User login
New-AFib risk may not rise with light drinking, may fall with wine
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Even 10 minutes of daily exercise beneficial after ICD implantation
Small increases in daily physical activity are associated with a boost in 1-year survival in patients with heart failure and coronary disease who received an implantable cardioverter defibrillator (ICD), new research suggests.
“Our study looked at how much exercise was necessary for a better outcome in patients with prior ICD implantation and, for every 10 minutes of exercise, we saw a 1% reduction in the likelihood of death or hospitalization, which is a pretty profound impact on outcome for just a small amount of additional physical activity per day,” lead author Brett Atwater, MD, told this news organization.
“These improvements were achieved outside of a formal cardiac rehabilitation program, suggesting that the benefits of increased physical activity obtained in cardiac rehabilitation programs may also be achievable at home,” he said.
Cardiac rehabilitation (CR) programs have been shown to improve short- and long-term outcomes in patients with heart failure (HF) but continue to be underutilized, especially by women, the elderly, and minorities. Home-based CR could help overcome this limitation but the science behind it is relatively new, noted Dr. Atwater, director of electrophysiology and electrophysiology research, Inova Heart and Vascular Institute, Fairfax, Va.
As reported in Circulation Cardiovascular Quality and Outcomes, the study involved 41,731 Medicare beneficiaries (mean age, 73.5 years) who received an ICD from 2014 to 2016.
ICD heart rate and activity sensor measurements were used to establish a personalized physical activity (PA) threshold for each patient in the first 3 weeks after ICD implantation. Thereafter, the ICD logged PA when the personalized PA threshold was exceeded. The mean baseline PA level was 128.9 minutes/day.
At 3 years’ follow-up, one-quarter of the patients had died and half had been hospitalized for HF. Of the total population, only 3.2% participated in CR.
Compared with nonparticipants, CR participants were more likely to be White (91.0% versus 87.3%), male (75.5% versus 72.2%), and to have diabetes (48.8% versus 44.1%), ischemic heart disease (91.4% versus 82.1%), or congestive heart failure (90.4% versus 83.4%).
CR participants attended a median of 24 sessions, during which time daily PA increased by a mean of 9.7 minutes per day. During the same time, PA decreased by a mean of 1.0 minute per day in non-CR participants (P < .001).
PA levels remained “relatively constant” for the first 36 months of follow-up among CR participants before showing a steep decline, whereas levels gradually declined throughout follow-up among nonparticipants, with a median annual change of –4.5 min/day.
In adjusted analysis, every 10 minutes of increased daily PA was associated with a 1.1% reduced risk for death (hazard ratio, 0.989; 95% confidence interval, 0.979-0.996) and a 1% reduced risk for HF hospitalization (HR, 0.99; 95% CI, 0.986-0.995) at 1-year follow-up (P < .001).
After propensity score was used to match CR participants with nonparticipants by demographic characteristics, comorbidities, and baseline PA level, CR participants had a significantly lower risk for death at 1 year (HR, 0.76; 95% CI, 0.69-0.85). This difference in risk remained at 2- and 3-year follow-ups.
However, when the researchers further adjusted for change in PA during CR or the same time period after device implantation, no differences in mortality were found between CR participants and nonparticipants at 1 year (HR, 1.00; 95% CI, 0.82-1.21) or at 2 or 3 years.
The risk for HF hospitalization did not differ between the two groups in either propensity score model.
Unlike wearable devices, implanted devices “don’t give that type of feedback to patients regarding PA levels – only to providers – and it will be interesting to discover whether providing feedback to patients can motivate them to do more physical activity,” Dr. Atwater commented.
The team is currently enrolling patients in a follow-up trial, in which patients will be given feedback from their ICD “to move these data from an interesting observation to something that can drive outcomes,” he said.
Commenting for this news organization, Melissa Tracy, MD, Rush University Medical Center, Chicago, said the study reiterates the “profound” underutilization of CR.
“Only about 3% of patients who should have qualified for cardiac rehabilitation actually attended, which is startling considering that it has class 1A level of evidence supporting its use,” she said.
Dr. Tracy, who is also a member of the American College of Cardiology’s Prevention of Cardiovascular Disease Section Leadership Council, described the study as “another notch in the belt of positive outcomes supporting the need for cardiac rehabilitation” and emphasizing the importance of a home-based alternative.
“One of the reasons women, minorities, and older patients don’t go to cardiac rehabilitation is they have to get there, rely on someone to drive them, or they have other responsibilities – especially women, who are often primary caretakers of others,” she said. “For women and men, the pressure to get back to work and support their families means they don’t have the luxury to go to cardiac rehabilitation.”
Dr. Tracy noted that home-based CR is covered by CMS until the end of 2021. “An important take-home is for providers and patients to understand that they do have a home-based option,” she stated.
Limitations of the study are that only 24% of patients were women, only 6% were Black, and the results might not be generalizable to patients younger than 65 years, note Dr. Atwater and colleagues. Also, previous implantation might have protected the cohort from experiencing arrhythmic death, and it remains unclear if similar results would be obtained in patients without a previous ICD.
