User login
EHR Tool Enhances Primary Aldosteronism Screening in Hypertensive Patients
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Continued Caution Needed Combining Nitrates With ED Drugs
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.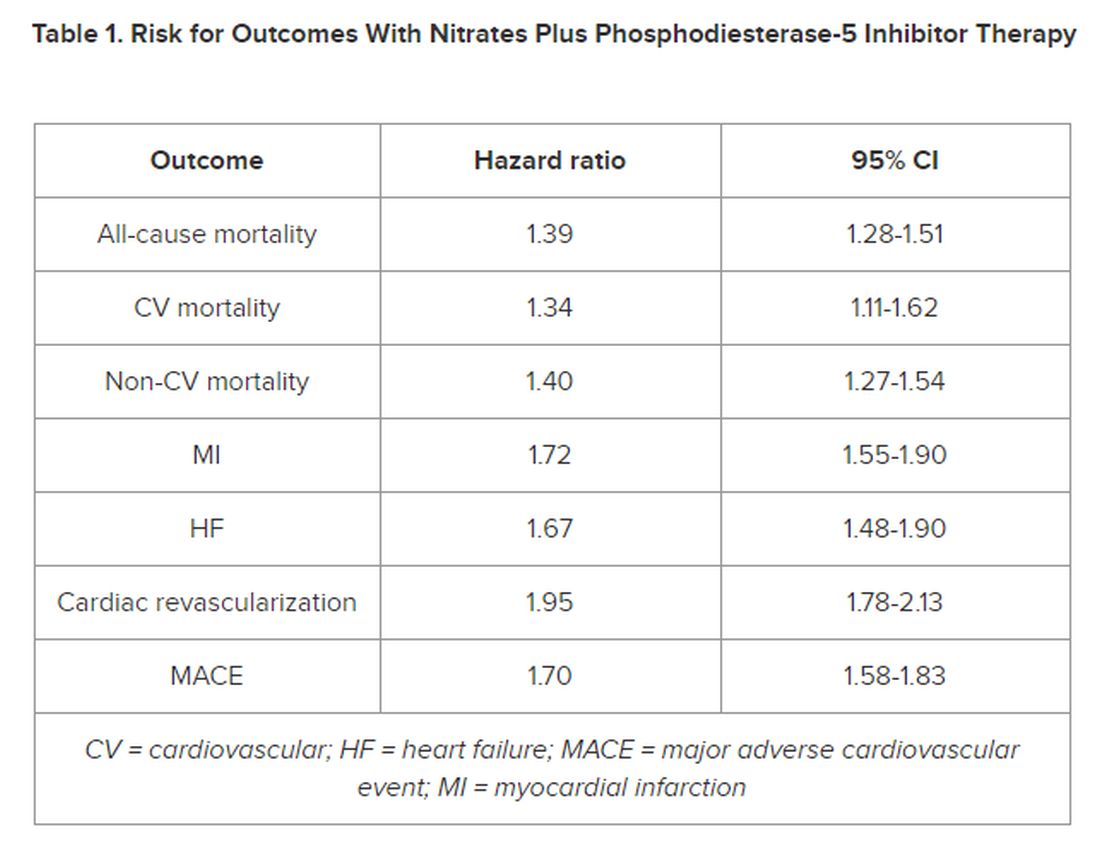
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New Insights Into Mortality in Takotsubo Syndrome
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Severely Irregular Sleep Patterns and OSA Prompt Increased Odds of Hypertension
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Temporary Higher Stroke Rate After TAVR
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.
Medical Cannabis for Chronic Pain Tied to Arrhythmia Risk
TOPLINE:
, mainly atrial fibrillation/flutter, a Danish registry study suggested. Cannabis use has been associated with increased cardiovascular (CV) risk, but data on CV side effects with use of medical cannabis for chronic pain are limited.
METHODOLOGY:
- To investigate, researchers identified 5391 patients with chronic pain (median age 59; 63% women) initiating first-time treatment with medical cannabis during 2018-2021 and matched them (1:5) to 26,941 control patients on age, sex, chronic pain diagnosis, and concomitant use of other noncannabis pain medication.
