User login
Changes in metabolism tied to risk of subsequent dementia
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2021
‘Staggering’ increase in global dementia cases predicted by 2050
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
From AAIC 2021
Coffee and the brain: ‘Concerning’ new data
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM NUTRITIONAL NEUROSCIENCE
Remote cognitive assessments get positive mark
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
FROM AAIC 2021
Reducing air pollution is linked to slowed brain aging and lower dementia risk
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
From AAIC 2021
First guidance on appropriate use of controversial Alzheimer’s drug
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2021
Dementia caregivers benefit from telehealth support
The program combines information, education, and skills training to help participants overcome specific challenges.
“It focuses on individualized problem solving and is tailored to the needs of the person. The focus is not just on educating caregivers, but working on strategies to maintain independence in the person with dementia and support them to remain active and engaged,” said Kate Laver, PhD, who presented the study at the annual meeting of the Alzheimer’s Association International Conference. Dr. Laver is an associate professor in the College of Medicine and Public Health at Flinders University in Adelaide, South Australia.
The program is called Care of Persons with Dementia in Their Environments (COPE), and has previously been demonstrated to improve outcomes when conducted through in-person home visits. Over a maximum of ten sessions in 4 months, COPE employs occupational therapists and individuals with nursing skills to identify environmental stressors that can be modified to reduce sensory, physical, and cognitive demands. It also looks for comorbidities in the person with dementia that could be contributing to poor functioning. The goal of COPE is to encourage the person with dementia to reengage in daily activities, and to reduce caregiver burden as a result.
In a 2020 study, Dr. Laver and colleagues showed that COPE is noninferior when delivered by telehealth compared with in-person delivery. They randomized 63 caregiver-patient dyads to telehealth or home visit delivery of the COPE program. Sixty percent of the persons with dementia were male, and the mean caregiver time was 32 months.
Similar improvements in outcomes were seen in both groups, with no statistically significant differences for the primary outcome of change in Caregiver Mastery Index score at 4 months (mean difference, 0.09; 95% confidence interval, –1.26 to 1.45). Similar changes were also seen in the Perceived Change Scale, which is a 13-item caregiver questionnaire that covers day-to-day care challenges, including feeling overwhelmed or upset, sleeping patterns, and availability of personal time.
Not surprisingly, telehealth implementation led to reduced mean travel time (77.2 minutes vs. 255.9 minutes; P < .0001). The face-to-face time was shorter in the telehealth group (308 vs. 337 minutes), though the difference was not statistically significant. Dr. Laver noted that the consent rate was high at 75%, but there were some missed sessions.
Lessons learned
During the presentation, Dr. Laver emphasized some lessons learned from conversion to a telehealth model. These included providing a tablet and stand on loan, a user guide with pictures, and an initial on-site training session. The first two sessions were conducted on site to do an in-person demonstration and to assess the participants and the home environment.
She noted that it was important to have an IT support person on call to help participants use the provided tablet if needed, though this was rarely used.
“Although few people (at the time) had their own devices, they were able to quickly master videoconferencing. We felt that it was important to ensure that the first couple of consultations were in person – this enabled the therapist to develop rapport, practice use of videoconferencing, and get a good idea of the person’s environment and relationship with the person with dementia,” said Dr. Laver.
She noted that telehealth can be more efficient, and even preferred, during times like the COVID-19 pandemic, as well as in rural settings. But home visits will always be needed. “They are important for developing rapport and enabling a comprehensive assessment of the person with dementia, relationships, and environment. They are also preferred by some caregivers,” said Dr. Laver.
The demonstration of equivalence to in-person delivery was welcome, said Ingo Kilimann, MD, who comoderated the session where Dr. Laver presented. “We have to bring help to the families where they are, and not just tell them where they can get the help, because some people are just not able to actually come to some specialists’ centers. So it’s very important information that it does work,” said Dr. Kilimann, who is a dementia neurologist and head of the memory clinic at the The German Center for Neurodegenerative Diseases in Bonn.
He added that mixing of on-site and remote sessions is a good model. “I think that is the way to be most effective – to have someone in person at the person with dementia’s house, and then have online support for the rest of the time, and then it can be as successful as a total in-person intervention,” said Dr. Kilimann.
Dr. Laver had no relevant financial disclosures.
The program combines information, education, and skills training to help participants overcome specific challenges.
“It focuses on individualized problem solving and is tailored to the needs of the person. The focus is not just on educating caregivers, but working on strategies to maintain independence in the person with dementia and support them to remain active and engaged,” said Kate Laver, PhD, who presented the study at the annual meeting of the Alzheimer’s Association International Conference. Dr. Laver is an associate professor in the College of Medicine and Public Health at Flinders University in Adelaide, South Australia.
The program is called Care of Persons with Dementia in Their Environments (COPE), and has previously been demonstrated to improve outcomes when conducted through in-person home visits. Over a maximum of ten sessions in 4 months, COPE employs occupational therapists and individuals with nursing skills to identify environmental stressors that can be modified to reduce sensory, physical, and cognitive demands. It also looks for comorbidities in the person with dementia that could be contributing to poor functioning. The goal of COPE is to encourage the person with dementia to reengage in daily activities, and to reduce caregiver burden as a result.
