User login
Ablation during mitral valve surgery offers up mixed results
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
AT ACC 15
Key clinical point: Surgical ablation of atrial fibrillation during mitral valve surgery decreases AF at 6 months and 1 year, but increases pacemaker implantations.
Major finding: Freedom from AF at both 6 months and 1 year was 63% with mitral valve surgery plus ablation and 29% for MVS alone.
Data source: Prospective, randomized study in 260 patients with persistent or longstanding persistent AF who required mitral valve surgery.
Disclosures: The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
Evidence builds for complete revascularization in STEMI
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
AT ACC/CRFi2
Key clinical point: Complete revascularization of multivessel disease in patients with STEMI improved long-term outcomes, but may not be the optimal strategy for all.
Major finding: Complete revascularization reduced the risk of the primary endpoint from 22% with infarct-only PCI to 13%.
Data source: Randomized trial in 627 STEMI patients with multivessel disease.
Disclosures: The presenter had no financial disclosures.
VTE with transient risk factors is being overtreated
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
FROM THROMBOSIS RESEARCH
Key clinical point: After venous thromboembolism, patients with transient or removable risk factors for VTE often underwent unneeded, prolonged anticoagulation therapy.
Major finding: Of patients with transient VTE risk factors, 42% underwent anticoagulation therapy for 12 months or longer.
Data source: Prospective cohort study of 6,944 patients with VTE.
Disclosures: Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
Palliative surgery eases pain at end of life
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
Over the past decade palliative surgery has been increasingly discussed and scrutinized, as the concept of palliative care has gained greater traction with medical professionals and the public. Not too long ago the designation “palliative” when applied to a surgical procedure had an apologetic connotation because the operation would fail to heal. In some cases the term was even used to describe positive tumor margins at the conclusion of a resection – something totally irrelevant when assessing the direct impact of the operation upon a patient’s self-designated symptoms.
Only recently has a more positive perspective emerged, helped by data such as these researchers have presented. Palliative surgery now is not failing to cure, but succeeding to comfort.
New perspectives, however, will raise new and necessary questions to better define the role of surgery in the greater context of relief of suffering in all its manifestations. In our wish to respond surgically to pain and other symptoms we must be vigilant against the temptation to “do something” when surgery for cure or palliation is unlikely to help in order to assuage our feelings of hopelessness. Hopelessness is not an indication for surgery – pain, obstruction, and saving life are. Indications for surgery must be more specific, as this article points out, and it is more likely to help with localized and pressing symptoms. A rule of thumb passed down to me from my surgeon grandfather who practiced in an era when the vast majority of operations were palliative, the more pressing and clear the indication for surgery, the better the result.
Dr. Geoffrey Dunn, an ACS Fellow based in Erie, Pa.
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
HOUSTON – Palliative surgery can alleviate pain and improve the quality of life for patients dying from advanced cancers, without compromising performance status, a study showed.
Among 202 patients with stage III or IV cancers who underwent surgery with palliation as the goal, pain scores were significantly improved after surgery, while Karnofsky Performance Status (KPS) scores remained unchanged, said Dr. Anne Falor, a surgical oncology fellow at City of Hope in Duarte, Calif.
“Surgical oncology has not been historically involved in palliative care. If a patient is deemed unresectable, his or her treatment is often the purview of medical or radiation oncology,” she said at the annual Society of Surgical Oncology Cancer Symposium.
But for patients who are likely to have prolonged disease-free intervals, palliative surgery can be performed with low morbidity, she said.
Dr. Falor and her colleagues reviewed their center’s experience with palliative surgery in 2011, during which time 202 patients with a predicted 5-year survival of less than 5% underwent a total of 247 palliative procedures.
The patients had malignancies at various sites, including the large intestine, lung, stomach, breast, prostate, lymph nodes, esophagus, pancreas, and ovaries.
The primary indications for the procedure included dysphagia, pain/wound problems, dyspnea, nausea and vomiting, and dysuria.
Most of the patients (83%) had a single procedure, but 13% had two operations, 4% had three operations, 1% had four procedures, and 0.4% had five or more interventions.
