User login
Venous thromboembolism common after heart transplant
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
Key clinical point: Venous thromboembolism (VTE) was common after heart transplant, especially when patients had relevant risk factors and were not on anticoagulants.
Major finding: Cumulative incidence of VTE was 8.5% during eight years of follow-up and was much higher during the first year after transplant.
Data source: Single-center retrospective cohort study of 635 heart transplant recipients.
Disclosures: The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
Fast-track protocol cuts lung resection complications, LOS
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
CHICAGO – An enhanced recovery pathway reduces short-term complications and hospital stays following cancer-related lung resection without raising readmissions or emergency visits after discharge, a study showed.
“A multimodal pathway for open, elective lobectomy seems to improve efficiency and quality of care,” Dr. Amin Madani, from McGill University in Montreal, said at the annual meeting of the Central Surgical Association (CSA).
Prior research suggests that an enhanced recovery pathway (ERP), also known as fast-track protocols, can improve surgical outcomes, but there is little evidence to support its use and effectiveness in lung resection.
Surgeons at McGill established an integrated, multimodal approach to perioperative care of these patients after creating a written, evidence-based, step-by-step pathway. Key elements are standardized preoperative patient education; removal of urine drains on postoperative day 1; removal of the last chest tube by postop (POD) day 3, if there is <300 cc of drainage in 24 hours and no air leak; ambulation goals of more than 75 m thrice-daily by POD 3; introduction of solid food on POD 1; and a target discharge of POD 4; Dr. Madani explained.
To examine the effectiveness of the pathway, the authors retrospectively analyzed outcomes in 127 patients undergoing elective lung resection for primary or secondary lung cancer receiving traditional care and 107 patients treated after the ERP was implemented in September 2012. At baseline, the two groups were similar with respect to age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) scores, pulmonary function, and smoking history.
Hospital length of stay was significantly reduced after the ERP from a median of 7 days with traditional care to 6 days (P < .01), driven largely by patients with an uncomplicated hospital course who were discharged after a median of 5 days after the pathway was implemented, Dr. Madani said.
It was not the case that patients went home too early, as readmissions (5% vs. 6%) and ED visits (3% vs. 5%) were similar between both groups, he added.
After the pathway was implemented, patients had earlier Foley catheter removal (POD 2 vs. 1), IV discontinuation (POD 3 vs. 2), ambulation (POD 2 vs. 1), last chest tube removal (POD 5 vs. 4), and epidural removal (POD 5 vs. 4).
The enhanced recovery pathway group had fewer overall complications than did the traditional care group (37% vs. 50%; P = .03), a threefold decrease in urinary tract infections (3% vs. 12%; P < .01), and a trend toward fewer pulmonary complications (25% vs. 31%; P = .38) and surgical site infections (1% vs. 6%; P = .07), he said.
Despite significantly earlier removal of chest tubes after the pathway, there was no difference in the incidence of pneumothorax or pleural effusion requiring tube re-insertion, affirming that “Chest tubes were not being removed too early, causing harm to patients,” Dr. Madani said.
In multivariate regression analysis adjusted for age, sex, BMI, and ASA score, there was a significant negative association between implementation of an enhanced recovery pathway and length of stay (beta, –0.18; P < .01) and complications (odds ratio, 0.46; P < .01), but not readmissions (OR, 1.59; P = .44).
Early removal of chest tubes and urinary catheter were independent predictors of decreased length of stay.
Dr. L. Michael Brunt, a discussant from Washington University in St. Louis, said the development of care pathways to enhance recovery after surgery is gaining a lot of interest in the surgical community, but went on to ask how much it cost to implement.
The overall cost of the surgeon-driven initiative, involving multiple pathways for various surgical procedures, is about $120,000 annually, or $100/patient for the 1,200 patients undergoing surgery using an ERP program at the McGill University Health Centre each year, Dr. Madani said. This cost also includes a full-time nurse practitioner now serving as the pathway coordinator and roughly $13,000 for patient education booklets, but no additional staff.
An audience member questioned whether the authors have identified factors predicting which patients would fail to meet pathway goals, observing that in the colorectal field, there are patients such as the 80-year-old, narcotic-naive woman with diabetes, who simply won’t progress.