In a 2020 study, Dr. Laver and colleagues showed that COPE is noninferior when delivered by telehealth compared with in-person delivery. They randomized 63 caregiver-patient dyads to telehealth or home visit delivery of the COPE program. Sixty percent of the persons with dementia were male, and the mean caregiver time was 32 months.
Similar improvements in outcomes were seen in both groups, with no statistically significant differences for the primary outcome of change in Caregiver Mastery Index score at 4 months (mean difference, 0.09; 95% confidence interval, –1.26 to 1.45). Similar changes were also seen in the Perceived Change Scale, which is a 13-item caregiver questionnaire that covers day-to-day care challenges, including feeling overwhelmed or upset, sleeping patterns, and availability of personal time.
Not surprisingly, telehealth implementation led to reduced mean travel time (77.2 minutes vs. 255.9 minutes; P < .0001). The face-to-face time was shorter in the telehealth group (308 vs. 337 minutes), though the difference was not statistically significant. Dr. Laver noted that the consent rate was high at 75%, but there were some missed sessions.
Lessons learned
During the presentation, Dr. Laver emphasized some lessons learned from conversion to a telehealth model. These included providing a tablet and stand on loan, a user guide with pictures, and an initial on-site training session. The first two sessions were conducted on site to do an in-person demonstration and to assess the participants and the home environment.
She noted that it was important to have an IT support person on call to help participants use the provided tablet if needed, though this was rarely used.
“Although few people (at the time) had their own devices, they were able to quickly master videoconferencing. We felt that it was important to ensure that the first couple of consultations were in person – this enabled the therapist to develop rapport, practice use of videoconferencing, and get a good idea of the person’s environment and relationship with the person with dementia,” said Dr. Laver.
She noted that telehealth can be more efficient, and even preferred, during times like the COVID-19 pandemic, as well as in rural settings. But home visits will always be needed. “They are important for developing rapport and enabling a comprehensive assessment of the person with dementia, relationships, and environment. They are also preferred by some caregivers,” said Dr. Laver.
The demonstration of equivalence to in-person delivery was welcome, said Ingo Kilimann, MD, who comoderated the session where Dr. Laver presented. “We have to bring help to the families where they are, and not just tell them where they can get the help, because some people are just not able to actually come to some specialists’ centers. So it’s very important information that it does work,” said Dr. Kilimann, who is a dementia neurologist and head of the memory clinic at the The German Center for Neurodegenerative Diseases in Bonn.
He added that mixing of on-site and remote sessions is a good model. “I think that is the way to be most effective – to have someone in person at the person with dementia’s house, and then have online support for the rest of the time, and then it can be as successful as a total in-person intervention,” said Dr. Kilimann.
Dr. Laver had no relevant financial disclosures.
The program combines information, education, and skills training to help participants overcome specific challenges.
“It focuses on individualized problem solving and is tailored to the needs of the person. The focus is not just on educating caregivers, but working on strategies to maintain independence in the person with dementia and support them to remain active and engaged,” said Kate Laver, PhD, who presented the study at the annual meeting of the Alzheimer’s Association International Conference. Dr. Laver is an associate professor in the College of Medicine and Public Health at Flinders University in Adelaide, South Australia.
The program is called Care of Persons with Dementia in Their Environments (COPE), and has previously been demonstrated to improve outcomes when conducted through in-person home visits. Over a maximum of ten sessions in 4 months, COPE employs occupational therapists and individuals with nursing skills to identify environmental stressors that can be modified to reduce sensory, physical, and cognitive demands. It also looks for comorbidities in the person with dementia that could be contributing to poor functioning. The goal of COPE is to encourage the person with dementia to reengage in daily activities, and to reduce caregiver burden as a result.
In a 2020 study, Dr. Laver and colleagues showed that COPE is noninferior when delivered by telehealth compared with in-person delivery. They randomized 63 caregiver-patient dyads to telehealth or home visit delivery of the COPE program. Sixty percent of the persons with dementia were male, and the mean caregiver time was 32 months.
Similar improvements in outcomes were seen in both groups, with no statistically significant differences for the primary outcome of change in Caregiver Mastery Index score at 4 months (mean difference, 0.09; 95% confidence interval, –1.26 to 1.45). Similar changes were also seen in the Perceived Change Scale, which is a 13-item caregiver questionnaire that covers day-to-day care challenges, including feeling overwhelmed or upset, sleeping patterns, and availability of personal time.
Not surprisingly, telehealth implementation led to reduced mean travel time (77.2 minutes vs. 255.9 minutes; P < .0001). The face-to-face time was shorter in the telehealth group (308 vs. 337 minutes), though the difference was not statistically significant. Dr. Laver noted that the consent rate was high at 75%, but there were some missed sessions.
Lessons learned
During the presentation, Dr. Laver emphasized some lessons learned from conversion to a telehealth model. These included providing a tablet and stand on loan, a user guide with pictures, and an initial on-site training session. The first two sessions were conducted on site to do an in-person demonstration and to assess the participants and the home environment.
She noted that it was important to have an IT support person on call to help participants use the provided tablet if needed, though this was rarely used.
“Although few people (at the time) had their own devices, they were able to quickly master videoconferencing. We felt that it was important to ensure that the first couple of consultations were in person – this enabled the therapist to develop rapport, practice use of videoconferencing, and get a good idea of the person’s environment and relationship with the person with dementia,” said Dr. Laver.
