User login
Psychiatric patients face inordinately long wait times in emergency departments
Individuals with psychiatric conditions are facing increasingly longer wait times in emergency departments across the country, including children, according to a pair of studies presented by the American College of Emergency Physicians.
“I really started doing this research because of my clinical experience,” explained Suzanne Catherine Lippert, MD, of Stanford (Calif.) University and the lead author of both studies during an Oct. 17 conference call, saying that seeing patients sit in the ED for days prompted her to finally look into this issue.
Patients with bipolar disorder had the highest likelihood of waiting more than 24 hours in the ED, with an odds ratio of 3.7 (95% confidence interval, 1.5-9.4). This was followed by patients with a diagnosis of psychosis, a dual diagnosis of psychiatric disorders, multiple psychiatric diagnoses, or depression. The most common diagnoses were substance abuse, anxiety, and depression, which constituted 41%, 26%, and 23% of the diagnoses, respectively. Patients with psychosis were admitted 34% of the time and transferred 24% of the time; those who self-harmed were admitted 33% of the time and transferred 29% of the time; and patients with bipolar disorder were admitted 29% of the time and transferred 40% of the time. Patients who had either two or three diagnoses were admitted 9% and 10% of the time, respectively.
“Further investigation of the systems affecting these patients, including placement of involuntary holds, availability of ED psychiatric consultants, or outpatient resources would delineate potential intervention points for the care of these vulnerable patients,” Dr. Lippert and her coauthors wrote.
The second study looked at the differences in waiting for care at EDs between psychiatric patients and medical patients. Length of stay was defined the same way it was in the previous study, with disposition meaning either “discharge, admission to medical or psychiatric bed, [or] transfer to any acute facility.” Length of stay was divided into the same three categories as the previous study, too.
Psychiatric patients were more likely than were medical patients to wait more than 6 hours for disposition, regardless of what the disposition itself ended up being, by a rate of 23% vs. 10%. Similarly, 7% of psychiatric patients vs. just 2.3% of medical patients had to wait longer than 12 hours in the ED, while 1.3% of psychiatric had to wait longer than 24 hours, compared with only 0.5% of medical patients. The average length of stay was significantly longer for psychiatric patients: 194 minutes vs. 138 minutes for medical patients (P less than .01).
Additionally, psychiatric patients were more likely to be uninsured, with 22% not having insurance, compared with 15% of medical patients being uninsured. Furthermore, 4.6% of the psychiatric patients’ previous visit to the ED had been within the prior 72 hours, compared with 3.6% of medical patients. A total of 21% of psychiatric patients required admittance, compared with 13% of medical patients, while 11% of psychiatric patients were transferred, compared with just 1.4% of medical patients.
“These results compel us to further investigate the potential causes of prolonged length of stay in psychiatric patients and to further characterize the population of psychiatric patients most at risk of prolonged stays,” Dr. Lippert and her coinvestigators concluded.
ACEP President Rebecca B. Parker, MD, chimed in during the conference call, explaining that a survey of more than 1,700 emergency physicians revealed some “troubling” findings about the state of emergency departments over the last year.
The nation’s dwindling mental health resources are having a direct impact on patients having psychiatric emergencies, including children,” Dr. Parker explained. “These patients are waiting longer for care, especially those patients who require hospitalization.”
Findings of the survey indicate that 48% of ED physicians witness psychiatric patients being “boarded” in their EDs at least once a day while they wait for a bed. Additionally, less than 17% of respondents said their ED has a psychiatrist on call to respond to psychiatric emergencies, with 11.7% responding that they have no psychiatrist on call to deal with such emergencies. And 52% of respondents said the mental health system in their community has gotten noticeably worse in just the last year.
In a separate statement, Dr. Parker voiced outrage about the situation. “Psychiatric patients wait in the emergency department for hours and even days for a bed, which delays the psychiatric care they so desperately need,” she said. “It also leads to delays in care and diminished resources for other emergency patients. The emergency department has become the dumping ground for these vulnerable patients who have been abandoned by every other part of the health care system.”
ACEP presented the findings during its annual meeting in Las Vegas. No funding sources for these studies were disclosed; Dr. Lippert did not report any financial disclosures.
Individuals with psychiatric conditions are facing increasingly longer wait times in emergency departments across the country, including children, according to a pair of studies presented by the American College of Emergency Physicians.
“I really started doing this research because of my clinical experience,” explained Suzanne Catherine Lippert, MD, of Stanford (Calif.) University and the lead author of both studies during an Oct. 17 conference call, saying that seeing patients sit in the ED for days prompted her to finally look into this issue.
Patients with bipolar disorder had the highest likelihood of waiting more than 24 hours in the ED, with an odds ratio of 3.7 (95% confidence interval, 1.5-9.4). This was followed by patients with a diagnosis of psychosis, a dual diagnosis of psychiatric disorders, multiple psychiatric diagnoses, or depression. The most common diagnoses were substance abuse, anxiety, and depression, which constituted 41%, 26%, and 23% of the diagnoses, respectively. Patients with psychosis were admitted 34% of the time and transferred 24% of the time; those who self-harmed were admitted 33% of the time and transferred 29% of the time; and patients with bipolar disorder were admitted 29% of the time and transferred 40% of the time. Patients who had either two or three diagnoses were admitted 9% and 10% of the time, respectively.
“Further investigation of the systems affecting these patients, including placement of involuntary holds, availability of ED psychiatric consultants, or outpatient resources would delineate potential intervention points for the care of these vulnerable patients,” Dr. Lippert and her coauthors wrote.
The second study looked at the differences in waiting for care at EDs between psychiatric patients and medical patients. Length of stay was defined the same way it was in the previous study, with disposition meaning either “discharge, admission to medical or psychiatric bed, [or] transfer to any acute facility.” Length of stay was divided into the same three categories as the previous study, too.
Psychiatric patients were more likely than were medical patients to wait more than 6 hours for disposition, regardless of what the disposition itself ended up being, by a rate of 23% vs. 10%. Similarly, 7% of psychiatric patients vs. just 2.3% of medical patients had to wait longer than 12 hours in the ED, while 1.3% of psychiatric had to wait longer than 24 hours, compared with only 0.5% of medical patients. The average length of stay was significantly longer for psychiatric patients: 194 minutes vs. 138 minutes for medical patients (P less than .01).
Additionally, psychiatric patients were more likely to be uninsured, with 22% not having insurance, compared with 15% of medical patients being uninsured. Furthermore, 4.6% of the psychiatric patients’ previous visit to the ED had been within the prior 72 hours, compared with 3.6% of medical patients. A total of 21% of psychiatric patients required admittance, compared with 13% of medical patients, while 11% of psychiatric patients were transferred, compared with just 1.4% of medical patients.
“These results compel us to further investigate the potential causes of prolonged length of stay in psychiatric patients and to further characterize the population of psychiatric patients most at risk of prolonged stays,” Dr. Lippert and her coinvestigators concluded.
ACEP President Rebecca B. Parker, MD, chimed in during the conference call, explaining that a survey of more than 1,700 emergency physicians revealed some “troubling” findings about the state of emergency departments over the last year.
The nation’s dwindling mental health resources are having a direct impact on patients having psychiatric emergencies, including children,” Dr. Parker explained. “These patients are waiting longer for care, especially those patients who require hospitalization.”
Findings of the survey indicate that 48% of ED physicians witness psychiatric patients being “boarded” in their EDs at least once a day while they wait for a bed. Additionally, less than 17% of respondents said their ED has a psychiatrist on call to respond to psychiatric emergencies, with 11.7% responding that they have no psychiatrist on call to deal with such emergencies. And 52% of respondents said the mental health system in their community has gotten noticeably worse in just the last year.
In a separate statement, Dr. Parker voiced outrage about the situation. “Psychiatric patients wait in the emergency department for hours and even days for a bed, which delays the psychiatric care they so desperately need,” she said. “It also leads to delays in care and diminished resources for other emergency patients. The emergency department has become the dumping ground for these vulnerable patients who have been abandoned by every other part of the health care system.”
ACEP presented the findings during its annual meeting in Las Vegas. No funding sources for these studies were disclosed; Dr. Lippert did not report any financial disclosures.
Individuals with psychiatric conditions are facing increasingly longer wait times in emergency departments across the country, including children, according to a pair of studies presented by the American College of Emergency Physicians.
“I really started doing this research because of my clinical experience,” explained Suzanne Catherine Lippert, MD, of Stanford (Calif.) University and the lead author of both studies during an Oct. 17 conference call, saying that seeing patients sit in the ED for days prompted her to finally look into this issue.
Patients with bipolar disorder had the highest likelihood of waiting more than 24 hours in the ED, with an odds ratio of 3.7 (95% confidence interval, 1.5-9.4). This was followed by patients with a diagnosis of psychosis, a dual diagnosis of psychiatric disorders, multiple psychiatric diagnoses, or depression. The most common diagnoses were substance abuse, anxiety, and depression, which constituted 41%, 26%, and 23% of the diagnoses, respectively. Patients with psychosis were admitted 34% of the time and transferred 24% of the time; those who self-harmed were admitted 33% of the time and transferred 29% of the time; and patients with bipolar disorder were admitted 29% of the time and transferred 40% of the time. Patients who had either two or three diagnoses were admitted 9% and 10% of the time, respectively.
“Further investigation of the systems affecting these patients, including placement of involuntary holds, availability of ED psychiatric consultants, or outpatient resources would delineate potential intervention points for the care of these vulnerable patients,” Dr. Lippert and her coauthors wrote.
The second study looked at the differences in waiting for care at EDs between psychiatric patients and medical patients. Length of stay was defined the same way it was in the previous study, with disposition meaning either “discharge, admission to medical or psychiatric bed, [or] transfer to any acute facility.” Length of stay was divided into the same three categories as the previous study, too.
Psychiatric patients were more likely than were medical patients to wait more than 6 hours for disposition, regardless of what the disposition itself ended up being, by a rate of 23% vs. 10%. Similarly, 7% of psychiatric patients vs. just 2.3% of medical patients had to wait longer than 12 hours in the ED, while 1.3% of psychiatric had to wait longer than 24 hours, compared with only 0.5% of medical patients. The average length of stay was significantly longer for psychiatric patients: 194 minutes vs. 138 minutes for medical patients (P less than .01).
Additionally, psychiatric patients were more likely to be uninsured, with 22% not having insurance, compared with 15% of medical patients being uninsured. Furthermore, 4.6% of the psychiatric patients’ previous visit to the ED had been within the prior 72 hours, compared with 3.6% of medical patients. A total of 21% of psychiatric patients required admittance, compared with 13% of medical patients, while 11% of psychiatric patients were transferred, compared with just 1.4% of medical patients.
“These results compel us to further investigate the potential causes of prolonged length of stay in psychiatric patients and to further characterize the population of psychiatric patients most at risk of prolonged stays,” Dr. Lippert and her coinvestigators concluded.
ACEP President Rebecca B. Parker, MD, chimed in during the conference call, explaining that a survey of more than 1,700 emergency physicians revealed some “troubling” findings about the state of emergency departments over the last year.
The nation’s dwindling mental health resources are having a direct impact on patients having psychiatric emergencies, including children,” Dr. Parker explained. “These patients are waiting longer for care, especially those patients who require hospitalization.”
Findings of the survey indicate that 48% of ED physicians witness psychiatric patients being “boarded” in their EDs at least once a day while they wait for a bed. Additionally, less than 17% of respondents said their ED has a psychiatrist on call to respond to psychiatric emergencies, with 11.7% responding that they have no psychiatrist on call to deal with such emergencies. And 52% of respondents said the mental health system in their community has gotten noticeably worse in just the last year.
In a separate statement, Dr. Parker voiced outrage about the situation. “Psychiatric patients wait in the emergency department for hours and even days for a bed, which delays the psychiatric care they so desperately need,” she said. “It also leads to delays in care and diminished resources for other emergency patients. The emergency department has become the dumping ground for these vulnerable patients who have been abandoned by every other part of the health care system.”
ACEP presented the findings during its annual meeting in Las Vegas. No funding sources for these studies were disclosed; Dr. Lippert did not report any financial disclosures.
FROM AN ACEP TELECONFERENCE
Key clinical point:
Major finding: Higher percentages of psychiatric patients have to wait more than a day before disposition, compared with medical patients.