- They calculated and compared absolute risks for first-time arrhythmia (atrial fibrillation/flutter, conduction disorders, paroxysmal tachycardias, and ventricular arrhythmias) and acute coronary syndrome (ACS) between groups.
TAKEAWAY:
- Within 180 days, 42 medical cannabis users and 107 control participants developed arrhythmia, most commonly atrial fibrillation/flutter.
- Medical cannabis users had a slightly elevated risk for new-onset arrhythmia compared with nonusers (180-day absolute risk, 0.8% vs 0.4%).
- The 180-day risk ratio with cannabis use was 2.07 (95% CI, 1.34-2.80), and the 1-year risk ratio was 1.36 (95% CI, 1.00-1.73).
- Adults with cancer or cardiometabolic disease had the highest risk for arrhythmia with cannabis use (180-day absolute risk difference, 1.1% and 0.8%). There was no significant association between medical cannabis use and ACS risk.
IN PRACTICE:
“With the investigated cohort’s low age and low prevalence of comorbidity in mind, the notable relative risk increase of new-onset arrhythmia, mainly driven by atrial fibrillation/flutter, could be a reason for concern, albeit the absolute risks in this study population were modest,” the authors wrote.
“Medical cannabis may not be a ‘one-size-fits-all’ therapeutic option for certain medical conditions and should be contextualized based on patient comorbidities and potential vulnerability to side effects,” added the author of an editorial.
SOURCE:
The study, led by Anders Holt, MD, Copenhagen University and Herlev-Gentofte Hospital, Hellerup, Denmark, was published online on January 11, 2024, in the European Heart Journal, with an editorial by Robert Page II, PharmD, MSPH, University of Colorado, Aurora.
LIMITATIONS:
Residual confounding is possible. The registers lack information on disease severity, clinical measures, blood tests, and lifestyle factors. The route of cannabis administration was not known.
DISCLOSURES:
The study was funded by external and independent medical research grants. Holt had no relevant disclosures. Some coauthors reported research grants and speakers’ fees from various drug companies.
A version of this article appeared on Medscape.com.
TOPLINE:
, mainly atrial fibrillation/flutter, a Danish registry study suggested. Cannabis use has been associated with increased cardiovascular (CV) risk, but data on CV side effects with use of medical cannabis for chronic pain are limited.
METHODOLOGY:
- To investigate, researchers identified 5391 patients with chronic pain (median age 59; 63% women) initiating first-time treatment with medical cannabis during 2018-2021 and matched them (1:5) to 26,941 control patients on age, sex, chronic pain diagnosis, and concomitant use of other noncannabis pain medication.
- They calculated and compared absolute risks for first-time arrhythmia (atrial fibrillation/flutter, conduction disorders, paroxysmal tachycardias, and ventricular arrhythmias) and acute coronary syndrome (ACS) between groups.
TAKEAWAY:
- Within 180 days, 42 medical cannabis users and 107 control participants developed arrhythmia, most commonly atrial fibrillation/flutter.
- Medical cannabis users had a slightly elevated risk for new-onset arrhythmia compared with nonusers (180-day absolute risk, 0.8% vs 0.4%).
- The 180-day risk ratio with cannabis use was 2.07 (95% CI, 1.34-2.80), and the 1-year risk ratio was 1.36 (95% CI, 1.00-1.73).
- Adults with cancer or cardiometabolic disease had the highest risk for arrhythmia with cannabis use (180-day absolute risk difference, 1.1% and 0.8%). There was no significant association between medical cannabis use and ACS risk.
IN PRACTICE:
“With the investigated cohort’s low age and low prevalence of comorbidity in mind, the notable relative risk increase of new-onset arrhythmia, mainly driven by atrial fibrillation/flutter, could be a reason for concern, albeit the absolute risks in this study population were modest,” the authors wrote.
“Medical cannabis may not be a ‘one-size-fits-all’ therapeutic option for certain medical conditions and should be contextualized based on patient comorbidities and potential vulnerability to side effects,” added the author of an editorial.
