User login
Ablation cuts AF recurrence 2.5-fold vs. amiodarone in heart failure
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.
But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
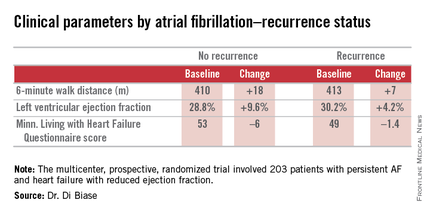
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.

But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.

But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
AT ACC 15
Key clinical point: Catheter ablation is hands down more effective than amiodarone for the treatment of persistent atrial fibrillation in patients with systolic heart failure.
Major finding: The rate of freedom from recurrent atrial fibrillation during 26 months of follow-up was 70% in patients randomized to catheter ablation, compared with 34% in those assigned to amiodarone.
Data source: The AATAC-AF study was a multicenter, randomized, prospective clinical trial inc 203 patients.
Disclosures: The trial was funded by the participating investigators and institutions without commercial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
VIDEO: Long-term PARTNER 1 data tip scales toward TAVR
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 15
Novel Watchman device approved as warfarin alternative in atrial fib
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
VIDEO: SAPIEN and CoreValve provide complementary TAVR options
SAN DIEGO – Long-term outcome results from the trials that compared the first-generation SAPIEN system and the CoreValve system for transcatheter aortic valve replacement (TAVR) have shown that both devices had excellent performance, compared with surgical aortic valve replacement.
The SAPIEN system continued to show similar performance, compared with surgery after 5-year follow-up, and the CoreValve showed even better superiority to surgery after 2 years, compared with first-year results.
But the performance of the two systems for TAVR should not be interpreted to suggest that CoreValve outperforms the SAPIEN system, cautioned Dr. Stephen Ramee during an interview at the annual meeting of the American College of Cardiology.
The CoreValve study began 4 years after the PARTNER trial that studied the SAPIEN valve, and during that intervening period, the cardiologists and cardiac surgeons who collaborate on TAVR learned important lessons on how to better select patients and how to avoid other risks during the procedure, said Dr. Ramee, an interventional cardiologist and director of the John Ochsner Heart and Vascular Institute in New Orleans.
As a consequence, the SAPIEN valve (currently the XT system) remains a very viable option, and Dr. Ramee said he generally used the SAPIEN XT system for about 60% of his cases. The major patient group best suited for CoreValve TAVR are patients with a highly calcified aortic-valve annulus, which is better suited to the self-expanding CoreValve because of a reduced risk for rupture of the annulus during balloon expansion with the SAPIEN valve.
Dr. Ramee has received honoraria from Edwards and Medtronic, the companies that respectively market the SAPIEN and CoreValve systems. He also has a financial interest in several companies developing new TAVR devices.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter mitchelzoler
SAN DIEGO – Long-term outcome results from the trials that compared the first-generation SAPIEN system and the CoreValve system for transcatheter aortic valve replacement (TAVR) have shown that both devices had excellent performance, compared with surgical aortic valve replacement.
The SAPIEN system continued to show similar performance, compared with surgery after 5-year follow-up, and the CoreValve showed even better superiority to surgery after 2 years, compared with first-year results.
But the performance of the two systems for TAVR should not be interpreted to suggest that CoreValve outperforms the SAPIEN system, cautioned Dr. Stephen Ramee during an interview at the annual meeting of the American College of Cardiology.
The CoreValve study began 4 years after the PARTNER trial that studied the SAPIEN valve, and during that intervening period, the cardiologists and cardiac surgeons who collaborate on TAVR learned important lessons on how to better select patients and how to avoid other risks during the procedure, said Dr. Ramee, an interventional cardiologist and director of the John Ochsner Heart and Vascular Institute in New Orleans.
As a consequence, the SAPIEN valve (currently the XT system) remains a very viable option, and Dr. Ramee said he generally used the SAPIEN XT system for about 60% of his cases. The major patient group best suited for CoreValve TAVR are patients with a highly calcified aortic-valve annulus, which is better suited to the self-expanding CoreValve because of a reduced risk for rupture of the annulus during balloon expansion with the SAPIEN valve.
Dr. Ramee has received honoraria from Edwards and Medtronic, the companies that respectively market the SAPIEN and CoreValve systems. He also has a financial interest in several companies developing new TAVR devices.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter mitchelzoler
SAN DIEGO – Long-term outcome results from the trials that compared the first-generation SAPIEN system and the CoreValve system for transcatheter aortic valve replacement (TAVR) have shown that both devices had excellent performance, compared with surgical aortic valve replacement.
The SAPIEN system continued to show similar performance, compared with surgery after 5-year follow-up, and the CoreValve showed even better superiority to surgery after 2 years, compared with first-year results.
But the performance of the two systems for TAVR should not be interpreted to suggest that CoreValve outperforms the SAPIEN system, cautioned Dr. Stephen Ramee during an interview at the annual meeting of the American College of Cardiology.
The CoreValve study began 4 years after the PARTNER trial that studied the SAPIEN valve, and during that intervening period, the cardiologists and cardiac surgeons who collaborate on TAVR learned important lessons on how to better select patients and how to avoid other risks during the procedure, said Dr. Ramee, an interventional cardiologist and director of the John Ochsner Heart and Vascular Institute in New Orleans.
As a consequence, the SAPIEN valve (currently the XT system) remains a very viable option, and Dr. Ramee said he generally used the SAPIEN XT system for about 60% of his cases. The major patient group best suited for CoreValve TAVR are patients with a highly calcified aortic-valve annulus, which is better suited to the self-expanding CoreValve because of a reduced risk for rupture of the annulus during balloon expansion with the SAPIEN valve.
Dr. Ramee has received honoraria from Edwards and Medtronic, the companies that respectively market the SAPIEN and CoreValve systems. He also has a financial interest in several companies developing new TAVR devices.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter mitchelzoler
EXPERT ANALYSIS FROM ACC 15
Everolimus-eluting stents linked to higher MI risk than CABG
Percutaneous coronary intervention with second-generation everolimus-eluting stents is associated with a similar risk of death but a higher risk of myocardial infarction and repeat revascularization than is coronary-artery bypass grafting.
An observational registry study of 34,819 patients with multivessel coronary artery disease who underwent one or the other procedure showed a similar risk of death at a mean follow-up of 2.9 years between patients who underwent PCI with everolimus-eluting stents and those who underwent CABG (3.1% and 2.9% per year, hazard ratio, 1.04; P = .50).
However, those in the percutaneous coronary intervention (PCI) group had a 51% greater risk of MI (95% confidence interval, 1.29-1.77; P < .001).
This increase in risk was significant only in patients who had incomplete revascularization and not in those with complete revascularization. In addition, the increase in risk largely was tied to spontaneous myocardial infarction, according to the findings.
“Randomized trials comparing PCI with CABG have not been typically powered to evaluate differences in the rates of myocardial infarction, stroke, and death from any cause; instead, they have been based on composite outcomes that include repeat revascularization,” wrote Dr. Sripal Bangalore of the cardiovascular clinical research center at the New York University, and his coauthors.