The majority of procedures performed were endoscopic interventions characterized as minor in nature, followed by minor genitourinary and thoracic interventions, although a nearly equal proportion of thoracic interventions (about 28%) were major procedures such as diverting ostomy.
When the investigators looked at 30-day outcomes following palliative surgery, they found that only 13% of patients needed an urgent care visit, 2% required a triage call, 22% were readmitted, and 60% had an institutional supportive care referral.
Total 30-day morbidity of any kind was seen in 37% of patients; 15% of patients died within 30 days of surgery.
Looking at quality of life outcomes, the investigators found no differences in the percentage of patients with KPS scores from 80 to 100 between the presurgery and postsurgery periods (78% and 70%, respectively, P = ns).
There were significant improvements, however, in pain scores, which dropped by a mean of 1.2 points from the preoperative period to discharge (P < .0001), and decreased by 0.6 points before surgery to the first follow-up visit (P = .0037).
Dr. Falor said that it’s important for patients and their care team to have a discussion regarding expectations for surgery and the goals of care.
The study was internally funded. Dr. Falor reported having no conflicts of interest.
Key clinical point: Palliative surgery in patients with advanced cancers can relieve pain with minimal morbidity.
Major finding: Pain scores improved significantly from the pre- to postoperative periods, without a significant decline in performance status scores.
Data source: Case series of 202 patients with stage III or IV malignancies who underwent 247 palliative procedures.
Disclosures: The study was internally funded. Dr. Falor reported having no conflicts of interest.
Few PE patients treated with catheter-directed interventions had complications
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
CoreValve receives first TAVR valve-in-valve indication
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
Cardiologists honor a fallen colleague
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
It’s extremely unusual for a cardiologist or cardiac surgeon to die in the line of duty, but that tragedy occurred last January in Boston when the enraged son of a patient mortally shot Dr. Michael J. Davidson while he was on the job as director of endovascular cardiac surgery at Brigham and Women’s Hospital in Boston.
Dr. Davidson had been an active coinvestigator in the PARTNER study since it began in 2007 to make the first direct comparison of a transcatheter aortic valve replacement (TAVR) system against aortic-valve replacement with conventional heart surgery.
Because of Dr. Davidson’s long and active involvement with the PARTNER trial, his colleagues decided to dedicate the study’s 5-year follow-up findings to him, making the announcement during the first public release of the 5-year results in mid-March at the annual meeting of the American College of Cardiology.
“On behalf of the PARTNER team, we would like to dedicate this study – the 5-year outcomes – to Mike Davidson,” said Dr. Michael J. Mack as he finished his podium presentation of the report.
Preceding Dr. Mack’s talk, the session began with brief remarks about Dr. Davidson from Dr. Martin B. Leon, coleader of the PARTNER trial, and then the airing of a 6-minute video featuring several of Dr. Davidson’s colleagues recalling his unique career and accomplishments.
Notable in their comments was the outline they provided of the unusual training and career path Dr. Davidson forged for himself, based on his remarkably prescient realization a decade or more ago that the future of cardiology and cardiac surgery lay in fusing the two into a hybrid discipline.
Dr. Davidson’s colleagues cited the training he undertook to become both a fully qualified cardiac surgeon and a skilled interventional cardiologist, turning himself into an embodiment of the “heart team.” Several in the video called him “visionary” for recognizing this fusion as an important step toward the future of treating heart disease.
The poignancy of the moment did not stop there.
After the video ended and before Dr. Mack delivered the session’s first talk, ACC president Dr. Patrick T. O’Gara presented a posthumous distinguished-service award from the ACC to Dr. Davidson – with Dr. O’Gara handing the award to the fallen surgeon’s parents, including his father, Dr. Robert M. Davidson, a long-time ACC fellow and former clinical chief of cardiology at Cedars-Sinai Medical Center in Los Angeles.
Following the award, Dr. Athena Poppas, chair of the meeting’s program committee and cochair for the latebreaker session on heart valve replacement, stressed that the entire session was dedicated to honor Dr. Michael Davidson.