SOURCE:
The study, led by Anders Holt, MD, Copenhagen University and Herlev-Gentofte Hospital, Hellerup, Denmark, was published online on January 11, 2024, in the European Heart Journal, with an editorial by Robert Page II, PharmD, MSPH, University of Colorado, Aurora.
LIMITATIONS:
Residual confounding is possible. The registers lack information on disease severity, clinical measures, blood tests, and lifestyle factors. The route of cannabis administration was not known.
DISCLOSURES:
The study was funded by external and independent medical research grants. Holt had no relevant disclosures. Some coauthors reported research grants and speakers’ fees from various drug companies.
A version of this article appeared on Medscape.com.
TOPLINE:
, mainly atrial fibrillation/flutter, a Danish registry study suggested. Cannabis use has been associated with increased cardiovascular (CV) risk, but data on CV side effects with use of medical cannabis for chronic pain are limited.
METHODOLOGY:
- To investigate, researchers identified 5391 patients with chronic pain (median age 59; 63% women) initiating first-time treatment with medical cannabis during 2018-2021 and matched them (1:5) to 26,941 control patients on age, sex, chronic pain diagnosis, and concomitant use of other noncannabis pain medication.
- They calculated and compared absolute risks for first-time arrhythmia (atrial fibrillation/flutter, conduction disorders, paroxysmal tachycardias, and ventricular arrhythmias) and acute coronary syndrome (ACS) between groups.
TAKEAWAY:
- Within 180 days, 42 medical cannabis users and 107 control participants developed arrhythmia, most commonly atrial fibrillation/flutter.
- Medical cannabis users had a slightly elevated risk for new-onset arrhythmia compared with nonusers (180-day absolute risk, 0.8% vs 0.4%).
- The 180-day risk ratio with cannabis use was 2.07 (95% CI, 1.34-2.80), and the 1-year risk ratio was 1.36 (95% CI, 1.00-1.73).
- Adults with cancer or cardiometabolic disease had the highest risk for arrhythmia with cannabis use (180-day absolute risk difference, 1.1% and 0.8%). There was no significant association between medical cannabis use and ACS risk.
IN PRACTICE:
“With the investigated cohort’s low age and low prevalence of comorbidity in mind, the notable relative risk increase of new-onset arrhythmia, mainly driven by atrial fibrillation/flutter, could be a reason for concern, albeit the absolute risks in this study population were modest,” the authors wrote.
“Medical cannabis may not be a ‘one-size-fits-all’ therapeutic option for certain medical conditions and should be contextualized based on patient comorbidities and potential vulnerability to side effects,” added the author of an editorial.
SOURCE:
The study, led by Anders Holt, MD, Copenhagen University and Herlev-Gentofte Hospital, Hellerup, Denmark, was published online on January 11, 2024, in the European Heart Journal, with an editorial by Robert Page II, PharmD, MSPH, University of Colorado, Aurora.
LIMITATIONS:
Residual confounding is possible. The registers lack information on disease severity, clinical measures, blood tests, and lifestyle factors. The route of cannabis administration was not known.
DISCLOSURES:
The study was funded by external and independent medical research grants. Holt had no relevant disclosures. Some coauthors reported research grants and speakers’ fees from various drug companies.
A version of this article appeared on Medscape.com.
What’s the Disease Burden From Plastic Exposure?
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF THE ENDOCRINE SOCIETY
Study Identifies Cardiovascular Comorbidities Associated With Dermatomyositis
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Delayed Meals Tied to Increased CVD Risk
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Preventing ASCVD Events: Using Coronary Artery Calcification Scores to Personalize Risk and Guide Statin Therapy
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
Lung cancer is the most common cause of cancer mortality, and cigarette smoking is the most significant risk factor. Several randomized clinical trials have shown that lung cancer screening (LCS) with nonelectrocardiogram (ECG)-gated low-dose computed tomography (LDCT) reduces both lung cancer and all-cause mortality.1,2 Hence, the US Preventive Screening Task Force (USPSTF) recommends annual screening with LDCT in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.3
Smoking is also an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), and LCS clinical trials acknowledge that mortality from ASCVD events exceeds that of lung cancer.4,5 In an analysis of asymptomatic individuals from the Framingham Heart Offspring study who were eligible for LCS, the ASCVD event rate during a median (IQR) follow-up of 11.4 (9.7-12.0) years was 12.6%.6 However, despite the high rate of ASCVD events in this population, primary prevention strategies are consistently underused. In a study of 5495 individuals who underwent LCS with LDCT, only 40% of those eligible for statins had one prescribed, underscoring the missed opportunity for preventing ASCVD events during LCS.7 Yet the interactions for shared decision making and the availability of coronary artery calcification (CAC) scores from the LDCT provide an ideal window for intervening and preventing ASCVD events during LCS.