Patients undergoing PCI with everolimus-eluting stents also had a greater than twofold increase in the risk of repeat revascularization (hazard ratio, 2.35; 95% CI, 2.14-2.58; P < .001), but particularly in patients with three-vessel as opposed to two-vessel disease.
However, those in the PCI group also had a 58% lower risk of stroke than did those in the CABG group (95% CI, 0.50-0.76; P < .001), which was driven largely by a reduced risk in the first 30 days after the procedure (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1412168]).
In the short term – in the hospital or within 30 days of the procedure – patients who underwent PCI showed significantly lower risk of death and stroke but no significant differences in MI risk.
“Thus, the choice between CABG and PCI with everolimus-eluting stents may depend on whether complete revascularization can be achieved with PCI,” the authors wrote. “If the answer is yes, the choice between PCI and CABG should be made on the basis of weighing the short-term risk of death and stroke against the long-term risk of revascularization with PCI.”
The authors acknowledged limitations of the study. It was a nonrandomized, observational trial; it did not examine variables such as smoking and other comorbidities; and it did not capture other neurologic events, such as transient ischemic attack.
The study was supported by Abbott Vascular. Dr. Bangalore has received consultant fees or honoraria from Abbott Vascular, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Pfizer, and Unique Pharmaceuticals.
CABG previously has been shown to be associated with fewer repeat vascularizations than has PCI, but questions have been raised about incremental improvements in stent technology that might narrow this gap.

|
Dr. Robert A. Harrington |
However, data from this and the BEST study show there are clearly trade-offs between the two revascularization strategies that need to be discussed with patients as part of the shared decision-making process.
The early risk of stroke with CABG may be unacceptable to some, while others may want to avoid the risk of a later myocardial infarction or repeat procedure associated with PCI.
Dr. Robert A. Harrington is Arthur L. Bloomfield Professor of Medicine at Stanford (Calif.) University. These comments are taken from an editorial (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMe1501045]) accompanying this and the BEST study, which was also presented at the annual meeting of the American College of Cardiology in San Diego (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]). Dr. Harrington has received consultant fees and/or research grants from numerous pharmaceutical and device companies, including Amgen, Medtronic, Merck, Novartis, GlaxoSmithKline, and the Medicines Company. He is a principal or has ownership interest in Element Science and MyoKardia.
CABG previously has been shown to be associated with fewer repeat vascularizations than has PCI, but questions have been raised about incremental improvements in stent technology that might narrow this gap.

|
Dr. Robert A. Harrington |
However, data from this and the BEST study show there are clearly trade-offs between the two revascularization strategies that need to be discussed with patients as part of the shared decision-making process.
The early risk of stroke with CABG may be unacceptable to some, while others may want to avoid the risk of a later myocardial infarction or repeat procedure associated with PCI.
Dr. Robert A. Harrington is Arthur L. Bloomfield Professor of Medicine at Stanford (Calif.) University. These comments are taken from an editorial (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMe1501045]) accompanying this and the BEST study, which was also presented at the annual meeting of the American College of Cardiology in San Diego (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]). Dr. Harrington has received consultant fees and/or research grants from numerous pharmaceutical and device companies, including Amgen, Medtronic, Merck, Novartis, GlaxoSmithKline, and the Medicines Company. He is a principal or has ownership interest in Element Science and MyoKardia.
CABG previously has been shown to be associated with fewer repeat vascularizations than has PCI, but questions have been raised about incremental improvements in stent technology that might narrow this gap.

|
Dr. Robert A. Harrington |
However, data from this and the BEST study show there are clearly trade-offs between the two revascularization strategies that need to be discussed with patients as part of the shared decision-making process.
The early risk of stroke with CABG may be unacceptable to some, while others may want to avoid the risk of a later myocardial infarction or repeat procedure associated with PCI.
Dr. Robert A. Harrington is Arthur L. Bloomfield Professor of Medicine at Stanford (Calif.) University. These comments are taken from an editorial (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMe1501045]) accompanying this and the BEST study, which was also presented at the annual meeting of the American College of Cardiology in San Diego (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]). Dr. Harrington has received consultant fees and/or research grants from numerous pharmaceutical and device companies, including Amgen, Medtronic, Merck, Novartis, GlaxoSmithKline, and the Medicines Company. He is a principal or has ownership interest in Element Science and MyoKardia.
Percutaneous coronary intervention with second-generation everolimus-eluting stents is associated with a similar risk of death but a higher risk of myocardial infarction and repeat revascularization than is coronary-artery bypass grafting.
An observational registry study of 34,819 patients with multivessel coronary artery disease who underwent one or the other procedure showed a similar risk of death at a mean follow-up of 2.9 years between patients who underwent PCI with everolimus-eluting stents and those who underwent CABG (3.1% and 2.9% per year, hazard ratio, 1.04; P = .50).
However, those in the percutaneous coronary intervention (PCI) group had a 51% greater risk of MI (95% confidence interval, 1.29-1.77; P < .001).
This increase in risk was significant only in patients who had incomplete revascularization and not in those with complete revascularization. In addition, the increase in risk largely was tied to spontaneous myocardial infarction, according to the findings.
“Randomized trials comparing PCI with CABG have not been typically powered to evaluate differences in the rates of myocardial infarction, stroke, and death from any cause; instead, they have been based on composite outcomes that include repeat revascularization,” wrote Dr. Sripal Bangalore of the cardiovascular clinical research center at the New York University, and his coauthors.
Patients undergoing PCI with everolimus-eluting stents also had a greater than twofold increase in the risk of repeat revascularization (hazard ratio, 2.35; 95% CI, 2.14-2.58; P < .001), but particularly in patients with three-vessel as opposed to two-vessel disease.
However, those in the PCI group also had a 58% lower risk of stroke than did those in the CABG group (95% CI, 0.50-0.76; P < .001), which was driven largely by a reduced risk in the first 30 days after the procedure (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1412168]).
In the short term – in the hospital or within 30 days of the procedure – patients who underwent PCI showed significantly lower risk of death and stroke but no significant differences in MI risk.
“Thus, the choice between CABG and PCI with everolimus-eluting stents may depend on whether complete revascularization can be achieved with PCI,” the authors wrote. “If the answer is yes, the choice between PCI and CABG should be made on the basis of weighing the short-term risk of death and stroke against the long-term risk of revascularization with PCI.”
The authors acknowledged limitations of the study. It was a nonrandomized, observational trial; it did not examine variables such as smoking and other comorbidities; and it did not capture other neurologic events, such as transient ischemic attack.
The study was supported by Abbott Vascular. Dr. Bangalore has received consultant fees or honoraria from Abbott Vascular, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Pfizer, and Unique Pharmaceuticals.
Percutaneous coronary intervention with second-generation everolimus-eluting stents is associated with a similar risk of death but a higher risk of myocardial infarction and repeat revascularization than is coronary-artery bypass grafting.
An observational registry study of 34,819 patients with multivessel coronary artery disease who underwent one or the other procedure showed a similar risk of death at a mean follow-up of 2.9 years between patients who underwent PCI with everolimus-eluting stents and those who underwent CABG (3.1% and 2.9% per year, hazard ratio, 1.04; P = .50).
However, those in the percutaneous coronary intervention (PCI) group had a 51% greater risk of MI (95% confidence interval, 1.29-1.77; P < .001).
