril (Vasotec).
The investigators found that among patients randomized to continue ACE inhibitor therapy, 7 of 7 on captopril and 9 of 14 on enalapril developed hypotension, defined as a systolic blood pressure (SBP) less than 90 mm Hg. In contrast, among patients assigned to the ACE-inhibitor hold protocol, only 2 of 11 on captopril and 4 of 19 on enalapril developed hypotension during surgery.
In a second randomized trial, investigators looked at 37 patients on an ARB who were randomly assigned to either discontinue ARB on the day before surgery (18 patients), or to receive their ARB 1 hour before anesthesia induction (19 patients).
The authors defined hypotension for their study as an SBP less than 80 mm Hg for more than 1 minute. They found that all 19 patients who continued on ARB had hypotension during surgery, compared with 12 of 18 who discontinued their ARB the day before. Patients who received their ARB on the day of surgery used significantly more vasoactive drugs. Despite the discontinuation of the ARB, there were no differences in hypertension between the groups in the recovery period. Postoperative cardiac complications occurred in 1 patient in each group.
In the final randomized study that Dr. Grant cited, 40 patients on an ACE inhibitor with good left-ventricular function were scheduled to undergo coronary artery bypass graft (CABG). They were randomly assigned to hold or continue on ACE inhibitors on the day of surgery.
Patients in whom the ACE inhibitors were held before CABG had higher mean blood pressures than patients who continued on the drugs, and they used less vasopressor during the surgery. In contrast, patients who continued on ACE inhibitors needed more vasodilators after CABG and in the recovery period. The authors of this trial did not study other clinical endpoints, Dr. Grant noted.
Evidence from two observational studies was more equivocal, however.
In a retrospective observational study, investigators studied the relationship between the timing of discontinuing ACE inhibitors and angiotensin II receptor subtype 1 antagonists (ARA) and the onset of hypotension in 267 patients scheduled for general surgery.
They found that patients exposed to an ACE inhibitor or ARA within 10 hours of anesthesia had an adjusted odds ratio of 1.74 for moderate hypotension (SBP 85 mm Hg or less; P = .04), but there was no difference in severe hypotension between these patients and those who discontinued the drugs more than 10 hours before surgery. There were no differences in either vasopressor use or postoperative complications, including unplanned intensive care unit stay, myocardial infarction, stroke, renal impairment, or death.
A second, smaller study compared 12 vascular surgery patients on ARB the day of surgery with matched cohorts of patients taking beta-blockers and/or calcium channel blockers the day of surgery, or ACE inhibitors held on the day of surgery.
Hypotension (SBP less than 90 mm Hg in this study) occurred in all of the patients on ARB but in only 60% (27 of 45) patients on the beta-blocker/calcium channel blockers, and in 67% (18 of 27) in the ACE-inhibitor hold cohort. The ARB patients were also less responsive to ephedrine and phenylephrine than other patients, and in some cases responded only to a vasopressin system agonist, Dr. Grant noted.
Finally, the authors of a random-effects meta-analysis of five studies with a total of 434 patients reported that patients receiving an immediate preoperative ACE inhibitor or ARA dose had a relative risk of 1.50 for developing hypotension requiring vasopressors at or shortly after induction of anesthesia, compared with patients who did not receive the drugs.
Dr. Grant noted that the American College of Physicians’ Smart Medicine guidelines on perioperative management of hypertensive patients recommend continuing ACE inhibitors "with caution," and they advise clinicians to avoid hypovolemia in patients maintained on ACE inhibitors during surgery. He said that in certain cases, it may be appropriate to continue surgical patients on ACE inhibitors or ARB, as in patients with hypertension that is difficult to control with multiple medications, or in those with severe heart disease who have adequate blood pressure.
Dr. Grant reported having no financial disclosures.

|
|
Dr. Vera A. DePalo comments:The findings of the studies
presented by Dr. Grant have important implications for understanding the
significant issue of hypotension that the postoperative patient may
face. However, the studies are relatively small, and in some cases the
results are conflicting. A larger randomized, controlled trial would
help shed light on how we can better identify the patients who can
benefit from these therapeutic choices.