This research was funded through the unrestricted Abbott Medical-Duke Health Strategic Alliance Research Grant. Dr. Atwater receives significant research support from Boston Scientific and Abbott Medical, and modest honoraria from Abbott Medical, Medtronic, and Biotronik. Coauthor disclosures are listed in the paper. Dr. Tracy has created cardiac prevention programs with Virtual Health Partners (VHP) and owns the intellectual property and consults with VHP but receives no monetary compensation.
A version of this article first appeared on Medscape.com.
Small increases in daily physical activity are associated with a boost in 1-year survival in patients with heart failure and coronary disease who received an implantable cardioverter defibrillator (ICD), new research suggests.
“Our study looked at how much exercise was necessary for a better outcome in patients with prior ICD implantation and, for every 10 minutes of exercise, we saw a 1% reduction in the likelihood of death or hospitalization, which is a pretty profound impact on outcome for just a small amount of additional physical activity per day,” lead author Brett Atwater, MD, told this news organization.
“These improvements were achieved outside of a formal cardiac rehabilitation program, suggesting that the benefits of increased physical activity obtained in cardiac rehabilitation programs may also be achievable at home,” he said.
Cardiac rehabilitation (CR) programs have been shown to improve short- and long-term outcomes in patients with heart failure (HF) but continue to be underutilized, especially by women, the elderly, and minorities. Home-based CR could help overcome this limitation but the science behind it is relatively new, noted Dr. Atwater, director of electrophysiology and electrophysiology research, Inova Heart and Vascular Institute, Fairfax, Va.
As reported in Circulation Cardiovascular Quality and Outcomes, the study involved 41,731 Medicare beneficiaries (mean age, 73.5 years) who received an ICD from 2014 to 2016.
ICD heart rate and activity sensor measurements were used to establish a personalized physical activity (PA) threshold for each patient in the first 3 weeks after ICD implantation. Thereafter, the ICD logged PA when the personalized PA threshold was exceeded. The mean baseline PA level was 128.9 minutes/day.
At 3 years’ follow-up, one-quarter of the patients had died and half had been hospitalized for HF. Of the total population, only 3.2% participated in CR.
Compared with nonparticipants, CR participants were more likely to be White (91.0% versus 87.3%), male (75.5% versus 72.2%), and to have diabetes (48.8% versus 44.1%), ischemic heart disease (91.4% versus 82.1%), or congestive heart failure (90.4% versus 83.4%).
CR participants attended a median of 24 sessions, during which time daily PA increased by a mean of 9.7 minutes per day. During the same time, PA decreased by a mean of 1.0 minute per day in non-CR participants (P < .001).
PA levels remained “relatively constant” for the first 36 months of follow-up among CR participants before showing a steep decline, whereas levels gradually declined throughout follow-up among nonparticipants, with a median annual change of –4.5 min/day.
In adjusted analysis, every 10 minutes of increased daily PA was associated with a 1.1% reduced risk for death (hazard ratio, 0.989; 95% confidence interval, 0.979-0.996) and a 1% reduced risk for HF hospitalization (HR, 0.99; 95% CI, 0.986-0.995) at 1-year follow-up (P < .001).
After propensity score was used to match CR participants with nonparticipants by demographic characteristics, comorbidities, and baseline PA level, CR participants had a significantly lower risk for death at 1 year (HR, 0.76; 95% CI, 0.69-0.85). This difference in risk remained at 2- and 3-year follow-ups.
However, when the researchers further adjusted for change in PA during CR or the same time period after device implantation, no differences in mortality were found between CR participants and nonparticipants at 1 year (HR, 1.00; 95% CI, 0.82-1.21) or at 2 or 3 years.
The risk for HF hospitalization did not differ between the two groups in either propensity score model.
Unlike wearable devices, implanted devices “don’t give that type of feedback to patients regarding PA levels – only to providers – and it will be interesting to discover whether providing feedback to patients can motivate them to do more physical activity,” Dr. Atwater commented.
The team is currently enrolling patients in a follow-up trial, in which patients will be given feedback from their ICD “to move these data from an interesting observation to something that can drive outcomes,” he said.
Commenting for this news organization, Melissa Tracy, MD, Rush University Medical Center, Chicago, said the study reiterates the “profound” underutilization of CR.
“Only about 3% of patients who should have qualified for cardiac rehabilitation actually attended, which is startling considering that it has class 1A level of evidence supporting its use,” she said.
Dr. Tracy, who is also a member of the American College of Cardiology’s Prevention of Cardiovascular Disease Section Leadership Council, described the study as “another notch in the belt of positive outcomes supporting the need for cardiac rehabilitation” and emphasizing the importance of a home-based alternative.
“One of the reasons women, minorities, and older patients don’t go to cardiac rehabilitation is they have to get there, rely on someone to drive them, or they have other responsibilities – especially women, who are often primary caretakers of others,” she said. “For women and men, the pressure to get back to work and support their families means they don’t have the luxury to go to cardiac rehabilitation.”
Dr. Tracy noted that home-based CR is covered by CMS until the end of 2021. “An important take-home is for providers and patients to understand that they do have a home-based option,” she stated.