This increase in risk was significant only in patients who had incomplete revascularization and not in those with complete revascularization. In addition, the increase in risk largely was tied to spontaneous myocardial infarction, according to the findings.
“Randomized trials comparing PCI with CABG have not been typically powered to evaluate differences in the rates of myocardial infarction, stroke, and death from any cause; instead, they have been based on composite outcomes that include repeat revascularization,” wrote Dr. Sripal Bangalore of the cardiovascular clinical research center at the New York University, and his coauthors.
Patients undergoing PCI with everolimus-eluting stents also had a greater than twofold increase in the risk of repeat revascularization (hazard ratio, 2.35; 95% CI, 2.14-2.58; P < .001), but particularly in patients with three-vessel as opposed to two-vessel disease.
However, those in the PCI group also had a 58% lower risk of stroke than did those in the CABG group (95% CI, 0.50-0.76; P < .001), which was driven largely by a reduced risk in the first 30 days after the procedure (N. Engl. J. Med. 2015 March 16 [doi:10.1056/NEJMoa1412168]).
In the short term – in the hospital or within 30 days of the procedure – patients who underwent PCI showed significantly lower risk of death and stroke but no significant differences in MI risk.
“Thus, the choice between CABG and PCI with everolimus-eluting stents may depend on whether complete revascularization can be achieved with PCI,” the authors wrote. “If the answer is yes, the choice between PCI and CABG should be made on the basis of weighing the short-term risk of death and stroke against the long-term risk of revascularization with PCI.”
The authors acknowledged limitations of the study. It was a nonrandomized, observational trial; it did not examine variables such as smoking and other comorbidities; and it did not capture other neurologic events, such as transient ischemic attack.
The study was supported by Abbott Vascular. Dr. Bangalore has received consultant fees or honoraria from Abbott Vascular, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Pfizer, and Unique Pharmaceuticals.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: PCI with second-generation everolimus-eluting stents is associated with a similar risk of death but a higher risk of MI and repeat revascularization than does CABG.
Major finding: PCI was associated with a 51% greater risk of MI at 2.9 years of follow-up, compared with CABG.
Data source: An observational registry study of 34,819 patients with multivessel coronary artery disease.
Disclosures: The study was supported by Abbott Vascular. Dr. Bangalore has received consultant fees or honoraria from Abbott Vascular, Boehringer Ingelheim, Daiichi Sankyo, Gilead Sciences, Pfizer, and Unique Pharmaceuticals.
PCI linked to higher rate of cardiovascular events than CABG
SAN DIEGO– Percutaneous coronary intervention with everolimus-eluting stents is associated with significantly higher major adverse cardiovascular events than is coronary artery bypass grafting in patients with multivessel coronary artery disease, according to results of the BEST trial.
In the randomized noninferiority trial of 880 patients, there was a 47% higher rate of the primary endpoint of death, myocardial infarction, or target vessel revascularization among patients randomized to percutaneous coronary intervention (PCI) with the new-generation drug-eluting stent than among those randomized to coronary artery bypass grafting (CABG), after a median of 4.6 years follow-up.
However, the differences in primary endpoint were not significant for noninferiority between the two groups at the 2-year follow-up mark, Dr. Seung-Jung Park said at the annual meeting of the American College of Cardiology.
The Xience everolimus-eluting stent used in BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease) is one of several bioabsorbable vascular scaffolds that have caught on in recent years. The working hypothesis behind the device is that by dissolving during a period of 12-24 months, the scaffold provides temporary bracing against restenosis but then disappears, allowing improved endovascular healing.
Patients were randomized after diagnostic coronary angiography to PCI (438 patients) or to CABG (442).
The study, which was terminated early because of slow enrollment, also found a significantly greater rate of the composite secondary endpoint of death, myocardial infarction, stroke, or repeat revascularization in the PCI group compared to the CABG group (19.9% vs. 13.3%, P = .01).
There were no significant differences between the two groups in the rate of the other secondary safety endpoint: a composite of death, MI, and stroke.
In total, 29 patients assigned to PCI died, compared with 22 assigned to CABG (6.6% vs. 5%, P = .30).
The rate of spontaneous myocardial infarction was significantly higher in the PCI group (4.3% vs. 1.6%, P = .02), as was the rate of repeat revascularization (11% vs. 5.4%, P = .003).
There were fewer incidences of major bleeding in the PCI group compared to the CABG group, although the rate of fatal major bleeding was similar for both arms of the study.
Diabetes status had a major negative impact on outcome for patients undergoing PCI, increasing the rate of the primary endpoint to 19.2%, compared to 9.1% in patients undergoing CABG (P = .007).
“In the BEST trial, PCI with everolimus-eluting stents was not shown to be noninferior to CABG with respect to the primary endpoint of death, myocardial infarction, or target vessel revascularization at 2 years,” wrote Dr. Park of the University of Ulsan College of Medicine, and his coauthors. The article was published online simultaneously with his presentation (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]).
“At longer-term follow-up (median 4.6 years), PCI was associated with a significant increase in the incidence of the primary endpoint, as compared to the incidence with CABG.”
The authors suggested this difference was largely attributable to the higher rate of repeat target-vessel revascularization in patients who had undergone PCI, as well as the spontaneous myocardial infarction and new lesion revascularization.
In contrast to previous studies, the researchers did not find a significant difference in the rate of stroke between the two groups.
“The reason for this discrepancy is not clear, but the use of off-pump CABG can avoid excessive manipulation of the aorta, and may have contributed to a reduced rate of stroke in the CABG group in our study,” the authors noted.
The researchers acknowledged that the trial was not powered to detect differences in individual endpoints and that they did experience enrollment difficulties.
The CardioVascular Research Foundation, Abbott Vascular, and the Korea Healthcare Technology Research and Development Project supported the study. Dr. Park disclosed ties with Abbott, Cordis, Boston Scientific, and Medtronic, and has an ownership interest in the Cardiovascular Research Foundation.
SAN DIEGO– Percutaneous coronary intervention with everolimus-eluting stents is associated with significantly higher major adverse cardiovascular events than is coronary artery bypass grafting in patients with multivessel coronary artery disease, according to results of the BEST trial.
In the randomized noninferiority trial of 880 patients, there was a 47% higher rate of the primary endpoint of death, myocardial infarction, or target vessel revascularization among patients randomized to percutaneous coronary intervention (PCI) with the new-generation drug-eluting stent than among those randomized to coronary artery bypass grafting (CABG), after a median of 4.6 years follow-up.
However, the differences in primary endpoint were not significant for noninferiority between the two groups at the 2-year follow-up mark, Dr. Seung-Jung Park said at the annual meeting of the American College of Cardiology.
The Xience everolimus-eluting stent used in BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease) is one of several bioabsorbable vascular scaffolds that have caught on in recent years. The working hypothesis behind the device is that by dissolving during a period of 12-24 months, the scaffold provides temporary bracing against restenosis but then disappears, allowing improved endovascular healing.
Patients were randomized after diagnostic coronary angiography to PCI (438 patients) or to CABG (442).
The study, which was terminated early because of slow enrollment, also found a significantly greater rate of the composite secondary endpoint of death, myocardial infarction, stroke, or repeat revascularization in the PCI group compared to the CABG group (19.9% vs. 13.3%, P = .01).
