User login
More than 23% of antibiotic fills deemed unnecessary
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.
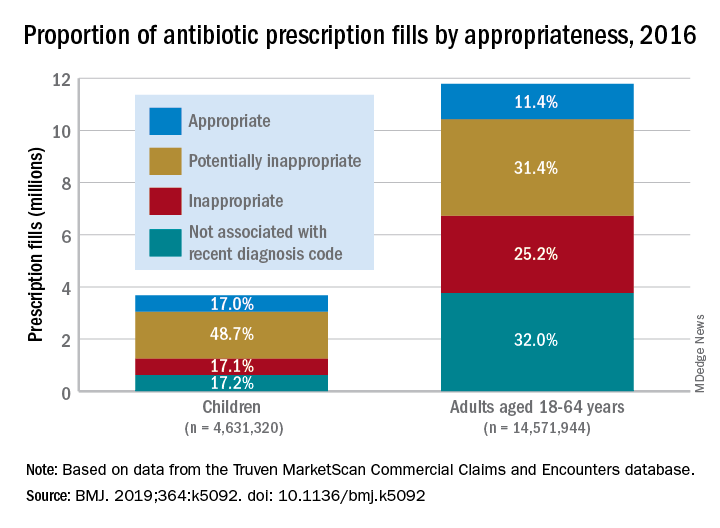
Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
FROM THE BMJ
How to assess an Antimicrobial Stewardship Program
A study compares the merits of DOT and DOTA
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
A study compares the merits of DOT and DOTA
A study compares the merits of DOT and DOTA
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
The currently recommended method for hospital antimicrobial stewardship programs (ASPs) to measure antibiotic use is Days of Therapy/1,000 patient-days, but there are a few disadvantages of using the DOT, said Maryrose Laguio-Vila, MD, coauthor of a recent study on stewardship.
“For accurate measurement, it requires information technology (IT) support to assist an ASP in generating reports of antibiotic prescriptions and administrations to patients, often from an electronic medical record (EMR). In hospitals where there is no EMR, DOT is probably not easily done and would have to be manually extracted (a herculean task),” she said. “Second, DOT tends to be an aggregate measurement of antibiotics used at an institution or hospital location; if an ASP does a specific intervention targeting a group of antibiotics or infectious indication, changes in the hospital-wide DOT or drug-class DOT may not accurately reflect the exact impact of an ASP’s intervention.”
The paper offers an alternative/supplemental method for ASPs to quantify their impact on antibiotic use without using an EMR or needing IT support: Days of Therapy Avoided. “DOTA can be tracked prospectively (or retrospectively) with each intervention an ASP makes, and calculates an exact amount of antibiotic use avoided,” Dr. Laguio-Vila said. “If the ASP also tracks the types of antibiotic recommendations made according to infectious indication, comparison of DOTA between indications – such as pneumonia versus UTI [urinary tract infection] – can lead to ideas of which type of indication needs clinical guidelines development, or order set revision, or which type of infection the ASP should target to reduce high-risk antibiotics.”
Also, she added, because most ASPs have several types of interventions at once (such as education on pneumonia guidelines, as well as penicillin-allergy assessment), aggregate assessments of institutional antibiotic use like the DOT cannot quantify how much impact a specific intervention has accomplished. DOTA may offer a fairer assessment of the direct changes in antibiotic use resulting from specific ASP activities, because tracking DOTA is extracted from each specific patient intervention.
“Now that the Joint Commission has a requirement that all hospitals seeking JC accreditation have some form of an ASP in place and measure antibiotic use in some way at their institution, there may be numerous hospitals facing the same challenges with calculating a DOT. DOTA would meet these requirements, but in a ‘low tech’ way,” Dr. Laguio-Vila said. “For hospitalists with interests in being the antibiotic steward or champion for their institution, DOTA is an option for measuring antibiotic use.”
Reference
Datta S et al. Days of therapy avoided: A novel method for measuring the impact of an antimicrobial stewardship program to stop antibiotics. J Hosp Med. 2018 Feb 8. doi: 10.12788/jhm.2927.