Dr. Vera A. DePalo is associate professor of medicine at Brown University, Providence, R.I. She is the deputy medical editor of CHEST Physician.

|
|
Dr. Vera A. DePalo comments:The findings of the studies
presented by Dr. Grant have important implications for understanding the
significant issue of hypotension that the postoperative patient may
face. However, the studies are relatively small, and in some cases the
results are conflicting. A larger randomized, controlled trial would
help shed light on how we can better identify the patients who can
benefit from these therapeutic choices.
Dr. Vera A. DePalo is associate professor of medicine at Brown University, Providence, R.I. She is the deputy medical editor of CHEST Physician.

|
|
Dr. Vera A. DePalo comments:The findings of the studies
presented by Dr. Grant have important implications for understanding the
significant issue of hypotension that the postoperative patient may
face. However, the studies are relatively small, and in some cases the
results are conflicting. A larger randomized, controlled trial would
help shed light on how we can better identify the patients who can
benefit from these therapeutic choices.
Dr. Vera A. DePalo is associate professor of medicine at Brown University, Providence, R.I. She is the deputy medical editor of CHEST Physician.
SCOTTSDALE, ARIZ. – When it comes to holding or continuing with ACE inhibitors before surgery, all bets are off, a perioperative medicine consultant suggested.
Patients on angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) have about a 50% risk of developing hypotension during surgery, and a significant proportion of those episodes could be severe, said Dr. Paul Grant, of the University of Michigan Health System in Ann Arbor.
"I recommend having some sort of standard approach [to perioperative ACE inhibitor use] at your institution if that’s at all possible, either for a certain surgery type or across the board," he said at a meeting on perioperative medicine sponsored by the University of Miami.
Evidence from a small number of randomized trials and observational studies suggests that continuing ACE inhibitors during cardiac surgery may result in less cardiac enzyme release, less kidney injury, and a lower incidence of atrial fibrillation. In vascular surgery, evidence suggests that patients on ACE inhibitors who are undergoing surgery have less of a drop in cardiac output and may have improved creatinine clearance.
On the other hand, patients who remain on ACE inhibitors during surgery can experience a "profound" drop in blood pressure requiring immediate intervention, he said.
Data to support the continue vs. hold debate are sparse, but include a trial of 51 patients randomized to continue ACE inhibitors on the day of surgery or to have the drugs held for 12-24 hours before surgery. In all, 33 of the patients were on captopril (Capoten), and 18 were on enalapril (Vasotec).
The investigators found that among patients randomized to continue ACE inhibitor therapy, 7 of 7 on captopril and 9 of 14 on enalapril developed hypotension, defined as a systolic blood pressure (SBP) less than 90 mm Hg. In contrast, among patients assigned to the ACE-inhibitor hold protocol, only 2 of 11 on captopril and 4 of 19 on enalapril developed hypotension during surgery.
In a second randomized trial, investigators looked at 37 patients on an ARB who were randomly assigned to either discontinue ARB on the day before surgery (18 patients), or to receive their ARB 1 hour before anesthesia induction (19 patients).
The authors defined hypotension for their study as an SBP less than 80 mm Hg for more than 1 minute. They found that all 19 patients who continued on ARB had hypotension during surgery, compared with 12 of 18 who discontinued their ARB the day before. Patients who received their ARB on the day of surgery used significantly more vasoactive drugs. Despite the discontinuation of the ARB, there were no differences in hypertension between the groups in the recovery period. Postoperative cardiac complications occurred in 1 patient in each group.
In the final randomized study that Dr. Grant cited, 40 patients on an ACE inhibitor with good left-ventricular function were scheduled to undergo coronary artery bypass graft (CABG). They were randomly assigned to hold or continue on ACE inhibitors on the day of surgery.
Patients in whom the ACE inhibitors were held before CABG had higher mean blood pressures than patients who continued on the drugs, and they used less vasopressor during the surgery. In contrast, patients who continued on ACE inhibitors needed more vasodilators after CABG and in the recovery period. The authors of this trial did not study other clinical endpoints, Dr. Grant noted.
Evidence from two observational studies was more equivocal, however.