CAC is a hallmark of atherosclerotic plaque development and is proportional to plaque burden and ASCVD risk.8 Because of the relationship between CAC, subclinical atherosclerosis, and ASCVD risk, there is an opportunity to use CAC detected by LDCT to predict ASCVD risk and guide recommendations for statin treatment in individuals enrolled in LCS. Traditionally, CAC has been visualized by ECG-gated noncontrast CT scans with imaging protocols specifically designed to visualize the coronary arteries, minimize motion artifacts, and reduce signal noise. These scans are specifically done for primary prevention risk assessment and report an Agatston score, a summed measure based on calcified plaque area and maximal density.9 Results are reported as an overall CAC score and an age-, sex-, and race-adjusted percentile of CAC. Currently, a CAC score ≥ 100 or above the 75th percentile for age, sex, and race is considered abnormal.
High-quality evidence supports CAC scores as a strong predictor of ASCVD risk independent of age, sex, race, and other traditional risk factors.10-12 In asymptomatic individuals, a CAC score of 0 is a strong, negative risk factor associated with very low annualized mortality rates and cardiovascular (CV) events, so intermediate-risk individuals can be reclassified to a lower risk group avoiding or delaying statin therapy.13 As a result, current primary prevention guidelines allow for CAC scoring in asymptomatic, intermediate-risk adults where the clinical benefits of statin therapy are uncertain, knowing the CAC score will aid in the clinical decision to delay or initiate statin therapy.
Unlike traditional ECG-gated CAC scoring, LDCT imaging protocols are non–ECG-gated and performed at variable energy and slice thickness to optimize the detection of lung nodules. Early studies suggested that CAC detected by LDCT could be used in lieu of traditional CAC scoring to personalize risk.14,15 Recently, multiple studies have validated the accuracy and reproducibility of LDCT to detect and quantify CAC. In both the NELSON and the National Lung Screening Trial (NLST) LCS trials, higher visual and quantitative measures of CAC were independently and incrementally associated with ASCVD risk.16,17 A subsequent review and meta-analysis of 6 LCS trials confirmed CAC detected by LDCT to be an independent predictor of ASCVD events regardless of the method used to measure CAC.18
There is now consensus that either an Agatston score or a visual estimate of CAC be reported on all noncontrast, noncardiac chest CT scans irrespective of the indication or technique, including LDCT scans for LCS using a uniform reporting system known as the Coronary Artery Calcium Data and Reporting System (CAC-DRS).19 The CAC-DRS simplifies reporting and adds modifiers indicating if the reported score is visual (V) or Agatston (A) and number of vessels involved. For example, CAC-DRS A0 or CAC-DRS V0 would indicate an Agatston score of 0 or a visual score of 0. CAC-DRS A1/N2 would indicate a total Agatston score of 1-99 in 2 coronary arteries. The currently agreed-on CAC-DRS risk groups are listed in the Table, along with their corresponding visual score or Agatston score and anticipated 10-year event rate, irrespective of other risk factors.20
As LCS efforts increase, primary care practitioners will receive LDCT reports that now incorporate an estimation of CAC (visual or quantitative). Thus, it will be increasingly important to know how to interpret and use these scores to guide clinical decisions regarding the initiation of statin therapy, referral for additional testing, and when to seek specialty cardiology care. For instance, does the absence of CAC (CAC = 0) on LDCT predict a low enough risk for statin therapy to be delayed or withdrawn? Does increasing CAC scores on follow-up LDCT in individuals on statin therapy represent treatment failure? When should CAC scores trigger additional testing, such as a stress test or referral to cardiology specialty care?