Data source: Two retrospective reviews of more than 65 million ED visits in the NHAMCS database from 2001-2011.
Disclosures: No funding sources or disclosures were reported.
An irritable, inattentive, and disruptive child: Is it ADHD or bipolar disorder?
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
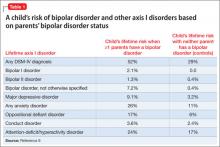
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
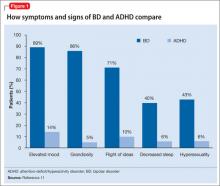
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
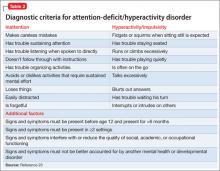
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
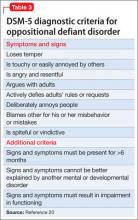
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Psychiatric risks quantified in siblings of mental disorder patients
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
AT THE ECNP CONGRESS
Key clinical point: Siblings of patients with schizophrenia or bipolar disorder are at sharply increased risk of subsequent hospitalization for a range of psychiatric disorders, not just what their sibling has.
Major finding: Siblings of schizophrenia patients were not only at 9.4-fold increased risk of subsequent hospitalization for schizophrenia in a national study, but also at 7.7-fold greater risk for bipolar disorder.
Data source: This nested case-control study compared psychiatric hospitalization rates for all siblings of 6,111 Israeli patients with schizophrenia, bipolar disorder, or unipolar depression to nearly 75,000 matched controls.
Disclosures: The study was conducted without external funding. The presenter reported having no financial conflicts of interest.
Type 2 diabetes peer-led intervention in primary care tied to improved depression symptoms
BETHESDA, MD. – A novel, peer- and nurse-led intervention in a primary care setting for type 2 diabetes in people with serious mental illness was associated with improvements in depression symptoms, global psychopathology, and overall health, a study has shown.
“The intervention really is patient self-management. It could be a nice complement to team-based, multidisciplinary care,” said Martha Sajatovic, MD, who presented the data in a poster at a National Institute of Mental Health conference on mental health services research. Dr. Sajatovic is the Willard W. Brown Chair and director of the Neurological & Behavioral Outcomes Center at University Hospitals Neurological Institute in Cleveland.
People with serious mental illness (SMI) have a significantly higher risk of premature death than do those in the general population, in part because this cohort experiences higher rates of metabolic disease, often exacerbated by higher rates of smoking, poor diet, substance abuse, and lack of exercise. However, in a 60-week randomized controlled trial of 200 people with SMI and comorbid type 2 diabetes, which was conducted in a primary care setting, those who were taught better self-care fared better than did those who received treatment as usual.
The group-based, psychosocial intervention – called “targeted training in illness” – blended psychoeducation, problem identification, goal setting, behavioral modeling, and care coordination around SMI and diabetes. In the first 12 weeks, groups of 6-10 people met in weekly, hour-long sessions co-led by a peer and nurse educator. Group discussions focused on self-management of diabetes through proper eating habits, regular exercise, tobacco cessation, and other forms of behavior modification.
Meeting as a group helped to “combat some of the social isolation that you see in this population,” Dr. Sajatovic said in an interview. “The peer leadership is really critical, too, because it empowers [the participants]. I believe peer support gives resilience ... and helps [the group] see you don’t have to be perfect to make progress.” In the study, the 3 months of group sessions were followed by weekly telephone maintenance sessions with either the peer or nurse educator for 48 weeks.
Half of the study’s participants – two-thirds of whom were women, and just over half of whom were black – had had a diagnosis of diabetes for at least 10 years; half of all participants used insulin. All had either schizophrenia, schizoaffective disorder, bipolar disorder, or major depressive disorder. Baseline rates of depression were high, and psychotic symptoms were minimal.
After assessments at baseline, 13, 30, and 60 weeks, the study arm was found to have improvements in depression, global psychopathology, and functional status, which Dr. Sajatovic said could be attributable to the group’s significantly improved knowledge about diabetes (P less than .01).
Glycemic control improved generally, a surprising finding that Dr. Sajatovic said could have been tied to the expansion of Medicaid in Ohio, where the study was done, and a “real concerted effort” to provide treatment by medical homes at this time.
While no significant difference between the groups was found overall, a post hoc analysis showed a difference in the 53% of the entire sample who had good to fair glycemic control (hemoglobin A1c equal to or less than 7.5) at baseline: At 60 weeks, those in the treatment arm achieved stable, long-term control compared with controls, whose values had worsened slightly (P = .024). Those people tended to be older, more likely to have schizophrenia, and less likely to be on insulin, and to have a shorter history of diabetes, said Dr. Sajatovic, professor of psychiatry and of neurology at Case Western Reserve University, Cleveland.
Compared with controls, the study arm had greater improvement at 60 weeks in Clinical Global Impression scores (P = .0008); Montgomery-Åsberg Depression Rating Scale scores (P = .0156); Global Assessment of Functioning scores (P = .0031); and knowledge of diabetes (P less than .0002), as well as an improvement trend in Sheehan Disability Scale scores (P = .0863). There was no difference between the groups on the Brief Psychiatric Rating Scale, the Short Form–36 or HbA1c values. By study’s end, Dr. Sajatovic said about a quarter had been lost to follow-up.
The intervention meets three important criteria, she said. “First, people need to know what to do. Then, they need to have confidence, or self-efficacy. The third thing is that the person has to believe in a given outcome based on a given behavior.”
Dr. Sajatovic did not have any relevant disclosures. The National Institutes of Health funded the study.
On Twitter @whitneymcknight
BETHESDA, MD. – A novel, peer- and nurse-led intervention in a primary care setting for type 2 diabetes in people with serious mental illness was associated with improvements in depression symptoms, global psychopathology, and overall health, a study has shown.
“The intervention really is patient self-management. It could be a nice complement to team-based, multidisciplinary care,” said Martha Sajatovic, MD, who presented the data in a poster at a National Institute of Mental Health conference on mental health services research. Dr. Sajatovic is the Willard W. Brown Chair and director of the Neurological & Behavioral Outcomes Center at University Hospitals Neurological Institute in Cleveland.
People with serious mental illness (SMI) have a significantly higher risk of premature death than do those in the general population, in part because this cohort experiences higher rates of metabolic disease, often exacerbated by higher rates of smoking, poor diet, substance abuse, and lack of exercise. However, in a 60-week randomized controlled trial of 200 people with SMI and comorbid type 2 diabetes, which was conducted in a primary care setting, those who were taught better self-care fared better than did those who received treatment as usual.
The group-based, psychosocial intervention – called “targeted training in illness” – blended psychoeducation, problem identification, goal setting, behavioral modeling, and care coordination around SMI and diabetes. In the first 12 weeks, groups of 6-10 people met in weekly, hour-long sessions co-led by a peer and nurse educator. Group discussions focused on self-management of diabetes through proper eating habits, regular exercise, tobacco cessation, and other forms of behavior modification.
Meeting as a group helped to “combat some of the social isolation that you see in this population,” Dr. Sajatovic said in an interview. “The peer leadership is really critical, too, because it empowers [the participants]. I believe peer support gives resilience ... and helps [the group] see you don’t have to be perfect to make progress.” In the study, the 3 months of group sessions were followed by weekly telephone maintenance sessions with either the peer or nurse educator for 48 weeks.
Half of the study’s participants – two-thirds of whom were women, and just over half of whom were black – had had a diagnosis of diabetes for at least 10 years; half of all participants used insulin. All had either schizophrenia, schizoaffective disorder, bipolar disorder, or major depressive disorder. Baseline rates of depression were high, and psychotic symptoms were minimal.
After assessments at baseline, 13, 30, and 60 weeks, the study arm was found to have improvements in depression, global psychopathology, and functional status, which Dr. Sajatovic said could be attributable to the group’s significantly improved knowledge about diabetes (P less than .01).
Glycemic control improved generally, a surprising finding that Dr. Sajatovic said could have been tied to the expansion of Medicaid in Ohio, where the study was done, and a “real concerted effort” to provide treatment by medical homes at this time.
While no significant difference between the groups was found overall, a post hoc analysis showed a difference in the 53% of the entire sample who had good to fair glycemic control (hemoglobin A1c equal to or less than 7.5) at baseline: At 60 weeks, those in the treatment arm achieved stable, long-term control compared with controls, whose values had worsened slightly (P = .024). Those people tended to be older, more likely to have schizophrenia, and less likely to be on insulin, and to have a shorter history of diabetes, said Dr. Sajatovic, professor of psychiatry and of neurology at Case Western Reserve University, Cleveland.
Compared with controls, the study arm had greater improvement at 60 weeks in Clinical Global Impression scores (P = .0008); Montgomery-Åsberg Depression Rating Scale scores (P = .0156); Global Assessment of Functioning scores (P = .0031); and knowledge of diabetes (P less than .0002), as well as an improvement trend in Sheehan Disability Scale scores (P = .0863). There was no difference between the groups on the Brief Psychiatric Rating Scale, the Short Form–36 or HbA1c values. By study’s end, Dr. Sajatovic said about a quarter had been lost to follow-up.
The intervention meets three important criteria, she said. “First, people need to know what to do. Then, they need to have confidence, or self-efficacy. The third thing is that the person has to believe in a given outcome based on a given behavior.”
Dr. Sajatovic did not have any relevant disclosures. The National Institutes of Health funded the study.
On Twitter @whitneymcknight
BETHESDA, MD. – A novel, peer- and nurse-led intervention in a primary care setting for type 2 diabetes in people with serious mental illness was associated with improvements in depression symptoms, global psychopathology, and overall health, a study has shown.
“The intervention really is patient self-management. It could be a nice complement to team-based, multidisciplinary care,” said Martha Sajatovic, MD, who presented the data in a poster at a National Institute of Mental Health conference on mental health services research. Dr. Sajatovic is the Willard W. Brown Chair and director of the Neurological & Behavioral Outcomes Center at University Hospitals Neurological Institute in Cleveland.
People with serious mental illness (SMI) have a significantly higher risk of premature death than do those in the general population, in part because this cohort experiences higher rates of metabolic disease, often exacerbated by higher rates of smoking, poor diet, substance abuse, and lack of exercise. However, in a 60-week randomized controlled trial of 200 people with SMI and comorbid type 2 diabetes, which was conducted in a primary care setting, those who were taught better self-care fared better than did those who received treatment as usual.
The group-based, psychosocial intervention – called “targeted training in illness” – blended psychoeducation, problem identification, goal setting, behavioral modeling, and care coordination around SMI and diabetes. In the first 12 weeks, groups of 6-10 people met in weekly, hour-long sessions co-led by a peer and nurse educator. Group discussions focused on self-management of diabetes through proper eating habits, regular exercise, tobacco cessation, and other forms of behavior modification.
Meeting as a group helped to “combat some of the social isolation that you see in this population,” Dr. Sajatovic said in an interview. “The peer leadership is really critical, too, because it empowers [the participants]. I believe peer support gives resilience ... and helps [the group] see you don’t have to be perfect to make progress.” In the study, the 3 months of group sessions were followed by weekly telephone maintenance sessions with either the peer or nurse educator for 48 weeks.
Half of the study’s participants – two-thirds of whom were women, and just over half of whom were black – had had a diagnosis of diabetes for at least 10 years; half of all participants used insulin. All had either schizophrenia, schizoaffective disorder, bipolar disorder, or major depressive disorder. Baseline rates of depression were high, and psychotic symptoms were minimal.
After assessments at baseline, 13, 30, and 60 weeks, the study arm was found to have improvements in depression, global psychopathology, and functional status, which Dr. Sajatovic said could be attributable to the group’s significantly improved knowledge about diabetes (P less than .01).
Glycemic control improved generally, a surprising finding that Dr. Sajatovic said could have been tied to the expansion of Medicaid in Ohio, where the study was done, and a “real concerted effort” to provide treatment by medical homes at this time.