In a retrospective observational study, investigators studied the relationship between the timing of discontinuing ACE inhibitors and angiotensin II receptor subtype 1 antagonists (ARA) and the onset of hypotension in 267 patients scheduled for general surgery.
They found that patients exposed to an ACE inhibitor or ARA within 10 hours of anesthesia had an adjusted odds ratio of 1.74 for moderate hypotension (SBP 85 mm Hg or less; P = .04), but there was no difference in severe hypotension between these patients and those who discontinued the drugs more than 10 hours before surgery. There were no differences in either vasopressor use or postoperative complications, including unplanned intensive care unit stay, myocardial infarction, stroke, renal impairment, or death.
A second, smaller study compared 12 vascular surgery patients on ARB the day of surgery with matched cohorts of patients taking beta-blockers and/or calcium channel blockers the day of surgery, or ACE inhibitors held on the day of surgery.
Hypotension (SBP less than 90 mm Hg in this study) occurred in all of the patients on ARB but in only 60% (27 of 45) patients on the beta-blocker/calcium channel blockers, and in 67% (18 of 27) in the ACE-inhibitor hold cohort. The ARB patients were also less responsive to ephedrine and phenylephrine than other patients, and in some cases responded only to a vasopressin system agonist, Dr. Grant noted.
Finally, the authors of a random-effects meta-analysis of five studies with a total of 434 patients reported that patients receiving an immediate preoperative ACE inhibitor or ARA dose had a relative risk of 1.50 for developing hypotension requiring vasopressors at or shortly after induction of anesthesia, compared with patients who did not receive the drugs.
Dr. Grant noted that the American College of Physicians’ Smart Medicine guidelines on perioperative management of hypertensive patients recommend continuing ACE inhibitors "with caution," and they advise clinicians to avoid hypovolemia in patients maintained on ACE inhibitors during surgery. He said that in certain cases, it may be appropriate to continue surgical patients on ACE inhibitors or ARB, as in patients with hypertension that is difficult to control with multiple medications, or in those with severe heart disease who have adequate blood pressure.
Dr. Grant reported having no financial disclosures.
SCOTTSDALE, ARIZ. – When it comes to holding or continuing with ACE inhibitors before surgery, all bets are off, a perioperative medicine consultant suggested.
Patients on angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) have about a 50% risk of developing hypotension during surgery, and a significant proportion of those episodes could be severe, said Dr. Paul Grant, of the University of Michigan Health System in Ann Arbor.
"I recommend having some sort of standard approach [to perioperative ACE inhibitor use] at your institution if that’s at all possible, either for a certain surgery type or across the board," he said at a meeting on perioperative medicine sponsored by the University of Miami.
Evidence from a small number of randomized trials and observational studies suggests that continuing ACE inhibitors during cardiac surgery may result in less cardiac enzyme release, less kidney injury, and a lower incidence of atrial fibrillation. In vascular surgery, evidence suggests that patients on ACE inhibitors who are undergoing surgery have less of a drop in cardiac output and may have improved creatinine clearance.
On the other hand, patients who remain on ACE inhibitors during surgery can experience a "profound" drop in blood pressure requiring immediate intervention, he said.
Data to support the continue vs. hold debate are sparse, but include a trial of 51 patients randomized to continue ACE inhibitors on the day of surgery or to have the drugs held for 12-24 hours before surgery. In all, 33 of the patients were on captopril (Capoten), and 18 were on enalapril (Vasotec).
The investigators found that among patients randomized to continue ACE inhibitor therapy, 7 of 7 on captopril and 9 of 14 on enalapril developed hypotension, defined as a systolic blood pressure (SBP) less than 90 mm Hg. In contrast, among patients assigned to the ACE-inhibitor hold protocol, only 2 of 11 on captopril and 4 of 19 on enalapril developed hypotension during surgery.
In a second randomized trial, investigators looked at 37 patients on an ARB who were randomly assigned to either discontinue ARB on the day before surgery (18 patients), or to receive their ARB 1 hour before anesthesia induction (19 patients).
