User login
ICU infections: Chlorhexidine wipes tame MRSA, CRE
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
REPORTING FROM ID WEEK 2018
Key clinical point: For high rates of MRSA and CRE in the ICU, consider decolonization instead of contact precautions.
Major finding: Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6; infection rates fell from 3.9 isolates to 2 per 10,000 patient-days.
Study details: Review of ICU quality improvement initiative
Disclosures: There was no funding for the work, and the investigators had no disclosures.
Source: Moss J et al. ID Week 2018, Abstract 32.
Point-of-care test for respiratory viruses lowers antibiotic use
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
Routine testing in the ED is advocated
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point:
Major finding: Of patients with a negative chest x-ray and low CRP level, 36% avoided hospital admission due to a positive test for a virus.
Study details: A case series.
Disclosures: Dr. Roy reports no financial relationships relevant to this study.
FDA attacks antibiotic resistance with new strategy
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
WASHINGTON – A strategy combining stewardship and science is needed to help combat antimicrobial resistance, and updated plans from the U.S. Food and Drug Administration include four key components to address all aspects of product development and use, FDA commissioner Scott Gottlieb, MD, said in a press briefing in Washington on Sept. 14.
“The FDA plays a unique role in advancing human and animal health” that provides a unique vantage point for coordinating all aspects of product development and application, he said.
The FDA’s comprehensive approach to the challenge of antimicrobial resistance (AMR) includes:
- Facilitating product development.
- Promoting antimicrobial stewardship.
- Supporting the development of new tools for surveillance.
- Advancing scientific initiatives, including research for the development of alternative treatments.
The FDA’s product development plan to combat AMR includes the creation of incentives for companies to develop new antibiotic products and create a robust pipeline, which is a challenge because of the lack of immediate economic gain, Dr. Gottlieb said.
“It necessary to change the perception that the costs and risks of antibiotic innovation are too high relative to their expected gains,” he emphasized.
Strategies to incentivize companies include fast track designation, priority review, and breakthrough therapy designation. In addition, the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) is designed to promote development of antimicrobial drugs for limited and underserved populations, Dr. Gottlieb said. The FDA plan also calls for pursuing reimbursement options with the Centers for Medicare & Medicaid Services.
Promoting antimicrobial stewardship remains an ongoing element of the FDA’s plan to reduce AMR. In conjunction with the release of the FDA’s updated approach to AMR, the FDA’s Center for Veterinary Medicine CVM released a 5-year action plan to promote and support antimicrobial stewardship in not only the agricultural arena, but in companion animals as well.
The FDA plans to bring all antimicrobials of medical importance that are approved for use in animals under the oversight of CVM, which will pursue the improve labeling on antimicrobial drugs used in the feed and water of food-producing animals, including defining durations of use, Dr. Gottlieb noted.
Supporting the development and improvement of surveillance tools is “essential to understanding the drivers of resistance in human and veterinary settings and formulating appropriate responses” to outbreaks, Dr. Gottlieb said.
To help meet this goal, the FDA will expand sampling via the National Antimicrobial Resistance Monitoring System (NARMS) database, he said. Other surveillance goals include supporting genomics research and expanding AMR monitoring to include pathogens associated with animal feed and companion animals, he added.
As part of the final component of the FDA’s AMR strategy to advance scientific initiatives, the FDA has released a new Request for Information “to obtain additional, external input on how best to develop an annual list of regulatory science initiatives specific for antimicrobial products,” Dr. Gottlieb announced. The FDA intends to use the information gained from clinicians and others in its creation of guidance documents and recommendations to streamline the antibiotic development process. He also cited the FDA’s ongoing support of partnerships with public and private organizations such as the Clinical Trials Transformation Initiative, which focuses on drug development for severe bacterial infections with current unmet medical need.
“We need to harness science and policy to help our public health systems and researchers become nimbler in the battle against drug-resistant pathogens,” Dr. Gottlieb concluded.
In a panel discussion following the briefing, several experts offered perspective on the FDA’s goals and on the challenges of AMR.
William Flynn, DVM, deputy director of science policy for the Center of Veterinary Medicine, noted some goals for reducing the use of antibiotics in the veterinary arena.
“We are trying to focus on the driver: What are the disease conditions that drive use of the product,” he said. Ideally, better management of disease conditions can reduce reliance on antibiotics, he added.
Also in the panel discussion, Steven Gitterman, MD, deputy director of the division of microbiology devices at the Center for Devices and Radiological Health, emphasized the value of sustainable trial databases so AMR research can continue on an ongoing basis. Finally, Carolyn Wilson, PhD, associate director of research at the Center for Biologics Evaluation and Research, noted that the FDA’s research and development efforts include antibiotic alternatives, including live biotherapeutic products, fecal microbiota transplantation, and bacteriophage therapy.