There were no significant differences between the two groups in the rate of the other secondary safety endpoint: a composite of death, MI, and stroke.
In total, 29 patients assigned to PCI died, compared with 22 assigned to CABG (6.6% vs. 5%, P = .30).
The rate of spontaneous myocardial infarction was significantly higher in the PCI group (4.3% vs. 1.6%, P = .02), as was the rate of repeat revascularization (11% vs. 5.4%, P = .003).
There were fewer incidences of major bleeding in the PCI group compared to the CABG group, although the rate of fatal major bleeding was similar for both arms of the study.
Diabetes status had a major negative impact on outcome for patients undergoing PCI, increasing the rate of the primary endpoint to 19.2%, compared to 9.1% in patients undergoing CABG (P = .007).
“In the BEST trial, PCI with everolimus-eluting stents was not shown to be noninferior to CABG with respect to the primary endpoint of death, myocardial infarction, or target vessel revascularization at 2 years,” wrote Dr. Park of the University of Ulsan College of Medicine, and his coauthors. The article was published online simultaneously with his presentation (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]).
“At longer-term follow-up (median 4.6 years), PCI was associated with a significant increase in the incidence of the primary endpoint, as compared to the incidence with CABG.”
The authors suggested this difference was largely attributable to the higher rate of repeat target-vessel revascularization in patients who had undergone PCI, as well as the spontaneous myocardial infarction and new lesion revascularization.
In contrast to previous studies, the researchers did not find a significant difference in the rate of stroke between the two groups.
“The reason for this discrepancy is not clear, but the use of off-pump CABG can avoid excessive manipulation of the aorta, and may have contributed to a reduced rate of stroke in the CABG group in our study,” the authors noted.
The researchers acknowledged that the trial was not powered to detect differences in individual endpoints and that they did experience enrollment difficulties.
The CardioVascular Research Foundation, Abbott Vascular, and the Korea Healthcare Technology Research and Development Project supported the study. Dr. Park disclosed ties with Abbott, Cordis, Boston Scientific, and Medtronic, and has an ownership interest in the Cardiovascular Research Foundation.
SAN DIEGO– Percutaneous coronary intervention with everolimus-eluting stents is associated with significantly higher major adverse cardiovascular events than is coronary artery bypass grafting in patients with multivessel coronary artery disease, according to results of the BEST trial.
In the randomized noninferiority trial of 880 patients, there was a 47% higher rate of the primary endpoint of death, myocardial infarction, or target vessel revascularization among patients randomized to percutaneous coronary intervention (PCI) with the new-generation drug-eluting stent than among those randomized to coronary artery bypass grafting (CABG), after a median of 4.6 years follow-up.
However, the differences in primary endpoint were not significant for noninferiority between the two groups at the 2-year follow-up mark, Dr. Seung-Jung Park said at the annual meeting of the American College of Cardiology.
The Xience everolimus-eluting stent used in BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease) is one of several bioabsorbable vascular scaffolds that have caught on in recent years. The working hypothesis behind the device is that by dissolving during a period of 12-24 months, the scaffold provides temporary bracing against restenosis but then disappears, allowing improved endovascular healing.
Patients were randomized after diagnostic coronary angiography to PCI (438 patients) or to CABG (442).
The study, which was terminated early because of slow enrollment, also found a significantly greater rate of the composite secondary endpoint of death, myocardial infarction, stroke, or repeat revascularization in the PCI group compared to the CABG group (19.9% vs. 13.3%, P = .01).
There were no significant differences between the two groups in the rate of the other secondary safety endpoint: a composite of death, MI, and stroke.
In total, 29 patients assigned to PCI died, compared with 22 assigned to CABG (6.6% vs. 5%, P = .30).
The rate of spontaneous myocardial infarction was significantly higher in the PCI group (4.3% vs. 1.6%, P = .02), as was the rate of repeat revascularization (11% vs. 5.4%, P = .003).
There were fewer incidences of major bleeding in the PCI group compared to the CABG group, although the rate of fatal major bleeding was similar for both arms of the study.
Diabetes status had a major negative impact on outcome for patients undergoing PCI, increasing the rate of the primary endpoint to 19.2%, compared to 9.1% in patients undergoing CABG (P = .007).
“In the BEST trial, PCI with everolimus-eluting stents was not shown to be noninferior to CABG with respect to the primary endpoint of death, myocardial infarction, or target vessel revascularization at 2 years,” wrote Dr. Park of the University of Ulsan College of Medicine, and his coauthors. The article was published online simultaneously with his presentation (N. Engl. J. Med. 2015 March 15 [doi:10.1056/NEJMoa1415447]).
“At longer-term follow-up (median 4.6 years), PCI was associated with a significant increase in the incidence of the primary endpoint, as compared to the incidence with CABG.”
The authors suggested this difference was largely attributable to the higher rate of repeat target-vessel revascularization in patients who had undergone PCI, as well as the spontaneous myocardial infarction and new lesion revascularization.
In contrast to previous studies, the researchers did not find a significant difference in the rate of stroke between the two groups.
“The reason for this discrepancy is not clear, but the use of off-pump CABG can avoid excessive manipulation of the aorta, and may have contributed to a reduced rate of stroke in the CABG group in our study,” the authors noted.
The researchers acknowledged that the trial was not powered to detect differences in individual endpoints and that they did experience enrollment difficulties.
The CardioVascular Research Foundation, Abbott Vascular, and the Korea Healthcare Technology Research and Development Project supported the study. Dr. Park disclosed ties with Abbott, Cordis, Boston Scientific, and Medtronic, and has an ownership interest in the Cardiovascular Research Foundation.
AT ACC 15
Key clinical point: PCI with everolimus-eluting stents is associated with significantly higher major adverse cardiovascular events than is CABG in patients with multivessel coronary artery disease.
Major finding: Patients undergoing PCI had a 47% higher rate of the primary endpoint of death, myocardial infarction, or target vessel revascularization among patients compared to those undergoing CABG.
Data source: BEST, a randomized noninferiority trial of 880 patients.
Disclosures: The CardioVascular Research Foundation, Abbott Vascular, and the Korea Healthcare Technology Research and Development Project supported the study. Dr. Park disclosed ties with Abbott, Cordis, Boston Scientific, and Medtronic, and has an ownership interest in the Cardiovascular Research Foundation.
Greater surgeon experience linked to better long-term survival in NSCLC
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:Insufficient lymph-node sampling by less-experienced surgeons may be a reason why patients with stage I NSCLC had better long-term survival if operated on by moderate- and high-experience surgeons.
Major finding: Short-term outcomes in pathologic stage I NSCLC were not affected by surgeon experience, but 5-year survival was significantly lower for the low-experience compared to the moderate-experience surgeons (76.9% vs. 67.5%).
Data source: An institutional database analysis was conducted of 800 operations on stage I NSCLC patients performed from 2000 to 2012.
Disclosures: The authors reported having no conflicts of interest.
Five-year PARTNER 1 results held up
SAN DIEGO – Five years out, transcatheter aortic valve replacement beat standard therapy in patients with severe, inoperable aortic stenosis, and measured up to surgery in high-risk patients.