The authors defined hypotension for their study as an SBP less than 80 mm Hg for more than 1 minute. They found that all 19 patients who continued on ARB had hypotension during surgery, compared with 12 of 18 who discontinued their ARB the day before. Patients who received their ARB on the day of surgery used significantly more vasoactive drugs. Despite the discontinuation of the ARB, there were no differences in hypertension between the groups in the recovery period. Postoperative cardiac complications occurred in 1 patient in each group.
In the final randomized study that Dr. Grant cited, 40 patients on an ACE inhibitor with good left-ventricular function were scheduled to undergo coronary artery bypass graft (CABG). They were randomly assigned to hold or continue on ACE inhibitors on the day of surgery.
Patients in whom the ACE inhibitors were held before CABG had higher mean blood pressures than patients who continued on the drugs, and they used less vasopressor during the surgery. In contrast, patients who continued on ACE inhibitors needed more vasodilators after CABG and in the recovery period. The authors of this trial did not study other clinical endpoints, Dr. Grant noted.
Evidence from two observational studies was more equivocal, however.
In a retrospective observational study, investigators studied the relationship between the timing of discontinuing ACE inhibitors and angiotensin II receptor subtype 1 antagonists (ARA) and the onset of hypotension in 267 patients scheduled for general surgery.
They found that patients exposed to an ACE inhibitor or ARA within 10 hours of anesthesia had an adjusted odds ratio of 1.74 for moderate hypotension (SBP 85 mm Hg or less; P = .04), but there was no difference in severe hypotension between these patients and those who discontinued the drugs more than 10 hours before surgery. There were no differences in either vasopressor use or postoperative complications, including unplanned intensive care unit stay, myocardial infarction, stroke, renal impairment, or death.
A second, smaller study compared 12 vascular surgery patients on ARB the day of surgery with matched cohorts of patients taking beta-blockers and/or calcium channel blockers the day of surgery, or ACE inhibitors held on the day of surgery.
Hypotension (SBP less than 90 mm Hg in this study) occurred in all of the patients on ARB but in only 60% (27 of 45) patients on the beta-blocker/calcium channel blockers, and in 67% (18 of 27) in the ACE-inhibitor hold cohort. The ARB patients were also less responsive to ephedrine and phenylephrine than other patients, and in some cases responded only to a vasopressin system agonist, Dr. Grant noted.
Finally, the authors of a random-effects meta-analysis of five studies with a total of 434 patients reported that patients receiving an immediate preoperative ACE inhibitor or ARA dose had a relative risk of 1.50 for developing hypotension requiring vasopressors at or shortly after induction of anesthesia, compared with patients who did not receive the drugs.
Dr. Grant noted that the American College of Physicians’ Smart Medicine guidelines on perioperative management of hypertensive patients recommend continuing ACE inhibitors "with caution," and they advise clinicians to avoid hypovolemia in patients maintained on ACE inhibitors during surgery. He said that in certain cases, it may be appropriate to continue surgical patients on ACE inhibitors or ARB, as in patients with hypertension that is difficult to control with multiple medications, or in those with severe heart disease who have adequate blood pressure.
Dr. Grant reported having no financial disclosures.
AT THE PERIOPERATIVE MEDICINE SUMMIT
Major finding: Among patients randomized to continue ACE inhibitor therapy during surgery, 16 of 21 patients developed hypotension, compared with only 6 of 30 patients who discontinued their ACE inhibitors the day before.
Data source: Review of evidence on the perioperative management of patients with hypertension treated with ACE inhibitors or angiotensin II receptor blockers.
Disclosures: Dr. Grant reported having no financial disclosures.
Hold the immunomodulators for surgery? Maybe yes, maybe no
SCOTTSDALE, ARIZ. – When patients on immunosuppressive therapies need surgery, the risks of disease flare and compromised postoperative recovery and rehabilitation must be weighed against the risk of increased infections and impaired wound healing.
"I’m not sure that there is necessarily a right answer, but I think most people would stop biologic [agents] beforehand," Dr. Paul Grant said at a meeting on perioperative medicine sponsored by the University of Miami.
The decision whether to suspend a disease-modifying antirheumatic drug before surgery may depend on the individual drug and on the patient, said Dr. Grant, director of perioperative and consultative medicine at the University of Michigan Health System in Ann Arbor.