“That’s a very good point, and I agree there are some patients whom you can’t fast track,” Dr. Madani replied. “Part of the deal here is that, yes, we have this protocolized pathway; however, the surgeon still has the right to change that if they feel it is important. We didn’t look at the specifics of which patient [factors] achieved adherence, but we could at some point in the future.”
CSA president and session moderator Christopher McHenry, from MetroHealth Medical Center in Cleveland, said he was impressed with the study and called the findings very believable.
“I think all of these recovery pathways can be very beneficial,” Dr. McHenry said in an interview. “It helps us re-look at how we’re managing our patients and see if there are ways that we can improve on their postoperative management that may lead to earlier discharge.”
The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: An enhanced recovery pathway reduces complications and hospital stay following lung cancer resection without raising readmissions or ED visits.
Major finding: Patients in the enhanced recovery pathway vs. traditional care had fewer overall complications (37% vs. 50%; P = .03) and threefold fewer UTIs (3% vs. 12%; P < .01).
Data source: Observational study of 234 patients undergoing lung resection.
Disclosures: The study was funded by an investigator-initiated research grant from Ethicon Canada. Dr. Madani, his coauthors, Dr. Brunt, and Dr. McHenry reported having no financial conflicts.
Ranolazine plus beta-blockers might prevent postop AF
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Ranolazine’s protective effect seems to come at the cost of symptomatic hypotension in the first 3 days after surgery.
Major finding: Postop atrial fibrillation occurred in 6 (10.5%) of 57 patients in the ranolazine group and 26 (45.6%) of 57 matched controls (P < .0001).
Data source: Retrospective cohort study of postop follow-up in 114 adults who had cardiac surgery.
Disclosures: There was no outside funding for the work. The investigators said they have no financial relationship with Gilead, maker of ranolazine (Ranexa).
CHADS2 predicts postop atrial fibrillation
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Postop atrial fibrillation is more likely if patients go into surgery with an elevated CHADS 2 score.
Major finding: For every unit increase in baseline CHADS2 score, there is a 17% increase in the risk of new-onset AF following major vascular or thoracic surgery (HR 1.17, 95% CI 1.04-1.31).
Data source: Retrospective chart review of 1,550 adult patients.
Disclosures: The investigators said they had no disclosures. No outside funding was reported for the work.
Hybrid revascularization remains a rare bird
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.
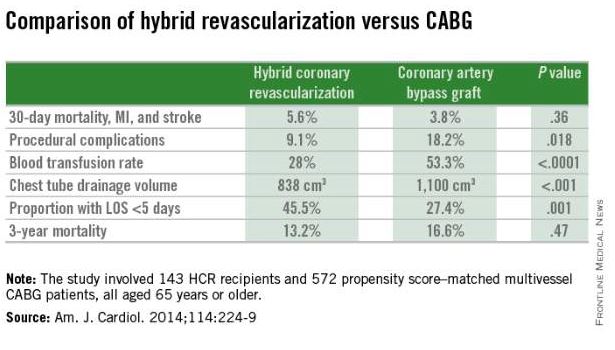
He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
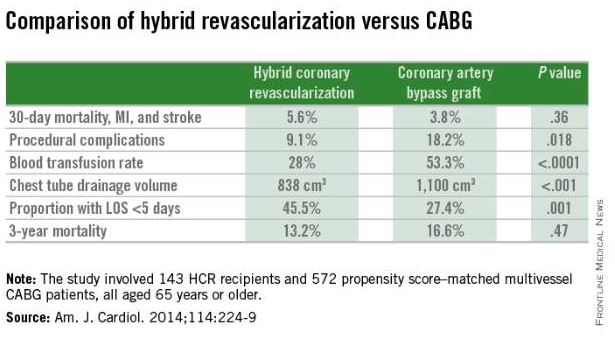
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Baseline QOL measures not associated with outcomes in high-risk operable lung cancer patients
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Baseline quality-of-life measures were not predictive of outcomes after lung cancer surgery in high-risk operable patients.
Major finding: A significantly greater improvement in the physical component of quality of life at 3 months and in dyspnea at 1 year was seen from using VATS, compared with thoracotomy.