Limitations of the study are that only 24% of patients were women, only 6% were Black, and the results might not be generalizable to patients younger than 65 years, note Dr. Atwater and colleagues. Also, previous implantation might have protected the cohort from experiencing arrhythmic death, and it remains unclear if similar results would be obtained in patients without a previous ICD.
This research was funded through the unrestricted Abbott Medical-Duke Health Strategic Alliance Research Grant. Dr. Atwater receives significant research support from Boston Scientific and Abbott Medical, and modest honoraria from Abbott Medical, Medtronic, and Biotronik. Coauthor disclosures are listed in the paper. Dr. Tracy has created cardiac prevention programs with Virtual Health Partners (VHP) and owns the intellectual property and consults with VHP but receives no monetary compensation.
A version of this article first appeared on Medscape.com.
Small increases in daily physical activity are associated with a boost in 1-year survival in patients with heart failure and coronary disease who received an implantable cardioverter defibrillator (ICD), new research suggests.
“Our study looked at how much exercise was necessary for a better outcome in patients with prior ICD implantation and, for every 10 minutes of exercise, we saw a 1% reduction in the likelihood of death or hospitalization, which is a pretty profound impact on outcome for just a small amount of additional physical activity per day,” lead author Brett Atwater, MD, told this news organization.
“These improvements were achieved outside of a formal cardiac rehabilitation program, suggesting that the benefits of increased physical activity obtained in cardiac rehabilitation programs may also be achievable at home,” he said.
Cardiac rehabilitation (CR) programs have been shown to improve short- and long-term outcomes in patients with heart failure (HF) but continue to be underutilized, especially by women, the elderly, and minorities. Home-based CR could help overcome this limitation but the science behind it is relatively new, noted Dr. Atwater, director of electrophysiology and electrophysiology research, Inova Heart and Vascular Institute, Fairfax, Va.
As reported in Circulation Cardiovascular Quality and Outcomes, the study involved 41,731 Medicare beneficiaries (mean age, 73.5 years) who received an ICD from 2014 to 2016.
ICD heart rate and activity sensor measurements were used to establish a personalized physical activity (PA) threshold for each patient in the first 3 weeks after ICD implantation. Thereafter, the ICD logged PA when the personalized PA threshold was exceeded. The mean baseline PA level was 128.9 minutes/day.
At 3 years’ follow-up, one-quarter of the patients had died and half had been hospitalized for HF. Of the total population, only 3.2% participated in CR.
Compared with nonparticipants, CR participants were more likely to be White (91.0% versus 87.3%), male (75.5% versus 72.2%), and to have diabetes (48.8% versus 44.1%), ischemic heart disease (91.4% versus 82.1%), or congestive heart failure (90.4% versus 83.4%).
CR participants attended a median of 24 sessions, during which time daily PA increased by a mean of 9.7 minutes per day. During the same time, PA decreased by a mean of 1.0 minute per day in non-CR participants (P < .001).
PA levels remained “relatively constant” for the first 36 months of follow-up among CR participants before showing a steep decline, whereas levels gradually declined throughout follow-up among nonparticipants, with a median annual change of –4.5 min/day.
In adjusted analysis, every 10 minutes of increased daily PA was associated with a 1.1% reduced risk for death (hazard ratio, 0.989; 95% confidence interval, 0.979-0.996) and a 1% reduced risk for HF hospitalization (HR, 0.99; 95% CI, 0.986-0.995) at 1-year follow-up (P < .001).
After propensity score was used to match CR participants with nonparticipants by demographic characteristics, comorbidities, and baseline PA level, CR participants had a significantly lower risk for death at 1 year (HR, 0.76; 95% CI, 0.69-0.85). This difference in risk remained at 2- and 3-year follow-ups.
However, when the researchers further adjusted for change in PA during CR or the same time period after device implantation, no differences in mortality were found between CR participants and nonparticipants at 1 year (HR, 1.00; 95% CI, 0.82-1.21) or at 2 or 3 years.
The risk for HF hospitalization did not differ between the two groups in either propensity score model.
Unlike wearable devices, implanted devices “don’t give that type of feedback to patients regarding PA levels – only to providers – and it will be interesting to discover whether providing feedback to patients can motivate them to do more physical activity,” Dr. Atwater commented.
The team is currently enrolling patients in a follow-up trial, in which patients will be given feedback from their ICD “to move these data from an interesting observation to something that can drive outcomes,” he said.
Commenting for this news organization, Melissa Tracy, MD, Rush University Medical Center, Chicago, said the study reiterates the “profound” underutilization of CR.
“Only about 3% of patients who should have qualified for cardiac rehabilitation actually attended, which is startling considering that it has class 1A level of evidence supporting its use,” she said.
Dr. Tracy, who is also a member of the American College of Cardiology’s Prevention of Cardiovascular Disease Section Leadership Council, described the study as “another notch in the belt of positive outcomes supporting the need for cardiac rehabilitation” and emphasizing the importance of a home-based alternative.
“One of the reasons women, minorities, and older patients don’t go to cardiac rehabilitation is they have to get there, rely on someone to drive them, or they have other responsibilities – especially women, who are often primary caretakers of others,” she said. “For women and men, the pressure to get back to work and support their families means they don’t have the luxury to go to cardiac rehabilitation.”