Designing a better EHR
Hospitals can create a more effective system
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
Hospitals can create a more effective system
Hospitals can create a more effective system
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
Be judicious with empiric antibiotics for febrile neutropenia
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
SAN FRANCISCO – Empiric antibiotic therapy for febrile neutropenia, a common and life-threatening complication of chemotherapy, hasn’t really changed much in 20 years, according to Alison Freifeld, MD, director of the section of oncology infectious diseases at the University of Nebraska, Omaha.
Antibiotic resistance has become a major problem over that time. Multidrug-resistant, gram-negative blood stream infections are not uncommon, particularly with extended-spectrum, beta-lactamase–producing Escherichia coli and Klebsiella pneumoniae. Carbapenemase-producing Enterobacteriaceae are also on the rise, among others.
“Our standard empiric antibiotics” – ceftazidime, cefepime, piperacillin/tazobactam, and carbapenems – “are generally not active against these organisms, putting us in a major dilemma about what to do” with patients who have them, Dr. Freifeld said.
“Our goal at the moment is to unpack this ship, take some of these loads of antibiotics off, and figure out how we can more effectively bridge the gap between risk factors and outcomes, with fewer and more stringently applied targeted antibiotics,” she said at ID Week, an annual scientific meeting on infectious diseases.
Dr. Freifeld shared her advice at the meeting on what to do as that plays out. The main driver is to protect the remaining potency of current antibiotics without sacrificing patient care while also keeping new options in reserve for the sickest patients, so “we do not overuse these precious commodities.”
For one thing, it’s okay to shorten treatment – traditionally around 2 weeks, until the absolute neutrophil count (ANC) tops 500 cells/mcg – once the fever abates and cultures turn negative, even if the ANC remains low.
A recent trial put the approach to the test. A total of 78 patients had their antibiotics stopped after they had been free of fever for 72 hours, with normal vital signs and no other signs of infection; 79 in the control group had usual care, continuing treatment until their ANC recovered.
Early withdrawal shortened treatment by about 3 days and there were no statistically significant differences in mortality, with one death in the short-arm group and three in the long-arm group. Over half of the patients in the short-arm group were neutropenic when antibiotics were discontinued.
Serious adverse events, meanwhile, were far less common in the short-arm group (18 vs. 38). The take-home lesson is that “interventions to shorten duration of empiric antibiotics are safe and effective and important to implement now,” Dr. Freifeld said (Lancet Haematol. 2017 Dec;4(12):e573-83).
Also, “use escalation and deescalation approaches,” she said. The basic idea is to begin with monotherapy – cefepime or piperacillin/tazobactam – in uncomplicated cases, bumped up as necessary, and, in complicated cases, to start with broad, multidrug regimens, deescalated as culture reports and other information comes in (Haematologica. 2013 Dec;98(12):1826-35).
Finally, fluoroquinolone prophylaxis, “once considered the wonder of the world,” Dr. Freifeld said, needs to be limited to the highest-risk patients, particularly those with neutropenia expected to last a week or more. It does seem to lower the rates of fever and bloodstream infections, but recent investigations have shown no mortality benefit, and fluoroquinolone prophylaxis makes patients more likely to be colonized by multidrug-resistant bacteria. Many centers have opted against it, even in higher-risk patients (J Infect. 2018 Jan;76(1):20-37).
Dr. Freifeld serves on a data adjudication committee for Merck, and reported research support from the company.
EXPERT ANALYSIS FROM IDWEEK 2018
Single-dose zoliflodacin successfully treats uncomplicated urogenital gonorrhea
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Cure rates of 96% and 100% were reported for urethral/cervical and rectal infections, respectively, and ranged from 50% to 82% in pharyngeal infections.
Study details: A randomized, open-label, phase 2 study including 181 patients with uncomplicated urogenital gonorrhea who received zoliflodacin or ceftriaxone.