Data source: Researchers reviewed self-assessment QOL data from 212 eligible high-risk operable patients from the ACSOG Z4032 trial who had sublobar resections with or without brachytherapy using VATS or thoracotomy.
Disclosures: The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
VIDEO: Cast a wider net for intimate partner and sexual violence
LAKE BUENA VISTA, FLA. – Screening for intimate partner and sexual violence occurs sporadically in trauma centers, typically among women and when there is a suspicious mechanism of injury.
In hopes of reaching a broader population, investigators at the Ryder Trauma Center at the University of Miami conducted a prospective pilot study to determine the feasibility of universal screening for intimate partner and sexual violence in all patients presenting at the level I trauma center.
In all, 399 consecutive patients were eligible, with 40% screened using a four-item questionnaire that asks patients how often their partner physically hurt, insulted, threatened with harm, and screamed at them (HITS) and the SAVE (screen, ask, validate, evaluate) method developed in Florida to screen for sexual violence. Both instruments were available in English, Spanish, and Haitian French.
Over a 4-month period, 14% of patients scored positive for physical and psychological abuse – and even more surprising, 8% scored positive for sexual violence, lead study author Dr. Tanya Zakrison reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
“Interestingly, there was no significant difference between men and women. They were all scoring at an equal rate,” Dr. Zakrison said. Patients who scored positive on HITS or SAVE were also found across all ethnicities and all age groups.
“The last thing that was quite surprising for us to find was that only 14% of patients who were HITS positive were actually admitted as a result of intimate partner or interpersonal violence, and none of the patients who scored positive for SAVE were admitted to trauma because of this,” she added. “Again, they’re coming in for the motor vehicle collisions, falls from standing, or other mechanisms of injury.”
To learn more about the intervention and its impact at Ryder Trauma, watch our video interview.
Dr. Zakrison and her coauthors reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAKE BUENA VISTA, FLA. – Screening for intimate partner and sexual violence occurs sporadically in trauma centers, typically among women and when there is a suspicious mechanism of injury.
In hopes of reaching a broader population, investigators at the Ryder Trauma Center at the University of Miami conducted a prospective pilot study to determine the feasibility of universal screening for intimate partner and sexual violence in all patients presenting at the level I trauma center.
In all, 399 consecutive patients were eligible, with 40% screened using a four-item questionnaire that asks patients how often their partner physically hurt, insulted, threatened with harm, and screamed at them (HITS) and the SAVE (screen, ask, validate, evaluate) method developed in Florida to screen for sexual violence. Both instruments were available in English, Spanish, and Haitian French.
Over a 4-month period, 14% of patients scored positive for physical and psychological abuse – and even more surprising, 8% scored positive for sexual violence, lead study author Dr. Tanya Zakrison reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
“Interestingly, there was no significant difference between men and women. They were all scoring at an equal rate,” Dr. Zakrison said. Patients who scored positive on HITS or SAVE were also found across all ethnicities and all age groups.
“The last thing that was quite surprising for us to find was that only 14% of patients who were HITS positive were actually admitted as a result of intimate partner or interpersonal violence, and none of the patients who scored positive for SAVE were admitted to trauma because of this,” she added. “Again, they’re coming in for the motor vehicle collisions, falls from standing, or other mechanisms of injury.”
To learn more about the intervention and its impact at Ryder Trauma, watch our video interview.
Dr. Zakrison and her coauthors reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAKE BUENA VISTA, FLA. – Screening for intimate partner and sexual violence occurs sporadically in trauma centers, typically among women and when there is a suspicious mechanism of injury.
In hopes of reaching a broader population, investigators at the Ryder Trauma Center at the University of Miami conducted a prospective pilot study to determine the feasibility of universal screening for intimate partner and sexual violence in all patients presenting at the level I trauma center.
In all, 399 consecutive patients were eligible, with 40% screened using a four-item questionnaire that asks patients how often their partner physically hurt, insulted, threatened with harm, and screamed at them (HITS) and the SAVE (screen, ask, validate, evaluate) method developed in Florida to screen for sexual violence. Both instruments were available in English, Spanish, and Haitian French.