Primary Prevention in LCS
The initial approach to primary prevention in LCS is no different from that recommended by the 2018 multisociety guidelines on the management of blood cholesterol, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline on primary prevention, or the 2022 USPTSF recommendations on statin use for primary prevention of CV disease in adults.21-23 For a baseline low-density lipoprotein cholesterol (LDL-C) ≥ 190 mg/dL, high-intensity statin therapy is recommended without further risk stratification. Individuals with diabetes also are at higher-than-average risk, and moderate-intensity statin therapy is recommended.
For individuals not in either group, a validated ASCVD risk assessment tool is recommended to estimate baseline risk. The most validated tool for estimating risk in the US population is the 2013 ACC/AHA Pooled Cohort Equation (PCE) which provides an estimate of the 10-year risk for fatal and myocardial infarction and fatal and nonfatal stroke.24 The PCE risk calculator uses age, presence of diabetes, sex, smoking history, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and treatment for hypertension to place individuals into 1 of 4 risk groups: low (< 5%), borderline (5% to < 7.5%), intermediate (≥ 7.5% to < 20%), and high (≥ 20%). Clinicians should be aware that the PCE only considers current smoking history and not prior smoking history or cumulative pack-year history. This differs from eligibility for LCS where recent smoking plays a larger role. All these risk factors are important to consider when evaluating risk and discussing risk-reducing strategies like statin therapy.
The 2018 multisociety guidelines and the 2019 primary prevention guidelines set the threshold for considering initiation of statin therapy at intermediate risk ≥ 7.5%.21,22 The 2020 US Department of Veterans Affairs/Department of Defense guidelines set the threshold for considering statin therapy at an estimated 10-year event rate of 12%, whereas the 2022 UPSTF recommendations set the threshold at 10% with additional risk factors as the threshold for statin therapy.23,25 The reasons for these differences are beyond the scope of this review, but all these guidelines use the PCE to estimate baseline risk as the starting point for clinical decision making.
The PCE was originally derived and validated in population studies dating to the 1960s when the importance of diet, exercise, and smoking cessation in reducing ASCVD events was not well appreciated. The application of the PCE in more contemporary populations shows that it overestimates risk, especially in older individuals and women.26,27 Overestimation of risk has the potential to result in the initiation of statin therapy in individuals in whom the actual clinical benefit would otherwise be small.
To address this issue, current guidelines allow the use of CAC scoring to refine risk in individuals who are classified as intermediate risk and who otherwise desire to avoid lifelong statin therapy. Using current recommendations, we make suggestions on how to use CAC scores from LDCT to aid in clinical decision making for individuals in LCS (Figure).
No Coronary Artery Calcification
Between 25% and 30% of LDCT done for LCS will show no CAC.14,16 In general population studies, a CAC score of 0 is a strong negative predictor when there are no other risk factors.13,28 In contrast, the negative predictive ability of a CAC score of 0 in individuals with a smoking history who are eligible for LCS is unproven. In multivariate modeling, a CAC score of 0 did not reduce the significant hazard of all-cause mortality in patients with diabetes or smokers.29 In an analysis of 44,042 individuals without known heart disease referred for CAC scoring, the frequency of a CAC score of 0 was only modestly lower in smokers (38%) compared with nonsmokers (42%), yet the all-cause mortality rate was significantly higher.30 In addition, Multi-Ethnic Study of Atherosclerosis (MESA) participants who were current smokers or eligible for LCS and had a CAC score of 0 had an observed 11-year ASCVD event rate of 13.4% and 20.8%, respectively, leading to the conclusion that a CAC score of 0 may not be predictive of minimal risk in smokers and those eligible for LCS.31 Additionally, in LCS-eligible individuals, the PCE underestimated event rates and incorporation of CAC scores did not significantly improve risk estimation. Finally, data from the NLST screening trial showed that the absence of CAC on LDCT was not associated with better survival or lower CV mortality compared with individuals with low CAC scores.32
The question of whether individuals undergoing LCS with LDCT who have no detectable CAC can avoid statin therapy is an unresolved issue; no contemporary studies have looked specifically at the relationship between estimated risk, a CAC score of 0, and ASCVD outcomes in individuals participating in LCS. For these reasons, we recommend moderate-intensity statin therapy when the estimated risk is intermediate because it is unclear that either an Agatston score of 0 reclassifies intermediate-risk LCS-eligible individuals to a lower risk group.
