User login
SCD-HeFT 10-year results: Primary-prevention ICD insights in nonischemic heart failure
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
AHA statement addresses genetic testing for CVD
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
FROM CIRCULATION: GENOMIC AND PRECISION MEDICINE
New oral anticoagulants drive ACC consensus on bleeding
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
How to reboot elective CV procedures after COVID-19 lockdown
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
COVID-19: ‘dramatic’ surge in out-of-hospital cardiac arrests in NYC
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
LAA Closure noninferior to DOACs to prevent AF-related events
Left atrial appendage closure was noninferior to use of direct oral anticoagulants for the prevention of atrial fibrillation (AFib)–related events in high-risk patients, based on data from 402 adults.
Given the limitations of vitamin K antagonists for preventing stroke in AFib, “a novel site-specific therapeutic alternative, mechanical left atrial appendage occlusion [LAAO], entered clinical practice,” but has not been compared with current safe and effective oral anticoagulants, wrote Pavel Osmancik, MD, of University Hospital Kralovske Vinohrady, Prague, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers randomized 201 moderate- or high-risk adults with nonvalvular AFib to LAAO and another 201 to direct oral anticoagulants (DOAC).
Patients in the LAAO group underwent transesophageal echocardiography to exclude left atrial thrombi and underwent implantation with Boston Scientific’s Watchman, Watchman-FLX, or Abbott’s Amulet devices. Patients in the DOAC group received rivaroxaban, apixaban, or dabigatran at the manufacturer-recommended dose.
The primary outcome was a composite of complications related to procedures or devices, thromboembolic events (including stroke), and clinically significant bleeding. After an average of 20 months follow-up, 35 patients in the LAAO group and 41 in the DOAC group met the primary outcome (11% per 100 patient-years vs. 13% per 100 patient-years).
In addition, no differences appeared between the groups for the endpoint components of all-stroke/transient ischemic attack event (subdistribution hazard ratio, 1.00), clinically significantly bleeding (sHR, 0.81), or cardiovascular death (sHR, 0.75).
Nine patients experienced major complications related to LAAO, including clinically significant bleeding (sHR, 0.81; 95% CI, 0.44-1.52) and cardiovascular death (sHR, 0.75; 95% CI, 0.34-1.62). Major LAAO-related complications occurred in nine (4.5%) patients, with a short-term (up to 7 days or hospital discharge) complication rate of 2.1% and a 2.7% late complication rate. The late complications included three pericardial effusions, one of which resulted in death, the researchers wrote.
The study findings were limited by several factors, including the inability to assess the differences among the components of the composite primary endpoint. For example, “Regarding the primary endpoint, stroke reduction may be more important than bleeding reduction,” the investigators wrote.
The results were strengthened, however, by the enrollment of a high-risk AF population and is the first known randomized trial to compare percutaneous LAAO and DOACs for stroke prevention in this group. But the late complication rate of 2.7% is “suboptimal” and safety issues reinforce the need for refinement of operator technique and device technology with LAAO, they concluded.
‘Important step forward,’ with caveats
“How LAAO might stack up against DOAC therapy has remained an open question: Compared with warfarin, DOACs are easier to use and are associated with a reduction in mortality, driven by a substantially lower risk of intracranial hemorrhage and fatal bleeding,” wrote Matthew J. Price, MD, of the Scripps Clinic in La Jolla, Calif., and Jacqueline Saw, MD, of Vancouver General Hospital, in an accompanying editorial.
Previous studies of LAAO have shown a reduced risk of gastrointestinal bleeding, but procedure hazards interfered with long-term benefits, they said. The current study findings of similar rates of stroke and lower bleeding rates with LAAO, compared with DOAC, “are provocative given the clinical consensus that DOACs are safer, well tolerated, and generally better than warfarin, which was an easy target for transcatheter LAAO, given warfarin’s extensive limitations,” the editorialists wrote. Although the findings lend support to the use of LAAO, clinicians should consider several caveats such as the inclusion of patients who were “not optimal candidates for long-term OAC but were selected because they were at high risk for bleeding or because OAC treatment had already failed.”