The final data from the PARTNER 1 data showed TAVR as an alternative to surgery for some high-risk surgical patients, Dr. Michael Mack reported at the annual scientific sessions of the American College of Cardiology. High-risk surgical patients had similar all-cause mortality, cardiovascular mortality, stroke, and hospital readmission rates, regardless of whether they underwent TAVR or surgical valve replacement, Dr. Mack of Baylor Scott & White Health in Plano, Texas, and his associates wrote in an article published online simultaneously with the presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60308-7]). “Functional outcomes were also similar and preservation of valve hemodynamics was equivalent in both groups,” they wrote.
The trial also showed “a sustained benefit of TAVR” for inoperable aortic stenosis, “as measured by all-cause mortality, cardiovascular mortality, repeat hospital admission, and functional status. Valves were durable, with no increase in transvalvular gradient, attrition of valve area, or worsening of aortic regurgitation,” Dr. Samir Kapadia at the Cleveland Clinic in Ohio and his associates reported in an related article that was not presented at the ACC meeting but was published at the same time as Dr. Mack’s presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60290-2]). Based on the findings, “TAVR should be strongly considered for patients who are not surgical candidates for aortic valve replacement,” the researchers added. “Appropriate selection of patients will help to maximize the benefit of TAVR and reduce mortality from coexisting severe comorbidities.”
The Placement of Aortic Transcatheter Valves (PARTNER 1) trial compared TAVR, standard nonsurgical treatment, and surgery in patients with severe, symptomatic aortic stenosis. The inoperable cohort included 358 patients who averaged 83 years of age. The high-risk cohort enrolled 699 patients whose overall average Society of Thoracic Surgeons (STS) Predicted Risk of Mortality Score was more than 11%. Inoperable patients were randomized to TAVR or standard treatment (usually including balloon aortic valvuloplasty), while high-risk patients were randomized to TAVR or surgical valve replacement.
Five years after treatment, almost 72% of TAVR patients in the inoperable cohort had died, compared with 94% of patients who received standard treatment (hazard ratio, 0.50; 95% confidence interval, 0.39-0.65; P < .0001), Dr. Kapadia and his associates reported. Notably, 86% (or 42 of 49) of surviving TAVR patients had New York Heart Association class 1 or 2 symptoms, compared with only 60% of patients who received standard treatment. Echocardiography did not reveal valve deterioration, the investigators said.
Patients in the high-risk group also faced substantial mortality – only about a third were alive 5 years after TAVR or surgery, Dr. Mack reported. Also, 14% of TAVR patients developed moderate to severe valvular regurgitation, compared with only 1% of the surgery group; P < .0001), and this complication was tied to lower survival, they wrote. “The clinical outcomes and valve performance in this trial might not reflect that of subsequent generations of balloon-expandable transcatheter valves, present operator expertise and experience, and more rigorous patient selection for TAVR,” he cautioned. “The patients selected for treatment in this trial, which started in 2007, are also representative of clinical practice at that time; clinicians have since refined patient selection, at least partly on the basis of early outcomes from this trial.”
Edwards Lifesciences funded the study. Several authors reported receiving travel reimbursements from Edwards Lifesciences and financial or consulting relationships with Abbott Vascular, Edwards Lifesciences, Medtronic, Thubrikar Aortic Valve, St Jude Medical, Philips Healthcare, Sorin Medical, DirectFlow, Boston Scientific, Cardiosolutions, ValvXchange, and Posthorax.
The PARTNER trial was perhaps unique in showing that the first generation of a medical device resulted in a substantial mortality benefit compared with standard treatment. However, some uncertainties remain. The conclusions apply only to appropriately selected patients because many more patients were screened than were enrolled. Other patient populations that might ultimately benefit most from treatment with these new technologies should become better defined over the coming years.
In patients with aortic stenosis who were unsuitable for surgery, transcatheter aortic valve replacement provided a survival benefit of almost 22% [compared with standard treatment]... and a 28% lower cardiovascular mortality. Even more important for elderly patients is quality of life, and 86% of the 49 survivors who received TAVR had New York Heart Association (NYHA) functional class 1 or 2. A benefit of this size is remarkable for inoperable old patients treated with a first-generation medical device. However, a concern is that 48% of the patients undergoing TAVR were readmitted to hospital … and 34% of deaths were noncardiovascular. To treat one disease process, only for another to take its place, is not the objective of an invasive and expensive treatment with complications.
For high-risk patients, the clinical results of TAVR equaled those of SAVR, and the valve showed itself to be durable. The findings challenge whether surgery can still be considered the gold standard for patients at high surgical risk. In 2008, Dr. Mack predicted that the benefits of new, less invasive procedures for percutaneous heart valve treatment would equal or surpass those of their open-surgery predecessors, and concluded that patients will choose a less invasive approach over a more invasive one even if there is uncertainty. With more than 150,000 implantations worldwide and the indication shifting towards intermediate-risk patients, this prediction has been met.
Arie P. Kappetein, M.D., Ph.D., is a cardiothoracic surgeon at Erasmus University in Rotterdam, the Netherlands. These comments were excerpted from his accompanying editorial (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60448-2]).
The PARTNER trial was perhaps unique in showing that the first generation of a medical device resulted in a substantial mortality benefit compared with standard treatment. However, some uncertainties remain. The conclusions apply only to appropriately selected patients because many more patients were screened than were enrolled. Other patient populations that might ultimately benefit most from treatment with these new technologies should become better defined over the coming years.
In patients with aortic stenosis who were unsuitable for surgery, transcatheter aortic valve replacement provided a survival benefit of almost 22% [compared with standard treatment]... and a 28% lower cardiovascular mortality. Even more important for elderly patients is quality of life, and 86% of the 49 survivors who received TAVR had New York Heart Association (NYHA) functional class 1 or 2. A benefit of this size is remarkable for inoperable old patients treated with a first-generation medical device. However, a concern is that 48% of the patients undergoing TAVR were readmitted to hospital … and 34% of deaths were noncardiovascular. To treat one disease process, only for another to take its place, is not the objective of an invasive and expensive treatment with complications.
For high-risk patients, the clinical results of TAVR equaled those of SAVR, and the valve showed itself to be durable. The findings challenge whether surgery can still be considered the gold standard for patients at high surgical risk. In 2008, Dr. Mack predicted that the benefits of new, less invasive procedures for percutaneous heart valve treatment would equal or surpass those of their open-surgery predecessors, and concluded that patients will choose a less invasive approach over a more invasive one even if there is uncertainty. With more than 150,000 implantations worldwide and the indication shifting towards intermediate-risk patients, this prediction has been met.
Arie P. Kappetein, M.D., Ph.D., is a cardiothoracic surgeon at Erasmus University in Rotterdam, the Netherlands. These comments were excerpted from his accompanying editorial (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60448-2]).
The PARTNER trial was perhaps unique in showing that the first generation of a medical device resulted in a substantial mortality benefit compared with standard treatment. However, some uncertainties remain. The conclusions apply only to appropriately selected patients because many more patients were screened than were enrolled. Other patient populations that might ultimately benefit most from treatment with these new technologies should become better defined over the coming years.