Disclosures: The study authors reported disclosures related to AstraZeneca (parent company of Entasis Therapeutics, which is developing zoliflodacin) and other pharmaceutical companies.
Source: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
H. pylori antibiotic resistance reaches ‘alarming levels’
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
The first-line treatment of individuals with Helicobacter pylori infection using clarithromycin-based triple therapies or, if penicillin allergic, bismuth-based quadruple therapies is generally effective. However, reports of declining therapeutic efficacy have led to published guidelines to recommend confirmation of H. pylori eradication after completing a course of antibiotics. It is believed that increasing antibiotic use in agriculture and medicine around the globe have contributed to the increasing H. pylori antibiotic resistance and declining efficacy of standard H. pylori regimens.
Indeed, most H. pylori guidelines recommend antibiotic sensitivity testing after failing two courses of treatment; however, performing such testing successfully may require sending fresh gastric biopsy samples to an in-house H. pylori culture lab within 1 hour, which is generally not available to most clinicians. Clearly, the gap in knowledge of local antibiotic resistance could be addressed by having a readily accessible culture facility and the testing should be reimbursed by health insurance.
Single-center experiences with antibiotic sensitivity–guided salvage therapy in the United States, however, registered a lower efficacy rate of approximately 50%, which indicates that other host factors (such as gastric acidity pH less than 5.5 or body mass index greater than 30 kg/m2) may affect the minimum inhibitory concentration (MIC) of the antibiotics against H. pylori.
In order to better study the effects of these host factors relative to the effect of antibiotic resistance on therapeutic efficacy, it is critical that we practice precision medicine by determining the antibiotic sensitivity of the H. pylori strain prior to initiating the antibiotic treatment. It may be possible to achieve more than 90% therapeutic efficacy given known antibiotic sensitivities of the bacteria and optimized host factors to lower the MIC. In addition, with the increasing awareness of the importance of gut microbiota in health and disease, clinicians should strive to narrow the antibiotic coverage that will be possible if antibiotic sensitivity is known (for example, use high-dose amoxicillin and proton-pump inhibitor dual therapy).
John Y. Kao, MD, AGAF, is the current chair of the AGA Institute Council Esophageal, Gastric and Duodenal Disorders Section, a physician investigator in the University of Michigan Center for Gastrointestinal Research, and an associate professor in the department of medicine in the division of gastroenterology & hepatology and an associate program director of the GI Fellowship Program at Michigan Medicine at the University of Michigan, Ann Arbor. He has no conflicts.
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
Over the past decade, Helicobacter pylori strains have reached “alarming levels” of antimicrobial resistance worldwide, investigators reported in the November issue of Gastroenterology.
In a large meta-analysis spanning 2007-2017, H. pylori isolates showed a 15% or higher pooled prevalence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin in almost all World Health Organization (WHO) regions. “Local surveillance networks are required to select appropriate eradication regimens for each region,” concluded Alessia Savoldi, MD, of the University of Tübingen (Germany) and her associates.
Typically, the threshold of antimicrobial resistance for choosing empiric regimens is 15%, Dr. Savoldi and her associates noted. Their systematic review and meta-analysis included 178 studies comprising 66,142 isolates from 65 countries. They defined H. pylori infection as a positive histology, serology, stool antigen, urea breath test, or rapid urease test. They excluded studies of fewer than 50 isolates, studies that only reported resistance as a percentage with no denominator, studies that failed to specify time frames or clustered data over more than 3 years, and data reported in guidelines, conference presentations, or letters without formal publication.
The prevalence of primary clarithromycin resistance exceeded 15% in the WHO European Region (18%; 95% confidence interval, 16%-20%), the Eastern Mediterranean Region (33%), and the Western Pacific Region (34%) and reached 10% in the Americas and the South East Asia region. Furthermore, primary resistance to metronidazole exceeded 15% in all WHO regions, ranging from 56% in the Eastern Mediterranean Region to 23% in the Americas. Resistance to levofloxacin was at least 15% in all WHO regions except the European region (11%).