For the few borderline risk (estimated risk, 5% to < 7.5%) LCS-eligible individuals, a CAC score of 0 might confer low short-term risk but the long-term benefit of statin therapy on reducing subsequent risk, the presence of other risk factors, and the willingness to stop smoking should all be considered. For these individuals who elect to avoid statin therapy, annual re-estimation of risk at the time of repeat LDCT is recommended. In these circumstances, referral for traditional Agatston scoring is not likely to change decision making because the sensitivity of the 2 techniques is very similar.
Agatston Score of 1-99 or CAC-DRS or Visual Score of 1
In general population studies, these scores correspond to borderline risk and an estimated 10-year event rate of just under 7.5%.20 In both the NELSON and NLST LCS trials, even low amounts of CAC regardless of the scoring method were associated with higher observed ASCVD mortality when adjusted for other baseline risk factors.32 Thus, in patients undergoing LCS with intermediate and borderline risk, a CAC score between 1 and 99 or a visual estimate of 1 indicates the presence of subclinical atherosclerosis, and moderate-intensity statin therapy is reasonable.
Agatston Score of 100-299 or CAC-DRS or Visual Score of 2
Across all ages, races, and sexes, CAC scores between 100 to 299 are associated with an event rate of about 15% over 10 years.20 In the NELSON LCS trial, the adjusted hazard ratio for ASCVD events with a nontraditional Agatston score of 101 to 400 was 6.58.33 Thus, in patients undergoing LCS with a CAC score of 100 to 299, regardless of the baseline risk estimate, the projected absolute event rate at 10 years would be about 20%. Moderate-intensity statin therapy is recommended to reduce the baseline LDL-C by 30% to 49%.
Agatston Score of > 300 or CAC-DRS or Visual Score of 3
Agatston CAC scores > 300 are consistent with a 10-year incidence of ASCVD events of > 15% regardless of age, sex, or race and ethnicity.20 In the Calcium Consortium, a CAC > 400 was correlated with an event rate of 13.6 events/1000 person-years.12 In a Walter Reed Military Medical Center study, a CAC score > 400 projected a cumulative incidence of ASCVD events of nearly 20% at 10 years.34 In smokers eligible for LCS, a CAC score > 300 projected a 10-year ASCVD event rate of 25%.29 In these patients, moderate-intensity statin therapy is recommended, although high-intensity statin therapy can be considered if there are other risk factors.
Agatston Score ≥ 1000
The 2018 consensus statement on CAC reporting categorizes all CAC scores > 300 into a single risk group because the recommended treatment options do not differ.19 However, recent data suggest this might not be the case since individuals with very high CAC scores experience high rates of events that might justify more aggressive intervention. In an analysis of individuals who participated in the CAC Consortium with a CAC score ≥ 1000, the all-cause mortality rate was 18.8 per 1000 person-years with a CV mortality rate of 8 per 1000 person-years.35 Individuals with very high levels of CAC > 1000 also have a greater number of diseased coronary arteries, higher involvement of the left main coronary artery, and significantly higher event rates compared with those with a CAC of 400 to 999.36 In an analysis of individuals from the NLST trial, nontraditionally measured Agatston score > 1000 was associated with a hazard ratio for coronary artery disease (CAD) mortality of 3.66 in men and 5.81 in women.17 These observed and projected levels of risk are like that seen in secondary prevention trials, and some experts have recommended the use of high-intensity statin therapy to reduce LDL-C to < 70 mg/dL.37
Primary Prevention in Individuals aged 76 to 80 years
LCS can continue through age 80 years, while the PCE and primary prevention guidelines are truncated at age 75 years. Because age is a major contributor to risk, many of these individuals will already be in the intermediate- to high-risk group. However, the net clinical benefit of statin therapy for primary prevention in this age group is not well established, and the few primary prevention trials in this group have not demonstrated net clinical benefit.38 As a result, current guidelines do not provide specific treatment recommendations for individuals aged > 75 years but recognize the value of shared decision making considering associated comorbidities, age-related risks of statin therapy, and the desires of the individual to avoid ASCVD-related events even if the net clinical benefit is low.