For example, it appears to be safe for patients on methotrexate to continue on therapy during elective orthopedic surgery. Evidence for this comes from a randomized clinical trial in which patients with rheumatoid arthritis (RA) were assigned to either continue on methotrexate (MTX) or suspend taking it for 2 weeks before and 2 weeks after surgery. The study also contained a control of patients with RA who were not on MTX (Ann. Rheum. Dis. 2001;60:214-7).
The investigators found that there were no significant differences in early complication rates or in complications up to 1 year of follow-up between patients who suspended or remained on MTX. Patients who stayed on the drug had significantly lower rates of RA flare.
Additionally, two systematic reviews, one looking at eight studies echoes the findings of the aforementioned randomized trial, and the other looking at four studies, in which the reviewer concluded that "continued MTX therapy appears to be safe perioperatively and seems also to be associated with a reduced risk of flares (Clin. Exp. Rheumatol. 2009;27:856-62) (Clin. Rheumatol. 2008;27:1217-20).None of the examined papers addresses the issue of safety in connection with comorbidities, age, or high doses of methotrexate."
"The bottom line here is that methotrexate should be continued for most surgeries. I think it might be reasonable to hold it in certain situations, for example if the patient has pretty bad kidney or liver disease, or if it’s surgery to treat a major infection," Dr. Grant said.
TNF-alpha antagonists
In contrast, the data on tumor necrosis factor–alpha (TNF-alpha) antagonists are fuzzier, with limited and conflicting information on perioperative use of these agents (etanercept, infliximab, adalimumab, certolizumab, golimumab).
"The major concern with these drugs is infection," Dr. Grant said. He pointed to a meta-analysis published in JAMA in 2006, which showed that taking the drugs doubled the risk of serious infections in general. The study did not specifically look at perioperative use of TNF-alpha antagonists (JAMA 2006;295:2275-85).
A retrospective cohort studyof 127 patients with RA who were undergoing various orthopedic procedures found that there were no differences in surgical site infections but more cases of wound dehiscence in patients who continued on the drugs, compared with those who interrupted their use perioperatively (Clin. Exp. Rheumatol. 2007;25:430-6).
A second, prospective study in 31 patients with RA undergoing foot/ankle surgery found that there were no significant differences in infection or healing between patients who interrupted therapy and those who did not (Foot Ankle Clin. 2007;12:509-24).
Other studies and systematic reviews in patients with RA or Crohn’s disease generally found no significant differences in serious infection rates, but they did detect a higher incidence of skin and soft-tissue infections among patients on anti-TNF-alpha agents vs. other disease-modifying antirheumatic drugs.
The risk of infections tends to be highest at the start of therapy with a TNF-alpha antagonist and stopping therapy is more likely to result in RA flares among patients with established disease, compared with those in the early stages of RA. Therefore, TNF blocker therapy should be restarted as soon as possible after surgery to prevent flare, Dr. Grant said.
The American College of Rheumatology and British Society of Rheumatology recommend holding TNF-alpha antagonists for one dosing cycle before major surgery. For etanercept (Enbrel), that translates to a 1-week before surgery hold, for infliximab (Remicade) 6-8 weeks, and for adalimumab (Humira) 2 weeks. These agents should also be held for 10-14 days after surgery or until wound healing is satisfactory.
"It’s probably safe to continue these medications for minor surgeries," Dr. Grant said.
Other agents
The anti-CD20 agent rituximab (Rituxan) – currently used to treat RA, vasculitis, hematologic malignancies, and other conditions – has a lower risk for bacterial infections than does TNF-alpha antagonists and has been shown to be safe in patients with a history of recurrent bacterial infections.
"Hydroxychloroquine (or Plaquenil) is felt to be safe during the preoperative period. It is recommended to continue this medication without stopping," Dr. Grant said.
There is conflicting information on infection risk with the use leflunomide (Arava), but it may be wise to stop therapy 2-4 weeks before nonurgent surgery in higher-risk patients.
There is consensus that sulfasalazine (Azulfidine) and azathioprine (Imuran) can be safely continued perioperatively, he said, although some advise holding sulfasalazine on the day of surgery.