In patients with aortic stenosis who were unsuitable for surgery, transcatheter aortic valve replacement provided a survival benefit of almost 22% [compared with standard treatment]... and a 28% lower cardiovascular mortality. Even more important for elderly patients is quality of life, and 86% of the 49 survivors who received TAVR had New York Heart Association (NYHA) functional class 1 or 2. A benefit of this size is remarkable for inoperable old patients treated with a first-generation medical device. However, a concern is that 48% of the patients undergoing TAVR were readmitted to hospital … and 34% of deaths were noncardiovascular. To treat one disease process, only for another to take its place, is not the objective of an invasive and expensive treatment with complications.
For high-risk patients, the clinical results of TAVR equaled those of SAVR, and the valve showed itself to be durable. The findings challenge whether surgery can still be considered the gold standard for patients at high surgical risk. In 2008, Dr. Mack predicted that the benefits of new, less invasive procedures for percutaneous heart valve treatment would equal or surpass those of their open-surgery predecessors, and concluded that patients will choose a less invasive approach over a more invasive one even if there is uncertainty. With more than 150,000 implantations worldwide and the indication shifting towards intermediate-risk patients, this prediction has been met.
Arie P. Kappetein, M.D., Ph.D., is a cardiothoracic surgeon at Erasmus University in Rotterdam, the Netherlands. These comments were excerpted from his accompanying editorial (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60448-2]).
SAN DIEGO – Five years out, transcatheter aortic valve replacement beat standard therapy in patients with severe, inoperable aortic stenosis, and measured up to surgery in high-risk patients.
The final data from the PARTNER 1 data showed TAVR as an alternative to surgery for some high-risk surgical patients, Dr. Michael Mack reported at the annual scientific sessions of the American College of Cardiology. High-risk surgical patients had similar all-cause mortality, cardiovascular mortality, stroke, and hospital readmission rates, regardless of whether they underwent TAVR or surgical valve replacement, Dr. Mack of Baylor Scott & White Health in Plano, Texas, and his associates wrote in an article published online simultaneously with the presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60308-7]). “Functional outcomes were also similar and preservation of valve hemodynamics was equivalent in both groups,” they wrote.
The trial also showed “a sustained benefit of TAVR” for inoperable aortic stenosis, “as measured by all-cause mortality, cardiovascular mortality, repeat hospital admission, and functional status. Valves were durable, with no increase in transvalvular gradient, attrition of valve area, or worsening of aortic regurgitation,” Dr. Samir Kapadia at the Cleveland Clinic in Ohio and his associates reported in an related article that was not presented at the ACC meeting but was published at the same time as Dr. Mack’s presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60290-2]). Based on the findings, “TAVR should be strongly considered for patients who are not surgical candidates for aortic valve replacement,” the researchers added. “Appropriate selection of patients will help to maximize the benefit of TAVR and reduce mortality from coexisting severe comorbidities.”
The Placement of Aortic Transcatheter Valves (PARTNER 1) trial compared TAVR, standard nonsurgical treatment, and surgery in patients with severe, symptomatic aortic stenosis. The inoperable cohort included 358 patients who averaged 83 years of age. The high-risk cohort enrolled 699 patients whose overall average Society of Thoracic Surgeons (STS) Predicted Risk of Mortality Score was more than 11%. Inoperable patients were randomized to TAVR or standard treatment (usually including balloon aortic valvuloplasty), while high-risk patients were randomized to TAVR or surgical valve replacement.
Five years after treatment, almost 72% of TAVR patients in the inoperable cohort had died, compared with 94% of patients who received standard treatment (hazard ratio, 0.50; 95% confidence interval, 0.39-0.65; P < .0001), Dr. Kapadia and his associates reported. Notably, 86% (or 42 of 49) of surviving TAVR patients had New York Heart Association class 1 or 2 symptoms, compared with only 60% of patients who received standard treatment. Echocardiography did not reveal valve deterioration, the investigators said.
Patients in the high-risk group also faced substantial mortality – only about a third were alive 5 years after TAVR or surgery, Dr. Mack reported. Also, 14% of TAVR patients developed moderate to severe valvular regurgitation, compared with only 1% of the surgery group; P < .0001), and this complication was tied to lower survival, they wrote. “The clinical outcomes and valve performance in this trial might not reflect that of subsequent generations of balloon-expandable transcatheter valves, present operator expertise and experience, and more rigorous patient selection for TAVR,” he cautioned. “The patients selected for treatment in this trial, which started in 2007, are also representative of clinical practice at that time; clinicians have since refined patient selection, at least partly on the basis of early outcomes from this trial.”
Edwards Lifesciences funded the study. Several authors reported receiving travel reimbursements from Edwards Lifesciences and financial or consulting relationships with Abbott Vascular, Edwards Lifesciences, Medtronic, Thubrikar Aortic Valve, St Jude Medical, Philips Healthcare, Sorin Medical, DirectFlow, Boston Scientific, Cardiosolutions, ValvXchange, and Posthorax.
SAN DIEGO – Five years out, transcatheter aortic valve replacement beat standard therapy in patients with severe, inoperable aortic stenosis, and measured up to surgery in high-risk patients.
The final data from the PARTNER 1 data showed TAVR as an alternative to surgery for some high-risk surgical patients, Dr. Michael Mack reported at the annual scientific sessions of the American College of Cardiology. High-risk surgical patients had similar all-cause mortality, cardiovascular mortality, stroke, and hospital readmission rates, regardless of whether they underwent TAVR or surgical valve replacement, Dr. Mack of Baylor Scott & White Health in Plano, Texas, and his associates wrote in an article published online simultaneously with the presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60308-7]). “Functional outcomes were also similar and preservation of valve hemodynamics was equivalent in both groups,” they wrote.
The trial also showed “a sustained benefit of TAVR” for inoperable aortic stenosis, “as measured by all-cause mortality, cardiovascular mortality, repeat hospital admission, and functional status. Valves were durable, with no increase in transvalvular gradient, attrition of valve area, or worsening of aortic regurgitation,” Dr. Samir Kapadia at the Cleveland Clinic in Ohio and his associates reported in an related article that was not presented at the ACC meeting but was published at the same time as Dr. Mack’s presentation (Lancet 2015 Mar. 15 [doi: 10.1016/ S0140-6736(15)60290-2]). Based on the findings, “TAVR should be strongly considered for patients who are not surgical candidates for aortic valve replacement,” the researchers added. “Appropriate selection of patients will help to maximize the benefit of TAVR and reduce mortality from coexisting severe comorbidities.”
The Placement of Aortic Transcatheter Valves (PARTNER 1) trial compared TAVR, standard nonsurgical treatment, and surgery in patients with severe, symptomatic aortic stenosis. The inoperable cohort included 358 patients who averaged 83 years of age. The high-risk cohort enrolled 699 patients whose overall average Society of Thoracic Surgeons (STS) Predicted Risk of Mortality Score was more than 11%. Inoperable patients were randomized to TAVR or standard treatment (usually including balloon aortic valvuloplasty), while high-risk patients were randomized to TAVR or surgical valve replacement.
Five years after treatment, almost 72% of TAVR patients in the inoperable cohort had died, compared with 94% of patients who received standard treatment (hazard ratio, 0.50; 95% confidence interval, 0.39-0.65; P < .0001), Dr. Kapadia and his associates reported. Notably, 86% (or 42 of 49) of surviving TAVR patients had New York Heart Association class 1 or 2 symptoms, compared with only 60% of patients who received standard treatment. Echocardiography did not reveal valve deterioration, the investigators said.