Perhaps most moving of all were the small white buttons that Dr. O’Gara, Dr. Davidson’s parents, and others wore on their lapels during the session, featuring a blue heart and the initials MJD. It combined for an affecting tribute to someone who had played a central role in transforming heart-valve replacement and then was murdered for doing this work.
On Twitter @mitchelzoler
Advanced age no barrier to aggressive heart attack treatment
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
SAN DIEGO – Patients aged 80 years and older benefit from more invasive early treatment after non-ST-elevation myocardial infarction or unstable angina, the After Eighty Trial showed.
After a median follow-up of 1.5 years, an invasive strategy that included coronary angiography significantly reduced the primary endpoint of myocardial infarction (MI), need for urgent revascularization, stroke, and death from 61% with optimal medical treatment to 41% (risk ratio, 0.48; P value < .00001).
That drop was driven primarily by significantly fewer MIs (17% vs. 30%; RR, 0.50; P = .0003) and urgent revascularizations (2% vs. 11%; RR, 0.19; P = .0001), lead author Dr. Nicolai Tegn reported at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
There were no significant differences between the invasive and conservative strategy groups in rates of stroke (3% vs. 6%; RR, 0.61; P = .26) or all-cause death (25% vs. 27%; RR, 0.87; P = .53).
The composite of death and MI, however, significantly favored the invasive group (35% vs. 48%; RR, 0.54; P < .0001), he said during a latebreaking clinical trial session.
After Eighty randomly assigned 457 patients, aged 80 years or older, to either optimal medical therapy with no invasive treatments or coronary angiography at a percutaneous coronary intervention (PCI) center the day after inclusion, plus optimal medical therapy after about 4-5 hours if PCI was not performed or about 6-18 hours if it was. Of the 225 patients receiving angiography, 48% went on to balloon angioplasty and/or coronary stenting, and 3% had bypass surgery.
Patients 80 years or older account for roughly one-third of all patients with non-STEMI and unstable angina, but they are underrepresented in clinical trials. As a result, the role of an early invasive strategy, and even an invasive strategy at all, in those elderly patients is still a subject of debate, observed Dr. Tegn, a cardiologist from Rikshospitalet, Oslo University Hospital, Oslo.
The study demonstrated that an invasive strategy is superior to a conservative strategy in patients at least 80 years with NSTEMI or unstable angina, he concluded.
After Eighty is a welcome study because of the under-representation of the elderly in clinical trials, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said during a press briefing at the meeting. But it raises the challenge of identifying patients in clinical practice with the same qualifying characteristics, he added, given that the study population represents only 10% of the entire screened population,
There was also a fairly high prevalence of patients who angiographically did not have significant coronary artery disease, yet MI and stroke rates were quite considerable.
That said, “the study in other ways reminds us that the coronary anatomy does not know the age of the patient, meaning that the findings of a benefit of an early invasive strategy seem consistent with previous studies we know across the management of patients with acute coronary syndromes,” Dr. Kandzari said.
After Eighty investigators screened 4,187 elderly patients presenting at 17 community hospitals in Norway with non-STEMI or unstable angina, with or without ST-segment depression in ECG, and normal or elevated troponin T or I levels. Patients had to have no chest pain or other ischemic symptoms after medical treatment and mobilization.
In all, 3,730 patients were excluded for life expectancy less than 12 months because of a serious comorbidity; ongoing or recent bleeding; inability to comply with protocol; clinically unstable including ongoing ischemia; refusal to participate; logistic reasons; or other reasons.
The average age was 84.7 years in the invasive group (range 80-93 years) and 84.9 years in the conservative group (range 80-94 years).
Medical treatment during the index admission included 75 mg aspirin in 97% of both the invasive and conservative groups, clopidogrel (85% vs. 82%), ticagrelor (both 5%), angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blockers (ARB) (43% vs. 50%), beta blocker (83% vs. 85%), statins (90% vs. 85%), loop or thiazide diuretics (41% vs. 33%), calcium channel blocker (20% vs. 21%), nitrates (45% vs. 55%), warfarin (17% vs. 9%), low molecular-weight heparin (both 76% ), and dabigatran in one patient in each group.