While no significant difference between the groups was found overall, a post hoc analysis showed a difference in the 53% of the entire sample who had good to fair glycemic control (hemoglobin A1c equal to or less than 7.5) at baseline: At 60 weeks, those in the treatment arm achieved stable, long-term control compared with controls, whose values had worsened slightly (P = .024). Those people tended to be older, more likely to have schizophrenia, and less likely to be on insulin, and to have a shorter history of diabetes, said Dr. Sajatovic, professor of psychiatry and of neurology at Case Western Reserve University, Cleveland.
Compared with controls, the study arm had greater improvement at 60 weeks in Clinical Global Impression scores (P = .0008); Montgomery-Åsberg Depression Rating Scale scores (P = .0156); Global Assessment of Functioning scores (P = .0031); and knowledge of diabetes (P less than .0002), as well as an improvement trend in Sheehan Disability Scale scores (P = .0863). There was no difference between the groups on the Brief Psychiatric Rating Scale, the Short Form–36 or HbA1c values. By study’s end, Dr. Sajatovic said about a quarter had been lost to follow-up.
The intervention meets three important criteria, she said. “First, people need to know what to do. Then, they need to have confidence, or self-efficacy. The third thing is that the person has to believe in a given outcome based on a given behavior.”
Dr. Sajatovic did not have any relevant disclosures. The National Institutes of Health funded the study.
On Twitter @whitneymcknight
AT AN NIMH CONFERENCE
Key clinical point: Targeted training in illness management effectively improves overall health outcomes in people with serious mental illness and comorbid type 2 diabetes.
Major finding: Compared with treatment as usual, peer-led intervention improved depression, overall health, and knowledge of diabetes at 60 weeks.
Data source: Randomized, controlled study of 200 people with serious mental illness and comorbid type 2 diabetes seen in primary care.
Disclosures: Dr. Sajatovic did not have any relevant disclosures. The National Institutes of Health funded the study.
New guidelines to focus on mixed features in depression, bipolar
WASHINGTON – A sea change is underway in how major depressive and bipolar disorders are diagnosed and treated.
Historically, the absence of an accurate, comprehensive nosology of depression has led to much suffering and confusion. People with bipolar disorder in particular are either not diagnosed early enough or are not diagnosed with the correct “flavor” of depression, according to Roger S. McIntyre, MD, author of updated treatment guidelines for bipolar depression, and the first-ever treatment guidelines for mixed features in major depressive disorder. “Twenty years ago, we would have described bipolar as episodic breakthroughs of mania and depression, with well intervals in between,” Dr. McIntyre said at Summit in Neurology & Psychiatry. “But now, we’ve really changed our fundamental thinking about bipolar disorder.”
Although reasons for the evolution in thinking are many, one of the strongest currents of change flows from the 2013 publication of the DSM-5, according to Dr. McIntyre, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at University Health Network in Toronto.
“The DSM-5’s authors took a neo-Kraepelinian view that mood disorders are dimensional,” Dr. McIntyre said in an interview. As a result, there’s been a reversal of what he called the “social construct imposed upon the cosmos of mood disorders by the DSM-III that divided that world into either depression or bipolar disorder.”
This return to thinking of mood disorders as existing on a continuum, as psychiatrist Emil Kraepelin, MD, theorized around the turn of the last century, pivots on the decision to do away with mixed states and to instead add the mixed features specifier.
“The move to mixed features is the necessary bridge between bipolar disease and major depressive disorder,” Dr. McIntyre said in the interview.
Therefore, for a period of time between the 1980 publication of the DSM-III and the DSM-5, “real-world” presentations of subsyndromal, opposite-pole symptoms that are common in major depressive disorder (MDD) and in bipolar disorder were not accounted for.
In practical terms, the addition of mixed features means that a patient with mania who presents with subsyndromal depressive symptoms would be seen, for example, to have mania with mixed features. A patient with a depressive episode who presents with subsyndromal hypomanic symptoms would be seen to have depression with mixed features. Therefore, depression with mixed features can be present not only in MDD, but in both bipolar I and II.
New treatment algorithms
This dimensional approach of assessing mixed features along a continuum could lead to better and earlier diagnosis of bipolar depression and more targeted therapies, according to Dr. McIntyre. What he thinks it won’t do is lead to an overzealousness in the overdiagnosis of bipolar depression.
“That is false. We wouldn’t say we’re not going to diagnose bowel cancer because we hear it’s overdiagnosed. But to get the diagnoses right, what we need is fidelity to diagnostic criteria.”
Enter the state of Florida. As part of its best practices for psychotherapeutic use in adults, Florida is the first state to have published evidence-based guidelines for depression with mixed features. Dr. McIntyre is one of the guidelines’ coauthors.
Antidepressants bad, olanzapine worse
Some changes to treatment algorithms might come as a surprise. Despite being among the most commonly prescribed treatments for bipolar disorder, monotherapy with antidepressants is not approved in the guidelines. “Period,” said Dr. McIntyre. “Many patients do well on antidepressants, but the most common outcome is inefficacy.”
It might be better to combine an antidepressant with an atypical antipsychotic, or a mood stabilizer to avoid treatment-emergent mania, or, more commonly, destabilization in patients who are susceptible to subsyndromal mania, he said.
In addition to mitigating symptoms of a depressive episode, atypicals can help suppress hypomanic symptoms. Dr. McIntyre said this is critical to remember, because patients with these combinations of symptoms are “the very persons who shouldn’t get an antidepressant but are also the ones most likely to be prescribed them,” according to old ways of thinking.
As for maintenance in bipolar disorder, Dr. McIntyre believes the axiom, “What gets you well keeps you well,” is a good rule of thumb when going through the algorithm. “It’s not always true, but it’s almost always true.”
Management of MDD with mixed features includes the introduction of atypicals or mood stabilizers such as lithium or lamotrigine in patients with any prior history of hypomania or mania, something Dr. McIntyre said already is beginning to happen in practice.
Olanzapine monotherapy as a first-line treatment of bipolar disorder initially was “demoted” by Dr. McIntyre and his coauthors in Florida’s bipolar treatment guidelines. In the updated version, the atypical remains a second-line therapy behind lurasidone as the recommended first-line therapy because of olanzapine’s tendency to interfere with metabolic processes. Quetiapine also is a first-line therapy, but with the qualification that it, too, could interfere with metabolic processes. Combination therapy with olanzapine plus fluoxetine is second-line.
“Lurasidone does not have the metabolic changes of quetiapine. It doesn’t make sense to treat mania and then erase 25 years of a person’s life because of weight gain,” Dr. McIntyre said.
The average lifespan of people with bipolar disorder is about 20 years shorter than it is for those without serious mental illness.
Inflammation harms cognition
The changes are indicative of how seriously the field has begun to take metabolic disturbance as an adverse event in serious mental illness. Literature on obesity as a “psycho-toxin” is growing, and Dr. McIntyre is among the pioneers.
One study by Dr. McIntyre and his colleagues, currently in press, explores how obesity-related inflammation disturbs the brain’s dopamine system, resulting in interference with executive function. Because it is well established that people with bipolar disorder are more likely to be obese (J Affect Disord. 2008 Sep;110[1-2]:149-55), they also are more susceptible to cognitive impairment than are people of normal weight, according to Dr. McIntyre.
In a 2013 study, Dr. McIntyre and his colleagues showed that a first episode of mania in a person with obesity creates the same level of cognitive impairment as that found in the brains of normal-weight individuals who have experienced five episodes of mania (Psychological Med. 2014 Feb;44[3]:533-41). “There is something about obesity that is brain toxic,” he said.
Proof of concept of this is that cognitive outcomes for people before bariatric surgery are worse than postsurgery outcomes (Am J Surg. 2014 Jun;207[6]:870-6).
Systemic inflammation elevates levels of C-reactive protein, interleukin-1, and other cytokines, and interferes with insulin signaling; all have deleterious effects on cognition, as well as metabolic health. Those disturbances negatively affect emotional regulation, sleep, appetite, and sex drive, as well as executive function, he said.
“Inflammation is a convergent system, implicated across many brain- and body-based disorders. People with bipolar disorder not only have systemic increases in inflammation, but also neuroinflammation,” Dr. McIntyre said.
Accordingly, controlling inflammation becomes essential to chronic management of depression in general and bipolar in particular since, with each successive episode of untreated mania, patients’ ability to think clearly takes a hit. “Cognitive function is the principal determinant of psychosocial function, of workplace visibility, [and] of quality of life in most patient-reported outcomes,” Dr. McIntyre said.
Cognitive impairment also can lead to a worsening of the ability to balance reward and impulse control, leading to higher rates of substance abuse or other psychiatric comorbidities after onset of bipolar disease; a vicious cycle can ensue.
“As cognitive difficulties rise, comorbidities rise. But also, some of the comorbidities we see are reflections of cognitive impairment,” Dr. McIntyre said. To wit, binge eating disorder and bulimia nervosa are common in people with bipolar disorder (J Affect Disord. 2016 Feb;191:216-2).
Citing a recent scientific statement from the American Heart Association recommending that bipolar disorder and MDD should be considered tier II risk factors for cardiovascular disease among youth, the Florida guidelines urge clinicians to regularly screen patients for cardiometabolic disorders – not only for their medical implications but for their potential to flag emergent psychiatric issues.
Pharmacotherapeutics specifically targeting neuroinflammation are not yet ready for clinical practice, but Dr. McIntyre said other, more conventional therapies are available for bipolar disorder that have anti-inflammatory properties, including selective serotonin reuptake inhibitors and lithium. “Lithium is also anti-amyloid and has an anti-suicide effect. It is a drug I would definitely use as first line.”
Some behavioral therapies also are protective against inflammation and are recommended in the guidelines. Those include attention to sleep hygiene, diet, and exercise. “Social rhythm therapy is underutilized. These patients need their day organized. They need aerobics; they need sleep,” Dr. McIntyre said.
Future is now
Although the “whole-person” approach is still nascent – seeing depression as a collection of what Dr. McIntyre said are alterations in the neural circuitry amounting to a series of “disconnection syndromes” – psychiatry already has entered a new era where disease models are more comprehensive, he said.
This new way of thinking can connect the dots between why, for example, so many people with bipolar depression also have drug and alcohol abuse. It also could help explain why in bipolar there is so much obesity, or why there is so much anxiety, he said. “We’ve moved away from the rather silly, overly simplistic notions that you have too much or too little serotonin. Or that there was too much or too little dopamine causing mania. It made for convenient sound bytes, but it was probably just superstition we were gravitating to.”
The guidelines have been peer reviewed and will be published in the Journal of Clinical Psychiatry later this year, Dr. McIntyre said.
The meeting was held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Dr. McIntyre disclosed that he has numerous industry relationships, including with AstraZeneca, Eli Lilly, Janssen Ortho, Lundbeck, Pfizer, and Shire.
On Twitter @whitneymcknight
This more holistic way of thinking about depression is one I endorse. It makes sense to conceptualize bipolar disorder as a whole body disorder rather than a condition that is specific to the brain. The clinical implications are that we need to consider integration of care approaches that can reduce stress and inflammation generally, and minimize the complications of medical conditions seen in people with bipolar disorder. Some behavioral therapies are protective against inflammation and are recommended in the guidelines. These include attention to sleep, diet, and exercise. Social rhythm therapy is underutilized, as are other types of psychosocial approaches. Appropriate access to and use of medical care to help manage medical conditions is important, and integrated medical care that considers both body and mind may be helpful.
Martha Sajatovic, MD, is the Willard Brown Chair in Neurological Outcomes Research, and director of the Neurological Outcomes Center at the University Hospitals Case Medical Center in Cleveland. She is professor of psychiatry and of neurology at Case Western Reserve University, also in Cleveland. Dr. Sajatovic reported she has several industry relationships, including with Janssen, Merck, Ortho-McNeil, and Pfizer.
This more holistic way of thinking about depression is one I endorse. It makes sense to conceptualize bipolar disorder as a whole body disorder rather than a condition that is specific to the brain. The clinical implications are that we need to consider integration of care approaches that can reduce stress and inflammation generally, and minimize the complications of medical conditions seen in people with bipolar disorder. Some behavioral therapies are protective against inflammation and are recommended in the guidelines. These include attention to sleep, diet, and exercise. Social rhythm therapy is underutilized, as are other types of psychosocial approaches. Appropriate access to and use of medical care to help manage medical conditions is important, and integrated medical care that considers both body and mind may be helpful.