She noted that telehealth can be more efficient, and even preferred, during times like the COVID-19 pandemic, as well as in rural settings. But home visits will always be needed. “They are important for developing rapport and enabling a comprehensive assessment of the person with dementia, relationships, and environment. They are also preferred by some caregivers,” said Dr. Laver.
The demonstration of equivalence to in-person delivery was welcome, said Ingo Kilimann, MD, who comoderated the session where Dr. Laver presented. “We have to bring help to the families where they are, and not just tell them where they can get the help, because some people are just not able to actually come to some specialists’ centers. So it’s very important information that it does work,” said Dr. Kilimann, who is a dementia neurologist and head of the memory clinic at the The German Center for Neurodegenerative Diseases in Bonn.
He added that mixing of on-site and remote sessions is a good model. “I think that is the way to be most effective – to have someone in person at the person with dementia’s house, and then have online support for the rest of the time, and then it can be as successful as a total in-person intervention,” said Dr. Kilimann.
Dr. Laver had no relevant financial disclosures.
FROM AAIC 2021
More on GRADE: Cognitive deficits linked to CV risk factors in T2D
In type 2 diabetes (T2D), a greater degree of hyperlipidemia and hypertension, although not hyperglycemia, was associated with measurable cognitive impairment even among patients with only a 4-year mean disease duration, according to a substudy of the GRADE trial.
The association of these cardiovascular (CV) risk factors with impairments in cognition has been reported before, but the findings are notable because the mean duration of T2D was short in a relatively healthy study population, reported a multicenter team of investigators.
The relative impairments in cognitive function “may not be clinically significant given the very small size of the differences,” conceded the authors of this study, led by José A. Luchsinger, MD, but they are consistent with previous reports of the same association in older patients with a longer duration of diabetes. In other words, the data suggest the risk of cognitive loss from CV risk factors in T2D patients begins early.
“A potential explanation for the small differences, compared with those previously reported, is that the GRADE cohort is relatively young with a healthier cardiovascular profile and shorter diabetes duration compared with other studies,” reported the investigators, whose results were published online July 20, 2021, in Diabetes Care.
99% complete cognitive assessments
In the GRADE (Glycemia Reduction Approaches in Diabetes: Comparative Effectiveness) trial, 5,018 (99.4%) of the 5,047 enrolled patients completed a battery of cognitive assessments at baseline. Patients were excluded from this study if they had any major CV event in the previous year, if they had T2D for more than 10 years, if they had significant renal impairment, and if they had any history of stage 3 or greater heart failure. Their mean age was 56.7 years.
By cross-sectional analysis, cognitive evaluations, including the Digit Symbol Substitution Test (DSST) and the Spanish English Verbal Learning Test, were evaluated in relation to baseline LDL cholesterol levels, systolic and diastolic blood pressure, hemoglobin A1c, and statin use.
Unlike previous studies in T2D patients, no relationship was observed between cognitive function and A1c level at baseline. However, LDL cholesterol greater than 100 mg/dL was associated with cognitive impairment as measured with the DSST after adjustment for age, sex, education, and general health. The mean difference relative to LDL cholesterol below 70 mg/dL was only 1.8 points, but this was highly significant (P < .001).
Similarly, significant but modest cognitive impairment on DSST score after adjustment for variables were seen for those with a systolic BP between 120 mg and <140 mg relative to either <120 mm Hg or at least 140 mm Hg (P = .014). The same was seen for diastolic BPs of 80 to <90 when compared with either <80 mm Hg or to 90 mm Hg or higher (P = .01).
For those taking statins versus no statins at baseline, there was a 1.4-point mean advantage in DSST score after adjusting for variables (P < .001).
Modest cognitive impairments recorded
Again, the absolute mean differences in the DSST cognitive scores, despite their statistical significance, were modest, according to the authors. In general, the mean difference was rarely greater than 2.0 points and often 1.0 point or less. The authors acknowledged that these changes are of an uncertain clinical significance, but they considered the findings consistent with the association of CV risk factors with cognitive deficits in older T2DM patients or T2DM patients with longer duration of disease.
One difference between this GRADE substudy and previous studies was the lack of an association between cognitive impairment and hyperglycemia. In the ACCORD trial for example, increased levels of blood glycemia were associated with lower performance on numerous tests of cognitive function.
In the Diabetes Control and Complications Trial (DCCT), poorer glycemic control was related to poorer performance on tests of executive function.
Both of those studies also linked hypertension and hyperlipidemia with cognitive deficits, but given that patients in ACCORD had T2DM of substantially longer duration and those in DCCT were older, “it seems reasonable to speculate that, in patients with diabetes duration of less than 10 years, the association between hyperglycemia and cognitive performance may not yet be evident,” the GRADE authors reported.
GRADE trial compares drugs in four classes
The GRADE trial was conducted to compare four classes of T2D therapies for long-term glycemic control as expressed by A1c control over time. The results of the trial, presented recently at the 2021 annual scientific sessions of the American Diabetes Association, found that insulin glargine and the glucagonlike peptide–1 receptor agonist liraglutide performed best on the primary endpoint of maintaining A1c below 7.0%. Both performed significantly better than the sulfonylurea glimepiride and the dipeptidyl peptidase–4 inhibitor sitagliptin.
This substudy of baseline cognitive function in the relatively large GRADE trial provided a unique opportunity to evaluate the impact of CV risk factors in patients with T2D of relatively short duration.