Dr. Tracy noted that home-based CR is covered by CMS until the end of 2021. “An important take-home is for providers and patients to understand that they do have a home-based option,” she stated.
Limitations of the study are that only 24% of patients were women, only 6% were Black, and the results might not be generalizable to patients younger than 65 years, note Dr. Atwater and colleagues. Also, previous implantation might have protected the cohort from experiencing arrhythmic death, and it remains unclear if similar results would be obtained in patients without a previous ICD.
This research was funded through the unrestricted Abbott Medical-Duke Health Strategic Alliance Research Grant. Dr. Atwater receives significant research support from Boston Scientific and Abbott Medical, and modest honoraria from Abbott Medical, Medtronic, and Biotronik. Coauthor disclosures are listed in the paper. Dr. Tracy has created cardiac prevention programs with Virtual Health Partners (VHP) and owns the intellectual property and consults with VHP but receives no monetary compensation.
A version of this article first appeared on Medscape.com.
Direct oral anticoagulants: Competition brought no cost relief
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.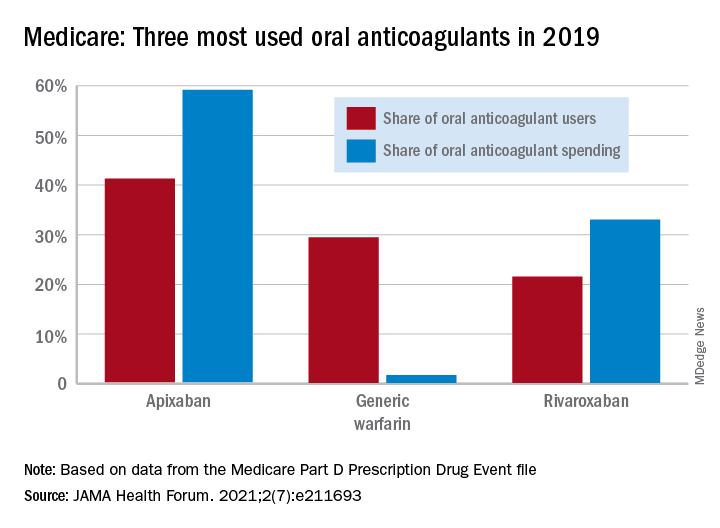
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
FROM JAMA HEALTH FORUM
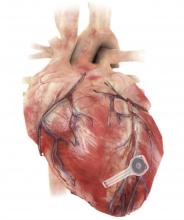
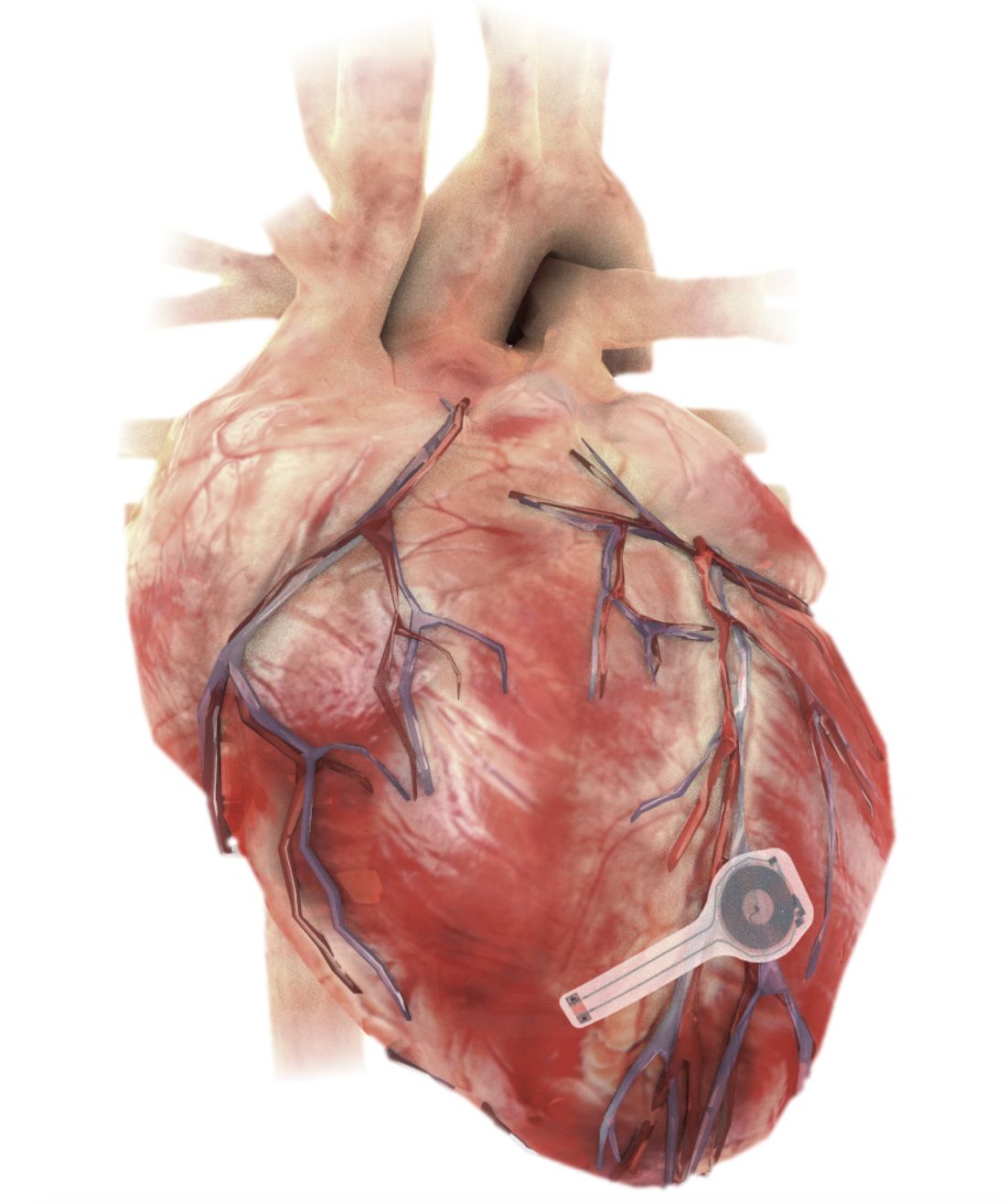
Dissolving pacemaker impressive in early research
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
Five risk factors may predict thrombus on LAA occlusion implants
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
Drinking coffee not linked to increased arrhythmia risk in new study
In fact, an adjusted analysis found that “each additional cup of coffee intake was associated with a 3% lower risk of incident arrhythmia,” Eun-jeong Kim, MD, of the division of cardiology at the University of California, San Francisco, and colleagues reported in JAMA Internal Medicine.
In addition, genetic differences that affect caffeine metabolism did not significantly influence the odds of arrhythmias, the researchers found.
Still, these findings should not necessarily encourage people to start drinking coffee if they don’t already, or to guzzle additional cups with abandon, they said.
“We certainly don’t want to say drink coffee and it will reduce your risk of arrhythmias,” study author Gregory M. Marcus, MD, MAS, associate chief of cardiology for research at UCSF Health, said in an interview. “But rather, we think the main point is that a blanket prohibition against coffee or caffeine to reduce the risk of arrhythmias among patients who have a diagnosis of arrhythmias is likely unwarranted. And given some evidence that coffee consumption may actually have other benefits regarding diabetes, mood, and perhaps overall mortality, it may be problematic to admonish patients to avoid coffee or caffeine when it is not really warranted.”
Methods and results