Visit www.fda.gov for a transcript of Dr. Gottlieb’s talk, and for the updated FDA website page with more details on the agency’s plans to combat antimicrobial resistance.
Dr. Gottlieb and the panelists had no financial conflicts to disclose.
NYC outbreak of Candida auris linked to 45% mortality
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
Mortality within 90 days of infection was 45% among 51 patients diagnosed with antibiotic-resistant Candida auris infections in a multihospital outbreak in New York City from 2012 to 2017.
Transmission is ongoing in health care facilities, primarily among patients with extensive health care exposures, according to a report published in Emerging Infectious Diseases.
“Intensive infection prevention and control efforts continue; the goals are delaying endemicity, preventing outbreaks within facilities, reducing transmission and geographic spread, and blunting the effect of C. auris in New York and the rest of the United States,” Eleanor Adams, MD, of the New York Health Department, and her colleagues wrote. “Among medically fragile patients in NYC who had a history of extensive contact with health care facilities, clinicians should include C. auris in the differential diagnosis for patients with symptoms compatible with bloodstream infection.”
In the intensive case-patient analysis conducted by the New York State Health Department, 21 cases were from seven hospitals in Brooklyn, 16 were from three hospitals and one private medical office in Queens, 12 were from five hospitals and one long-term acute care hospital in Manhattan, and 1 was from a hospital in the Bronx. The remaining clinical case was identified in a western New York hospital in a patient who had recently been admitted to an involved Brooklyn hospital.
Among these patients, 31 (61%) had resided in long-term care facilities immediately before being admitted to the hospital in which their infection was diagnosed, and 19 of these 31 resided in skilled nursing facilities with ventilator beds; 1 (2%) resided in a long-term acute care hospital; 5 (10%) had been transferred from another hospital; and 4 (8%) had traveled internationally within 5 years before diagnosis, according to the investigators.
Isolates from 50 patients (98%) were resistant to fluconazole and 13 (25%) were resistant to fluconazole and amphotericin B. No initial isolates were resistant to echinocandins, although subsequent isolates obtained from 3 persons who had received an echinocandin acquired resistance to it, according to the researchers. Whole-genome sequencing performed at The Centers for Disease Control and Prevention indicated that 50 of 51 isolates belonged to a South Asia clade; the remaining isolate was the only one susceptible to fluconazole.
The work was supported by the CDC. No disclosures were reported.
SOURCE: Adams E et al. Emerg Infect Dis. 2018 Sep 12; 24(10); ID: 18-0649.
FROM EMERGING INFECTIOUS DISEASES
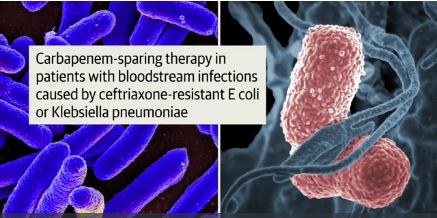
Piperacillin-tazobactam fails to outperform meropenem in bloodstream infections
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
There may be no greater present-day antibiotic resistance threat than the prospect of nonsusceptibility developing among patients with blood poisoning who receive carbapenems for infections caused by E. coli or K. pneumonia. New antibiotics are being developed and researchers are taking a second look at existing drugs.
The new study aims to shed light on the effectiveness of piperacillin-tazobactam in this population compared to carbapenems. Surprisingly, the researchers failed to show a mortality benefit for the drug vs. meropenem. What now? Future research could shed light on newer beta-lactam/beta-lactamase inhibitors, and studies may also offer insight into alternatives such as short-term antibiotic therapy. The upcoming availability of electronic decision support tools may be helpful, and it remains important to prevent infections in the first place.
This commentary was taken from an editorial by Mary K. Hayden, MD, and Sarah Y. Won, MD, MPH, of Rush University Medical Center (JAMA 2018 Sep 11;320[10]:979-81). Dr. Hayden reports research funding from Colorox and serving as an investigator on research products that received product support from Sage Corporation, Molnlycke, Clorox, OpGen and Medline.
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
A new study finds that piperacillin-tazobactam doesn’t improve mortality compared to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae. The findings were so striking that the study was ended early.

Courtesy: JAMA
“These findings do not support use of piperacillin-tazobactam in this setting,” wrote the authors. The report was published Sept. 11 in JAMA (2018;320[10]:984-94.)
According to the Centers for Disease Control and Prevention, an estimated 1,700 deaths in the United States in 2011 were caused by gram-negative bacteria that produce extended-spectrum beta-lactamase enzymes.
