User login
High-dose statins don’t prevent postop AF
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.
The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
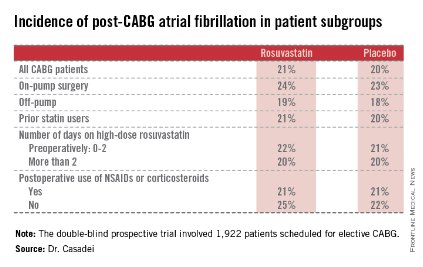
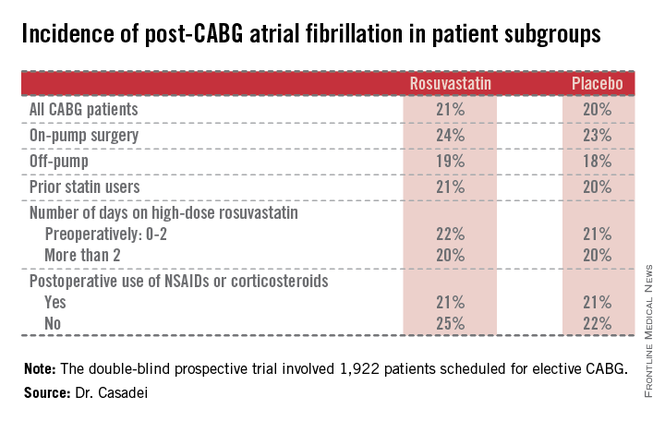
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
AT THE ESC CONGRESS 2014
Key clinical point: Perioperative statin therapy in patients undergoing CABG failed to protect against new-onset postop atrial fibrillation.
Major finding: The incidence of postop atrial fibrillation within 5 days post-CABG was 21% in patients randomized to 20 mg/day of rosuvastatin and 20% in placebo-treated controls.
Data source: The multicenter STICS trial included 1,922 randomized patients scheduled for elective CABG.
Disclosures: STICS was funded by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. The presenter reported having received a research grant from AstraZeneca.
Anticoagulation options in trauma are expanding
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation ( J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin- 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio, and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-9). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk

|
|
Dr. Frank Podbielski, FCCP, comments: Replacement of blood clotting factors in trauma (and other medical settings) has long been a challenge given the logistics of transfusion and shelf life of blood products. Advances in technology that have yielded powdered forms of blood clotting factors offer clinicians a greater degree of latitude in resuscitation of these patients.
Dr. Francis J. Podbielski, FCCP, is Visiting Clinical Associate Professor of Surgery at the University of Illinois at Chicago - College of Medicine and the Medical Director of the lung cancer program at Jordan Hospital in Plymouth, Massachusetts.

|
|
Dr. Frank Podbielski, FCCP, comments: Replacement of blood clotting factors in trauma (and other medical settings) has long been a challenge given the logistics of transfusion and shelf life of blood products. Advances in technology that have yielded powdered forms of blood clotting factors offer clinicians a greater degree of latitude in resuscitation of these patients.
Dr. Francis J. Podbielski, FCCP, is Visiting Clinical Associate Professor of Surgery at the University of Illinois at Chicago - College of Medicine and the Medical Director of the lung cancer program at Jordan Hospital in Plymouth, Massachusetts.

|
|
Dr. Frank Podbielski, FCCP, comments: Replacement of blood clotting factors in trauma (and other medical settings) has long been a challenge given the logistics of transfusion and shelf life of blood products. Advances in technology that have yielded powdered forms of blood clotting factors offer clinicians a greater degree of latitude in resuscitation of these patients.
Dr. Francis J. Podbielski, FCCP, is Visiting Clinical Associate Professor of Surgery at the University of Illinois at Chicago - College of Medicine and the Medical Director of the lung cancer program at Jordan Hospital in Plymouth, Massachusetts.
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation ( J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin- 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio, and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-9). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation ( J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin- 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio, and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-9). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
Guideline adds clarity on perioperative beta-blockers
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious, and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Pprofessor ofAnesthesiology anesthesiology andCritical criticalCare care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI) and the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert

|
|
Dr. Jun Chiong, FCCP, comments: The largest randomized controlled trial (RCT) ever undertaken in perioperative medicine, PeriOperative Ischemia Study Evaluation trial (POISE), showed that perioperative beta-blockade decreased cardiac risks but increased all-cause mortality and the risk of disabling stroke.
These findings called for a thorough review of previous guidelines and accepted practice.
Several editorials and comments followed the publication of POISE. As clinicians, we have to keep in mind that guidelines also advocate the careful assessment of patient- and surgery-specific risk factors in determining who should receive therapy that may benefit or, conversely, be exposed to harm by the introduction of beta-blockade before non-cardiac surgery.
Jun Chiong, M.D., FCCP, is an Associate Clinical Professor of Medicine, Pharmacy, and Outcomes Science at Loma Linda University, Loma Linda, CA.