The conventional wisdom that caffeine increases arrhythmic risk has not been well substantiated. To further examine whether moderate, habitual coffee drinking relates to arrhythmia risk, and whether certain genetic variants influence the association, Dr. Kim and colleagues analyzed data from the UK Biobank. They focused on longitudinal data collected between 2006 and 2018 from 386,258 people who did not have a prior diagnosis of arrhythmia.
Participants had an average age of 56 years, and about 52% were female. They provided information about their coffee consumption, and the researchers grouped the participants into eight categories based on their daily coffee intake: 0, less than 1, 1, 2, 3, 4, 5, and 6 or more cups per day.
Over an average follow-up of 4.5 years, 16,979 participants developed an incident arrhythmia. After adjusting for demographic characteristics, comorbid conditions, and lifestyle habits, the decreased risk with each cup of coffee was similar for atrial fibrillation or flutter (hazard ratio, 0.97) and supraventricular tachycardia (HR, 0.96).
Taking into account genetic variations that relate to caffeine metabolism did not modify the findings. Mendelian randomization analyses that used a polygenic score of inherited caffeine metabolism patterns “failed to provide evidence that caffeine consumption leads to a greater risk of arrhythmias,” the researchers said.
Professional society guidelines have suggested staying away from caffeinated products to reduce the risk of arrhythmia, but this guidance has “relied on assumed mechanisms and a small observational study from 1980,” the authors wrote. Subsequent research has indicated that coffee’s reputation of increasing the risk of arrhythmia may be undeserved.
“The investigators should be commended on performing a high-quality observational study to try to further understand the association between coffee consumption and arrhythmias, or the lack of one,” commented Zachary D. Goldberger, MD, MS, with the division of cardiovascular medicine at the University of Wisconsin–Madison, who was not involved in the study. “This is not a randomized, controlled trial, and coffee consumption was self-reported, but the methods employed are rigorous, despite these and other important limitations. However, we need to be extremely cautious in how we interpret these findings, and not use these data as a prescription for more coffee. It’s important to recognize that this study is not telling us to drink more coffee, or start drinking coffee, to protect against developing arrhythmias. However, it should offer more reassurance that moderate coffee consumption is not necessarily harmful, and will not always lead to arrhythmias. This is important, given the widespread notion that coffee is universally proarrhythmic.”
A call for personalized guidance
“As the investigators note, there are definitely biologically plausible reasons how coffee and caffeine may not cause arrhythmias, and may be possibly protective in some, despite being a stimulant,” Dr. Goldberger said. “However, if your patient is reporting palpitations or symptoms of an arrhythmia, and feels they be related to coffee or caffeine, we should not use this study to tell them that coffee may not be the culprit. We need to listen to our patients, and the decision to reduce coffee consumption to reduce these symptoms needs to be personalized.”