However, “despite its imperfections, PRAGUE-17 is an important step forward and reinforces the role of transcatheter LAAO as a stroke-prevention strategy for patients with [AFib] at high risk of bleeding or medical treatment failure, even in the modern era of the DOACs,” they concluded. “Going forward, successful enrollment in ongoing and planned clinical trials while avoiding off-label procedures will be critical to define the appropriate use of transcatheter LAAO in expanded patient populations.”
The study was supported by the Ministry of Health of the Czech Republic. Dr. Osmancik disclosed speaking honoraria from Bayer and Abbot. Dr. Price’s financial disclosures included honoraria, speaker bureau fees, and/or research grants from Abbott Vascular, AstraZeneca, Boston Scientific, Chiesi USA, Daiichi Sankyo, and Medtronic. Dr. Saw disclosed receiving unrestricted research grant support several Canadian research institutes and fees and honoraria from AstraZeneca, Abbott Vascular, Boston Scientific, and Servier, among other drug companies.
SOURCES: Osmancik P et al. J Am Coll Cardiol. 2020;75:3122-35; Price MJ, Saw J. J Am Coll Cardiol. 2020;75:3136-9.
Left atrial appendage closure was noninferior to use of direct oral anticoagulants for the prevention of atrial fibrillation (AFib)–related events in high-risk patients, based on data from 402 adults.
Given the limitations of vitamin K antagonists for preventing stroke in AFib, “a novel site-specific therapeutic alternative, mechanical left atrial appendage occlusion [LAAO], entered clinical practice,” but has not been compared with current safe and effective oral anticoagulants, wrote Pavel Osmancik, MD, of University Hospital Kralovske Vinohrady, Prague, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers randomized 201 moderate- or high-risk adults with nonvalvular AFib to LAAO and another 201 to direct oral anticoagulants (DOAC).
Patients in the LAAO group underwent transesophageal echocardiography to exclude left atrial thrombi and underwent implantation with Boston Scientific’s Watchman, Watchman-FLX, or Abbott’s Amulet devices. Patients in the DOAC group received rivaroxaban, apixaban, or dabigatran at the manufacturer-recommended dose.
The primary outcome was a composite of complications related to procedures or devices, thromboembolic events (including stroke), and clinically significant bleeding. After an average of 20 months follow-up, 35 patients in the LAAO group and 41 in the DOAC group met the primary outcome (11% per 100 patient-years vs. 13% per 100 patient-years).
In addition, no differences appeared between the groups for the endpoint components of all-stroke/transient ischemic attack event (subdistribution hazard ratio, 1.00), clinically significantly bleeding (sHR, 0.81), or cardiovascular death (sHR, 0.75).
Nine patients experienced major complications related to LAAO, including clinically significant bleeding (sHR, 0.81; 95% CI, 0.44-1.52) and cardiovascular death (sHR, 0.75; 95% CI, 0.34-1.62). Major LAAO-related complications occurred in nine (4.5%) patients, with a short-term (up to 7 days or hospital discharge) complication rate of 2.1% and a 2.7% late complication rate. The late complications included three pericardial effusions, one of which resulted in death, the researchers wrote.
The study findings were limited by several factors, including the inability to assess the differences among the components of the composite primary endpoint. For example, “Regarding the primary endpoint, stroke reduction may be more important than bleeding reduction,” the investigators wrote.
The results were strengthened, however, by the enrollment of a high-risk AF population and is the first known randomized trial to compare percutaneous LAAO and DOACs for stroke prevention in this group. But the late complication rate of 2.7% is “suboptimal” and safety issues reinforce the need for refinement of operator technique and device technology with LAAO, they concluded.
‘Important step forward,’ with caveats
“How LAAO might stack up against DOAC therapy has remained an open question: Compared with warfarin, DOACs are easier to use and are associated with a reduction in mortality, driven by a substantially lower risk of intracranial hemorrhage and fatal bleeding,” wrote Matthew J. Price, MD, of the Scripps Clinic in La Jolla, Calif., and Jacqueline Saw, MD, of Vancouver General Hospital, in an accompanying editorial.
Previous studies of LAAO have shown a reduced risk of gastrointestinal bleeding, but procedure hazards interfered with long-term benefits, they said. The current study findings of similar rates of stroke and lower bleeding rates with LAAO, compared with DOAC, “are provocative given the clinical consensus that DOACs are safer, well tolerated, and generally better than warfarin, which was an easy target for transcatheter LAAO, given warfarin’s extensive limitations,” the editorialists wrote. Although the findings lend support to the use of LAAO, clinicians should consider several caveats such as the inclusion of patients who were “not optimal candidates for long-term OAC but were selected because they were at high risk for bleeding or because OAC treatment had already failed.”