While carbapenems such as meropenem (Merrem) are “regarded as the treatment of choice for serious infections,” the MERINO trial (NCT02176122) authors wrote, their rising use could lead to drug resistance.
One alternate option is to embrace beta-lactam/beta-lactamase inhibitors such as piperacillin-tazobactam (Zosyn), the researchers noted, but research has produced conflicting results.
Piperacillin-tazobactam is an injected penicillin antibiotic used to treat conditions such as severe pneumonia, complicated urinary tract infections and complicated skin and soft tissue infections.
For the new study, researchers led by Patrick N. A. Harris, MBBS, of the University of Queensland, randomly assigned 188 patients to intravenous piperacillin-tazobactam (4.5 g every 6 hours) and 191 patients to meropenem (1 g every 8 hours) for 4-14 days, depending on clinician’s preference. (12 other patients did not continue with the study after initial randomization due to factors such as errors).
All patients were adults and had at least one blood test showing they were positive for E. coli or K. pneumoniae. They all had to be nonsusceptible to ceftriaxone (Rocephin) but susceptible to piperacillin-tazobactam.
The study was ceased prior to enrollment because of the risk of harm. Interim findings suggested the study was unlikely to show higher effectiveness for piperacillin-tazobactam
The primary analysis included 379 patients (mean age 67 years, 48% were women), and the primary outcome analysis included 378 patients.
A total of 23 (12.3%) of 187 patients in the piperacillin-tazobactam group died by 30 days compared to 7 (3.7%) of 191 in the meropenem group (risk difference: 8.6%, P = .90 for noninferiority).
By day 4, 68% of the piperacillin-tazobactam group and 75% of the meropenem group achieved clinical and microbiological resolution.
Serious adverse effects other than death were rare, occurring in around 3% of the piperacillin-tazobactam group and nearly 2% of the meropenem group.
The researchers note various limitations, including the unblinded nature of the study and the fact that it’s not known if extended or continuous infusions of piperacillin-tazobactam would boost the drug’s effectiveness. They also note that delays resulted in some patients initially receiving treatment with one of the study’s two drugs before being randomized to the other.
The study authors caution that it’s not clear if newer beta-lactam/beta-lactamase inhibitors agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be effective in this population.
The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures, including funding from drugmakers such as Pfizer, maker of Zosyn (through its subsidiary Wyeth) and Merrem.
SOURCE: Harris PNA et al. JAMA 2018 Sep 11;320[10]:984-94. doi: 10.1001/jama.2018.12163.
FROM JAMA
Key clinical point: Piperacillin-tazobactam isn’t a superior alternative to meropenem in patients with ceftriaxone-resistant blood poisoning caused by E. coli or K. pneumoniae.
Major finding: By 30 days, 12% of patients in the piperacillin-tazobactam group died compared to 4% of the meropenem group.
Study details: Unblinded, randomized, noninferiority trial of 379 patients with bloodstream infection caused by ceftriaxone-nonsusceptible E. coli or K. pneumoniae who received piperacillin-tazobactam (n=188) or meropenem (n = 191).
Disclosures: The study was funded by the University of Queensland, Australian Society for Antimicrobials, International Society for Chemotherapy, and National University Hospital Singapore. Various organizations funded the researchers and the study’s whole-genome sequencing. The study authors report various disclosures.
Source: Harris PNA et al. JAMA 2018 Sep 11. doi: 10.1001/jama.2018.12163.
SHM aids national infection prevention and control effort
Multidisciplinary teams celebrate achievements in getting to zero
The Society of Hospital Medicine is pleased to share successes and resources from a 3-year national quality improvement program called STRIVE (States Targeting Reduction in Infections Via Engagement). This program targeted opportunities to streamline and enhance infection prevention and control efforts in participating hospitals.
SHM was a key partner in the STRIVE program, which was managed by the Health Research & Educational Trust, the not-for-profit research and education affiliate of the American Hospital Association. Other partners included the American Society for Healthcare Engineering, Association for Professionals in Infection Control and Epidemiology, University of Michigan, Ann Arbor, and experts from academic institutions and professional societies such as Cornell University, Ithaca, N.Y.; Rush University, Chicago; and the Association for the Healthcare Environment. SHM provided specific knowledge and experience on HAI prevention and helped develop the STRIVE curriculum and resources. Faculty coaches from SHM also supported STRIVE hospitals by presenting on webinars, attending in-person meetings, and consulting on calls.