The effect size was small, and only about 4% of the participants developed an arrhythmia, Dr. Goldberger and Rodney A. Hayward, MD, wrote in an invited commentary on the study in JAMA Internal Medicine. Dr. Hayward is a professor of public health and internal medicine at the University of Michigan, Ann Arbor, and a senior investigator at the Ann Arbor Veterans Affairs Center for Clinical Management Research.
“Unfortunately, coffee consumption was self-reported at a single time point. Not only can this lead to recall bias, but subsequent and substantial changes in coffee consumption are also possible, including reductions due to new signs or symptoms,” they said.
No evidence that coffee ups risk for developing arrhythmias
Another recent study suggests that people may alter their coffee consumption depending on their baseline cardiovascular health, according to the commentary.
Overall, the results “strengthen the evidence that caffeine is not proarrhythmic, but they should not be taken as proving that coffee is an antiarrhythmic—this distinction is of paramount importance,” Dr. Goldberger and Dr. Hayward wrote. “Health care professionals can reassure patients that there is no evidence that drinking coffee increases the risk for developing arrhythmias. This is particularly important for the many patients with benign palpitations who are devastated when they think, or are told, that they have to stop drinking coffee. Given current evidence, this is entirely a patient-preference decision, not a medical one.”
Dr. Marcus, a cardiac electrophysiologist, sees patients with arrhythmias all the time. They tend to “come in fairly convinced that caffeine is to be avoided when they have arrhythmias,” he said. “Often, they been told by their primary care physician or their general cardiologist to avoid caffeine because they have an arrhythmia.
“What I suggest to my patients is that they feel free to go ahead and experiment and try coffee,” Dr. Marcus said.
Still, Dr. Marcus suspects that there are some individuals in whom caffeine is a trigger for the arrhythmia. But evidence indicates these cases likely are rare, and avoiding caffeine need not apply to the general population, particularly “given the potential health benefits of benefits of coffee and also, frankly, just the enhanced quality of life that people can enjoy drinking a good cup of coffee.”
The research was conducted using the UK Biobank resource, which was established by the Wellcome Trust, the Medical Research Council, the U.K. Department of Health, and the Scottish government. The UK Biobank has received funding from other agencies and foundations as well. Dr. Marcus disclosed grants from Baylis, Medtronic, and Eight Sleep outside the submitted work. In addition, he reported consulting for Johnson & Johnson and InCarda, and holding equity in InCarda. A coauthor received salary support from the National Institutes of Health during the study. Dr. Goldberger and Dr. Hayward disclosed no conflicts of interest.
In fact, an adjusted analysis found that “each additional cup of coffee intake was associated with a 3% lower risk of incident arrhythmia,” Eun-jeong Kim, MD, of the division of cardiology at the University of California, San Francisco, and colleagues reported in JAMA Internal Medicine.
In addition, genetic differences that affect caffeine metabolism did not significantly influence the odds of arrhythmias, the researchers found.
Still, these findings should not necessarily encourage people to start drinking coffee if they don’t already, or to guzzle additional cups with abandon, they said.
“We certainly don’t want to say drink coffee and it will reduce your risk of arrhythmias,” study author Gregory M. Marcus, MD, MAS, associate chief of cardiology for research at UCSF Health, said in an interview. “But rather, we think the main point is that a blanket prohibition against coffee or caffeine to reduce the risk of arrhythmias among patients who have a diagnosis of arrhythmias is likely unwarranted. And given some evidence that coffee consumption may actually have other benefits regarding diabetes, mood, and perhaps overall mortality, it may be problematic to admonish patients to avoid coffee or caffeine when it is not really warranted.”
Methods and results
The conventional wisdom that caffeine increases arrhythmic risk has not been well substantiated. To further examine whether moderate, habitual coffee drinking relates to arrhythmia risk, and whether certain genetic variants influence the association, Dr. Kim and colleagues analyzed data from the UK Biobank. They focused on longitudinal data collected between 2006 and 2018 from 386,258 people who did not have a prior diagnosis of arrhythmia.
Participants had an average age of 56 years, and about 52% were female. They provided information about their coffee consumption, and the researchers grouped the participants into eight categories based on their daily coffee intake: 0, less than 1, 1, 2, 3, 4, 5, and 6 or more cups per day.
Over an average follow-up of 4.5 years, 16,979 participants developed an incident arrhythmia. After adjusting for demographic characteristics, comorbid conditions, and lifestyle habits, the decreased risk with each cup of coffee was similar for atrial fibrillation or flutter (hazard ratio, 0.97) and supraventricular tachycardia (HR, 0.96).
Taking into account genetic variations that relate to caffeine metabolism did not modify the findings. Mendelian randomization analyses that used a polygenic score of inherited caffeine metabolism patterns “failed to provide evidence that caffeine consumption leads to a greater risk of arrhythmias,” the researchers said.
Professional society guidelines have suggested staying away from caffeinated products to reduce the risk of arrhythmia, but this guidance has “relied on assumed mechanisms and a small observational study from 1980,” the authors wrote. Subsequent research has indicated that coffee’s reputation of increasing the risk of arrhythmia may be undeserved.