Martha Sajatovic, MD, is the Willard Brown Chair in Neurological Outcomes Research, and director of the Neurological Outcomes Center at the University Hospitals Case Medical Center in Cleveland. She is professor of psychiatry and of neurology at Case Western Reserve University, also in Cleveland. Dr. Sajatovic reported she has several industry relationships, including with Janssen, Merck, Ortho-McNeil, and Pfizer.
This more holistic way of thinking about depression is one I endorse. It makes sense to conceptualize bipolar disorder as a whole body disorder rather than a condition that is specific to the brain. The clinical implications are that we need to consider integration of care approaches that can reduce stress and inflammation generally, and minimize the complications of medical conditions seen in people with bipolar disorder. Some behavioral therapies are protective against inflammation and are recommended in the guidelines. These include attention to sleep, diet, and exercise. Social rhythm therapy is underutilized, as are other types of psychosocial approaches. Appropriate access to and use of medical care to help manage medical conditions is important, and integrated medical care that considers both body and mind may be helpful.
Martha Sajatovic, MD, is the Willard Brown Chair in Neurological Outcomes Research, and director of the Neurological Outcomes Center at the University Hospitals Case Medical Center in Cleveland. She is professor of psychiatry and of neurology at Case Western Reserve University, also in Cleveland. Dr. Sajatovic reported she has several industry relationships, including with Janssen, Merck, Ortho-McNeil, and Pfizer.
WASHINGTON – A sea change is underway in how major depressive and bipolar disorders are diagnosed and treated.
Historically, the absence of an accurate, comprehensive nosology of depression has led to much suffering and confusion. People with bipolar disorder in particular are either not diagnosed early enough or are not diagnosed with the correct “flavor” of depression, according to Roger S. McIntyre, MD, author of updated treatment guidelines for bipolar depression, and the first-ever treatment guidelines for mixed features in major depressive disorder. “Twenty years ago, we would have described bipolar as episodic breakthroughs of mania and depression, with well intervals in between,” Dr. McIntyre said at Summit in Neurology & Psychiatry. “But now, we’ve really changed our fundamental thinking about bipolar disorder.”
Although reasons for the evolution in thinking are many, one of the strongest currents of change flows from the 2013 publication of the DSM-5, according to Dr. McIntyre, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at University Health Network in Toronto.
“The DSM-5’s authors took a neo-Kraepelinian view that mood disorders are dimensional,” Dr. McIntyre said in an interview. As a result, there’s been a reversal of what he called the “social construct imposed upon the cosmos of mood disorders by the DSM-III that divided that world into either depression or bipolar disorder.”
This return to thinking of mood disorders as existing on a continuum, as psychiatrist Emil Kraepelin, MD, theorized around the turn of the last century, pivots on the decision to do away with mixed states and to instead add the mixed features specifier.
“The move to mixed features is the necessary bridge between bipolar disease and major depressive disorder,” Dr. McIntyre said in the interview.
Therefore, for a period of time between the 1980 publication of the DSM-III and the DSM-5, “real-world” presentations of subsyndromal, opposite-pole symptoms that are common in major depressive disorder (MDD) and in bipolar disorder were not accounted for.
In practical terms, the addition of mixed features means that a patient with mania who presents with subsyndromal depressive symptoms would be seen, for example, to have mania with mixed features. A patient with a depressive episode who presents with subsyndromal hypomanic symptoms would be seen to have depression with mixed features. Therefore, depression with mixed features can be present not only in MDD, but in both bipolar I and II.
New treatment algorithms
This dimensional approach of assessing mixed features along a continuum could lead to better and earlier diagnosis of bipolar depression and more targeted therapies, according to Dr. McIntyre. What he thinks it won’t do is lead to an overzealousness in the overdiagnosis of bipolar depression.
“That is false. We wouldn’t say we’re not going to diagnose bowel cancer because we hear it’s overdiagnosed. But to get the diagnoses right, what we need is fidelity to diagnostic criteria.”
Enter the state of Florida. As part of its best practices for psychotherapeutic use in adults, Florida is the first state to have published evidence-based guidelines for depression with mixed features. Dr. McIntyre is one of the guidelines’ coauthors.
Antidepressants bad, olanzapine worse
Some changes to treatment algorithms might come as a surprise. Despite being among the most commonly prescribed treatments for bipolar disorder, monotherapy with antidepressants is not approved in the guidelines. “Period,” said Dr. McIntyre. “Many patients do well on antidepressants, but the most common outcome is inefficacy.”
It might be better to combine an antidepressant with an atypical antipsychotic, or a mood stabilizer to avoid treatment-emergent mania, or, more commonly, destabilization in patients who are susceptible to subsyndromal mania, he said.
In addition to mitigating symptoms of a depressive episode, atypicals can help suppress hypomanic symptoms. Dr. McIntyre said this is critical to remember, because patients with these combinations of symptoms are “the very persons who shouldn’t get an antidepressant but are also the ones most likely to be prescribed them,” according to old ways of thinking.
As for maintenance in bipolar disorder, Dr. McIntyre believes the axiom, “What gets you well keeps you well,” is a good rule of thumb when going through the algorithm. “It’s not always true, but it’s almost always true.”
Management of MDD with mixed features includes the introduction of atypicals or mood stabilizers such as lithium or lamotrigine in patients with any prior history of hypomania or mania, something Dr. McIntyre said already is beginning to happen in practice.
Olanzapine monotherapy as a first-line treatment of bipolar disorder initially was “demoted” by Dr. McIntyre and his coauthors in Florida’s bipolar treatment guidelines. In the updated version, the atypical remains a second-line therapy behind lurasidone as the recommended first-line therapy because of olanzapine’s tendency to interfere with metabolic processes. Quetiapine also is a first-line therapy, but with the qualification that it, too, could interfere with metabolic processes. Combination therapy with olanzapine plus fluoxetine is second-line.
“Lurasidone does not have the metabolic changes of quetiapine. It doesn’t make sense to treat mania and then erase 25 years of a person’s life because of weight gain,” Dr. McIntyre said.
The average lifespan of people with bipolar disorder is about 20 years shorter than it is for those without serious mental illness.
Inflammation harms cognition
The changes are indicative of how seriously the field has begun to take metabolic disturbance as an adverse event in serious mental illness. Literature on obesity as a “psycho-toxin” is growing, and Dr. McIntyre is among the pioneers.
One study by Dr. McIntyre and his colleagues, currently in press, explores how obesity-related inflammation disturbs the brain’s dopamine system, resulting in interference with executive function. Because it is well established that people with bipolar disorder are more likely to be obese (J Affect Disord. 2008 Sep;110[1-2]:149-55), they also are more susceptible to cognitive impairment than are people of normal weight, according to Dr. McIntyre.
In a 2013 study, Dr. McIntyre and his colleagues showed that a first episode of mania in a person with obesity creates the same level of cognitive impairment as that found in the brains of normal-weight individuals who have experienced five episodes of mania (Psychological Med. 2014 Feb;44[3]:533-41). “There is something about obesity that is brain toxic,” he said.
Proof of concept of this is that cognitive outcomes for people before bariatric surgery are worse than postsurgery outcomes (Am J Surg. 2014 Jun;207[6]:870-6).
Systemic inflammation elevates levels of C-reactive protein, interleukin-1, and other cytokines, and interferes with insulin signaling; all have deleterious effects on cognition, as well as metabolic health. Those disturbances negatively affect emotional regulation, sleep, appetite, and sex drive, as well as executive function, he said.
“Inflammation is a convergent system, implicated across many brain- and body-based disorders. People with bipolar disorder not only have systemic increases in inflammation, but also neuroinflammation,” Dr. McIntyre said.
Accordingly, controlling inflammation becomes essential to chronic management of depression in general and bipolar in particular since, with each successive episode of untreated mania, patients’ ability to think clearly takes a hit. “Cognitive function is the principal determinant of psychosocial function, of workplace visibility, [and] of quality of life in most patient-reported outcomes,” Dr. McIntyre said.
Cognitive impairment also can lead to a worsening of the ability to balance reward and impulse control, leading to higher rates of substance abuse or other psychiatric comorbidities after onset of bipolar disease; a vicious cycle can ensue.
“As cognitive difficulties rise, comorbidities rise. But also, some of the comorbidities we see are reflections of cognitive impairment,” Dr. McIntyre said. To wit, binge eating disorder and bulimia nervosa are common in people with bipolar disorder (J Affect Disord. 2016 Feb;191:216-2).
Citing a recent scientific statement from the American Heart Association recommending that bipolar disorder and MDD should be considered tier II risk factors for cardiovascular disease among youth, the Florida guidelines urge clinicians to regularly screen patients for cardiometabolic disorders – not only for their medical implications but for their potential to flag emergent psychiatric issues.
Pharmacotherapeutics specifically targeting neuroinflammation are not yet ready for clinical practice, but Dr. McIntyre said other, more conventional therapies are available for bipolar disorder that have anti-inflammatory properties, including selective serotonin reuptake inhibitors and lithium. “Lithium is also anti-amyloid and has an anti-suicide effect. It is a drug I would definitely use as first line.”
Some behavioral therapies also are protective against inflammation and are recommended in the guidelines. Those include attention to sleep hygiene, diet, and exercise. “Social rhythm therapy is underutilized. These patients need their day organized. They need aerobics; they need sleep,” Dr. McIntyre said.
Future is now
Although the “whole-person” approach is still nascent – seeing depression as a collection of what Dr. McIntyre said are alterations in the neural circuitry amounting to a series of “disconnection syndromes” – psychiatry already has entered a new era where disease models are more comprehensive, he said.
This new way of thinking can connect the dots between why, for example, so many people with bipolar depression also have drug and alcohol abuse. It also could help explain why in bipolar there is so much obesity, or why there is so much anxiety, he said. “We’ve moved away from the rather silly, overly simplistic notions that you have too much or too little serotonin. Or that there was too much or too little dopamine causing mania. It made for convenient sound bytes, but it was probably just superstition we were gravitating to.”
The guidelines have been peer reviewed and will be published in the Journal of Clinical Psychiatry later this year, Dr. McIntyre said.
The meeting was held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Dr. McIntyre disclosed that he has numerous industry relationships, including with AstraZeneca, Eli Lilly, Janssen Ortho, Lundbeck, Pfizer, and Shire.
On Twitter @whitneymcknight
WASHINGTON – A sea change is underway in how major depressive and bipolar disorders are diagnosed and treated.
Historically, the absence of an accurate, comprehensive nosology of depression has led to much suffering and confusion. People with bipolar disorder in particular are either not diagnosed early enough or are not diagnosed with the correct “flavor” of depression, according to Roger S. McIntyre, MD, author of updated treatment guidelines for bipolar depression, and the first-ever treatment guidelines for mixed features in major depressive disorder. “Twenty years ago, we would have described bipolar as episodic breakthroughs of mania and depression, with well intervals in between,” Dr. McIntyre said at Summit in Neurology & Psychiatry. “But now, we’ve really changed our fundamental thinking about bipolar disorder.”
Although reasons for the evolution in thinking are many, one of the strongest currents of change flows from the 2013 publication of the DSM-5, according to Dr. McIntyre, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at University Health Network in Toronto.
“The DSM-5’s authors took a neo-Kraepelinian view that mood disorders are dimensional,” Dr. McIntyre said in an interview. As a result, there’s been a reversal of what he called the “social construct imposed upon the cosmos of mood disorders by the DSM-III that divided that world into either depression or bipolar disorder.”
This return to thinking of mood disorders as existing on a continuum, as psychiatrist Emil Kraepelin, MD, theorized around the turn of the last century, pivots on the decision to do away with mixed states and to instead add the mixed features specifier.
“The move to mixed features is the necessary bridge between bipolar disease and major depressive disorder,” Dr. McIntyre said in the interview.
Therefore, for a period of time between the 1980 publication of the DSM-III and the DSM-5, “real-world” presentations of subsyndromal, opposite-pole symptoms that are common in major depressive disorder (MDD) and in bipolar disorder were not accounted for.
In practical terms, the addition of mixed features means that a patient with mania who presents with subsyndromal depressive symptoms would be seen, for example, to have mania with mixed features. A patient with a depressive episode who presents with subsyndromal hypomanic symptoms would be seen to have depression with mixed features. Therefore, depression with mixed features can be present not only in MDD, but in both bipolar I and II.