Following the U.S. experience with Ebola, the Centers for Disease Control and Infection identified the critical importance of enhancing infection control for all infectious threats to protect both patients and health care personnel. The CDC also recognized that many state and regional organizations and agencies work with the same health care facilities in order to coordinate efforts to address infectious threats. The STRIVE program provided tools and resources to help communities strengthen the relationships among diverse organizations to maximize improvement and coordination.
Closely aligned with SHM’s mission to promote exceptional care for hospitalized patients, the CDC’s STRIVE program goals were as follows:
- To expand the CDC’s Targeting Assessment for Prevention (TAP) strategy of using surveillance data to identify hospitals with a disproportionately high burden of health care–associated infections (HAIs),
- To build and strengthen relationships between state and regional organizations that help hospitals with infection control and prevention, and
- To provide technical assistance to hospitals to improve implementation of infection control practices in existing and newly constructed health care facilities.
The participants in this program included 449 hospitals from 28 states and the District of Columbia. Short-stay and long-term acute care hospitals that had a high burden of Clostridium difficile infection, and a high burden of one or more of the following HAIs – central line–associated bloodstream infection, catheter associated urinary tract infection, and health care–associated methicillin-resistant Staphylococcus aureus (MRSA) bacteremia – were targeted. Each participant had access to specific education modules, webinars, and learning networks designed to enhance collaboration, performance improvement, and understanding of the successes and barriers to coordinating hospital- and community-based services. Hospitals joined the program in cohorts and engaged in a year-long effort to reduce infection burden. During the program implementation period, many hospitals showed measurable improvement by achieving an HAI-specific relative rate reduction or maintenance of a rate of zero between baseline and intervention periods.
Key successes of the program centered around development of multidisciplinary teams that engaged not only the infection preventionists but also such areas as environmental services and other departments that may not have traditionally been included in infection prevention efforts. These teams focused on establishing competency-based trainings and processes for auditing competencies. One series of STRIVE resources helped hospitals learn new ways to implement best practices and communicate with diverse departments so every team member could participate in removing barriers to infection prevention in the hospital.
SHM was especially pleased to be a part of a program that brought together state health departments, state hospital associations, quality innovation network–quality improvement organizations, and other agencies and health systems committed to infection prevention. The collaboration and partnerships among the STRIVE program participants helped minimize duplication of work and improve efficiency and effectiveness of infection prevention efforts lead by hospitals.
To learn more about the STRIVE resources, visit www.hret.org/quality/projects/strive.shtml.
Multidisciplinary teams celebrate achievements in getting to zero
Multidisciplinary teams celebrate achievements in getting to zero
The Society of Hospital Medicine is pleased to share successes and resources from a 3-year national quality improvement program called STRIVE (States Targeting Reduction in Infections Via Engagement). This program targeted opportunities to streamline and enhance infection prevention and control efforts in participating hospitals.
SHM was a key partner in the STRIVE program, which was managed by the Health Research & Educational Trust, the not-for-profit research and education affiliate of the American Hospital Association. Other partners included the American Society for Healthcare Engineering, Association for Professionals in Infection Control and Epidemiology, University of Michigan, Ann Arbor, and experts from academic institutions and professional societies such as Cornell University, Ithaca, N.Y.; Rush University, Chicago; and the Association for the Healthcare Environment. SHM provided specific knowledge and experience on HAI prevention and helped develop the STRIVE curriculum and resources. Faculty coaches from SHM also supported STRIVE hospitals by presenting on webinars, attending in-person meetings, and consulting on calls.
Following the U.S. experience with Ebola, the Centers for Disease Control and Infection identified the critical importance of enhancing infection control for all infectious threats to protect both patients and health care personnel. The CDC also recognized that many state and regional organizations and agencies work with the same health care facilities in order to coordinate efforts to address infectious threats. The STRIVE program provided tools and resources to help communities strengthen the relationships among diverse organizations to maximize improvement and coordination.
Closely aligned with SHM’s mission to promote exceptional care for hospitalized patients, the CDC’s STRIVE program goals were as follows:
- To expand the CDC’s Targeting Assessment for Prevention (TAP) strategy of using surveillance data to identify hospitals with a disproportionately high burden of health care–associated infections (HAIs),
- To build and strengthen relationships between state and regional organizations that help hospitals with infection control and prevention, and
- To provide technical assistance to hospitals to improve implementation of infection control practices in existing and newly constructed health care facilities.