“The investigators should be commended on performing a high-quality observational study to try to further understand the association between coffee consumption and arrhythmias, or the lack of one,” commented Zachary D. Goldberger, MD, MS, with the division of cardiovascular medicine at the University of Wisconsin–Madison, who was not involved in the study. “This is not a randomized, controlled trial, and coffee consumption was self-reported, but the methods employed are rigorous, despite these and other important limitations. However, we need to be extremely cautious in how we interpret these findings, and not use these data as a prescription for more coffee. It’s important to recognize that this study is not telling us to drink more coffee, or start drinking coffee, to protect against developing arrhythmias. However, it should offer more reassurance that moderate coffee consumption is not necessarily harmful, and will not always lead to arrhythmias. This is important, given the widespread notion that coffee is universally proarrhythmic.”
A call for personalized guidance
“As the investigators note, there are definitely biologically plausible reasons how coffee and caffeine may not cause arrhythmias, and may be possibly protective in some, despite being a stimulant,” Dr. Goldberger said. “However, if your patient is reporting palpitations or symptoms of an arrhythmia, and feels they be related to coffee or caffeine, we should not use this study to tell them that coffee may not be the culprit. We need to listen to our patients, and the decision to reduce coffee consumption to reduce these symptoms needs to be personalized.”
The effect size was small, and only about 4% of the participants developed an arrhythmia, Dr. Goldberger and Rodney A. Hayward, MD, wrote in an invited commentary on the study in JAMA Internal Medicine. Dr. Hayward is a professor of public health and internal medicine at the University of Michigan, Ann Arbor, and a senior investigator at the Ann Arbor Veterans Affairs Center for Clinical Management Research.
“Unfortunately, coffee consumption was self-reported at a single time point. Not only can this lead to recall bias, but subsequent and substantial changes in coffee consumption are also possible, including reductions due to new signs or symptoms,” they said.
No evidence that coffee ups risk for developing arrhythmias
Another recent study suggests that people may alter their coffee consumption depending on their baseline cardiovascular health, according to the commentary.
Overall, the results “strengthen the evidence that caffeine is not proarrhythmic, but they should not be taken as proving that coffee is an antiarrhythmic—this distinction is of paramount importance,” Dr. Goldberger and Dr. Hayward wrote. “Health care professionals can reassure patients that there is no evidence that drinking coffee increases the risk for developing arrhythmias. This is particularly important for the many patients with benign palpitations who are devastated when they think, or are told, that they have to stop drinking coffee. Given current evidence, this is entirely a patient-preference decision, not a medical one.”
Dr. Marcus, a cardiac electrophysiologist, sees patients with arrhythmias all the time. They tend to “come in fairly convinced that caffeine is to be avoided when they have arrhythmias,” he said. “Often, they been told by their primary care physician or their general cardiologist to avoid caffeine because they have an arrhythmia.
“What I suggest to my patients is that they feel free to go ahead and experiment and try coffee,” Dr. Marcus said.
Still, Dr. Marcus suspects that there are some individuals in whom caffeine is a trigger for the arrhythmia. But evidence indicates these cases likely are rare, and avoiding caffeine need not apply to the general population, particularly “given the potential health benefits of benefits of coffee and also, frankly, just the enhanced quality of life that people can enjoy drinking a good cup of coffee.”
The research was conducted using the UK Biobank resource, which was established by the Wellcome Trust, the Medical Research Council, the U.K. Department of Health, and the Scottish government. The UK Biobank has received funding from other agencies and foundations as well. Dr. Marcus disclosed grants from Baylis, Medtronic, and Eight Sleep outside the submitted work. In addition, he reported consulting for Johnson & Johnson and InCarda, and holding equity in InCarda. A coauthor received salary support from the National Institutes of Health during the study. Dr. Goldberger and Dr. Hayward disclosed no conflicts of interest.
In fact, an adjusted analysis found that “each additional cup of coffee intake was associated with a 3% lower risk of incident arrhythmia,” Eun-jeong Kim, MD, of the division of cardiology at the University of California, San Francisco, and colleagues reported in JAMA Internal Medicine.
In addition, genetic differences that affect caffeine metabolism did not significantly influence the odds of arrhythmias, the researchers found.
Still, these findings should not necessarily encourage people to start drinking coffee if they don’t already, or to guzzle additional cups with abandon, they said.
“We certainly don’t want to say drink coffee and it will reduce your risk of arrhythmias,” study author Gregory M. Marcus, MD, MAS, associate chief of cardiology for research at UCSF Health, said in an interview. “But rather, we think the main point is that a blanket prohibition against coffee or caffeine to reduce the risk of arrhythmias among patients who have a diagnosis of arrhythmias is likely unwarranted. And given some evidence that coffee consumption may actually have other benefits regarding diabetes, mood, and perhaps overall mortality, it may be problematic to admonish patients to avoid coffee or caffeine when it is not really warranted.”
Methods and results
The conventional wisdom that caffeine increases arrhythmic risk has not been well substantiated. To further examine whether moderate, habitual coffee drinking relates to arrhythmia risk, and whether certain genetic variants influence the association, Dr. Kim and colleagues analyzed data from the UK Biobank. They focused on longitudinal data collected between 2006 and 2018 from 386,258 people who did not have a prior diagnosis of arrhythmia.