Over a 4-month period, 14% of patients scored positive for physical and psychological abuse – and even more surprising, 8% scored positive for sexual violence, lead study author Dr. Tanya Zakrison reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma (EAST).
“Interestingly, there was no significant difference between men and women. They were all scoring at an equal rate,” Dr. Zakrison said. Patients who scored positive on HITS or SAVE were also found across all ethnicities and all age groups.
“The last thing that was quite surprising for us to find was that only 14% of patients who were HITS positive were actually admitted as a result of intimate partner or interpersonal violence, and none of the patients who scored positive for SAVE were admitted to trauma because of this,” she added. “Again, they’re coming in for the motor vehicle collisions, falls from standing, or other mechanisms of injury.”
To learn more about the intervention and its impact at Ryder Trauma, watch our video interview.
Dr. Zakrison and her coauthors reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EAST SCIENTIFIC ASSEMBLY
ECMO alone before lung transplant linked to good survival rates
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
SAN DIEGO – Extracorporeal membrane oxygenation with spontaneous breathing is the optimal bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantation, based on data from over 18,000 patients who received lung transplants.
In the study, patients on extracorporeal membrane oxygenation (ECMO) alone had outcomes that were comparable to those of patients requiring no invasive support prior to transplantation, Dr. Matthew Schechter of Duke University in Durham, N.C., reported at the annual meeting of the Society of Thoracic Surgeons.
Dr. Schechter and his colleagues analyzed the United Network for Organ Sharing database for all adult patients who underwent lung transplantations between January 2000 and September 2013.
The 18,392 patients selected for study inclusion were divided into cohorts based on the type of preoperative support they received: ECMO with mechanical ventilation; ECMO only; ventilation only; and no support of any kind. Nearly 95% of the patients received no invasive preoperative support. Over 4% received mechanical ventilation alone, less than 1% received ECMO with mechanical ventilation, and about 0.5%) received ECMO only.
By using Kaplan-Meier survival analyses with log-rank testing, Dr. Schechter and his associates were able to compare survival rates for each type of preoperative support. Cox regression models were used to ascertain whether any particular type of preoperative support could definitively be associated with mortality.
At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively. Survival rates at 3 years after transplant were 38% in patients who received ECMO and mechanical ventilation and 52% in patients who received mechanical ventilation alone. The survival advantage in the ECMO only and no support groups was significantly better when compared to the ECMO and mechanical ventilation and the mechanical ventilation alone cohorts (P < .0001).
The findings held up after a multivariate analysis; the hazard ratio was 1.96 (95% confidence interval, 1.36-2.84) for ECMO with mechanical ventilation and 1.52 (95% CI, 1.31-1.78) for mechanical ventilation only (P < .0001 for both).
ECMO alone was not associated with any significant change in survival rate (HR = 1.07; 95% CI, 0.57-2.01; P = .843).
Patients who received just ECMO had the shortest lengths of stay after lung transplant. They also had the lowest rate of acute rejection prior to discharge, although not to an extent that was statistically significant. The incidence of new-onset dialysis was highest in patients who received ECMO with mechanical ventilation.
“ECMO alone may provide a survival advantage over other bridging strategies,” Dr. Schechter concluded. “One advantage of using ECMO only is an avoidance of the risks that come with mechanical ventilation, [which] include generalized muscle atrophy, maladapted muscle fiber remodeling in the diaphragm – which leads to a decrease in the overall durability of this muscle – as well as the induction of the pulmonary and systemic inflammatory risk responses, [all of which] have been shown to affect outcomes following lung transplantation.”
Dr. Schechter explained that patients receiving ECMO without mechanical ventilation can actively rehabilitate themselves post transplantation since nonintubated ECMO patients can participate in physical therapy.
Further study is needed to find an optimal way of assessing patients and determining exactly which ones would be best suited for ECMO with spontaneous breathing support, he said.
Dr, Schechter had no relevant financial disclosures.
FROM THE STS ANNUAL MEETING
Key clinical point: ECMO with spontaneous breathing should be considered the preferred bridging strategy for patients who have rapidly advancing pulmonary disease and are awaiting lung transplantations.
Major finding: At 3 years post transplantation, the survival rates of patients on ECMO alone and of those who received no preoperative support of any kind were comparable at 66% and 65%, respectively.