New treatment algorithms
This dimensional approach of assessing mixed features along a continuum could lead to better and earlier diagnosis of bipolar depression and more targeted therapies, according to Dr. McIntyre. What he thinks it won’t do is lead to an overzealousness in the overdiagnosis of bipolar depression.
“That is false. We wouldn’t say we’re not going to diagnose bowel cancer because we hear it’s overdiagnosed. But to get the diagnoses right, what we need is fidelity to diagnostic criteria.”
Enter the state of Florida. As part of its best practices for psychotherapeutic use in adults, Florida is the first state to have published evidence-based guidelines for depression with mixed features. Dr. McIntyre is one of the guidelines’ coauthors.
Antidepressants bad, olanzapine worse
Some changes to treatment algorithms might come as a surprise. Despite being among the most commonly prescribed treatments for bipolar disorder, monotherapy with antidepressants is not approved in the guidelines. “Period,” said Dr. McIntyre. “Many patients do well on antidepressants, but the most common outcome is inefficacy.”
It might be better to combine an antidepressant with an atypical antipsychotic, or a mood stabilizer to avoid treatment-emergent mania, or, more commonly, destabilization in patients who are susceptible to subsyndromal mania, he said.
In addition to mitigating symptoms of a depressive episode, atypicals can help suppress hypomanic symptoms. Dr. McIntyre said this is critical to remember, because patients with these combinations of symptoms are “the very persons who shouldn’t get an antidepressant but are also the ones most likely to be prescribed them,” according to old ways of thinking.
As for maintenance in bipolar disorder, Dr. McIntyre believes the axiom, “What gets you well keeps you well,” is a good rule of thumb when going through the algorithm. “It’s not always true, but it’s almost always true.”
Management of MDD with mixed features includes the introduction of atypicals or mood stabilizers such as lithium or lamotrigine in patients with any prior history of hypomania or mania, something Dr. McIntyre said already is beginning to happen in practice.
Olanzapine monotherapy as a first-line treatment of bipolar disorder initially was “demoted” by Dr. McIntyre and his coauthors in Florida’s bipolar treatment guidelines. In the updated version, the atypical remains a second-line therapy behind lurasidone as the recommended first-line therapy because of olanzapine’s tendency to interfere with metabolic processes. Quetiapine also is a first-line therapy, but with the qualification that it, too, could interfere with metabolic processes. Combination therapy with olanzapine plus fluoxetine is second-line.
“Lurasidone does not have the metabolic changes of quetiapine. It doesn’t make sense to treat mania and then erase 25 years of a person’s life because of weight gain,” Dr. McIntyre said.
The average lifespan of people with bipolar disorder is about 20 years shorter than it is for those without serious mental illness.
Inflammation harms cognition
The changes are indicative of how seriously the field has begun to take metabolic disturbance as an adverse event in serious mental illness. Literature on obesity as a “psycho-toxin” is growing, and Dr. McIntyre is among the pioneers.
One study by Dr. McIntyre and his colleagues, currently in press, explores how obesity-related inflammation disturbs the brain’s dopamine system, resulting in interference with executive function. Because it is well established that people with bipolar disorder are more likely to be obese (J Affect Disord. 2008 Sep;110[1-2]:149-55), they also are more susceptible to cognitive impairment than are people of normal weight, according to Dr. McIntyre.
In a 2013 study, Dr. McIntyre and his colleagues showed that a first episode of mania in a person with obesity creates the same level of cognitive impairment as that found in the brains of normal-weight individuals who have experienced five episodes of mania (Psychological Med. 2014 Feb;44[3]:533-41). “There is something about obesity that is brain toxic,” he said.
Proof of concept of this is that cognitive outcomes for people before bariatric surgery are worse than postsurgery outcomes (Am J Surg. 2014 Jun;207[6]:870-6).
Systemic inflammation elevates levels of C-reactive protein, interleukin-1, and other cytokines, and interferes with insulin signaling; all have deleterious effects on cognition, as well as metabolic health. Those disturbances negatively affect emotional regulation, sleep, appetite, and sex drive, as well as executive function, he said.
“Inflammation is a convergent system, implicated across many brain- and body-based disorders. People with bipolar disorder not only have systemic increases in inflammation, but also neuroinflammation,” Dr. McIntyre said.
Accordingly, controlling inflammation becomes essential to chronic management of depression in general and bipolar in particular since, with each successive episode of untreated mania, patients’ ability to think clearly takes a hit. “Cognitive function is the principal determinant of psychosocial function, of workplace visibility, [and] of quality of life in most patient-reported outcomes,” Dr. McIntyre said.
Cognitive impairment also can lead to a worsening of the ability to balance reward and impulse control, leading to higher rates of substance abuse or other psychiatric comorbidities after onset of bipolar disease; a vicious cycle can ensue.
“As cognitive difficulties rise, comorbidities rise. But also, some of the comorbidities we see are reflections of cognitive impairment,” Dr. McIntyre said. To wit, binge eating disorder and bulimia nervosa are common in people with bipolar disorder (J Affect Disord. 2016 Feb;191:216-2).
Citing a recent scientific statement from the American Heart Association recommending that bipolar disorder and MDD should be considered tier II risk factors for cardiovascular disease among youth, the Florida guidelines urge clinicians to regularly screen patients for cardiometabolic disorders – not only for their medical implications but for their potential to flag emergent psychiatric issues.
Pharmacotherapeutics specifically targeting neuroinflammation are not yet ready for clinical practice, but Dr. McIntyre said other, more conventional therapies are available for bipolar disorder that have anti-inflammatory properties, including selective serotonin reuptake inhibitors and lithium. “Lithium is also anti-amyloid and has an anti-suicide effect. It is a drug I would definitely use as first line.”
Some behavioral therapies also are protective against inflammation and are recommended in the guidelines. Those include attention to sleep hygiene, diet, and exercise. “Social rhythm therapy is underutilized. These patients need their day organized. They need aerobics; they need sleep,” Dr. McIntyre said.
Future is now
Although the “whole-person” approach is still nascent – seeing depression as a collection of what Dr. McIntyre said are alterations in the neural circuitry amounting to a series of “disconnection syndromes” – psychiatry already has entered a new era where disease models are more comprehensive, he said.
This new way of thinking can connect the dots between why, for example, so many people with bipolar depression also have drug and alcohol abuse. It also could help explain why in bipolar there is so much obesity, or why there is so much anxiety, he said. “We’ve moved away from the rather silly, overly simplistic notions that you have too much or too little serotonin. Or that there was too much or too little dopamine causing mania. It made for convenient sound bytes, but it was probably just superstition we were gravitating to.”
The guidelines have been peer reviewed and will be published in the Journal of Clinical Psychiatry later this year, Dr. McIntyre said.
The meeting was held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
Dr. McIntyre disclosed that he has numerous industry relationships, including with AstraZeneca, Eli Lilly, Janssen Ortho, Lundbeck, Pfizer, and Shire.
On Twitter @whitneymcknight
EXPERT ANALYSIS AT SUMMIT IN NEUROLOGY & PSYCHIATRY
AUDIO: New bipolar disorder algorithm changes ranking of first-line therapies
WASHINGTON – In 2015, the Florida Agency for Health Care Administration published clinical guidelines for numerous psychiatric conditions, including bipolar disorder, demoting several first-line therapies, and promoting others.
Because the authors of the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults agreed that inflammation is a mechanism of action in bipolar disorder, they adopted an approach to care that seeks to avoid inflammation at all costs.
“Some medications create metabolic disturbances, which can be disruptive to the inflammatory milieu,” said Roger McIntyre, MD, a professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at the University Health Network, Toronto. Dr. McIntyre, one of the coauthors of the guidelines, discussed why the combination of olanzapine and fluoxetine has been deferred in the algorithm, why other medications have moved further up, why antidepressants also are lower in the order of priority, and why psychoeducation, social rhythm therapy, and lifestyle changes have been emphasized more than ever before.
“There is no way our bipolar patients are going to achieve their goals with medication alone,” Dr. McIntyre said at the meeting, held by the Global Academy for Medical Education. In addition, Dr. McIntyre outlined why adding bipolar screening in the primary care setting is critical in 2016, and called the new recommendations “the most up-to-date guidelines for treating bipolar disorder, and the new nosology of major depression disorder with mixed features.”
To access the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults online, visit the Florida Medicaid Drug Therapy Management Program for Behavioral Health website.
Dr. McIntyre has numerous industry relationships, including research funding from Eli Lilly, Janssen-Ortho, Astra-Zeneca; Pfizer, and Lundbeck. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
WASHINGTON – In 2015, the Florida Agency for Health Care Administration published clinical guidelines for numerous psychiatric conditions, including bipolar disorder, demoting several first-line therapies, and promoting others.
Because the authors of the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults agreed that inflammation is a mechanism of action in bipolar disorder, they adopted an approach to care that seeks to avoid inflammation at all costs.
“Some medications create metabolic disturbances, which can be disruptive to the inflammatory milieu,” said Roger McIntyre, MD, a professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at the University Health Network, Toronto. Dr. McIntyre, one of the coauthors of the guidelines, discussed why the combination of olanzapine and fluoxetine has been deferred in the algorithm, why other medications have moved further up, why antidepressants also are lower in the order of priority, and why psychoeducation, social rhythm therapy, and lifestyle changes have been emphasized more than ever before.
“There is no way our bipolar patients are going to achieve their goals with medication alone,” Dr. McIntyre said at the meeting, held by the Global Academy for Medical Education. In addition, Dr. McIntyre outlined why adding bipolar screening in the primary care setting is critical in 2016, and called the new recommendations “the most up-to-date guidelines for treating bipolar disorder, and the new nosology of major depression disorder with mixed features.”
To access the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults online, visit the Florida Medicaid Drug Therapy Management Program for Behavioral Health website.
Dr. McIntyre has numerous industry relationships, including research funding from Eli Lilly, Janssen-Ortho, Astra-Zeneca; Pfizer, and Lundbeck. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
WASHINGTON – In 2015, the Florida Agency for Health Care Administration published clinical guidelines for numerous psychiatric conditions, including bipolar disorder, demoting several first-line therapies, and promoting others.
Because the authors of the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults agreed that inflammation is a mechanism of action in bipolar disorder, they adopted an approach to care that seeks to avoid inflammation at all costs.
“Some medications create metabolic disturbances, which can be disruptive to the inflammatory milieu,” said Roger McIntyre, MD, a professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit at the University Health Network, Toronto. Dr. McIntyre, one of the coauthors of the guidelines, discussed why the combination of olanzapine and fluoxetine has been deferred in the algorithm, why other medications have moved further up, why antidepressants also are lower in the order of priority, and why psychoeducation, social rhythm therapy, and lifestyle changes have been emphasized more than ever before.
“There is no way our bipolar patients are going to achieve their goals with medication alone,” Dr. McIntyre said at the meeting, held by the Global Academy for Medical Education. In addition, Dr. McIntyre outlined why adding bipolar screening in the primary care setting is critical in 2016, and called the new recommendations “the most up-to-date guidelines for treating bipolar disorder, and the new nosology of major depression disorder with mixed features.”
To access the Florida Best Practice Psychotherapeutic Medication Guidelines for Adults online, visit the Florida Medicaid Drug Therapy Management Program for Behavioral Health website.
Dr. McIntyre has numerous industry relationships, including research funding from Eli Lilly, Janssen-Ortho, Astra-Zeneca; Pfizer, and Lundbeck. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
AT SUMMIT IN NEUROLOGY & PSYCHIATRY
Using lipid guidelines to manage metabolic syndrome for patients taking an antipsychotic
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
Your patient who has schizophrenia, Mr. W, age 48, requests that you switch him from olanzapine, 10 mg/d, to another antipsychotic because he gained 25 lb over 1 month taking the drug. He now weighs 275 lb. Mr. W reports smoking at least 2 packs of cigarettes a day and takes lisinopril, 20 mg/d, for hypertension. You decide to start risperidone, 1 mg/d. First, however, your initial work-up includes:
- high-density lipoprotein (HDL), 24 mg/dL
- total cholesterol, 220 mg/dL
- blood pressure, 154/80 mm Hgwaist circumference, 39 in
- body mass index (BMI), 29
- hemoglobin A1c, of 5.6%.