Participants had an average age of 56 years, and about 52% were female. They provided information about their coffee consumption, and the researchers grouped the participants into eight categories based on their daily coffee intake: 0, less than 1, 1, 2, 3, 4, 5, and 6 or more cups per day.
Over an average follow-up of 4.5 years, 16,979 participants developed an incident arrhythmia. After adjusting for demographic characteristics, comorbid conditions, and lifestyle habits, the decreased risk with each cup of coffee was similar for atrial fibrillation or flutter (hazard ratio, 0.97) and supraventricular tachycardia (HR, 0.96).
Taking into account genetic variations that relate to caffeine metabolism did not modify the findings. Mendelian randomization analyses that used a polygenic score of inherited caffeine metabolism patterns “failed to provide evidence that caffeine consumption leads to a greater risk of arrhythmias,” the researchers said.
Professional society guidelines have suggested staying away from caffeinated products to reduce the risk of arrhythmia, but this guidance has “relied on assumed mechanisms and a small observational study from 1980,” the authors wrote. Subsequent research has indicated that coffee’s reputation of increasing the risk of arrhythmia may be undeserved.
“The investigators should be commended on performing a high-quality observational study to try to further understand the association between coffee consumption and arrhythmias, or the lack of one,” commented Zachary D. Goldberger, MD, MS, with the division of cardiovascular medicine at the University of Wisconsin–Madison, who was not involved in the study. “This is not a randomized, controlled trial, and coffee consumption was self-reported, but the methods employed are rigorous, despite these and other important limitations. However, we need to be extremely cautious in how we interpret these findings, and not use these data as a prescription for more coffee. It’s important to recognize that this study is not telling us to drink more coffee, or start drinking coffee, to protect against developing arrhythmias. However, it should offer more reassurance that moderate coffee consumption is not necessarily harmful, and will not always lead to arrhythmias. This is important, given the widespread notion that coffee is universally proarrhythmic.”
A call for personalized guidance
“As the investigators note, there are definitely biologically plausible reasons how coffee and caffeine may not cause arrhythmias, and may be possibly protective in some, despite being a stimulant,” Dr. Goldberger said. “However, if your patient is reporting palpitations or symptoms of an arrhythmia, and feels they be related to coffee or caffeine, we should not use this study to tell them that coffee may not be the culprit. We need to listen to our patients, and the decision to reduce coffee consumption to reduce these symptoms needs to be personalized.”
The effect size was small, and only about 4% of the participants developed an arrhythmia, Dr. Goldberger and Rodney A. Hayward, MD, wrote in an invited commentary on the study in JAMA Internal Medicine. Dr. Hayward is a professor of public health and internal medicine at the University of Michigan, Ann Arbor, and a senior investigator at the Ann Arbor Veterans Affairs Center for Clinical Management Research.
“Unfortunately, coffee consumption was self-reported at a single time point. Not only can this lead to recall bias, but subsequent and substantial changes in coffee consumption are also possible, including reductions due to new signs or symptoms,” they said.
No evidence that coffee ups risk for developing arrhythmias
Another recent study suggests that people may alter their coffee consumption depending on their baseline cardiovascular health, according to the commentary.
Overall, the results “strengthen the evidence that caffeine is not proarrhythmic, but they should not be taken as proving that coffee is an antiarrhythmic—this distinction is of paramount importance,” Dr. Goldberger and Dr. Hayward wrote. “Health care professionals can reassure patients that there is no evidence that drinking coffee increases the risk for developing arrhythmias. This is particularly important for the many patients with benign palpitations who are devastated when they think, or are told, that they have to stop drinking coffee. Given current evidence, this is entirely a patient-preference decision, not a medical one.”
Dr. Marcus, a cardiac electrophysiologist, sees patients with arrhythmias all the time. They tend to “come in fairly convinced that caffeine is to be avoided when they have arrhythmias,” he said. “Often, they been told by their primary care physician or their general cardiologist to avoid caffeine because they have an arrhythmia.
“What I suggest to my patients is that they feel free to go ahead and experiment and try coffee,” Dr. Marcus said.
Still, Dr. Marcus suspects that there are some individuals in whom caffeine is a trigger for the arrhythmia. But evidence indicates these cases likely are rare, and avoiding caffeine need not apply to the general population, particularly “given the potential health benefits of benefits of coffee and also, frankly, just the enhanced quality of life that people can enjoy drinking a good cup of coffee.”
The research was conducted using the UK Biobank resource, which was established by the Wellcome Trust, the Medical Research Council, the U.K. Department of Health, and the Scottish government. The UK Biobank has received funding from other agencies and foundations as well. Dr. Marcus disclosed grants from Baylis, Medtronic, and Eight Sleep outside the submitted work. In addition, he reported consulting for Johnson & Johnson and InCarda, and holding equity in InCarda. A coauthor received salary support from the National Institutes of Health during the study. Dr. Goldberger and Dr. Hayward disclosed no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
St. Jude to pay $27 million to end DOJ suit over faulty ICDs
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
CABANA: Ablation bests drugs for AFib in racial/ethnic minorities
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.