Regarding perioperative steroids, Dr. Grant recommended determining the patient’s steroid exposure over the past year.
"Stress dose steroids are not routinely needed as long as the patients continue their normal dose. That’s really the important piece: If someone’s taking prednisone every day, make sure they take at least that dose on the day of surgery," he said.
Dr. Grant reported having no financial disclosures.
Dr. Lary Robinson, FCCP, comments: Surgeons are rightfully
concerned about the preoperative use of any medicines that might
increase the risk of bleeding, infections or wound healing. The topic of
immunomodulating drugs was explored by evaluating the published
evidence about their safety in the perioperative period. These agents,
used in patients with autoimmune/inflammatory diseases such as
rheumatoid arthritis and Crohn\'s disease, are generally safe to
continue although most authorities generally recommend holding the
TNF-alpha antagonists prior to and after major surgery due to potential
wound healing and infectious problems.
Dr. Lary Robinson, FCCP, comments: Surgeons are rightfully
concerned about the preoperative use of any medicines that might
increase the risk of bleeding, infections or wound healing. The topic of
immunomodulating drugs was explored by evaluating the published
evidence about their safety in the perioperative period. These agents,
used in patients with autoimmune/inflammatory diseases such as
rheumatoid arthritis and Crohn\'s disease, are generally safe to
continue although most authorities generally recommend holding the
TNF-alpha antagonists prior to and after major surgery due to potential
wound healing and infectious problems.
Dr. Lary Robinson, FCCP, comments: Surgeons are rightfully
concerned about the preoperative use of any medicines that might
increase the risk of bleeding, infections or wound healing. The topic of
immunomodulating drugs was explored by evaluating the published
evidence about their safety in the perioperative period. These agents,
used in patients with autoimmune/inflammatory diseases such as
rheumatoid arthritis and Crohn\'s disease, are generally safe to
continue although most authorities generally recommend holding the
TNF-alpha antagonists prior to and after major surgery due to potential
wound healing and infectious problems.
SCOTTSDALE, ARIZ. – When patients on immunosuppressive therapies need surgery, the risks of disease flare and compromised postoperative recovery and rehabilitation must be weighed against the risk of increased infections and impaired wound healing.
"I’m not sure that there is necessarily a right answer, but I think most people would stop biologic [agents] beforehand," Dr. Paul Grant said at a meeting on perioperative medicine sponsored by the University of Miami.
The decision whether to suspend a disease-modifying antirheumatic drug before surgery may depend on the individual drug and on the patient, said Dr. Grant, director of perioperative and consultative medicine at the University of Michigan Health System in Ann Arbor.
For example, it appears to be safe for patients on methotrexate to continue on therapy during elective orthopedic surgery. Evidence for this comes from a randomized clinical trial in which patients with rheumatoid arthritis (RA) were assigned to either continue on methotrexate (MTX) or suspend taking it for 2 weeks before and 2 weeks after surgery. The study also contained a control of patients with RA who were not on MTX (Ann. Rheum. Dis. 2001;60:214-7).
The investigators found that there were no significant differences in early complication rates or in complications up to 1 year of follow-up between patients who suspended or remained on MTX. Patients who stayed on the drug had significantly lower rates of RA flare.
Additionally, two systematic reviews, one looking at eight studies echoes the findings of the aforementioned randomized trial, and the other looking at four studies, in which the reviewer concluded that "continued MTX therapy appears to be safe perioperatively and seems also to be associated with a reduced risk of flares (Clin. Exp. Rheumatol. 2009;27:856-62) (Clin. Rheumatol. 2008;27:1217-20).None of the examined papers addresses the issue of safety in connection with comorbidities, age, or high doses of methotrexate."
"The bottom line here is that methotrexate should be continued for most surgeries. I think it might be reasonable to hold it in certain situations, for example if the patient has pretty bad kidney or liver disease, or if it’s surgery to treat a major infection," Dr. Grant said.
TNF-alpha antagonists
In contrast, the data on tumor necrosis factor–alpha (TNF-alpha) antagonists are fuzzier, with limited and conflicting information on perioperative use of these agents (etanercept, infliximab, adalimumab, certolizumab, golimumab).