In most regions, H. pylori also accrued substantially more antimicrobial resistance over time, the investigators said. Clarithromycin resistance rose from 13% during 2006 through 2008 to 21% during 2012 through 2016 (P less than .001). Levofloxacin resistance in the Western Pacific region increased from 12% to 31% during the same two time periods (P less than .001). Several other WHO regions showed less significant trends toward increasing resistance. Multidrug resistance also rose. Resistance to both clarithromycin and metronidazole increased markedly in all WHO areas with available data, reaching 14% in the Eastern Mediterranean and Western Pacific regions and 23% in the European region.
Secondary analyses linked resistance with dramatic increases in the odds of treatment failure. For example, clarithromycin resistance conferred a sevenfold increase in the odds of treatment failure for regimens containing clarithromycin (odds ratio, 7.0; 95% CI, 5.2 to 9.3; P less than .001). Corresponding ORs were 8.2 for levofloxacin resistance, 2.5 for metronidazole resistance, and 9.4 for dual clarithromycin-metronidazole resistance.
The investigators acknowledged several limitations. Of publications in this meta-analysis, 85% represented single-center studies with limited sample sizes, they wrote. Studies often excluded demographic and endoscopic details. Furthermore, only three studies provided prevalence data for the WHO Africa Region and these only provided overall estimates without stratifying by resistance type.
The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
SOURCE: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007.
FROM GASTROENTEROLOGY
Key clinical point: Helicobacter pylori now shows significant levels of antibiotic resistance worldwide, complicating choices of empiric therapy.
Major finding: Primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin was 15% or more in all WHO regions except for primary clarithromycin resistance in the Americas (10%) and South East Asia (10%) and primary levofloxacin resistance in Europe (11%).
Study details: Meta-analysis of 178 studies comprising 66,142 isolates from 65 countries.
Disclosures: The German Center for Infection Research, Clinical Research Unit, and the WHO Priority List Pathogens project helped fund the work. One coinvestigator disclosed ties to RedHill Biopharma, BioGaia, and Takeda related to novel H. pylori therapies.