While the data support the adverse impact of inadequately controlled modifiable risk factors on cognitive function in T2D patients, David R. Matthews, DPhil, BM, BCh, emeritus professor of diabetes medicine at the University of Oxford (England), noted that the association was weak and advised a cautious interpretation.
“The effect size is very small indeed. The data are found as a subset of multiple testing,” he said in an interview. He suggested the associations might be the result of “data farming,” and he emphasized that the relationships between these risk factors and cognitive deficits are associations that do not imply causation.
Nevertheless, and despite their unclear clinical implications, Dr. Matthews said that these data might still have a message.
“It is another reminder that for many reasons we all need to be alert to the need for lowering hyperlipidemia and hypertension to normal levels – the benefits may not just be limited to cardiovascular outcome,” Dr. Matthews stated.
The lead author of the study, Dr. Luchsinger, also cautioned against overinterpreting the data.
While the data show that “lipid and blood pressure control within recommended guidelines are associated with marginally better cognitive function in patients with type 2 diabetes of less than 5 years duration on average,” he added that “the study is limited by its cross-sectional nature.”
He indicated that further analysis will be helpful in assessing the implications.
“Longitudinal analyses of the same group of individuals will be conducted next year,” noted Dr. Luchsinger, associate professor of medicine and epidemiology, Columbia University Medical Center, New York.
Dr. Luchsinger reported financial relationships with vTv therapeutics. Dr. Matthews reported no potential conflicts of interest.
In type 2 diabetes (T2D), a greater degree of hyperlipidemia and hypertension, although not hyperglycemia, was associated with measurable cognitive impairment even among patients with only a 4-year mean disease duration, according to a substudy of the GRADE trial.
The association of these cardiovascular (CV) risk factors with impairments in cognition has been reported before, but the findings are notable because the mean duration of T2D was short in a relatively healthy study population, reported a multicenter team of investigators.
The relative impairments in cognitive function “may not be clinically significant given the very small size of the differences,” conceded the authors of this study, led by José A. Luchsinger, MD, but they are consistent with previous reports of the same association in older patients with a longer duration of diabetes. In other words, the data suggest the risk of cognitive loss from CV risk factors in T2D patients begins early.
“A potential explanation for the small differences, compared with those previously reported, is that the GRADE cohort is relatively young with a healthier cardiovascular profile and shorter diabetes duration compared with other studies,” reported the investigators, whose results were published online July 20, 2021, in Diabetes Care.
99% complete cognitive assessments
In the GRADE (Glycemia Reduction Approaches in Diabetes: Comparative Effectiveness) trial, 5,018 (99.4%) of the 5,047 enrolled patients completed a battery of cognitive assessments at baseline. Patients were excluded from this study if they had any major CV event in the previous year, if they had T2D for more than 10 years, if they had significant renal impairment, and if they had any history of stage 3 or greater heart failure. Their mean age was 56.7 years.
By cross-sectional analysis, cognitive evaluations, including the Digit Symbol Substitution Test (DSST) and the Spanish English Verbal Learning Test, were evaluated in relation to baseline LDL cholesterol levels, systolic and diastolic blood pressure, hemoglobin A1c, and statin use.
Unlike previous studies in T2D patients, no relationship was observed between cognitive function and A1c level at baseline. However, LDL cholesterol greater than 100 mg/dL was associated with cognitive impairment as measured with the DSST after adjustment for age, sex, education, and general health. The mean difference relative to LDL cholesterol below 70 mg/dL was only 1.8 points, but this was highly significant (P < .001).
Similarly, significant but modest cognitive impairment on DSST score after adjustment for variables were seen for those with a systolic BP between 120 mg and <140 mg relative to either <120 mm Hg or at least 140 mm Hg (P = .014). The same was seen for diastolic BPs of 80 to <90 when compared with either <80 mm Hg or to 90 mm Hg or higher (P = .01).
For those taking statins versus no statins at baseline, there was a 1.4-point mean advantage in DSST score after adjusting for variables (P < .001).
Modest cognitive impairments recorded
Again, the absolute mean differences in the DSST cognitive scores, despite their statistical significance, were modest, according to the authors. In general, the mean difference was rarely greater than 2.0 points and often 1.0 point or less. The authors acknowledged that these changes are of an uncertain clinical significance, but they considered the findings consistent with the association of CV risk factors with cognitive deficits in older T2DM patients or T2DM patients with longer duration of disease.
One difference between this GRADE substudy and previous studies was the lack of an association between cognitive impairment and hyperglycemia. In the ACCORD trial for example, increased levels of blood glycemia were associated with lower performance on numerous tests of cognitive function.
In the Diabetes Control and Complications Trial (DCCT), poorer glycemic control was related to poorer performance on tests of executive function.
Both of those studies also linked hypertension and hyperlipidemia with cognitive deficits, but given that patients in ACCORD had T2DM of substantially longer duration and those in DCCT were older, “it seems reasonable to speculate that, in patients with diabetes duration of less than 10 years, the association between hyperglycemia and cognitive performance may not yet be evident,” the GRADE authors reported.