"The major concern with these drugs is infection," Dr. Grant said. He pointed to a meta-analysis published in JAMA in 2006, which showed that taking the drugs doubled the risk of serious infections in general. The study did not specifically look at perioperative use of TNF-alpha antagonists (JAMA 2006;295:2275-85).
A retrospective cohort studyof 127 patients with RA who were undergoing various orthopedic procedures found that there were no differences in surgical site infections but more cases of wound dehiscence in patients who continued on the drugs, compared with those who interrupted their use perioperatively (Clin. Exp. Rheumatol. 2007;25:430-6).
A second, prospective study in 31 patients with RA undergoing foot/ankle surgery found that there were no significant differences in infection or healing between patients who interrupted therapy and those who did not (Foot Ankle Clin. 2007;12:509-24).
Other studies and systematic reviews in patients with RA or Crohn’s disease generally found no significant differences in serious infection rates, but they did detect a higher incidence of skin and soft-tissue infections among patients on anti-TNF-alpha agents vs. other disease-modifying antirheumatic drugs.
The risk of infections tends to be highest at the start of therapy with a TNF-alpha antagonist and stopping therapy is more likely to result in RA flares among patients with established disease, compared with those in the early stages of RA. Therefore, TNF blocker therapy should be restarted as soon as possible after surgery to prevent flare, Dr. Grant said.
The American College of Rheumatology and British Society of Rheumatology recommend holding TNF-alpha antagonists for one dosing cycle before major surgery. For etanercept (Enbrel), that translates to a 1-week before surgery hold, for infliximab (Remicade) 6-8 weeks, and for adalimumab (Humira) 2 weeks. These agents should also be held for 10-14 days after surgery or until wound healing is satisfactory.
"It’s probably safe to continue these medications for minor surgeries," Dr. Grant said.
Other agents
The anti-CD20 agent rituximab (Rituxan) – currently used to treat RA, vasculitis, hematologic malignancies, and other conditions – has a lower risk for bacterial infections than does TNF-alpha antagonists and has been shown to be safe in patients with a history of recurrent bacterial infections.
"Hydroxychloroquine (or Plaquenil) is felt to be safe during the preoperative period. It is recommended to continue this medication without stopping," Dr. Grant said.
There is conflicting information on infection risk with the use leflunomide (Arava), but it may be wise to stop therapy 2-4 weeks before nonurgent surgery in higher-risk patients.
There is consensus that sulfasalazine (Azulfidine) and azathioprine (Imuran) can be safely continued perioperatively, he said, although some advise holding sulfasalazine on the day of surgery.
Regarding perioperative steroids, Dr. Grant recommended determining the patient’s steroid exposure over the past year.
"Stress dose steroids are not routinely needed as long as the patients continue their normal dose. That’s really the important piece: If someone’s taking prednisone every day, make sure they take at least that dose on the day of surgery," he said.
Dr. Grant reported having no financial disclosures.
SCOTTSDALE, ARIZ. – When patients on immunosuppressive therapies need surgery, the risks of disease flare and compromised postoperative recovery and rehabilitation must be weighed against the risk of increased infections and impaired wound healing.
"I’m not sure that there is necessarily a right answer, but I think most people would stop biologic [agents] beforehand," Dr. Paul Grant said at a meeting on perioperative medicine sponsored by the University of Miami.
The decision whether to suspend a disease-modifying antirheumatic drug before surgery may depend on the individual drug and on the patient, said Dr. Grant, director of perioperative and consultative medicine at the University of Michigan Health System in Ann Arbor.
For example, it appears to be safe for patients on methotrexate to continue on therapy during elective orthopedic surgery. Evidence for this comes from a randomized clinical trial in which patients with rheumatoid arthritis (RA) were assigned to either continue on methotrexate (MTX) or suspend taking it for 2 weeks before and 2 weeks after surgery. The study also contained a control of patients with RA who were not on MTX (Ann. Rheum. Dis. 2001;60:214-7).
The investigators found that there were no significant differences in early complication rates or in complications up to 1 year of follow-up between patients who suspended or remained on MTX. Patients who stayed on the drug had significantly lower rates of RA flare.