The participants in this program included 449 hospitals from 28 states and the District of Columbia. Short-stay and long-term acute care hospitals that had a high burden of Clostridium difficile infection, and a high burden of one or more of the following HAIs – central line–associated bloodstream infection, catheter associated urinary tract infection, and health care–associated methicillin-resistant Staphylococcus aureus (MRSA) bacteremia – were targeted. Each participant had access to specific education modules, webinars, and learning networks designed to enhance collaboration, performance improvement, and understanding of the successes and barriers to coordinating hospital- and community-based services. Hospitals joined the program in cohorts and engaged in a year-long effort to reduce infection burden. During the program implementation period, many hospitals showed measurable improvement by achieving an HAI-specific relative rate reduction or maintenance of a rate of zero between baseline and intervention periods.
Key successes of the program centered around development of multidisciplinary teams that engaged not only the infection preventionists but also such areas as environmental services and other departments that may not have traditionally been included in infection prevention efforts. These teams focused on establishing competency-based trainings and processes for auditing competencies. One series of STRIVE resources helped hospitals learn new ways to implement best practices and communicate with diverse departments so every team member could participate in removing barriers to infection prevention in the hospital.
SHM was especially pleased to be a part of a program that brought together state health departments, state hospital associations, quality innovation network–quality improvement organizations, and other agencies and health systems committed to infection prevention. The collaboration and partnerships among the STRIVE program participants helped minimize duplication of work and improve efficiency and effectiveness of infection prevention efforts lead by hospitals.
To learn more about the STRIVE resources, visit www.hret.org/quality/projects/strive.shtml.
The Society of Hospital Medicine is pleased to share successes and resources from a 3-year national quality improvement program called STRIVE (States Targeting Reduction in Infections Via Engagement). This program targeted opportunities to streamline and enhance infection prevention and control efforts in participating hospitals.
SHM was a key partner in the STRIVE program, which was managed by the Health Research & Educational Trust, the not-for-profit research and education affiliate of the American Hospital Association. Other partners included the American Society for Healthcare Engineering, Association for Professionals in Infection Control and Epidemiology, University of Michigan, Ann Arbor, and experts from academic institutions and professional societies such as Cornell University, Ithaca, N.Y.; Rush University, Chicago; and the Association for the Healthcare Environment. SHM provided specific knowledge and experience on HAI prevention and helped develop the STRIVE curriculum and resources. Faculty coaches from SHM also supported STRIVE hospitals by presenting on webinars, attending in-person meetings, and consulting on calls.
Following the U.S. experience with Ebola, the Centers for Disease Control and Infection identified the critical importance of enhancing infection control for all infectious threats to protect both patients and health care personnel. The CDC also recognized that many state and regional organizations and agencies work with the same health care facilities in order to coordinate efforts to address infectious threats. The STRIVE program provided tools and resources to help communities strengthen the relationships among diverse organizations to maximize improvement and coordination.
Closely aligned with SHM’s mission to promote exceptional care for hospitalized patients, the CDC’s STRIVE program goals were as follows:
- To expand the CDC’s Targeting Assessment for Prevention (TAP) strategy of using surveillance data to identify hospitals with a disproportionately high burden of health care–associated infections (HAIs),
- To build and strengthen relationships between state and regional organizations that help hospitals with infection control and prevention, and
- To provide technical assistance to hospitals to improve implementation of infection control practices in existing and newly constructed health care facilities.
The participants in this program included 449 hospitals from 28 states and the District of Columbia. Short-stay and long-term acute care hospitals that had a high burden of Clostridium difficile infection, and a high burden of one or more of the following HAIs – central line–associated bloodstream infection, catheter associated urinary tract infection, and health care–associated methicillin-resistant Staphylococcus aureus (MRSA) bacteremia – were targeted. Each participant had access to specific education modules, webinars, and learning networks designed to enhance collaboration, performance improvement, and understanding of the successes and barriers to coordinating hospital- and community-based services. Hospitals joined the program in cohorts and engaged in a year-long effort to reduce infection burden. During the program implementation period, many hospitals showed measurable improvement by achieving an HAI-specific relative rate reduction or maintenance of a rate of zero between baseline and intervention periods.
Key successes of the program centered around development of multidisciplinary teams that engaged not only the infection preventionists but also such areas as environmental services and other departments that may not have traditionally been included in infection prevention efforts. These teams focused on establishing competency-based trainings and processes for auditing competencies. One series of STRIVE resources helped hospitals learn new ways to implement best practices and communicate with diverse departments so every team member could participate in removing barriers to infection prevention in the hospital.
SHM was especially pleased to be a part of a program that brought together state health departments, state hospital associations, quality innovation network–quality improvement organizations, and other agencies and health systems committed to infection prevention. The collaboration and partnerships among the STRIVE program participants helped minimize duplication of work and improve efficiency and effectiveness of infection prevention efforts lead by hospitals.
To learn more about the STRIVE resources, visit www.hret.org/quality/projects/strive.shtml.