Older individuals with elevated CAC scores should be informed about the risk of ASCVD events and the potential but unproven benefit of moderate-intensity statin therapy. Older individuals with a CAC score of 0 likely have low short-term risk of ASCVD events and withholding statin therapy is not unreasonable.
CAC Scores on Annual LDCT Scans
Because LCS requires annual LDCT scans, primary care practitioners and patients need to understand the significance of changing CAC scores over time. For individuals not on statin therapy, increasing calcification is a marker of progression of subclinical atherosclerosis. Patients undergoing LCS not on statin who have progressive increases in their CAC should consider initiating statin therapy. Individuals who opted not to initiate statin therapy who subsequently develop CAC should be re-engaged in a discussion about the significance of the finding and the clinically proven benefits of statin therapy in individuals with subclinical atherosclerosis. These considerations do not apply to individuals already on statin therapy. Statins convert lipid-rich plaques to lipid-depleted plaques, resulting in increasing calcification. As a result, CAC scores do not decrease and may increase with statin therapy.39 Individuals participating in annual LCS should be informed of this possibility. Also, in these individuals, referral to specialty care as a treatment failure is not supported by the literature.
Furthermore, serial CAC scoring to titrate the intensity of statin therapy is not currently recommended. The goal with moderate-intensity statin therapy is a 30% to 49% reduction from baseline LDL-C. If this milestone is not achieved, the statin dose can be escalated. For high-intensity statin therapy, the goal is a > 50% reduction. If this milestone is not achieved, then additional lipid-lowering agents, such as ezetimibe, can be added.
Further ASCVD Testing
LCS with LDCT is associated with improved health outcomes, and LDCT is the preferred imaging modality. The ability of LDCT to detect and quantify CAC is sufficient for clinical decision making. Therefore, obtaining a traditional CAC score increases radiation exposure without additional clinical benefits.
Furthermore, although referral for additional testing in those with nonzero CAC scores is common, current evidence does not support this practice in asymptomatic individuals. Indeed, the risks of LCS include overdiagnosis, excessive testing, and overtreatment secondary to the discovery of other findings, such as benign pulmonary nodules and CAC. With respect to CAD, randomized controlled trials do not support a strategy of coronary angiography and intervention in asymptomatic individuals, even with moderate-to-severe ischemia on functional testing.40 As a result, routine stress tests to diagnose CAD or to confirm the results of CAC scores in asymptomatic individuals are not recommended. The only potential exception would be in select cases where the CAC score is > 1000 and when calcium is predominately located in the left main coronary artery.
Conclusions
LCS provides smokers at risk for lung cancer with the best probability to survive that diagnosis, and coincidentally LCS may also provide the best opportunity to prevent ASCVD events and mortality. Before initiating LCS, clinicians should initiate a shared decision making conversation about the benefits and risks of LDCT scans. In addition to relevant education about smoking, during shared decision making, the initial ASCVD risk estimate should be done using the PCE and when appropriate the benefits of statin therapy discussed. Individuals also should be informed of the potential for identifying CAC and counseled on its significance and how it might influence the decision to recommend statin therapy.
In patients undergoing LCS with an estimated risk of ≥ 7.5% to < 20%, moderate-intensity statin therapy is indicated. In this setting, a CAC score > 0 indicates subclinical atherosclerosis and should be used to help direct patients toward initiating statin therapy. Unfortunately, in patients undergoing LCS a CAC score of 0 might not provide protection against ASCVD, and until there is more information to the contrary, these individuals should at least participate in shared decision making about the long-term benefits of statin therapy in reducing ASCVD risk. Because LDCT scanning is done annually, there are opportunities to review the importance of prevention and to adjust therapy as needed to achieve the greatest reduction in ASCVD. Reported elevated CAC scores on LDCT provide an opportunity to re-engage the patient in the discussion about the benefits of statin therapy if they are not already on a statin, or consideration for high-intensity statin if the CAC score is > 1000 or reduction in baseline LDL-C is < 30% on the current statin dose.