Additionally, two systematic reviews, one looking at eight studies echoes the findings of the aforementioned randomized trial, and the other looking at four studies, in which the reviewer concluded that "continued MTX therapy appears to be safe perioperatively and seems also to be associated with a reduced risk of flares (Clin. Exp. Rheumatol. 2009;27:856-62) (Clin. Rheumatol. 2008;27:1217-20).None of the examined papers addresses the issue of safety in connection with comorbidities, age, or high doses of methotrexate."
"The bottom line here is that methotrexate should be continued for most surgeries. I think it might be reasonable to hold it in certain situations, for example if the patient has pretty bad kidney or liver disease, or if it’s surgery to treat a major infection," Dr. Grant said.
TNF-alpha antagonists
In contrast, the data on tumor necrosis factor–alpha (TNF-alpha) antagonists are fuzzier, with limited and conflicting information on perioperative use of these agents (etanercept, infliximab, adalimumab, certolizumab, golimumab).
"The major concern with these drugs is infection," Dr. Grant said. He pointed to a meta-analysis published in JAMA in 2006, which showed that taking the drugs doubled the risk of serious infections in general. The study did not specifically look at perioperative use of TNF-alpha antagonists (JAMA 2006;295:2275-85).
A retrospective cohort studyof 127 patients with RA who were undergoing various orthopedic procedures found that there were no differences in surgical site infections but more cases of wound dehiscence in patients who continued on the drugs, compared with those who interrupted their use perioperatively (Clin. Exp. Rheumatol. 2007;25:430-6).
A second, prospective study in 31 patients with RA undergoing foot/ankle surgery found that there were no significant differences in infection or healing between patients who interrupted therapy and those who did not (Foot Ankle Clin. 2007;12:509-24).
Other studies and systematic reviews in patients with RA or Crohn’s disease generally found no significant differences in serious infection rates, but they did detect a higher incidence of skin and soft-tissue infections among patients on anti-TNF-alpha agents vs. other disease-modifying antirheumatic drugs.
The risk of infections tends to be highest at the start of therapy with a TNF-alpha antagonist and stopping therapy is more likely to result in RA flares among patients with established disease, compared with those in the early stages of RA. Therefore, TNF blocker therapy should be restarted as soon as possible after surgery to prevent flare, Dr. Grant said.
The American College of Rheumatology and British Society of Rheumatology recommend holding TNF-alpha antagonists for one dosing cycle before major surgery. For etanercept (Enbrel), that translates to a 1-week before surgery hold, for infliximab (Remicade) 6-8 weeks, and for adalimumab (Humira) 2 weeks. These agents should also be held for 10-14 days after surgery or until wound healing is satisfactory.
"It’s probably safe to continue these medications for minor surgeries," Dr. Grant said.
Other agents
The anti-CD20 agent rituximab (Rituxan) – currently used to treat RA, vasculitis, hematologic malignancies, and other conditions – has a lower risk for bacterial infections than does TNF-alpha antagonists and has been shown to be safe in patients with a history of recurrent bacterial infections.
"Hydroxychloroquine (or Plaquenil) is felt to be safe during the preoperative period. It is recommended to continue this medication without stopping," Dr. Grant said.
There is conflicting information on infection risk with the use leflunomide (Arava), but it may be wise to stop therapy 2-4 weeks before nonurgent surgery in higher-risk patients.
There is consensus that sulfasalazine (Azulfidine) and azathioprine (Imuran) can be safely continued perioperatively, he said, although some advise holding sulfasalazine on the day of surgery.
Regarding perioperative steroids, Dr. Grant recommended determining the patient’s steroid exposure over the past year.
"Stress dose steroids are not routinely needed as long as the patients continue their normal dose. That’s really the important piece: If someone’s taking prednisone every day, make sure they take at least that dose on the day of surgery," he said.
Dr. Grant reported having no financial disclosures.
AT THE PERIOPERATIVE MEDICINE SUMMIT
Major finding: Some immunomodulating agents for inflammatory and autoimmune diseases can be safely continued in the perioperative period.
Data source: A review of evidence on the use of various immunomodulators.
Disclosures: Dr. Grant reported having no financial disclosures.