GRADE trial compares drugs in four classes
The GRADE trial was conducted to compare four classes of T2D therapies for long-term glycemic control as expressed by A1c control over time. The results of the trial, presented recently at the 2021 annual scientific sessions of the American Diabetes Association, found that insulin glargine and the glucagonlike peptide–1 receptor agonist liraglutide performed best on the primary endpoint of maintaining A1c below 7.0%. Both performed significantly better than the sulfonylurea glimepiride and the dipeptidyl peptidase–4 inhibitor sitagliptin.
This substudy of baseline cognitive function in the relatively large GRADE trial provided a unique opportunity to evaluate the impact of CV risk factors in patients with T2D of relatively short duration.
While the data support the adverse impact of inadequately controlled modifiable risk factors on cognitive function in T2D patients, David R. Matthews, DPhil, BM, BCh, emeritus professor of diabetes medicine at the University of Oxford (England), noted that the association was weak and advised a cautious interpretation.
“The effect size is very small indeed. The data are found as a subset of multiple testing,” he said in an interview. He suggested the associations might be the result of “data farming,” and he emphasized that the relationships between these risk factors and cognitive deficits are associations that do not imply causation.
Nevertheless, and despite their unclear clinical implications, Dr. Matthews said that these data might still have a message.
“It is another reminder that for many reasons we all need to be alert to the need for lowering hyperlipidemia and hypertension to normal levels – the benefits may not just be limited to cardiovascular outcome,” Dr. Matthews stated.
The lead author of the study, Dr. Luchsinger, also cautioned against overinterpreting the data.
While the data show that “lipid and blood pressure control within recommended guidelines are associated with marginally better cognitive function in patients with type 2 diabetes of less than 5 years duration on average,” he added that “the study is limited by its cross-sectional nature.”
He indicated that further analysis will be helpful in assessing the implications.
“Longitudinal analyses of the same group of individuals will be conducted next year,” noted Dr. Luchsinger, associate professor of medicine and epidemiology, Columbia University Medical Center, New York.
Dr. Luchsinger reported financial relationships with vTv therapeutics. Dr. Matthews reported no potential conflicts of interest.
In type 2 diabetes (T2D), a greater degree of hyperlipidemia and hypertension, although not hyperglycemia, was associated with measurable cognitive impairment even among patients with only a 4-year mean disease duration, according to a substudy of the GRADE trial.
The association of these cardiovascular (CV) risk factors with impairments in cognition has been reported before, but the findings are notable because the mean duration of T2D was short in a relatively healthy study population, reported a multicenter team of investigators.
The relative impairments in cognitive function “may not be clinically significant given the very small size of the differences,” conceded the authors of this study, led by José A. Luchsinger, MD, but they are consistent with previous reports of the same association in older patients with a longer duration of diabetes. In other words, the data suggest the risk of cognitive loss from CV risk factors in T2D patients begins early.
“A potential explanation for the small differences, compared with those previously reported, is that the GRADE cohort is relatively young with a healthier cardiovascular profile and shorter diabetes duration compared with other studies,” reported the investigators, whose results were published online July 20, 2021, in Diabetes Care.
99% complete cognitive assessments
In the GRADE (Glycemia Reduction Approaches in Diabetes: Comparative Effectiveness) trial, 5,018 (99.4%) of the 5,047 enrolled patients completed a battery of cognitive assessments at baseline. Patients were excluded from this study if they had any major CV event in the previous year, if they had T2D for more than 10 years, if they had significant renal impairment, and if they had any history of stage 3 or greater heart failure. Their mean age was 56.7 years.
By cross-sectional analysis, cognitive evaluations, including the Digit Symbol Substitution Test (DSST) and the Spanish English Verbal Learning Test, were evaluated in relation to baseline LDL cholesterol levels, systolic and diastolic blood pressure, hemoglobin A1c, and statin use.
Unlike previous studies in T2D patients, no relationship was observed between cognitive function and A1c level at baseline. However, LDL cholesterol greater than 100 mg/dL was associated with cognitive impairment as measured with the DSST after adjustment for age, sex, education, and general health. The mean difference relative to LDL cholesterol below 70 mg/dL was only 1.8 points, but this was highly significant (P < .001).
Similarly, significant but modest cognitive impairment on DSST score after adjustment for variables were seen for those with a systolic BP between 120 mg and <140 mg relative to either <120 mm Hg or at least 140 mm Hg (P = .014). The same was seen for diastolic BPs of 80 to <90 when compared with either <80 mm Hg or to 90 mm Hg or higher (P = .01).
For those taking statins versus no statins at baseline, there was a 1.4-point mean advantage in DSST score after adjusting for variables (P < .001).
Modest cognitive impairments recorded
Again, the absolute mean differences in the DSST cognitive scores, despite their statistical significance, were modest, according to the authors. In general, the mean difference was rarely greater than 2.0 points and often 1.0 point or less. The authors acknowledged that these changes are of an uncertain clinical significance, but they considered the findings consistent with the association of CV risk factors with cognitive deficits in older T2DM patients or T2DM patients with longer duration of disease.
One difference between this GRADE substudy and previous studies was the lack of an association between cognitive impairment and hyperglycemia. In the ACCORD trial for example, increased levels of blood glycemia were associated with lower performance on numerous tests of cognitive function.
In the Diabetes Control and Complications Trial (DCCT), poorer glycemic control was related to poorer performance on tests of executive function.