Medical therapy at discharge in the invasive and conservative groups was aspirin (both 93%), clopidogrel (both 72%), ticagrelor (both 4%), ACE inhibitor/ARB (52% vs. 54%), beta blockers (both 84%), statins (90% vs. 86%), diuretics (45% vs. 38%), calcium channel blocker (24% vs. 23%), nitrates (34% vs. 48%), warfarin (21% vs. 14%), rivaroxaban (three patients in both groups), and dabigatran (one patient vs. six patients).
There were no differences in bleeding rates between the two groups, Dr. Tegn said. Four major bleeding events were reported in both groups, while 23 patients in the invasive group and 16 patients in the conservative group had minor bleeds.
The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandazari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
AT ACC/CRF i2 SUMMIT
Key clinical point: An early invasive treatment strategy improved most outcomes in patients aged 80 years and older with acute coronary syndromes.
Major finding: Myocardial infarction, need for urgent revascularization, stroke, and death were significantly lower with invasive vs. conservative care (41% vs. 61%; risk ratio, 0.48; P < .00001).
Data source: Randomized study in 457 patients aged 80 years or older with non-STEMI or unstable angina.
Disclosures: The Norwegian Health Association sponsored the study. Dr. Tegn reported nothing to disclose. Dr. Kandzari reported research and grant support from Abbott Vascular, Biotronic, Boston Scientific, and Medtronic, and minor consulting honoraria from Boston Scientific and Medtronic.
Catheter treatment for PAH outperforms medication
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.
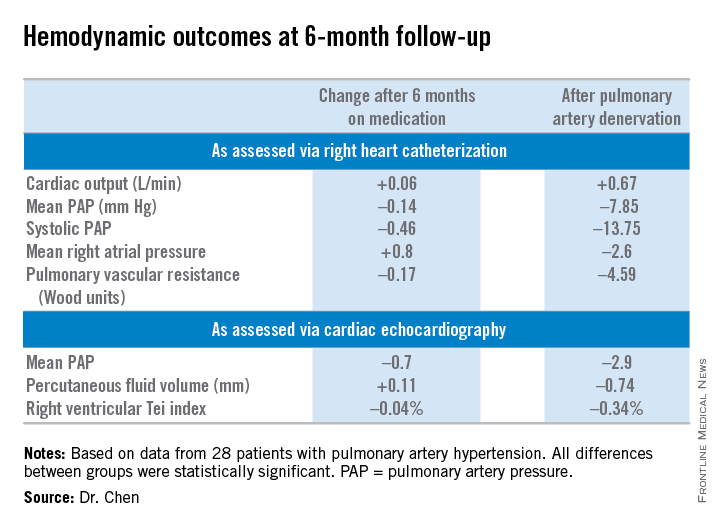
He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.

He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
SAN DIEGO – Percutaneous pulmonary artery denervation for the treatment of pulmonary arterial hypertension safely resulted in significantly greater improvement in functional capacity and hemodynamics compared with medication, in a controlled before-and-after study.
A particularly noteworthy secondary finding in the study was that rehospitalizations during the first 6 months after pulmonary artery denervation (PADN) occurred just one-third as frequently as in the 6-month preprocedural period on standard medications, Dr. Shao-Liang Chen said at the annual meeting of the American College of Cardiology.

He and his coinvestigators, including Dr. Gregg W. Stone of Columbia University in New York, developed a percutaneous catheter-based method of destroying the pulmonary baroreceptor structure located at the bifurcation area of the middle pulmonary artery. Along the way, they redefined the understanding of the pathogenesis of pulmonary artery hypertension (PAH) by demonstrating that local sympathetic nerve activity plays a pivotal role in modulating the elevations of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), which are the disease hallmarks.
Dr. Chen and coinvestigators previously reported the first-in-man study of PADN, which demonstrated safety and short-term efficacy (J. Am. Coll. Cardiol. 2013;62:1092-100). At ACC 15, Dr. Chen presented the findings of the new PADN-2 study, which expands upon the first study by including more patients and longer and more comprehensive follow-up.