CDC reports Salmonella outbreak
A total of 90 people in 26 states have been infected with multidrug-resistant Salmonella in an outbreak linked to raw turkey products, according to the Centers for Disease Control and Prevention.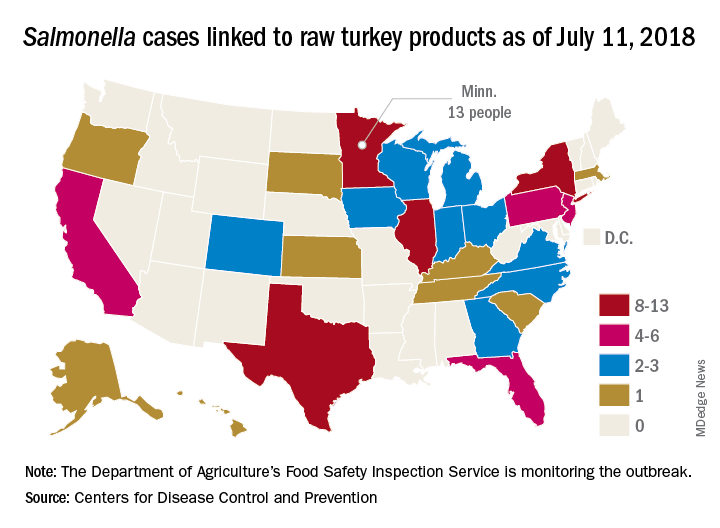
As of July 11, 2018, 40 of the 78 people with available information who were infected with the outbreak strain of Salmonella Reading have been hospitalized, but no deaths have been reported. Of the 61 ill people who have been interviewed, most have reported preparing or eating turkey products from a number of sources, although two lived in households where raw turkey was given to pets: No common supplier has been identified, the CDC reported in an investigation notice posted July 19.
The first illness in this outbreak started on Nov. 20, 2017, and the most recent one started on June 29, 2018. The U.S. Department of Agriculture’s Food Safety Inspection Service is monitoring the outbreak, and public health and regulatory agency efforts are being coordinated by the CDC through its PulseNet national subtyping network. DNA fingerprinting “performed on Salmonella from ill people in this outbreak showed that they are closely related genetically. This means that the ill people are more likely to share a common source of infection,” the CDC said.
Consumers should handle raw turkey carefully and cook it thoroughly to prevent Salmonella, the CDC advised. Raw food of any type should not be given to pets. At this time, the CDC said that it is “not advising that consumers avoid eating properly cooked turkey products, or that retailers stop selling raw turkey products.”
A total of 90 people in 26 states have been infected with multidrug-resistant Salmonella in an outbreak linked to raw turkey products, according to the Centers for Disease Control and Prevention.
As of July 11, 2018, 40 of the 78 people with available information who were infected with the outbreak strain of Salmonella Reading have been hospitalized, but no deaths have been reported. Of the 61 ill people who have been interviewed, most have reported preparing or eating turkey products from a number of sources, although two lived in households where raw turkey was given to pets: No common supplier has been identified, the CDC reported in an investigation notice posted July 19.
The first illness in this outbreak started on Nov. 20, 2017, and the most recent one started on June 29, 2018. The U.S. Department of Agriculture’s Food Safety Inspection Service is monitoring the outbreak, and public health and regulatory agency efforts are being coordinated by the CDC through its PulseNet national subtyping network. DNA fingerprinting “performed on Salmonella from ill people in this outbreak showed that they are closely related genetically. This means that the ill people are more likely to share a common source of infection,” the CDC said.
Consumers should handle raw turkey carefully and cook it thoroughly to prevent Salmonella, the CDC advised. Raw food of any type should not be given to pets. At this time, the CDC said that it is “not advising that consumers avoid eating properly cooked turkey products, or that retailers stop selling raw turkey products.”
A total of 90 people in 26 states have been infected with multidrug-resistant Salmonella in an outbreak linked to raw turkey products, according to the Centers for Disease Control and Prevention.
As of July 11, 2018, 40 of the 78 people with available information who were infected with the outbreak strain of Salmonella Reading have been hospitalized, but no deaths have been reported. Of the 61 ill people who have been interviewed, most have reported preparing or eating turkey products from a number of sources, although two lived in households where raw turkey was given to pets: No common supplier has been identified, the CDC reported in an investigation notice posted July 19.
The first illness in this outbreak started on Nov. 20, 2017, and the most recent one started on June 29, 2018. The U.S. Department of Agriculture’s Food Safety Inspection Service is monitoring the outbreak, and public health and regulatory agency efforts are being coordinated by the CDC through its PulseNet national subtyping network. DNA fingerprinting “performed on Salmonella from ill people in this outbreak showed that they are closely related genetically. This means that the ill people are more likely to share a common source of infection,” the CDC said.