Source: Savoldi A et al. Gastroenterology. 2018 Nov. doi: 10.1053/j.gastro.2018.07.007
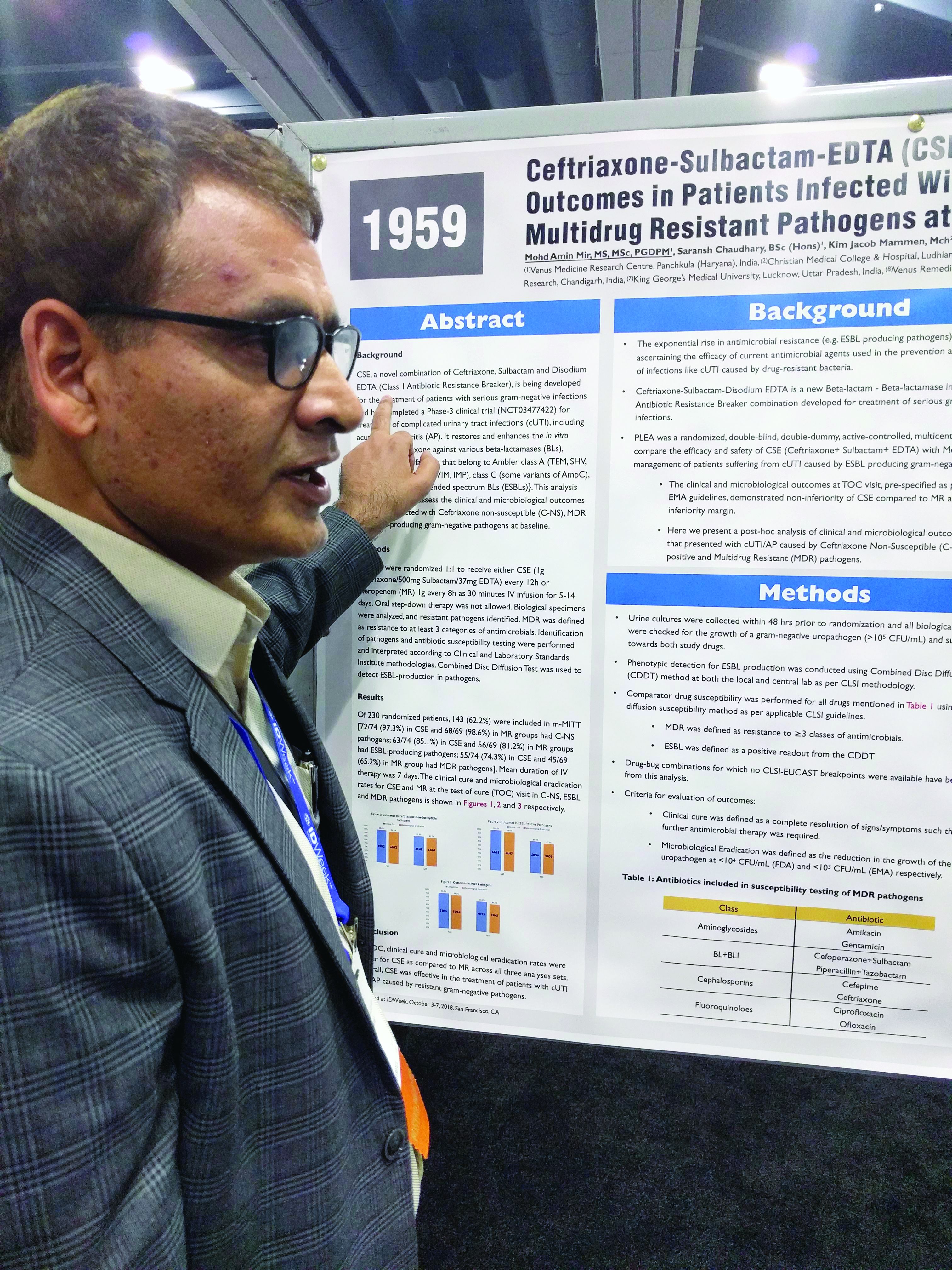
Three-drug combo proves effective against multidrug-resistant UTIs
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: The combination was noninferior in the context of different resistant subtypes.
Study details: Posthoc analysis of a randomized, controlled trial (n = 143).
Disclosures: The study was funded by Venus Medicine Research Center, which employs Dr. Mir.
Source: Mir MA et al. IDWeek 2018, Abstract 1959.
Daptomycin/fosfomycin: A new standard for MRSA bacteremia?
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
REPORTING FROM ID WEEK 2018
Key clinical point:
Major finding: At day 93% of the combination patients were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, vs. 77% on monotherapy.
Study details: Randomized, open label trial in 155 patients with MRSA bacteremia.
Disclosures: The work was funded by the Spanish government. The lead investigator said he had no relevant disclosures.
Source: Pujol M et al. 2018 ID Week, Abstract LB3
Stepdown to oral ciprofloxacin looks safe in gram-negative bloodstream infections
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
REPORTING FROM IDWEEK 2018
Key clinical point: Stepping down to oral ciprofloxacin at 48 hours is likely safe in stable patients.
Major finding: The 90-day treatment failure rate was 1.9% in patients switched to oral ciprofloxacin.
Study details: Retrospective analysis of 193 cases.
Disclosures: The study was not funded. Dr. Cook declared no financial conflicts of interest.
Source: ID Week 2018. Abstract 39.
In C. difficile, metronidazole may not benefit ICU patients on vancomycin
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
REPORTING FROM ID WEEK 2018
Key clinical point: The jury is still out on whether adding IV metronidazole helps C. difficile patients already on oral vancomycin in the ICU. Consider fecal transplant.
Major finding: Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm (P = .338).
Study details: Review of 101 ICU patients with severe C. difficile infections
Disclosures: There was no industry funding for the work, and the investigators didn’t have any disclosures.
Source: Vega AD. ID Week 2018, Abstract 488.