A prolactin level is pending.
How do you interpret these values?
Metabolic syndrome is defined as the cluster of central obesity, insulin resistance, hypertension, and dyslipidemia. Metabolic syndrome increases a patient's risk of diabetes 5-fold and cardiovascular disease 3-fold.1 Physical inactivity and eating high-fat foods typically precede weight gain and obesity that, in turn, develop into insulin resistance, hypertension, and dyslipidemia.1
Patients with severe psychiatric illness have an increased rate of mortality from cardiovascular disease, compared with the general population.2-4 The cause of this phenomenon is multifactorial: In general, patients with severe mental illness receive insufficient preventive health care, do not eat a balanced diet, and are more likely to smoke cigarettes than other people.2-4
Also, compared with the general population, the diet of men with schizophrenia contains less vegetables and grains and women with schizophrenia consume less grains. An estimated 70% of patients with schizophrenia smoke.4 As measured by BMI, 86% of women with schizophrenia and 70% of men with schizophrenia are overweight or obese.4
Antipsychotics used to treat severe mental illness also have been implicated in metabolic syndrome, specifically second-generation antipsychotics (SGAs).5 Several theories aim to explain how antipsychotics lead to metabolic alterations.
Oxidative stress. One theory centers on the production of oxidative stress and the consequent reactive oxygen species that form after SGA treatment.6
Mitochondrial function. Another theory assesses the impact of antipsychotic treatment on mitochondrial function. Mitochondrial dysfunction causes decreased fatty acid oxidation, leading to lipid accumulation.7
The culminating affect of severe mental illness alone as well as treatment-emergent side effects of antipsychotics raises the question of how to best treat the dyslipidemia component of metabolic syndrome. This article will:
- review which antipsychotics impact lipids the most
- provide an overview of the most recent lipid guidelines
- describe how to best manage patients to prevent and treat dyslipidemia.
Impact of antipsychotics on lipids
Antipsychotic treatment can lead to metabolic syndrome; SGAs are implicated in most cases.8 A study by Liao et al9 investigated the risk of developing type 2 diabetes mellitus, hypertension, and hyperlipidemia in patients with schizophrenia who received treatment with a first-generation antipsychotic (FGA) compared with patients who received a SGA. The significance-adjusted hazard ratio for the development of hyperlipidemia in patients treated with a SGA was statistically significant compared with the general population (1.41; 95% CI, 1.09-1.83). The risk of hyperlipidemia in patients treated with a FGA was not significant.
Studies have aimed to describe which SGAs carry the greatest risk of hyperlipidemia.10,11 To summarize findings, in 2004 the American Diabetes Association (ADA) and American Psychiatric Association released a consensus statement on the impact of antipsychotic medications on obesity and diabetes.12 The statement listed the following antipsychotics in order of greatest to least impact on hyperlipidemia:
- clozapine
- olanzapine
- quetiapine
- risperidone
- ziprasidone
- aripiprazole.
To evaluate newer SGAs, a systematic review and meta-analysis by De Hert et al13 aimed to assess the metabolic risks associated with asenapine, iloperidone, lurasidone, and paliperidone. In general, the studies included in the meta-analysis showed little or no clinically meaningful differences among these newer agents in terms of total cholesterol in short-term trials, except for asenapine and iloperidone.
Asenapine was found to increase the total cholesterol level in long-term trials (>12 weeks) by an average of 6.53 mg/dL. These trials also demonstrated a decrease in HDL cholesterol (−0.13 mg/dL) and a decrease in low-density lipoprotein cholesterol (LDL-C) (−1.72 mg/dL to −0.86 mg/dL). The impact of asenapine on these lab results does not appear to be clinically significant.13,14
Iloperidone. A study evaluating the impact iloperidone on lipid values showed a statistically significant increase in total cholesterol, HDL, and LDL-C levels after 12 weeks.13,15
Overview: Latest lipid guidelines
Current literature lacks information regarding statin use for overall prevention of metabolic syndrome. However, the most recent update to the American Heart Association's guideline on treating blood cholesterol to reduce atherosclerotic cardiovascular risk in adults describes the role of statin therapy to address dyslipidemia, which is one component of metabolic syndrome.16,17
Some of the greatest changes seen with the latest blood cholesterol guidelines include:
- focus on atherosclerotic cardiovascular disease (ASCVD) risk reduction to identify 4 statin benefit groups
- transition away from treating to a target LDL value
- use of the Pooled Cohort Equation to estimate 10-year ASCVD risk, rather than the Framingham Risk Score.
Placing patients in 1 of 4 statin benefit groups
Unlike the 2002 National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines, the latest guidelines have identified 4 statin treatment benefit groups:
- patients with clinical ASCVD (including those who have had acute coronary syndrome, stroke, or myocardial infarction, or who have stable or unstable angina, transient ischemic attacks, or peripheral artery disease, or a combination of these findings)patients with LDL-C >190 mg/dL
- patients age 40 to 75 with type 1 or type 2 diabetes mellitus
- patients with an estimated 10-year ASCVD risk of ≥7.5% that was estimated using the Pooled Cohort Equation.16,17
Table 1 represents each statin benefit group and recommended treatment options.
Selected statin therapy for each statin benefit group is further delineated into low-, moderate-, and high-intensity therapy. Intensity of statin therapy represents the expected LDL lowering capacity of selected statins. Low-intensity statin therapy, on average, is expected to lower LDL-C by <30%. Moderate-intensity statin therapy is expected to lower LDL-C by 30% to <50%. High-intensity statin therapy is expected to lower LDL-C by >50%.
When selecting treatment for patients, it is important to first determine the statin benefit group that the patient falls under, and then select the appropriate statin intensity. The categorization of the different statins based on LDL-C lowering capacity is described in Table 2.
Whenever a patient is started on statin therapy, order a liver function test and lipid profile at baseline. Repeat these tests 4 to 12 weeks after statin initiation, then every 3 to 12 months.
Transition away from treating to a target LDL-C goal
ATP III guidelines suggested that elevated LDL was the leading cause of coronary heart disease and recommended therapy with LDL-lowering medications.18 The panel that developed the 2013 lipid guideline concluded that there was no evidence that showed benefit in treating to a designated LDL-C goal.16,17 Arguably, treating to a target may lead to overtreatment in some patients and under-treatment in others. Treatment is now recommended based on statin intensity.
Using the Pooled Cohort Equation
In moving away from the Framingham Risk Score, the latest lipid guidelines established a new calculation to assess cardiovascular disease. The Pooled Cohort Equation estimates the 10-year ASCVD risk for patients based on selected risk factors: age, sex, race, lipids, diabetes, smoking status, and blood pressure. Although other potential cardiovascular disease risk factors have been identified, the Pooled Cohort Equation focused on those risk factors that have been correlated with cardiovascular disease since the 1960s.16,17,19 The Pooled Cohort Equation is intended to (1) more accurately identify higher-risk patients and (2) assess who would best benefit from statin therapy.
Recommended lab tests and subsequent treatment
With the new lipid guidelines in place to direct dyslipidemia treatment and a better understanding of how certain antipsychotics impact lipid values, the next step is monitoring parameters for patients. Before initiating antipsychotic treatment and in accordance with the 2014 National Institute for Health and Care Excellence (NICE) guidelines, baseline measurements should include weight, waist circumference, pulse, blood pressure, fasting blood glucose, hemoglobin A1c, blood lipid profile, and, if risperidone or paliperidone is initiated, prolactin level.20 Additionally, patients should be assessed at baseline for any movement disorders as well as current nutritional status, diet, and level of physical activity.
Once treatment is selected on a patient-specific basis, weight should be measured weekly for the first 6 weeks, again at 12 weeks and 1 year, and then annually. Pulse and blood pressure should be obtained 12 weeks after treatment initiation and at 1 year. Fasting blood glucose, hemoglobin A1c, and blood lipid levels should be collected 12 weeks after treatment onset, then at the 1-year mark.20 These laboratory parameters should be measured annually while the patient is receiving antipsychotic treatment.
Alternately, you can follow the monitoring parameters in the more dated 2004 ADA consensus statement:
- baseline assessment to include BMI, waist circumference, blood pressure, fasting plasma glucose, fasting lipid profile, and personal and family history
- BMI measured again at 4 weeks, 8 weeks, 12 weeks, and then quarterly
- 12-week follow-up measurement of fasting plasma glucose, fasting lipids, and blood pressure
- annual measurement of fasting blood glucose, blood pressure, and waist circumference.12
In addition to the NICE guidelines and the ADA consensus statement, use of the current lipid guidelines and the Pooled Cohort Equation to assess 10-year ASCVD risk should be obtained at baseline and throughout antipsychotic treatment. If you identify an abnormality in the lipid profile, you have several options:
- Decrease the antipsychotic dosage
- Switch to an antipsychotic considered to be less risky
- Discontinue therapy
- Implement diet and exercise
- Refer the patient to a dietitian or other clinician skilled in managing overweight or obesity and hyperlipidemia.21
Furthermore, patients identified as being in 1 of the 4 statin benefit groups should be started on appropriate pharmacotherapy. Non-statin therapy as adjunct or in lieu of statin therapy is not considered to be first-line.16
CASE CONTINUED
After reviewing Mr. W's lab results, you calculate that he has a 24% ten-year ASCVD risk, using the Pooled Cohort Equation. Following the treatment algorithm for statin benefit groups, you see that Mr. W meets criteria for high-intensity statin therapy. You stop olanzapine, switch to risperidone, 1 mg/d, and initiate atorvastatin, 40 mg/d. You plan to assess Mr. W's weight weekly over the next 6 weeks and order a liver profile and lipid profile in 6 weeks.
Related Resource
- AHA/ACC 2013 Prevention Guidelines Tools CV Risk Calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_Prevention-Guidelines.jsp.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Atorvastatin • Lipitor
Clozapine • Clozaril
Fluvastatin • Lescol
Iloperidone • Fanapt
Lovastatin • Mevacor
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Pitavastatin • Livalo
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Rosuvastatin • Crestor
Simvastatin • Zocor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
1. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12.
2. McCreadie RG; Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534-539.
3. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;7(12):1350-1363.
4. Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176.
5. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
6. Baig MR, Navaira E, Escamilla MA, et al. Clozapine treatment causes oxidation of proteins involved in energy metabolism in lymphoblastoid cells: a possible mechanism for antipsychotic-induced metabolic alterations. J Psychiatr Pract. 2010;16(5):325-333.
7. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):266-271.
8. Watanabe J, Suzuki Y, Someya T. Lipid effects of psychiatric medications. Curr Atheroscler Rep. 2013;15(1):292.
9. Liao HH, Chang CS, Wei WC, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126(1-3):110-116.
10. Lidenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290-296.
11. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825.
12. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone, and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
14. Kemp DE, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiary. 2014;75(3):238-245.
15. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo-and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-S28.
16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S72.
18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
19. Ioannidis JP. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463-464.
20. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management: the NICE Guideline on Treatment and Management. https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565. Published 2014. Accessed June 8, 2016.
21. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
Scopolamine-induced mania: ‘Theoretically possible, but statistically improbable'
Dr. Emjay Tan’s case study of a 36-year-old man who became “Manic after taking a vacation” (Cases That Test Your Skills, Current Psychiatry. April 2016, p. 45-50) is off the mark by attributing the manic episode to scopolamine—theoretically possible, but statistically improbable.
Dr. Tan may be unaware of a more frequent event: vacation hypomania. About one-third of my bipolar disorder patients had their first manic episode while on an overseas vacation or upon their return. It isn’t the fun, excitement, or novelty of a vacation that triggers the episode, but sleep deprivation, which is part and parcel of such events, particularly when they involve a holiday in a substantially different time zone.
Few people get to sleep more than a few hours the night before departing on a vacation; there’s so much to do: packing, getting to the airport hours before the flight, etc. Not many people sleep soundly on the plane, and many experience the effects of jet lag both during the first few days of vacation and when the vacationer returns home. Many vacations come with substantial and protracted sleep deprivation, and sleep deprivation is an excellent way to trigger a hypomanic episode. I suspect that is why Dr. Tan’s patient, who did not have a history of psychiatric symptoms, but who might have been genetically predisposed, became manifestly symptomatic shortly following his return from an overseas holiday.