|
|
Dr. Jun Chiong, FCCP, comments: The largest randomized controlled trial (RCT) ever undertaken in perioperative medicine, PeriOperative Ischemia Study Evaluation trial (POISE), showed that perioperative beta-blockade decreased cardiac risks but increased all-cause mortality and the risk of disabling stroke.
These findings called for a thorough review of previous guidelines and accepted practice.
Several editorials and comments followed the publication of POISE. As clinicians, we have to keep in mind that guidelines also advocate the careful assessment of patient- and surgery-specific risk factors in determining who should receive therapy that may benefit or, conversely, be exposed to harm by the introduction of beta-blockade before non-cardiac surgery.
Jun Chiong, M.D., FCCP, is an Associate Clinical Professor of Medicine, Pharmacy, and Outcomes Science at Loma Linda University, Loma Linda, CA.

|
|
Dr. Jun Chiong, FCCP, comments: The largest randomized controlled trial (RCT) ever undertaken in perioperative medicine, PeriOperative Ischemia Study Evaluation trial (POISE), showed that perioperative beta-blockade decreased cardiac risks but increased all-cause mortality and the risk of disabling stroke.
These findings called for a thorough review of previous guidelines and accepted practice.
Several editorials and comments followed the publication of POISE. As clinicians, we have to keep in mind that guidelines also advocate the careful assessment of patient- and surgery-specific risk factors in determining who should receive therapy that may benefit or, conversely, be exposed to harm by the introduction of beta-blockade before non-cardiac surgery.
Jun Chiong, M.D., FCCP, is an Associate Clinical Professor of Medicine, Pharmacy, and Outcomes Science at Loma Linda University, Loma Linda, CA.
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious, and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Pprofessor ofAnesthesiology anesthesiology andCritical criticalCare care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI) and the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious, and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Pprofessor ofAnesthesiology anesthesiology andCritical criticalCare care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI) and the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
Ex vivo lung perfusion device preserves donor organs
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lungs to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating them, "which oxygenates the cells and makes it possible for the transplant team to examine the lung’s’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end-stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About 1 in 5 donor lungs meets the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage.
"Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.

|
|
Dr. Jennifer Cox, FCCP, comments: This is exciting news given the shortage of available lungs that meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in post operative complications. Also, how significant will the financial impact be using the device. I look forward to the results of long- term studies. Hopefully this will be a viable option for our patients.
Dr. Jennifer D. Cox, FCCP, is an Assistant Professor of Pulmonary and Critical Care Medicine and clerkship director for the fourth-year medical student Critical Care Selective, Morsani College of Medicine, University of South Florida, in Tampa, Florida.

|
|
Dr. Jennifer Cox, FCCP, comments: This is exciting news given the shortage of available lungs that meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in post operative complications. Also, how significant will the financial impact be using the device. I look forward to the results of long- term studies. Hopefully this will be a viable option for our patients.
Dr. Jennifer D. Cox, FCCP, is an Assistant Professor of Pulmonary and Critical Care Medicine and clerkship director for the fourth-year medical student Critical Care Selective, Morsani College of Medicine, University of South Florida, in Tampa, Florida.