Both of those studies also linked hypertension and hyperlipidemia with cognitive deficits, but given that patients in ACCORD had T2DM of substantially longer duration and those in DCCT were older, “it seems reasonable to speculate that, in patients with diabetes duration of less than 10 years, the association between hyperglycemia and cognitive performance may not yet be evident,” the GRADE authors reported.
GRADE trial compares drugs in four classes
The GRADE trial was conducted to compare four classes of T2D therapies for long-term glycemic control as expressed by A1c control over time. The results of the trial, presented recently at the 2021 annual scientific sessions of the American Diabetes Association, found that insulin glargine and the glucagonlike peptide–1 receptor agonist liraglutide performed best on the primary endpoint of maintaining A1c below 7.0%. Both performed significantly better than the sulfonylurea glimepiride and the dipeptidyl peptidase–4 inhibitor sitagliptin.
This substudy of baseline cognitive function in the relatively large GRADE trial provided a unique opportunity to evaluate the impact of CV risk factors in patients with T2D of relatively short duration.
While the data support the adverse impact of inadequately controlled modifiable risk factors on cognitive function in T2D patients, David R. Matthews, DPhil, BM, BCh, emeritus professor of diabetes medicine at the University of Oxford (England), noted that the association was weak and advised a cautious interpretation.
“The effect size is very small indeed. The data are found as a subset of multiple testing,” he said in an interview. He suggested the associations might be the result of “data farming,” and he emphasized that the relationships between these risk factors and cognitive deficits are associations that do not imply causation.
Nevertheless, and despite their unclear clinical implications, Dr. Matthews said that these data might still have a message.
“It is another reminder that for many reasons we all need to be alert to the need for lowering hyperlipidemia and hypertension to normal levels – the benefits may not just be limited to cardiovascular outcome,” Dr. Matthews stated.
The lead author of the study, Dr. Luchsinger, also cautioned against overinterpreting the data.
While the data show that “lipid and blood pressure control within recommended guidelines are associated with marginally better cognitive function in patients with type 2 diabetes of less than 5 years duration on average,” he added that “the study is limited by its cross-sectional nature.”
He indicated that further analysis will be helpful in assessing the implications.
“Longitudinal analyses of the same group of individuals will be conducted next year,” noted Dr. Luchsinger, associate professor of medicine and epidemiology, Columbia University Medical Center, New York.
Dr. Luchsinger reported financial relationships with vTv therapeutics. Dr. Matthews reported no potential conflicts of interest.
FROM DIABETES CARE
Common parasite now tied to impaired cognitive function
Investigators reviewed and conducted a meta-analysis of 13 studies that encompassed more than 13,000 healthy adults and found a modest but significant association between T. gondii seropositivity and impaired performance on cognitive tests of processing speed, working memory, short-term verbal memory, and executive function. The average age of the persons in the studies was close to 50 years.
“Our findings show that T. gondii could have a negative but small effect on cognition,” study investigator Arjen Sutterland, MD, of the Amsterdam Neuroscience Research Institute and the Amsterdam Institute for Infection and Immunity, University of Amsterdam, said in an interview.
The study was published online July 14, 2021, in JAMA Psychiatry.
Mental illness link
T. gondii is “an intracellular parasite that produces quiescent infection in approximately 30% of humans worldwide,” the authors wrote. The parasite that causes the infection not only settles in muscle and liver tissue but also can cross the blood-brain barrier and settle quiescently in brain tissue. It can be spread through contact with cat feces or by consuming contaminated meat.
Previous research has shown that neurocognitive changes associated with toxoplasmosis can occur in humans, and meta-analyses suggest an association with neuropsychiatric disorders. Some research has also tied T. gondii infection to increased motor vehicle crashes and suicide attempts.
Dr. Sutterland said he had been inspired by the work of E. Fuller Torrey and Bob Yolken, who proposed the connection between T. gondii and schizophrenia.
Some years ago, Dr. Sutterland and his group analyzed the mental health consequences of T. gondii infection and found “several interesting associations,” but they were unable to “rule out reverse causation – i.e., people with mental health disorders more often get these infections – as well as determine the impact on the population of this common infection.”
For the current study, the investigators analyzed studies that examined specifically cognitive functioning in otherwise healthy individuals in relation to T. gondii infection, “because reverse causation would be less likely in this population and a grasp of global impact would become more clear.”
The researchers conducted a literature search of studies conducted through June 7, 2019, that analyzed cognitive function among healthy participants for whom data on T. gondii seropositivity were available.
A total of 13 studies (n = 13,289 participants; mean age, 46.7 years; 49.6% male) were used in the review and meta-analysis. Some of the studies enrolled a healthy population sample; other studies compared participants with and those without psychiatric disorders. From these, the researchers extracted only the data concerning healthy participants.
The studies analyzed four cognitive domains: processing speed, working memory, short-term verbal memory, and executive functioning.
All cognitive domains affected
Of all the participants, 22.6% had antibodies against T. gondii.