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
1. de Koning HJ, van der Aalst CM, Oudkerk M. Lung-cancer screening and the NELSON Trial. Reply. N Engl J Med. 2020;382(22):2165-2166. doi:10.1056/NEJMc2004224
2. Aberle T, Adams DR, Berg AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):396-409. doi:10.1056/NEJMoa1102873
3. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;25(10):962-970. doi:10.1001/jama.2021.1117
4. Jha P, Ramasundarahettige C, Landsman V. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350. doi:10.1056/NEJMsa1211128
5. Khan SS, Ning H, Sinha A, et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J Am Heart Assoc. 2021;10(23):e021751. doi:10.1161/JAHA.121.021751
6. Lu MT, Onuma OK, Massaro JM, et al. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
7. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
8. Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11(1):127-142. doi:10.1016/j.jcmg.2017.10.012
10. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15): 1657-1668. doi:10.1016/j.jacc.2015.07.066
11. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi:10.1056/NEJMoa072100
12. Grandhi GR, Mirbolouk M, Dardari ZA. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD Mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(5):1175-1186. doi:10.1016/j.jcmg.2019.08.024
13. Blaha M, Budoff MJ, Shaw J. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692-700. doi:10.1016/j.jcmg.2009.03.009
14. Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging. 2006;30(3):181-185. doi:10.1016/j.clinimag.2005.11.002
15. Shemesh J, Henschke C, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541-548. doi:10.1148/radiol.10100383
16. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
17. Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808-1817. doi:10.1016/j.jcmg.2018.10.026
18. Gendarme S, Goussault H, Assie JB, et al. Impact on all-cause and cardiovascular mortality rates of coronary artery calcifications detected during organized, low-dose, computed-tomography screening for lung cancer: systematic literature review and meta-analysis. Cancers (Basel). 2021;13(7):1553. doi:10.3390/cancers13071553
19. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185-191. doi:10.1016/j.jcct.2018.03.008
20. Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi:10.1093/eurheartj/ehy217
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046-e1081. doi:10.1161/CIR.0000000000000624
22. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
23. Mangione CM, Barry MJ, Nicholson WK, et al. US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2022;328(8):746-753. doi:10.1001/jama.2022.13044
24. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934. doi:10.1016/j.jacc.2013.11.002
 25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
25. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline. Updated August 25, 2021. Accessed November 3, 2023. https://www.healthquality.va.gov/guidelines/cd/lipids
26. DeFilippis AP, Young, R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266-275. doi:10.7326/M14-1281
27. Rana JS, Tabada GH, Solomon, MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
28. Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi:10.1016/j.jcmg.2008.12.031
29. McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging. 2012;5(10):1037-1045. doi:10.1016/j.jcmg.2012.02.017
30. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
31. Garg PK, Jorgensen NW, McClelland RL, et al. Use of coronary artery calcium testing to improve coronary heart disease risk assessment in lung cancer screening population: The Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomagr. 2018;12(6):439-400.
32. Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi:10.1148/radiol.15142062
33. Takx RA, Isgum I, Willemink MJ, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr. 2015;9(1):50-57. doi:10.1016/j.jcct.2014.11.006
34. Mitchell JD, Paisley R, Moon P, et al. Coronary artery calcium and long-term risk of death, myocardial infarction, and stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11(12):1799-1806. doi:10.1016/j.jcmg.2017.09.003
35. Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC >/=1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2019;13(1, pt 1):83-93. doi:10.1016/j.jcmg.2019.02.005
36. Peng AW, Dardari ZA. Blumenthal RS, et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571-1583. doi:10.1161/CIRCULATIONAHA.120.050545
37. Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33-60. doi:10.1016/j.jacl.2020.12.005
38. Sheperd J, Blauw GJ, Murphy MB, et al. PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease. (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623-1630. doi:10.1016/s0140-6736(02)11600-x
39. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273-1282. doi:10.1016/j.jacc.2015.01.036
40. Maron D.J, Hochman J S, Reynolds HR, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922