The study comprised 28 patients with PAH, including 11 with idiopathic PAH and 8 with pulmonary hypertension caused by left ventricular disease. All of them underwent medication washout followed by right heart catheterization and echocardiography for baseline off-drug hemodynamic measurements as well as a 6-minute walk distance test of their functional capacity. Then they went back on medications for 6 months, after which they underwent repeat testing. Then their medications were discontinued and they underwent PADN. Six months after the procedure, still off medications, they were retested once again.
The primary study endpoint was change in 6-minute walk distance. After 6 months of medication it improved from 361 to 373 meters, a modest 3.9% gain over off-drug baseline. In contrast, 6-minute walk distance grew from 358 to 423 meters 6 months after PADN, a clinically important 23.9% improvement, reported Dr. Chen, a cardiologist at First Hospital of Nanjing (China) Medical University.
Multiple secondary hemodynamic endpoints also showed significantly greater improvement with PADN than medical therapy.
Twelve predefined clinical events – mostly involving worsening PAH – occurred during medical management, compared with three in the 6 months following PADN.
In addition, there were 12 hospitalizations during the 6 months on medical management compared with only 4 after the same patients underwent PADN. Health care costs averaged $35,000 per patient during the 6-month study period on medication compared with $6,000 per patient in the first 6 months after PADN.
There were no deaths, aneurysms, access site hematomas, or thrombotic events during either study period.
Further randomized, controlled trials are planned to explore the possibility that the benefits seen in the PADN-2 trial will result in reduced mortality in patients with PAH, according to Dr. Chen.
The PADN-2 trial was sponsored by Nanjing Medical University. Dr. Chen reported serving as a consultant to MicroPort.
AT ACC 15
Key clinical point: Pulmonary artery denervation for PAH showed promise in a small study.
Major finding: Six-minute walk distance improved by 3.9% after 6 months of medication, compared with 23.9% in testing done 6 months after percutaneous pulmonary artery denervation.
Data source: The PADN-2 trial, a prospective before-and-after study of 28 patients with pulmonary arterial hypertension.
Disclosures: Nanjing Medical University funded the study. The presenter reported serving as a consultant to MicroPort.
VIDEO: Data support switching to transradial PCI access
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO – Cardiologists should switch from transfemoral to transradial access in acute coronary syndrome patients undergoing percutaneous coronary intervention, given the reduced mortality rates associated with the transradial approach in the MATRIX study and other studies, Dr. Cindy L. Grines said at the annual meeting of the American College of Cardiology.
Because U.S. interventionalists are “under the clock” when treating patients with ST-elevation myocardial infarction, “many physicians have been unwilling to risk having a difficult transradial case that would take too much time,” explained Dr. Grines, an interventional cardiologist at the Detroit Medical Center.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For that and other reasons, American interventionalists have been “slow adopters” of the transradial approach, currently using it for about 20% of PCIs, compared with a worldwide rate of about 70%.
It may take instituting incentives to get U.S. cardiologists to change their practice, Dr. Grines suggested in an interview. That could involve increased reimbursement for PCIs done transradially, an increased allowance on acceptable door-to-balloon times for STEMI patients treated transradially, or imposition of new standards for quality assurance that mandate use of transradial in a certain percentage of PCI cases, she said.
The MATRIX study included a second, independent, prespecified analysis that compared outcomes in patients randomized to treatment with two different antithrombin drugs, either bivalirudin (Angiomax) or unfractionated heparin.
That part of the study showed that while treatment with either of the two drugs resulted in no statistically significant difference in the study’s two primary endpoints, treatment with bivalirudin led to statistically significant reductions in all-cause death and cardiovascular death, as well as in major bleeding events, compared with patients treated with unfractionated heparin (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
Although bivalirudin has generally been the more commonly used antithrombin drug in this clinical setting by U.S. interventionalists in recent years, results reported last year from the HEAT-PCI trial (Lancet 2014;384:1849-58) that showed better outcomes with unfractionated heparin have led to reduced use of bivalirudin, Dr. Grines said.
The new results from MATRIX coupled with results from other trials that compared those drugs can make clinicians “more confident about the benefit of bivalirudin,” she said.
Dr. Grines has been a consultant to and received honoraria from the Medicines Company, which markets Angiomax, and from Abbott Vascular, Merck, and the Volcano Group.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM ACC 15