Participants who were seropositive for T. gondii had less favorable functioning in all cognitive domains, with “small but significant” differences. 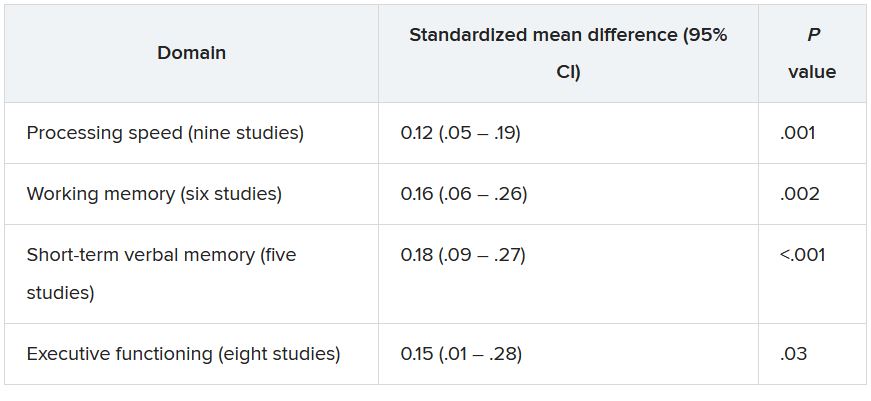
The researchers conducted a meta-regression analysis of mean age in the analysis of executive functioning and found greater effect sizes as age increased (Q = 6.17; R2 = 81%; P = .01).
The studies were of “high quality,” and there was “little suggestion of publication bias was detected,” the authors noted.
“Although the extent of the associations was modest, the ubiquitous prevalence of the quiescent infection worldwide ... suggests that the consequences for cognitive function of the population as a whole may be substantial, although it is difficult to quantify the global impact,” they wrote.
They note that because the studies were cross-sectional in nature, causality cannot be established.
Nevertheless, Dr. Sutterland suggested several possible mechanisms through which T. gondii might affect neurocognition.
“We know the parasite forms cysts in the brain and can influence dopaminergic neurotransmission, which, in turn, affects neurocognition. Alternatively, it is also possible that the immune response to the infection in the brain causes cognitive impairment. This remains an important question to explore further,” he said.
He noted that clinicians can reassure patients who test positive for T. gondii that although the infection can have a negative impact on cognition, the effect is “small.”
Prevention programs warranted
Commenting on the study in an interview, Shawn D. Gale, PhD, associate professor, department of psychology and neuroscience center, Brigham Young University, Provo, Utah, called it a “great meta-analysis.” He noted that his group is researching the subject and has obtained similar findings. A big plus is that the researchers assessed several cognitive domains, not just one.
Although the data showed “mild effects,” the findings could be important on a population level. Because 30% of the world’s population are seropositive for T. gondii, a potentially large number of people are at risk for cognitive impairment, noted Dr. Gale, who was not involved with the study.
“If you look at the United States, perhaps 10%-15% of people might test positive [for T. gondii], but in Germany and France, the number comes closer to 50%, and in other places in the world – especially countries that have a harder time economically – the rates are even higher. So if it can affect cognition, even a small effect is a big deal,” Dr. Gale said.
“I think prevention will be the most important thing, and perhaps down the road, I hope that a vaccine will be considered,” Dr. Gale added.
“These findings indicate that primary prevention of the infection could have substantial global impact on mental health” and that public health programs to prevent T. gondii “are warranted.”
These programs might consist of hygienic measures, especially after human contact with contaminated sources, as well as research into vaccine development.
No source of funding for the study was listed. The authors and Dr. Gale reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators reviewed and conducted a meta-analysis of 13 studies that encompassed more than 13,000 healthy adults and found a modest but significant association between T. gondii seropositivity and impaired performance on cognitive tests of processing speed, working memory, short-term verbal memory, and executive function. The average age of the persons in the studies was close to 50 years.
“Our findings show that T. gondii could have a negative but small effect on cognition,” study investigator Arjen Sutterland, MD, of the Amsterdam Neuroscience Research Institute and the Amsterdam Institute for Infection and Immunity, University of Amsterdam, said in an interview.
The study was published online July 14, 2021, in JAMA Psychiatry.
Mental illness link
T. gondii is “an intracellular parasite that produces quiescent infection in approximately 30% of humans worldwide,” the authors wrote. The parasite that causes the infection not only settles in muscle and liver tissue but also can cross the blood-brain barrier and settle quiescently in brain tissue. It can be spread through contact with cat feces or by consuming contaminated meat.
Previous research has shown that neurocognitive changes associated with toxoplasmosis can occur in humans, and meta-analyses suggest an association with neuropsychiatric disorders. Some research has also tied T. gondii infection to increased motor vehicle crashes and suicide attempts.
Dr. Sutterland said he had been inspired by the work of E. Fuller Torrey and Bob Yolken, who proposed the connection between T. gondii and schizophrenia.
Some years ago, Dr. Sutterland and his group analyzed the mental health consequences of T. gondii infection and found “several interesting associations,” but they were unable to “rule out reverse causation – i.e., people with mental health disorders more often get these infections – as well as determine the impact on the population of this common infection.”
For the current study, the investigators analyzed studies that examined specifically cognitive functioning in otherwise healthy individuals in relation to T. gondii infection, “because reverse causation would be less likely in this population and a grasp of global impact would become more clear.”
The researchers conducted a literature search of studies conducted through June 7, 2019, that analyzed cognitive function among healthy participants for whom data on T. gondii seropositivity were available.
A total of 13 studies (n = 13,289 participants; mean age, 46.7 years; 49.6% male) were used in the review and meta-analysis. Some of the studies enrolled a healthy population sample; other studies compared participants with and those without psychiatric disorders. From these, the researchers extracted only the data concerning healthy participants.