Of course, it isn’t just first episodes of hypomania that are triggered by sleep deprivation in patients with undiagnosed bipolar disorder; the event is common in the lives of people who already receive treatment. Accordingly, my patients know that I might increase their lithium dosage for at least a few days to give them added protection as they head overseas, coupled with advice to do their best to get proper sleep.
Despite such prophylaxis, many of my bipolar disorder patients have taken a long flight overseas and, then, after half a day in the air, continued “flying.” To the best of my knowledge, none ever took scopolamine.
Martin Blinder, MD
Past Assistant Clinical Professor of Psychiatry
University of California, San Francisco
Past Adjunct Professor of Law
University of California
Hastings College of Law
San Francisco, California
Dr. Emjay Tan’s case study of a 36-year-old man who became “Manic after taking a vacation” (Cases That Test Your Skills, Current Psychiatry. April 2016, p. 45-50) is off the mark by attributing the manic episode to scopolamine—theoretically possible, but statistically improbable.
Dr. Tan may be unaware of a more frequent event: vacation hypomania. About one-third of my bipolar disorder patients had their first manic episode while on an overseas vacation or upon their return. It isn’t the fun, excitement, or novelty of a vacation that triggers the episode, but sleep deprivation, which is part and parcel of such events, particularly when they involve a holiday in a substantially different time zone.
Few people get to sleep more than a few hours the night before departing on a vacation; there’s so much to do: packing, getting to the airport hours before the flight, etc. Not many people sleep soundly on the plane, and many experience the effects of jet lag both during the first few days of vacation and when the vacationer returns home. Many vacations come with substantial and protracted sleep deprivation, and sleep deprivation is an excellent way to trigger a hypomanic episode. I suspect that is why Dr. Tan’s patient, who did not have a history of psychiatric symptoms, but who might have been genetically predisposed, became manifestly symptomatic shortly following his return from an overseas holiday.
Of course, it isn’t just first episodes of hypomania that are triggered by sleep deprivation in patients with undiagnosed bipolar disorder; the event is common in the lives of people who already receive treatment. Accordingly, my patients know that I might increase their lithium dosage for at least a few days to give them added protection as they head overseas, coupled with advice to do their best to get proper sleep.
Despite such prophylaxis, many of my bipolar disorder patients have taken a long flight overseas and, then, after half a day in the air, continued “flying.” To the best of my knowledge, none ever took scopolamine.
Martin Blinder, MD
Past Assistant Clinical Professor of Psychiatry
University of California, San Francisco
Past Adjunct Professor of Law
University of California
Hastings College of Law
San Francisco, California
Dr. Emjay Tan’s case study of a 36-year-old man who became “Manic after taking a vacation” (Cases That Test Your Skills, Current Psychiatry. April 2016, p. 45-50) is off the mark by attributing the manic episode to scopolamine—theoretically possible, but statistically improbable.
Dr. Tan may be unaware of a more frequent event: vacation hypomania. About one-third of my bipolar disorder patients had their first manic episode while on an overseas vacation or upon their return. It isn’t the fun, excitement, or novelty of a vacation that triggers the episode, but sleep deprivation, which is part and parcel of such events, particularly when they involve a holiday in a substantially different time zone.
Few people get to sleep more than a few hours the night before departing on a vacation; there’s so much to do: packing, getting to the airport hours before the flight, etc. Not many people sleep soundly on the plane, and many experience the effects of jet lag both during the first few days of vacation and when the vacationer returns home. Many vacations come with substantial and protracted sleep deprivation, and sleep deprivation is an excellent way to trigger a hypomanic episode. I suspect that is why Dr. Tan’s patient, who did not have a history of psychiatric symptoms, but who might have been genetically predisposed, became manifestly symptomatic shortly following his return from an overseas holiday.
Of course, it isn’t just first episodes of hypomania that are triggered by sleep deprivation in patients with undiagnosed bipolar disorder; the event is common in the lives of people who already receive treatment. Accordingly, my patients know that I might increase their lithium dosage for at least a few days to give them added protection as they head overseas, coupled with advice to do their best to get proper sleep.
Despite such prophylaxis, many of my bipolar disorder patients have taken a long flight overseas and, then, after half a day in the air, continued “flying.” To the best of my knowledge, none ever took scopolamine.
Martin Blinder, MD
Past Assistant Clinical Professor of Psychiatry
University of California, San Francisco
Past Adjunct Professor of Law
University of California
Hastings College of Law
San Francisco, California
Treated with a mood stabilizer, he becomes incontinent and walks oddly
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
CASE Rapid decline
Mr. X, age 67, is a businessman who had a diagnosis of bipolar depression 8 years ago, and who is being evaluated now for new-onset cognitive impairment, gait disturbance that resembles child-like steps, dyskinesia, and urinary incontinence of approximately 2 months’ duration. He has been treated for bipolar depression with valproic acid, 1,000 mg/d, and venlafaxine, 150 mg/d, without complaint until now, since the diagnosis was made 8 years ago. The serum valproic acid level, tested every month, is within the therapeutic range; liver function tests, ordered every 6 months, also are within the normal range.
Mr. X has become confined to his bedroom and needs assistance to walk. He has to be lifted to a standing position by 2 attendants, who bear his weight and instruct him to take one step at a time. He wears a diaper and needs assistance shaving, showering, and getting dressed. When the treatment team asks him about his condition, Mr. X turns to his wife to respond on his behalf. He is slow to speak and struggles to remember the details about his condition or the duration of his disability.
Mr. X is referred to a neurologist, based on cognitive impairment and gait disturbance, who orders an MRI scan of the brain that shows enlarged ventricles and some cortical atrophy (Figure 1). A neurosurgeon removes approximately 25 mL of CSF as a diagnostic and therapeutic intervention.
Videography of his ambulation, recorded before and after the CSF tap, shows slight improvement in gait. Mr. X is seen by a neurosurgery team, who recommends that he receive a ventriculoperitoneal shunt for hydrocephalus.
While awaiting surgical treatment, Mr. X’s psychotropic medications are withheld, and he is closely monitored for reemergence of psychiatric symptoms. Mr. X shows gradual but significant improvement in his gait within 8 to 10 weeks. His dyskinesia improves significantly, as does his cognitive function.
What additional testing is recommended beyond MRI?
a) complete blood count with differential
b) blood ammonia level
c) neuropsychological evaluation
d) APOE-e4 genetic testing
e) all the above
The authors’ observations
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, or urinary incontinence that is associated with dilation of the brain’s ventricular system with normal opening CSF pressure (Table 1). Several studies have reported that patients with NPH might exhibit neuropsychiatric symptoms,1-4 possibly related to alterations in central neurotransmitter activity.5 NPH patients could present with symptoms reflecting frontal dominance (Table 2,6-9). In a study of 35 patients with idiopathic NPH in a tertiary hospital in Brazil,10 psychiatric symptoms were established by formal psychiatric evaluation in 71%, notably anxiety, depression, and psychotic syndromes.
Mechanism responsible for gait disturbance
Gait disturbance typically is the first and most prominent symptom of the NPH triad. Gait disturbance in NPH can be progressive because of expansion of the ventricular system, mainly the lateral ventricles, leading to pressure on the corticospinal motor fibers descending to the lumbosacral spinal cord. Although there is no one type of gait disturbance indicative of NPH, it often is described as shuffling, magnetic, and wide-based.11 Slowness of gait and gait imbalance or disequilibrium are common and more likely to respond to shunting.12
Drug-induced gait disturbance is likely to result in parkinsonian symptoms.13 A possible mechanism involves inhibition of neurite outgrowth. Qian et al14 found that therapeutic plasma levels of valproic acid reduced cell proliferation and neurite outgrowth, using SY5Y neuroblastoma cells as a neuronal model. Researchers also reported that valproic acid reduced mRNA and protein levels of neurofilament 160; a possible mechanistic explanation involves inhibition of neurite outgrowth that leads to gait disturbance. These effects reversed 2 days after stopping valproic acid.
Another possible mechanism is related to γ-aminobutyric acid (GABA) pathway disturbance leading to dopamine inhibition. This postulates that valproic acid or a metabolite of valproic acid, such as Δ-2-valproate, which may be a more potent inhibitor of the GABA-degrading enzyme than valproic acid, could cause a transient inhibitory effect on dopaminergic pathways.15
Mechanism of mood stabilizer action
Valproic acid is incorporated into neuronal membranes in a saturable manner and appears to displace naturally occurring branched-chain phospholipids.16 Chronic valproic acid use reduces protein kinase C (PKC) activity in patients with mania.17 Elevated PKC activity has been observed in patients with mania and in animal models of mania.18 Valproic acid has antioxidant effects and has reversed early DNA damage caused by amphetamine in an animal model of mania.19 Valproic acid and lithium both reduce inositol biosynthesis; the mechanism of action for valproic acid is unique, however, resulting from decreased myo-inositol-1-phosphate synthase inhibition.20
There is not a strong correlation between serum valproic acid levels and antimanic effects, but levels in the range of 50 to 150 μg/mL generally are required for therapeutic effect.
Neuropsychiatric adverse effects of valproic acid
With most antiepileptic drugs, adverse effects mainly are dose-related and include sedation, drowsiness, incoordination, nausea, and fatigue. Careful dose titration can reduce the risk of these adverse effects. Research on mothers with epilepsy has shown an association between valproic acid exposure in utero and lower IQ and a higher prevalence of autism spectrum disorder in children.21
Adverse effects on cognitive functioning are infrequent; valproic acid improves cognition in select patients.22 In a 20-week randomized, observer-blinded, parallel-group trial, adding valproic acid to carbamazepine resulted in improvement in short-term verbal memory.23 In a group of geriatric patients (mean age 77 years), no adverse cognitive effects were observed with valproic acid use.24
Masmoudi et al25 evaluated dementia and extrapyramidal symptoms associated with long-term valproic acid use. Among the side effects attributed to valproic acid, parkinsonian syndromes and cognitive impairment were not commonly reported. In a prospective study, Armon et al26 found several abnormal symptoms and signs related to motor and cognitive function impairment in patients on long-term valproic acid therapy. These side effects might be related to a disturbance in the GABAergic pathways in the basal ganglia system. Note that Δ2-valproic acid, a metabolite of valproic acid, preferentially accumulates in select areas of the brain: the substantia nigra, superior and inferior colliculus, hippocampus, and medulla.
What is the next best step in management?
a) surgically implant a shunt
b) adjust the dosage of valproic acid
c) switch to monotherapy
d) switch to an alternative psychotropic medication
e) provide observation and follow-up
The authors’ observations
Unusual appearances of NPH symptoms could hinder early diagnosis and proper treatment. Mr. X was taking valproic acid and venlafaxine for bipolar depression, without any complaints, and was asymptomatic for 8 years—until he developed symptoms of NPH.
In patients who have what can be considered classic symptoms of NPH and are taking valproic acid, consider discontinuing the drug on a trial basis before resorting to a more invasive procedure. This strategy could significantly reduce the cost of health care and contribute to the overall well-being of the patient.
NPH associated with chronic valproic acid use is rare, supported by only 1 case report13 in our literature review. Based on the severity of symptoms and chance for misdiagnosis, it is essential to identify such cases and differentiate them from others with underlying neuropathology or a secondary cause, such as age-related dementia or Parkinson’s disease, to avoid the burden of unnecessary diagnostic testing on the patient and physician.
Family history also is important in cases presenting with sensorineural hearing loss,13 which follows a pattern of maternal inheritance. Consider genetic testing in such cases.
Earlier diagnosis of valproic acid-induced NPH enables specific interventions and treatment. Treatment of NPH includes one of several forms of shunting and appropriate neuroleptic therapy for behavioral symptoms. Although there is a significant risk (40% to 50%) of psychiatric and behavioral symptoms as a shunt-related complication, as many as 60% of operated patients showed objective improvement. This makes the diagnosis of NPH, and referral for appropriate surgical treatment of NPH, an important challenge to the psychiatrist.27
OUTCOME No reemergence
Findings on a repeat MRI 2.5 months after the CSF tap remain unchanged. Surgery is cancelled and medications are discontinued. Mr. X is advised to continue outpatient follow-up for monitoring of re-emerging symptoms of bipolar depression.