Consumers should handle raw turkey carefully and cook it thoroughly to prevent Salmonella, the CDC advised. Raw food of any type should not be given to pets. At this time, the CDC said that it is “not advising that consumers avoid eating properly cooked turkey products, or that retailers stop selling raw turkey products.”
Urgent care and retail clinics fuel inappropriate antibiotic prescribing
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
This study suggests urgent care and retail clinics are “underrecognized” contributors to the ongoing problem of inappropriate antibiotic prescribing, according to authors of an invited commentary.
The urgent care sector, a $15 billion business representing more than 10,000 U.S. high-volume clinics, is growing very rapidly due to convenient locations, same-day access to care, and lower out-of-pocket expenditures versus emergency departments, the authors said.
“Lowering barriers for an office visit to such a degree may prompt frequent visits for mild self-resolving illnesses that would be better treated with rest and symptom management at home,” they wrote.
Innovations such as telephone triage lines could help reduce inappropriate antibiotic prescribing, but might “conflict with the business model” of urgent care and retail clinics, they added.
“Unfortunately, we all pay – in increased insurance premiums and increased antibiotic resistance – from the overprescribing of antibiotics for upper respiratory tract infections,” they wrote.
Michael A. Incze, MD, MSEd, and Rita F. Redberg, MD, MSc, are with the department of medicine, University of California, San Francisco. Mitchell H. Katz, MD, is with New York City Health and Hospitals. These comments are based on their invited commentary appearing in JAMA Internal Medicine . All three authors reported having no conflicts of interest.
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
When it comes to inappropriate antibiotic prescribing, urgent care centers and retail clinics may have an outsized impact, a review of commercial insurance claims suggests.
Antibiotic prescription rates were at least twice as high in those settings, compared with emergency departments and medical office visits, according to the retrospective analysis.
The issue may be particularly pronounced in urgent care centers, based on this study, in which nearly half of visits for antibiotic-inappropriate respiratory diagnoses resulted in antibiotic prescribing.
Those findings suggest a need for “antibiotic stewardship interventions” to reduce unnecessary prescribing of antibiotics in ambulatory care settings, authors of the analysis reported in a research letter to JAMA Internal Medicine.
“Efforts targeting urgent care centers are urgently needed,” wrote Danielle L. Palms, MPH, of the Centers for Disease Control and Prevention, Atlanta, and her coauthors.
The retrospective study by Ms. Palms and her colleagues included claims from 2014 in a database of individuals 65 years of age or younger with employer-sponsored insurance.
The researchers included encounters in which medical and prescription coverage data were captured, including approximately 2.7 million urgent care center visits, 58,000 retail clinic visits, 4.8 million emergency department visits, and 148.5 million medical office visits.[[{"fid":"194045","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html","field_file_image_credit[und][0][value]":"Sheep purple/flickr/CC BY 2.0 /en.wikipedia/CC BY-SA 4.0"}}}]]
They found antibiotic prescriptions linked to 39.0% of urgent care and 36.4% of retail clinic visits, compared with 13.8% of emergency department visits and 7.1% of medical office visits.
For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits, compared with 24.6% of emergency department, 17.0% of medical office visits, and 14.4% of retail clinic visits.
Those data show “substantial variability” that suggests case mix differences and evidence of antibiotic overuse, particularly in the urgent care setting, the researchers said in their letter.
In another recent study, looking at the 2010-2011 period, at least 30% of antibiotic prescriptions written in U.S. physician offices and emergency departments were unnecessary.
“The finding of the present study that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in urgent care centers suggests that unnecessary antibiotic prescribing nationally in all outpatient settings may be higher than the estimated 30%,” wrote Ms. Palms and her coinvestigators.
The research was funded by the Centers for Disease Control and Prevention. Ms. Palms and her coauthors reported no conflicts of interest.
SOURCE: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: For respiratory diagnoses where antibiotics would be inappropriate, such as viral upper respiratory infections, antibiotics were nevertheless prescribed in 45.7% of urgent care visits.
Study details: A retrospective cohort study including claims from 2014 in a database for individuals 65 years of age or younger with employer-sponsored insurance.
Disclosures: The research was funded by the Centers for Disease Control and Prevention. The authors reported no conflicts of interest.
Source: Palms DL et al. JAMA Intern Med. 2018 Jul 16.