Data source: Retrospective analysis of 18,392 adult patients in the United Network for Organ Sharing database.
Disclosures: Dr. Schechter had no relevant financial disclosures.
FDA approves vagal blocking device for obesity
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.
Bundled intervention tackles S. aureus SSIs
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
PHILADELPHIA – A bundled intervention including Staphylococcus aureus screening, decolonization, and targeted perioperative prophylaxis significantly decreased the rate of complex S. aureus surgical site infections in a multicenter quasi-experimental effectiveness study of patients undergoing cardiac operations or total joint arthroplasty.
The pooled rate of complex S. aureus surgical site infections (SSIs) decreased from 0.36% following 28,218 procedures performed during the preintervention period to 0.20% after 14,316 procedures performed during the intervention period (rate ratio, 0.58), Dr. Loreen A. Herwaldt of the University of Iowa, Iowa City, reported at an annual scientific meeting on infectious diseases.
Further, the number of months with no complex S. aureus SSIs increased from 2 of 39 months (5.1%) to 8 of 22 months (36.4%) Dr. Herwaldt said, noting that the median rate and range of complex SSIs became zero by intervention month 4.
The decrease in SSIs was greatest for joint arthroplasties, she said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Subgroup analyses also demonstrated significantly lower rates of complex SSIs for scheduled vs. nonscheduled or emergent operations (rate ratio, 0.55), fully adherent vs. partially or nonadherent operations (rate ratio, 0.26), and for operations in which the surgeon (in accordance with hospital participation) implemented at least some bundle elements vs. no bundle elements (rate ratio, 0.54), she said, explaining that surgeons could opt out of the study even if a hospital was participating.
The rate of complex SSIs caused by any pathogen also was reduced (rate ratio, 0.67).
“We were very pleased to note that gram negative SSIs did not increase. The rate ratio was 0.86, and the confidence interval did cross 1 and the P value was 0.67,” she said.
The study, known as STOP SSI, was conducted at 20 Hospital Corporation of America (HCA) hospitals in nine states from March 1, 2009, to March 31, 2014. Patients who tested positive for methicillin-resistant or methicillin-susceptible S. aureus on a preoperative nares screen within 30 days before surgery were asked to apply mupirocin intranasally twice daily for 5 days and to bathe with chlorhexidine gluconate once daily for 5 days prior to their operation, including on the night before and the morning of surgery. Those who tested negative for MRSA and MSSA bathed with chlorhexidine gluconate only on the night before surgery and the morning of surgery.
Those with MRSA were treated with vancomycin and cefazolin perioperatively, and those without MRSA received only cefazolin.
If the patient’s status was unknown at the time of the operation, the goal was to have the patient bathe in chlorhexidine and to give as many intranasal doses of mupirocin as possible before surgery. The patient was treated perioperatively with vancomycin and cefazolin, and if it was later determined that the patient was positive for MRSA, the mupirocin was continued after surgery until the patient had been treated for 5 days.
After a 3-month phase-in period, 48% of the hospitals were fully compliant with this protocol, and 20% were partially compliant.
The use of a bundled intervention similar to the one used in this study was shown in a recent meta-analysis (BMJ 2013;346:f2743) to be likely to reduce the rate of S. aureus SSIs, but the approach had not been studied in a multicenter trial, Dr. Herwaldt said.
“Implementation of this SSI bundle was associated with significantly lower rates of complex S. aureus SSIs in the total cohort and in the hip and knee arthroplasty group. It was not associated with an increase in gram-negative SSIs, and thus we feel that if people actually did implement this bundle, it could substantially reduce patient morbidity and the cost of care,” she concluded, noting that the effect was seen only with implementation of the full bundle.
The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.
AT IDWEEK 2014
Key clinical point: Implementing a bundled intervention reduced S. aureus SSIs and could reduce patient morbidity and costs.
Major finding: The pooled S. aureus SSI rate decreased from 0.36% to 0.20% (rate ratio, 0.58).
Data source: A multicenter quasi-experimental effectiveness study of 42,534 procedures.
Disclosures: The Agency for Healthcare Research and Quality funded the study. Dr. Herwaldt reported having no disclosures.