At a follow-up visit, Mr. X’s condition has returned to baseline. He ambulates spontaneously and responds to questions without evidence of cognitive deficit. He no longer is incontinent.
Follow-up MRI is performed and indicated normal results.
Neuropsychological testing is deemed unnecessary because Mr. X has fully recovered from cognitive clouding (and there would be no baseline results against which to compare current findings). Based on the medication history, the team concludes that prolonged use of valproic acid may have led to development of signs and symptoms of an NPH-like syndrome.
The authors’ observations
Awareness of an association of NPH with neuropsychiatric changes is important for clinical psychiatrists because early assessment and appropriate intervention can prevent associated long-term complications. Valproic acid is considered a relatively safe medication with few neurologic side effects, but the association of an NPH-like syndrome with chronic valproic acid use, documented in this case report, emphasizes the importance of studying long-term consequences of using valproic acid in geriatric patients. More such case reports need to be evaluated to study the association of neuropsychiatric complications with chronic valproic use in the geriatric population.
Mr. X apparently had cerebral atrophy with enlarged ventricles that was consistently evident for 10 years (Figure 2), although he has been maintained on valproic acid for 8 years. What is intriguing in this case is that discontinuing valproic acid relieved the triad of incontinence, imbalance, and memory deficits indicative of NPH. Mr. X remains free of these symptoms.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.
1. Pinner G, Johnson H, Bouman WP, et al. Psychiatric manifestations of normal-pressure hydrocephalus: a short review and unusual case. Int Psychogeriatr. 1997;9(4):465-470.
2. Alao AO, Naprawa SA. Psychiatric complications of hydrocephalus. Int J Psychiatry Med. 2001;31(3):337-340.
3. Lindqvist G, Andersson H, Bilting M, et al. Normal pressure hydrocephalus: psychiatric findings before and after shunt operation classified in a new diagnostic system for organic psychiatry. Acta Psychiatr Scand Suppl. 1993;373:18-32.
4. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21(3):165-174.
5. Markianos M, Lafazanos S, Koutsis G, et al. CSF neurotransmitter metabolites and neuropsychiatric symptomatology in patients with normal pressure hydrocephalus. Clin Neurol Neurosurg. 2009;111(3):231-234.
6. McIntyre AW, Emsley RA. Shoplifting associated with normal-pressure hydrocephalus: report of a case. J Geriatr Psychiatry Neurol. 1990;3(4):229-230.
7. Kwentus JA, Hart RP. Normal pressure hydrocephalus presenting as mania. J Nerv Ment Dis. 1987;175(8):500-502.
8. Bloom KK, Kraft WA. Paranoia—an unusual presentation of hydrocephalus. Am J Phys Med Rehabil. 1998;77(2):157-159.
9. Yusim A, Anbarasan D, Bernstein C, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008;165(9):1119-1125.
10. Oliveira MF, Oliveira JR, Rotta JM, et al. Psychiatric symptoms are present in most of the patients with idiopathic normal pressure hydrocephalus. Arq Neuropsiquiatr. 2014;72(6):435-438.
11. Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987-997.
12. Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13(7):434-437.
13. Evans MD, Shinar R, Yaari R. Reversible dementia and gait disturbance after prolonged use of valproic acid. Seizure. 2011;20(6):509-511.
14. Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21-33.
15. Löscher W. Pharmacological, toxicological and neurochemical effects of delta 2(E)-valproate in animals. Pharm Weekbl Sci. 1992;14(3A):139-143.
16. Siafaka-Kapadai A, Patiris M, Bowden C, et al. Incorporation of [3H]-valproic acid into lipids in GT1-7 neurons. Biochem Pharmacol. 1998;56(2):207-212.
17. Hahn CG, Umapathy, Wagn HY, et al. Lithium and valproic acid treatments reduce PKC activation and receptor-G-protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39(4):35-63.
18. Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59(12):1160-1171.
19. Andreazza AC, Frey BN, Stertz L, et al. Effects of lithium and valproate on DNA damage and oxidative stress markers in an animal model of mania [abstract P10]. Bipolar Disord. 2007;9(suppl 1):16.
20. Galit S, Shirley M, Ora K, et al. Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol Rep. 2007;59(4):402-407.
21. Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
22. Prevey ML, Delaney RC, Cramer JA, et al. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veteran Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53(10):1008-1016.
23. Aldenkamp AP, Baker G, Mulder OG, et al. A multicenter randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41(9):1167-1178.
24. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-390.
25. Masmoudi K, Gras-Champel V, Bonnet I, et al. Dementia and extrapyramidal problems caused by long-term valproic acid [in French]. Therapie. 2000;55(5):629-634.
26. Armon C, Shin C, Miller P, et al. Reversible parkinsonism and cognitive impairment with chronic valproate use. Neurology. 1996;47(3):626-635.
27. Price TR, Tucker GJ. Psychiatric and behavioral manifestations of normal pressure hydrocephalus. A case report and brief review. J Nerv Ment Dis. 1977;164(1):51-55.
SIMPle smartphone app shows promise for bipolar disorder psychoeducation
ATLANTA – A new smartphone application aimed at providing psychoeducation to patients with bipolar disorder was well received and showed promise for improving outcomes in a feasibility study, according to Dr. Eduard Vieta.
Early results of the study showed that adherence was quite high, with retention at 76% among 49 patients with bipolar disorder who tested the SIMPle app (Self Monitoring and Psychoeducation in Bipolar Patients with a Smartphone Application), Dr. Vieta said at the annual meeting of the American Psychiatric Association.
The app, currently available for free for Android and iPhones, is an interactive educational program that includes weekly and daily tests, with alerts for patients to take medications or see their doctor.
The patients in the study were representative of generally stable bipolar disorder patients in a real-world setting, as the app ideally would be used by those who are “in near remission or at least not acutely ill,” said Dr. Vieta of the University of Barcelona.
“People like the app and did follow the daily and weekly tests, which is a good sign,” he said, noting that satisfaction was high, and good correlation between test scores and mood changes suggested that the app is reliable for monitoring mood changes.
There were 10 suicide alerts during the study that were quickly addressed because messages were received immediately, he said.
The latest version of the app includes simpler navigation, rewards for fulfilling the daily and weekly testing, and medication reminders.
Dr. Vieta and his colleagues at the University of Barcelona previously have demonstrated the value of psychoeducation among patients with bipolar disorder. They developed a successful psychoeducation program in the late 1990s, which led to a trial published in 2003 and development of a training manual in 2006 that has been translated into numerous languages.
The program and trial showed that adding psychoeducation to medication in patients with bipolar disorder improves outcomes in terms of relapse and hospitalizations: the rate of relapses in the study was reduced by nearly 80%, Dr. Vieta said.
Providing such education, however, which has become a standard of care, is limited by the need for personnel training and by staffing and financial resource constraints.
“We wanted something nonstigmatizing but also linked to the clinical care team. … Clearly, we would like to make this intervention more widely available without compromising quality,” Dr. Vieta said, describing the impetus for the SIMPle app.
A phase II, randomized controlled study of the app is now underway, he said, adding that “this is really quite exciting. … There is huge potential for things we can do with this app.”
Dr. Vieta is a consultant or adviser for several pharmaceutical companies. He also has received research grants, honoraria, or consulting fees from numerous entities.
ATLANTA – A new smartphone application aimed at providing psychoeducation to patients with bipolar disorder was well received and showed promise for improving outcomes in a feasibility study, according to Dr. Eduard Vieta.
Early results of the study showed that adherence was quite high, with retention at 76% among 49 patients with bipolar disorder who tested the SIMPle app (Self Monitoring and Psychoeducation in Bipolar Patients with a Smartphone Application), Dr. Vieta said at the annual meeting of the American Psychiatric Association.
The app, currently available for free for Android and iPhones, is an interactive educational program that includes weekly and daily tests, with alerts for patients to take medications or see their doctor.
The patients in the study were representative of generally stable bipolar disorder patients in a real-world setting, as the app ideally would be used by those who are “in near remission or at least not acutely ill,” said Dr. Vieta of the University of Barcelona.
“People like the app and did follow the daily and weekly tests, which is a good sign,” he said, noting that satisfaction was high, and good correlation between test scores and mood changes suggested that the app is reliable for monitoring mood changes.
There were 10 suicide alerts during the study that were quickly addressed because messages were received immediately, he said.
The latest version of the app includes simpler navigation, rewards for fulfilling the daily and weekly testing, and medication reminders.
Dr. Vieta and his colleagues at the University of Barcelona previously have demonstrated the value of psychoeducation among patients with bipolar disorder. They developed a successful psychoeducation program in the late 1990s, which led to a trial published in 2003 and development of a training manual in 2006 that has been translated into numerous languages.
The program and trial showed that adding psychoeducation to medication in patients with bipolar disorder improves outcomes in terms of relapse and hospitalizations: the rate of relapses in the study was reduced by nearly 80%, Dr. Vieta said.
Providing such education, however, which has become a standard of care, is limited by the need for personnel training and by staffing and financial resource constraints.
“We wanted something nonstigmatizing but also linked to the clinical care team. … Clearly, we would like to make this intervention more widely available without compromising quality,” Dr. Vieta said, describing the impetus for the SIMPle app.
A phase II, randomized controlled study of the app is now underway, he said, adding that “this is really quite exciting. … There is huge potential for things we can do with this app.”
Dr. Vieta is a consultant or adviser for several pharmaceutical companies. He also has received research grants, honoraria, or consulting fees from numerous entities.
ATLANTA – A new smartphone application aimed at providing psychoeducation to patients with bipolar disorder was well received and showed promise for improving outcomes in a feasibility study, according to Dr. Eduard Vieta.
Early results of the study showed that adherence was quite high, with retention at 76% among 49 patients with bipolar disorder who tested the SIMPle app (Self Monitoring and Psychoeducation in Bipolar Patients with a Smartphone Application), Dr. Vieta said at the annual meeting of the American Psychiatric Association.
The app, currently available for free for Android and iPhones, is an interactive educational program that includes weekly and daily tests, with alerts for patients to take medications or see their doctor.
The patients in the study were representative of generally stable bipolar disorder patients in a real-world setting, as the app ideally would be used by those who are “in near remission or at least not acutely ill,” said Dr. Vieta of the University of Barcelona.
“People like the app and did follow the daily and weekly tests, which is a good sign,” he said, noting that satisfaction was high, and good correlation between test scores and mood changes suggested that the app is reliable for monitoring mood changes.
There were 10 suicide alerts during the study that were quickly addressed because messages were received immediately, he said.
The latest version of the app includes simpler navigation, rewards for fulfilling the daily and weekly testing, and medication reminders.
Dr. Vieta and his colleagues at the University of Barcelona previously have demonstrated the value of psychoeducation among patients with bipolar disorder. They developed a successful psychoeducation program in the late 1990s, which led to a trial published in 2003 and development of a training manual in 2006 that has been translated into numerous languages.
The program and trial showed that adding psychoeducation to medication in patients with bipolar disorder improves outcomes in terms of relapse and hospitalizations: the rate of relapses in the study was reduced by nearly 80%, Dr. Vieta said.
Providing such education, however, which has become a standard of care, is limited by the need for personnel training and by staffing and financial resource constraints.
“We wanted something nonstigmatizing but also linked to the clinical care team. … Clearly, we would like to make this intervention more widely available without compromising quality,” Dr. Vieta said, describing the impetus for the SIMPle app.
A phase II, randomized controlled study of the app is now underway, he said, adding that “this is really quite exciting. … There is huge potential for things we can do with this app.”
Dr. Vieta is a consultant or adviser for several pharmaceutical companies. He also has received research grants, honoraria, or consulting fees from numerous entities.
AT THE APA ANNUAL MEETING
Key clinical point: A new smartphone application aimed at providing psychoeducation to patients with bipolar disorder was well received and showed promise for improving outcomes in a feasibility study.
Major finding: Adherence was good and retention was high at 76%.
Data source: A feasibility study involving 49 patients.
Disclosures: Dr. Vieta is a consultant or advisor for several pharmaceutical companies. He also has received research grants, honoraria, or consulting fees from numerous entities.