Patients in the high-risk group also faced substantial mortality – only about a third were alive 5 years after TAVR or surgery, Dr. Mack reported. Also, 14% of TAVR patients developed moderate to severe valvular regurgitation, compared with only 1% of the surgery group; P < .0001), and this complication was tied to lower survival, they wrote. “The clinical outcomes and valve performance in this trial might not reflect that of subsequent generations of balloon-expandable transcatheter valves, present operator expertise and experience, and more rigorous patient selection for TAVR,” he cautioned. “The patients selected for treatment in this trial, which started in 2007, are also representative of clinical practice at that time; clinicians have since refined patient selection, at least partly on the basis of early outcomes from this trial.”
Edwards Lifesciences funded the study. Several authors reported receiving travel reimbursements from Edwards Lifesciences and financial or consulting relationships with Abbott Vascular, Edwards Lifesciences, Medtronic, Thubrikar Aortic Valve, St Jude Medical, Philips Healthcare, Sorin Medical, DirectFlow, Boston Scientific, Cardiosolutions, ValvXchange, and Posthorax.
Key clinical point:Transcatheter aortic valve replacement is an acceptable alternative to standard treatment in some patients with aortic stenosis.
Major finding:For inoperable patients, TAVR had lower five-year mortality than did standard therapy (P < .0001). For high-risk patients, TAVR and surgical mortality rates were similar.
Data source: Five-year data from the randomized Placement of Aortic Transcatheter Valves (PARTNER 1) trial.
Disclosures: Edwards Lifesciences funded the study. Several authors reported receiving travel reimbursements from Edwards Lifesciences and financial or consulting relationships with Abbott Vascular, Edwards Lifesciences, Medtronic, Thubrikar Aortic Valve, St Jude Medical, Philips Healthcare, Sorin Medical, DirectFlow, Boston Scientific, Cardiosolutions, ValvXchange, and Posthorax.
Low-volume centers using ECMO have poorest survival rates
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
AT THE STS ANNUAL MEETING
Key clinical point: Low-volume lung transplantation centers in the United States typically have the poorest survival rates compared to those with higher volumes when using ECMO.
Major finding: Of 85 LTx subjects bridged via ECMO, 20 (23.5%) of these were bridged in low, 30 (35.3%) in medium, and 35 (41.2%) in high-volume centers; in the ECMO cohort, the lowest 5-year survival rate (13.61%) was observed at low-volume centers.
Data source: Retrospective analysis of 16,603 adult LTx recipients in the International Registry for Heart and Lung Transplantation during 2005-2010.
Disclosures: Dr. Hayanga reported no financial conflicts of interest.
Restrictive vs. liberal transfusion after cardiac surgery
After cardiac surgery, using a restrictive transfusion threshold – forgoing transfusion until hemoglobin level drops to 7.5 g/dL – does not decrease morbidity or costs of care, compared with using a liberal transfusion threshold of 9 g/dL, according to a report published online March 12 in the New England Journal of Medicine.
Several blood management guidelines and health policy statements recommend the restrictive approach in the hope that it will reduce the increasing demand on blood services and the high costs of storing, handling, and administering red-cell units, and also because transfusions following cardiac surgery have been linked to infection, low cardiac output, acute kidney injury, and increased mortality. Clinicians remain uncertain about a safe threshold for transfusions in this setting, which is evidenced by the striking variation in transfusion rates among cardiac centers in the United States (8%-93%) and the United Kingdom (25%-75%), said Dr. Gavin J. Murphy of the British Heart Foundation and department of cardiovascular sciences, University of Leicester (England), and his associates.
They performed the Transfusion Indication Threshold Reduction (TITRe2) study to test the hypothesis that the restrictive approach is superior to the liberal approach regarding both postoperative morbidity and health care costs. Adults undergoing nonemergency cardiac surgery at 17 specialty centers in the United Kingdom were randomly assigned to a restricted (1,000 patients) or a liberal (1,003 patients) transfusion threshold. The median patient age was 70 years, and 68% were men. Most of the procedures were CABG or valve surgeries.
Contrary to expectations, the primary outcome – a composite of serious infection or an ischemic event such as stroke, MI, gut infarction, or acute kidney injury within 3 months – occurred in 35.1% of patients in the restrictive-threshold group and 33.0% in the liberal-threshold group. Secondary outcomes, including length of ICU stay and rates of clinically significant pulmonary complications, also were similar between the two study groups. Rates of other serious postoperative complications were similar, at 35.7% and 34.2%, as was general health status as assessed via the EuroQol Group 5-Dimension Self-Report Questionnaire, further contradicting the study hypothesis.
Mean health care costs were similar between the two study groups: the equivalent of $17,762 U.S. dollars with restrictive-threshhold transfusions and $18,059 with liberal-threshold transfusions, Dr. Murphy and his associates noted (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMoa1403612]).
Unexpectedly, 3-month mortality was significantly higher with restrictive- than with liberal-threshold transfusions (4.2% vs 2.6%). This association persisted in sensitivity analyses and “is a cause for concern,” but it may be due to chance alone, the investigators added.
Findings like those of Murphy et al. provide a great opportunity for discussion and debate, which could lead to development of a consensus on the best postoperative care for these patients. Cardiac surgery departments should review the TITRe2 trial results and decide which threshold they deem to be the most appropriate for transfusion.
The extreme range in hospitals’ rates of transfusion in cardiac surgery – from less than 5% to more than 90% – is extraordinary. Having clinicians actively debate the evidence presented in TITRe2, create transparent interpretations, develop protocols, and hold themselves accountable for following those protocols would represent important steps for improving patient care.
John Spertus, M.D., is at the University of Missouri-Kansas City and Saint Luke’s Mid America Heart Institute, Kansas City. He reported receiving grant support from Lilly, Gilead, Amorcyte, Genentech, and Abbott Vascular; receiving personal fees from United Healthcare, Novartis, and Amgen; having an equity interest in Health Outcomes Sciences; and owning copyrights to the Seattle Angina Questionnaire, the Kansas City Cardiomyopathy Questionnaire, and the Peripheral Artery Questionnaire. Dr. Spertus made these remarks in an editorial accompanying Dr. Murphy’s report (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMe1415394]).
Findings like those of Murphy et al. provide a great opportunity for discussion and debate, which could lead to development of a consensus on the best postoperative care for these patients. Cardiac surgery departments should review the TITRe2 trial results and decide which threshold they deem to be the most appropriate for transfusion.
The extreme range in hospitals’ rates of transfusion in cardiac surgery – from less than 5% to more than 90% – is extraordinary. Having clinicians actively debate the evidence presented in TITRe2, create transparent interpretations, develop protocols, and hold themselves accountable for following those protocols would represent important steps for improving patient care.
John Spertus, M.D., is at the University of Missouri-Kansas City and Saint Luke’s Mid America Heart Institute, Kansas City. He reported receiving grant support from Lilly, Gilead, Amorcyte, Genentech, and Abbott Vascular; receiving personal fees from United Healthcare, Novartis, and Amgen; having an equity interest in Health Outcomes Sciences; and owning copyrights to the Seattle Angina Questionnaire, the Kansas City Cardiomyopathy Questionnaire, and the Peripheral Artery Questionnaire. Dr. Spertus made these remarks in an editorial accompanying Dr. Murphy’s report (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMe1415394]).