|
|
Dr. Jennifer Cox, FCCP, comments: This is exciting news given the shortage of available lungs that meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in post operative complications. Also, how significant will the financial impact be using the device. I look forward to the results of long- term studies. Hopefully this will be a viable option for our patients.
Dr. Jennifer D. Cox, FCCP, is an Assistant Professor of Pulmonary and Critical Care Medicine and clerkship director for the fourth-year medical student Critical Care Selective, Morsani College of Medicine, University of South Florida, in Tampa, Florida.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lungs to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating them, "which oxygenates the cells and makes it possible for the transplant team to examine the lung’s’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end-stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About 1 in 5 donor lungs meets the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage.
"Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lungs to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating them, "which oxygenates the cells and makes it possible for the transplant team to examine the lung’s’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end-stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About 1 in 5 donor lungs meets the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage.
"Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
VEGF-A value may stratify risk in pediatric heart transplant recipients
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
This was a very nice preliminary study but extremely limited in scope, as it has few patients and limited mechanistic studies. Most importantly, there was no validation cohort as is required to have confidence that a biomarker is predictive. A lot more work will be necessary before significance can be assigned to the use of VEGF-A as a potential biomarker.
Dr. Daniel R. Salomon is a professor and program medical director at the Scripps Center for Organ Transplantation, Scripps Research Institute, La Jolla, Calif. He was the cochair at the session where the research was presented, and made his remarks in an interview. He had no relevant conflicts of interest.
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
SAN FRANCISCO – Monitoring plasma vascular endothelial growth factor A (VEGF-A) may help identify pediatric heart transplant patients who are at increased risk for poor outcomes, according to a study reported at the 2014 World Transplant Congress.
"Cardiac allograft vasculopathy [CAV] remains the leading cause of chronic allograft failure after heart transplantation. ... Therefore, it’s important for us to be able to anticipate the development of CAV and open up a therapeutic window," said Dr. Kevin P. Daly of Harvard Medical School and Boston Children’s Hospital.
"Our pilot data suggest that plasma VEGF-A levels below 90 pg/mL identify a low-risk patient population in whom a decreased frequency of coronary angiography can be considered. Future studies are needed to determine if using plasma VEGF-A levels to modify CAV screening frequency results in equivalent patient outcomes, with decreased resource utilization and improved quality of life," Dr. Daly commented at the congress, which was sponsored by the American Society of Transplant Surgeons.
As the vascular endothelium is the primary target of the immune response in CAV, the researchers hypothesized that VEGF-A likely contributes to an inflammatory cycle that leads to vascular damage and occlusion in the graft.
Participants in the single-center prospective cohort study were 44 consecutive children aged 2 years or older who were at least 18 months (median, 6 years) out from heart transplantation. They were scheduled for routine annual screening coronary angiography during 2009, and had no or mild CAV.
Moderate or severe CAV developed in 32% of patients who had VEGF-A values above the median value at baseline (90 pg/mL), compared with 5% of patients who had VEGF-A values below the median level (P = .02). Patients who developed this vasculopathy were more likely to die (38% vs. 0%), undergo retransplantation (38% vs. 0%), experience a myocardial infarction (12% vs. 0%), and be listed for retransplantation (12% vs. 0%).
"While this is a biomarker and we have shown it is associated with CAV, we have not shown that it is causal," Dr. Daly cautioned. Any treatment directed against VEGF would have to be conducted in the context of a clinical trial to assess its impact.
A subset of patients becomes nonadherent to therapy; a subset that is highly sensitized before transplant may have donor-specific antibody, Dr. Daly said. So "we don’t think we fully understand the inciting event, ... [but] VEGF-A has been shown before to be elevated in antibody-mediated rejection, so it’s not surprising to see this association."
"We didn’t have these data available clinically because it was all a research study, so we didn’t intervene on any of the patients in this cohort. But we have started to think about whether or not we could use VEGF-A levels at least in our ... patients who might not have arterial access, and it might be difficult to survey them for CAV. I think in order to really understand the appropriate way to use it, we would need a larger study," he remarked.
Dr. Daly disclosed that he had no conflicts of interest relevant to the study.
AT THE 2014 WORLD TRANSPLANT CONGRESS
Key clinical point: Plasma VEGF-A levels may be a biomarker of risk for pediatric heart transplant patients.
Major finding: Patients with plasma VEGF-A levels above the median value of 90 pg/mL had a 32% rate of moderate or severe cardiac allograft vasculopathy within 5 years.
Data source: A prospective cohort study of 44 consecutive children who had undergone heart transplantation.
Disclosures: Dr. Daly disclosed no relevant conflicts of interest.
Many surgical residents consider quitting during training
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
FROM JAMA Surgery
Major finding: More than half of survey respondents (58%) considered quitting their general surgery residency, an issue more persistent with female respondents.
Data source: Analysis of 288 responses to a survey of general surgery residents in 13 residency programs across different regions (West, Southwest, Midwest, and Northeast) and training centers (university programs, independent programs, or hybrid university-affiliated programs without an onsite university or medical school).
Disclosures: The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Findings support endovascular-first approach for ruptured VAAs
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a meeting hosted by the Society for Vascular Surgery.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures.
AT THE 2014 VASCULAR ANNUAL MEETING
Key clinical point: The researchers recommend aggressive treatment of visceral artery pseudoaneurysms and true aneurysms, with an endovascular-first approach to treating ruptured aneurysms.
Major finding: Thirty-day mortality was 7.4% vs. 26% with endovascular vs. open repair of ruptured VAAs.
Data source: A retrospective chart review involving 174 cases.
Disclosures: Dr. Shukla reported having no disclosures.
VIDEO: Consider cognitive function in elderly before surgery
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
AT AAIC 2014
Achieving aortic arch replacement without deep hypothermic circulatory arrest
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
AT AATS AORTIC SYMPOSIUM 2014
Key clinical point: By maintaining cardiac and distal body perfusion, complex reconstructions can be accomplished without concern about organ protection, allowing complete correction of aortic pathology.
Major finding: A method that avoids deep hypothermic circulatory arrest was associated with low mortality (3.2%) and low cerebral morbidity (5%).
Data source: Cohort study of 62 patients.
Disclosures: Dr. Matalanis reported having no relevant financial disclosures.
Stentless aortic bioprosthesis: Good 1-year outcomes, ‘remarkable’ functional improvement
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
AT THE AATS ANNUAL MEETING
Key clinical point: The valve is implanted with a single suture line in a supra-annular position, with no contact with the native annulus.
Major finding: Thirty-day mortality and 1-year mortality after implantation of the stentless Freedom Solo bioprosthesis were 1.4% and 4.4%, respectively.
Data source: Nonrandomized, prospective study of 616 patients at 18 centers in eight European countries.
Disclosures: Dr. Thalmann is a consultant for the Sorin Group, which funded the study.