However, “despite its imperfections, PRAGUE-17 is an important step forward and reinforces the role of transcatheter LAAO as a stroke-prevention strategy for patients with [AFib] at high risk of bleeding or medical treatment failure, even in the modern era of the DOACs,” they concluded. “Going forward, successful enrollment in ongoing and planned clinical trials while avoiding off-label procedures will be critical to define the appropriate use of transcatheter LAAO in expanded patient populations.”
The study was supported by the Ministry of Health of the Czech Republic. Dr. Osmancik disclosed speaking honoraria from Bayer and Abbot. Dr. Price’s financial disclosures included honoraria, speaker bureau fees, and/or research grants from Abbott Vascular, AstraZeneca, Boston Scientific, Chiesi USA, Daiichi Sankyo, and Medtronic. Dr. Saw disclosed receiving unrestricted research grant support several Canadian research institutes and fees and honoraria from AstraZeneca, Abbott Vascular, Boston Scientific, and Servier, among other drug companies.
SOURCES: Osmancik P et al. J Am Coll Cardiol. 2020;75:3122-35; Price MJ, Saw J. J Am Coll Cardiol. 2020;75:3136-9.
Left atrial appendage closure was noninferior to use of direct oral anticoagulants for the prevention of atrial fibrillation (AFib)–related events in high-risk patients, based on data from 402 adults.
Given the limitations of vitamin K antagonists for preventing stroke in AFib, “a novel site-specific therapeutic alternative, mechanical left atrial appendage occlusion [LAAO], entered clinical practice,” but has not been compared with current safe and effective oral anticoagulants, wrote Pavel Osmancik, MD, of University Hospital Kralovske Vinohrady, Prague, and colleagues.
In a study published in the Journal of the American College of Cardiology, the researchers randomized 201 moderate- or high-risk adults with nonvalvular AFib to LAAO and another 201 to direct oral anticoagulants (DOAC).
Patients in the LAAO group underwent transesophageal echocardiography to exclude left atrial thrombi and underwent implantation with Boston Scientific’s Watchman, Watchman-FLX, or Abbott’s Amulet devices. Patients in the DOAC group received rivaroxaban, apixaban, or dabigatran at the manufacturer-recommended dose.
The primary outcome was a composite of complications related to procedures or devices, thromboembolic events (including stroke), and clinically significant bleeding. After an average of 20 months follow-up, 35 patients in the LAAO group and 41 in the DOAC group met the primary outcome (11% per 100 patient-years vs. 13% per 100 patient-years).
In addition, no differences appeared between the groups for the endpoint components of all-stroke/transient ischemic attack event (subdistribution hazard ratio, 1.00), clinically significantly bleeding (sHR, 0.81), or cardiovascular death (sHR, 0.75).
Nine patients experienced major complications related to LAAO, including clinically significant bleeding (sHR, 0.81; 95% CI, 0.44-1.52) and cardiovascular death (sHR, 0.75; 95% CI, 0.34-1.62). Major LAAO-related complications occurred in nine (4.5%) patients, with a short-term (up to 7 days or hospital discharge) complication rate of 2.1% and a 2.7% late complication rate. The late complications included three pericardial effusions, one of which resulted in death, the researchers wrote.
The study findings were limited by several factors, including the inability to assess the differences among the components of the composite primary endpoint. For example, “Regarding the primary endpoint, stroke reduction may be more important than bleeding reduction,” the investigators wrote.
The results were strengthened, however, by the enrollment of a high-risk AF population and is the first known randomized trial to compare percutaneous LAAO and DOACs for stroke prevention in this group. But the late complication rate of 2.7% is “suboptimal” and safety issues reinforce the need for refinement of operator technique and device technology with LAAO, they concluded.
‘Important step forward,’ with caveats
“How LAAO might stack up against DOAC therapy has remained an open question: Compared with warfarin, DOACs are easier to use and are associated with a reduction in mortality, driven by a substantially lower risk of intracranial hemorrhage and fatal bleeding,” wrote Matthew J. Price, MD, of the Scripps Clinic in La Jolla, Calif., and Jacqueline Saw, MD, of Vancouver General Hospital, in an accompanying editorial.