Findings like those of Murphy et al. provide a great opportunity for discussion and debate, which could lead to development of a consensus on the best postoperative care for these patients. Cardiac surgery departments should review the TITRe2 trial results and decide which threshold they deem to be the most appropriate for transfusion.
The extreme range in hospitals’ rates of transfusion in cardiac surgery – from less than 5% to more than 90% – is extraordinary. Having clinicians actively debate the evidence presented in TITRe2, create transparent interpretations, develop protocols, and hold themselves accountable for following those protocols would represent important steps for improving patient care.
John Spertus, M.D., is at the University of Missouri-Kansas City and Saint Luke’s Mid America Heart Institute, Kansas City. He reported receiving grant support from Lilly, Gilead, Amorcyte, Genentech, and Abbott Vascular; receiving personal fees from United Healthcare, Novartis, and Amgen; having an equity interest in Health Outcomes Sciences; and owning copyrights to the Seattle Angina Questionnaire, the Kansas City Cardiomyopathy Questionnaire, and the Peripheral Artery Questionnaire. Dr. Spertus made these remarks in an editorial accompanying Dr. Murphy’s report (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMe1415394]).
After cardiac surgery, using a restrictive transfusion threshold – forgoing transfusion until hemoglobin level drops to 7.5 g/dL – does not decrease morbidity or costs of care, compared with using a liberal transfusion threshold of 9 g/dL, according to a report published online March 12 in the New England Journal of Medicine.
Several blood management guidelines and health policy statements recommend the restrictive approach in the hope that it will reduce the increasing demand on blood services and the high costs of storing, handling, and administering red-cell units, and also because transfusions following cardiac surgery have been linked to infection, low cardiac output, acute kidney injury, and increased mortality. Clinicians remain uncertain about a safe threshold for transfusions in this setting, which is evidenced by the striking variation in transfusion rates among cardiac centers in the United States (8%-93%) and the United Kingdom (25%-75%), said Dr. Gavin J. Murphy of the British Heart Foundation and department of cardiovascular sciences, University of Leicester (England), and his associates.
They performed the Transfusion Indication Threshold Reduction (TITRe2) study to test the hypothesis that the restrictive approach is superior to the liberal approach regarding both postoperative morbidity and health care costs. Adults undergoing nonemergency cardiac surgery at 17 specialty centers in the United Kingdom were randomly assigned to a restricted (1,000 patients) or a liberal (1,003 patients) transfusion threshold. The median patient age was 70 years, and 68% were men. Most of the procedures were CABG or valve surgeries.
Contrary to expectations, the primary outcome – a composite of serious infection or an ischemic event such as stroke, MI, gut infarction, or acute kidney injury within 3 months – occurred in 35.1% of patients in the restrictive-threshold group and 33.0% in the liberal-threshold group. Secondary outcomes, including length of ICU stay and rates of clinically significant pulmonary complications, also were similar between the two study groups. Rates of other serious postoperative complications were similar, at 35.7% and 34.2%, as was general health status as assessed via the EuroQol Group 5-Dimension Self-Report Questionnaire, further contradicting the study hypothesis.
Mean health care costs were similar between the two study groups: the equivalent of $17,762 U.S. dollars with restrictive-threshhold transfusions and $18,059 with liberal-threshold transfusions, Dr. Murphy and his associates noted (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMoa1403612]).
Unexpectedly, 3-month mortality was significantly higher with restrictive- than with liberal-threshold transfusions (4.2% vs 2.6%). This association persisted in sensitivity analyses and “is a cause for concern,” but it may be due to chance alone, the investigators added.
After cardiac surgery, using a restrictive transfusion threshold – forgoing transfusion until hemoglobin level drops to 7.5 g/dL – does not decrease morbidity or costs of care, compared with using a liberal transfusion threshold of 9 g/dL, according to a report published online March 12 in the New England Journal of Medicine.
Several blood management guidelines and health policy statements recommend the restrictive approach in the hope that it will reduce the increasing demand on blood services and the high costs of storing, handling, and administering red-cell units, and also because transfusions following cardiac surgery have been linked to infection, low cardiac output, acute kidney injury, and increased mortality. Clinicians remain uncertain about a safe threshold for transfusions in this setting, which is evidenced by the striking variation in transfusion rates among cardiac centers in the United States (8%-93%) and the United Kingdom (25%-75%), said Dr. Gavin J. Murphy of the British Heart Foundation and department of cardiovascular sciences, University of Leicester (England), and his associates.
They performed the Transfusion Indication Threshold Reduction (TITRe2) study to test the hypothesis that the restrictive approach is superior to the liberal approach regarding both postoperative morbidity and health care costs. Adults undergoing nonemergency cardiac surgery at 17 specialty centers in the United Kingdom were randomly assigned to a restricted (1,000 patients) or a liberal (1,003 patients) transfusion threshold. The median patient age was 70 years, and 68% were men. Most of the procedures were CABG or valve surgeries.
Contrary to expectations, the primary outcome – a composite of serious infection or an ischemic event such as stroke, MI, gut infarction, or acute kidney injury within 3 months – occurred in 35.1% of patients in the restrictive-threshold group and 33.0% in the liberal-threshold group. Secondary outcomes, including length of ICU stay and rates of clinically significant pulmonary complications, also were similar between the two study groups. Rates of other serious postoperative complications were similar, at 35.7% and 34.2%, as was general health status as assessed via the EuroQol Group 5-Dimension Self-Report Questionnaire, further contradicting the study hypothesis.
Mean health care costs were similar between the two study groups: the equivalent of $17,762 U.S. dollars with restrictive-threshhold transfusions and $18,059 with liberal-threshold transfusions, Dr. Murphy and his associates noted (N. Engl. J. Med. 2015 March 12 [doi:10.1056/NEJMoa1403612]).
Unexpectedly, 3-month mortality was significantly higher with restrictive- than with liberal-threshold transfusions (4.2% vs 2.6%). This association persisted in sensitivity analyses and “is a cause for concern,” but it may be due to chance alone, the investigators added.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: After cardiac surgery, using a restrictive transfusion threshold – forgoing transfusion unless hemoglobin level drops to 7.5 g/dL – doesn’t decrease morbidity or costs, compared with using a liberal transfusion threshold (9 g/dL).
Major finding: Contrary to expectations, the primary outcome, a composite of serious infection or an ischemic event such as stroke, MI, gut infarction, or acute kidney injury within 3 months, occurred in 35% of patients in the restrictive-threshold group and 33% in the liberal-threshold group.
Data source: A multicenter randomized controlled trial comparing restrictive and liberal transfusion thresholds in 2,003 cardiac surgery patients in the United Kingdom who were followed for 3 months for the development of serious complications.
Disclosures: The National Institute for Health Research’s Health Technology Assessment Program, the NIHR Bristol Biomedical Research Unit in Cardiovascular Disease, and the British Heart Foundation supported the study. Dr. Murphy and his associates reported having no financial disclosures.