Primary cirrhotic prophylaxis of bacterial peritonitis falls short
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
WASHINGTON –
The mortality rate during follow-up of cirrhotic patients hospitalized while on primary prophylaxis against spontaneous bacterial peritonitis (SBP) was 19%, compared with a 9% death rate among cirrhotic patients hospitalized while on secondary prophylaxis, Jasmohan S. Bajaj, MD, said at the annual Digestive Disease Week®.
Although the findings raised questions about the value of primary prophylaxis with an antibiotic in cirrhotic patients for preventing a first episode of SBP, secondary prophylaxis remains an important precaution.
“There is clear benefit from secondary prophylaxis; please use it. The data supporting it are robust,” said Dr. Bajaj, a hepatologist at Virginia Commonwealth University, Richmond. In contrast, the evidence supporting benefits from primary prophylaxis is weaker, he said. The findings were also counterintuitive, because patients who experience repeat episodes of SBP might be expected to fare worse than those hit by SBP just once.
Dr. Bajaj also acknowledged the substantial confounding that distinguishes patients with cirrhosis receiving primary or secondary prophylaxis, and the difficulty of fully adjusting for all this confounding by statistical analyses. “There is selective bias for secondary prevention, and there is no way to correct for this,” he explained. Patients who need secondary prophylaxis have “weathered the storm” of a first episode of SBP, which might have exerted selection pressure, and might have triggered important immunologic changes, Dr. Bajaj suggested.
The findings also raised concerns about the appropriateness of existing antibiotic prophylaxis for SBP. The patients included in the study all received similar regimens regardless of whether they were on primary or secondary prophylaxis. Three-quarters of primary prophylaxis patients received a fluoroquinolone, as did 81% on secondary prophylaxis. All other patients received trimethoprim-sulfamethoxazole. These regimens are aimed at preventing gram-negative infections; however, an increasing number of SBP episodes are caused by either gram-positive pathogens or strains of gram-negative bacteria or fungi resistant to standard antibiotics.
Clinicians “absolutely” need to rethink their approach to both primary and secondary prophylaxis, Dr. Bajaj said. “As fast as treatment evolves, bacteria evolve 20 times faster. We need to find ways to prevent infections without antibiotic prophylaxis, whatever that might be.”
The study used data collected prospectively from patients with cirrhosis at any of 12 U.S. and 2 Canadian centers that belonged to the North American Consortium for the Study of End-Stage Liver Disease. Among 2,731 cirrhotic patients admitted nonelectively, 492 (18%) were on antibiotic prophylaxis at the time of their admission, 305 for primary prophylaxis and 187 for secondary prophylaxis. Dr. Bajaj and his associates used both the baseline model for end-stage liver disease score and serum albumin level of each patient to focus on a group of 154 primary prophylaxis and 154 secondary prophylaxis patients who were similar by these two criteria. Despite this matching, the two subgroups showed statistically significant differences at the time of their index hospitalization for several important clinical measures.
The secondary prophylaxis patients were significantly more likely to have been hospitalized within the previous 6 months, significantly more likely to be on treatment for hepatic encephalopathy at the time of their index admission, and significantly less likely to have systemic inflammatory response syndrome on admission.
Also, at the time of admission, secondary prophylaxis patients were significantly more likely to have an infection of any type at a rate of 40%, compared with 24% among those on primary prophylaxis, as well as a significantly higher rate of SBP at 16%, compared with a 9% rate among the primary prophylaxis patients. During hospitalization, nosocomial SBP occurred significantly more often among the secondary prophylaxis patients at a rate of 6%, compared with a 0.5% rate among those on primary prophylaxis.
Despite these between-group differences, the average duration of hospitalization, and the average incidence of acute-on-chronic liver failure during follow-up out to 30 days post discharge was similar in the two subgroups. And the patients on secondary prophylaxis showed better outcomes by two important parameters: mortality during hospitalization and 30 days post discharge; and the incidence of ICU admission during hospitalization, which was significantly greater for primary prophylaxis patients at 31%, compared with 21% among the secondary prophylaxis patients, Dr. Bajaj reported.
Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
REPORTING FROM DDW 2018
Key clinical point: Antibiotic prophylaxis for bacterial peritonitis showed limitations, especially for primary prophylaxis.
Major finding: Mortality was 19% among primary prophylaxis patients and 9% among secondary prophylaxis patients during hospitalization and 30 days following.
Study details: An analysis of data from 308 cirrhotic patients on antibiotic prophylaxis at 14 North American centers.
Disclosures: Dr. Bajaj has been a consultant for Norgine and Salix Pharmaceuticals and has received research support from Grifols and Salix Pharmaceuticals.
CDC concerned about multidrug-resistant Shigella
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.