Previous studies of LAAO have shown a reduced risk of gastrointestinal bleeding, but procedure hazards interfered with long-term benefits, they said. The current study findings of similar rates of stroke and lower bleeding rates with LAAO, compared with DOAC, “are provocative given the clinical consensus that DOACs are safer, well tolerated, and generally better than warfarin, which was an easy target for transcatheter LAAO, given warfarin’s extensive limitations,” the editorialists wrote. Although the findings lend support to the use of LAAO, clinicians should consider several caveats such as the inclusion of patients who were “not optimal candidates for long-term OAC but were selected because they were at high risk for bleeding or because OAC treatment had already failed.”
However, “despite its imperfections, PRAGUE-17 is an important step forward and reinforces the role of transcatheter LAAO as a stroke-prevention strategy for patients with [AFib] at high risk of bleeding or medical treatment failure, even in the modern era of the DOACs,” they concluded. “Going forward, successful enrollment in ongoing and planned clinical trials while avoiding off-label procedures will be critical to define the appropriate use of transcatheter LAAO in expanded patient populations.”
The study was supported by the Ministry of Health of the Czech Republic. Dr. Osmancik disclosed speaking honoraria from Bayer and Abbot. Dr. Price’s financial disclosures included honoraria, speaker bureau fees, and/or research grants from Abbott Vascular, AstraZeneca, Boston Scientific, Chiesi USA, Daiichi Sankyo, and Medtronic. Dr. Saw disclosed receiving unrestricted research grant support several Canadian research institutes and fees and honoraria from AstraZeneca, Abbott Vascular, Boston Scientific, and Servier, among other drug companies.
SOURCES: Osmancik P et al. J Am Coll Cardiol. 2020;75:3122-35; Price MJ, Saw J. J Am Coll Cardiol. 2020;75:3136-9.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: A composite primary outcome including stroke and death was not significantly different in high-risk patients randomized to left atrial appendage occlusion or direct oral anticoagulants at roughly 20 months’ follow-up (11% vs. 13%, respectively).
Study details: The data come from the PRAGUE-17 study, a randomized trial of 402 adults at increased risk for atrial fibrillation.
Disclosures: The study was supported by the Ministry of Health of the Czech Republic. Dr. Osmancik disclosed speaking honoraria from Bayer and Abbot.
Sources: Osmancik P et al. J Am Coll Cardiol. 2020;75:3122-35; Price MJ, Saw J. J Am Coll Cardiol. 2020;75:3136-9.
Preventing arrhythmias and QTc prolongation in COVID-19 patients on psychotropics
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Smart phones boosted compliance for cardiac device data transmission
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
FROM HEART RHYTHM 2020
Ventricular tachycardia storm responds to magnetic stimulation
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.
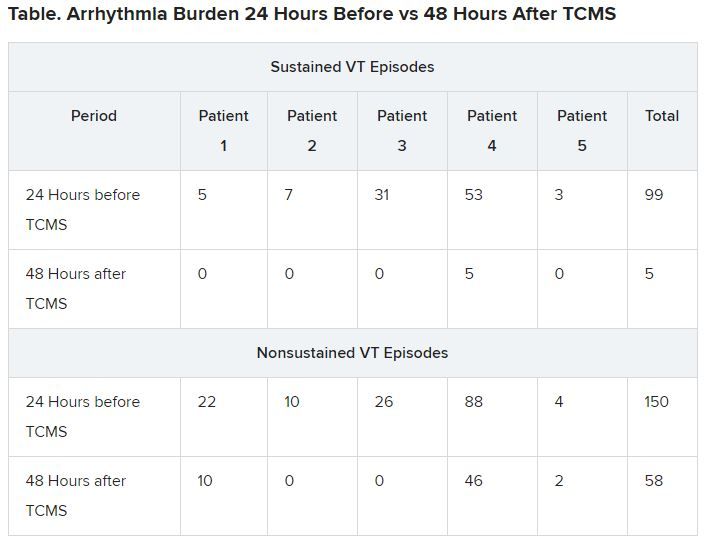
In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.

