The studies analyzed four cognitive domains: processing speed, working memory, short-term verbal memory, and executive functioning.
All cognitive domains affected
Of all the participants, 22.6% had antibodies against T. gondii.
Participants who were seropositive for T. gondii had less favorable functioning in all cognitive domains, with “small but significant” differences. 
The researchers conducted a meta-regression analysis of mean age in the analysis of executive functioning and found greater effect sizes as age increased (Q = 6.17; R2 = 81%; P = .01).
The studies were of “high quality,” and there was “little suggestion of publication bias was detected,” the authors noted.
“Although the extent of the associations was modest, the ubiquitous prevalence of the quiescent infection worldwide ... suggests that the consequences for cognitive function of the population as a whole may be substantial, although it is difficult to quantify the global impact,” they wrote.
They note that because the studies were cross-sectional in nature, causality cannot be established.
Nevertheless, Dr. Sutterland suggested several possible mechanisms through which T. gondii might affect neurocognition.
“We know the parasite forms cysts in the brain and can influence dopaminergic neurotransmission, which, in turn, affects neurocognition. Alternatively, it is also possible that the immune response to the infection in the brain causes cognitive impairment. This remains an important question to explore further,” he said.
He noted that clinicians can reassure patients who test positive for T. gondii that although the infection can have a negative impact on cognition, the effect is “small.”
Prevention programs warranted
Commenting on the study in an interview, Shawn D. Gale, PhD, associate professor, department of psychology and neuroscience center, Brigham Young University, Provo, Utah, called it a “great meta-analysis.” He noted that his group is researching the subject and has obtained similar findings. A big plus is that the researchers assessed several cognitive domains, not just one.
Although the data showed “mild effects,” the findings could be important on a population level. Because 30% of the world’s population are seropositive for T. gondii, a potentially large number of people are at risk for cognitive impairment, noted Dr. Gale, who was not involved with the study.
“If you look at the United States, perhaps 10%-15% of people might test positive [for T. gondii], but in Germany and France, the number comes closer to 50%, and in other places in the world – especially countries that have a harder time economically – the rates are even higher. So if it can affect cognition, even a small effect is a big deal,” Dr. Gale said.
“I think prevention will be the most important thing, and perhaps down the road, I hope that a vaccine will be considered,” Dr. Gale added.
“These findings indicate that primary prevention of the infection could have substantial global impact on mental health” and that public health programs to prevent T. gondii “are warranted.”
These programs might consist of hygienic measures, especially after human contact with contaminated sources, as well as research into vaccine development.
No source of funding for the study was listed. The authors and Dr. Gale reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators reviewed and conducted a meta-analysis of 13 studies that encompassed more than 13,000 healthy adults and found a modest but significant association between T. gondii seropositivity and impaired performance on cognitive tests of processing speed, working memory, short-term verbal memory, and executive function. The average age of the persons in the studies was close to 50 years.
“Our findings show that T. gondii could have a negative but small effect on cognition,” study investigator Arjen Sutterland, MD, of the Amsterdam Neuroscience Research Institute and the Amsterdam Institute for Infection and Immunity, University of Amsterdam, said in an interview.
The study was published online July 14, 2021, in JAMA Psychiatry.
Mental illness link
T. gondii is “an intracellular parasite that produces quiescent infection in approximately 30% of humans worldwide,” the authors wrote. The parasite that causes the infection not only settles in muscle and liver tissue but also can cross the blood-brain barrier and settle quiescently in brain tissue. It can be spread through contact with cat feces or by consuming contaminated meat.
Previous research has shown that neurocognitive changes associated with toxoplasmosis can occur in humans, and meta-analyses suggest an association with neuropsychiatric disorders. Some research has also tied T. gondii infection to increased motor vehicle crashes and suicide attempts.
Dr. Sutterland said he had been inspired by the work of E. Fuller Torrey and Bob Yolken, who proposed the connection between T. gondii and schizophrenia.
Some years ago, Dr. Sutterland and his group analyzed the mental health consequences of T. gondii infection and found “several interesting associations,” but they were unable to “rule out reverse causation – i.e., people with mental health disorders more often get these infections – as well as determine the impact on the population of this common infection.”
For the current study, the investigators analyzed studies that examined specifically cognitive functioning in otherwise healthy individuals in relation to T. gondii infection, “because reverse causation would be less likely in this population and a grasp of global impact would become more clear.”
The researchers conducted a literature search of studies conducted through June 7, 2019, that analyzed cognitive function among healthy participants for whom data on T. gondii seropositivity were available.
A total of 13 studies (n = 13,289 participants; mean age, 46.7 years; 49.6% male) were used in the review and meta-analysis. Some of the studies enrolled a healthy population sample; other studies compared participants with and those without psychiatric disorders. From these, the researchers extracted only the data concerning healthy participants.
The studies analyzed four cognitive domains: processing speed, working memory, short-term verbal memory, and executive functioning.
All cognitive domains affected
Of all the participants, 22.6% had antibodies against T. gondii.
Participants who were seropositive for T. gondii had less favorable functioning in all cognitive domains, with “small but significant” differences. 
The researchers conducted a meta-regression analysis of mean age in the analysis of executive functioning and found greater effect sizes as age increased (Q = 6.17; R2 = 81%; P = .01).
The studies were of “high quality,” and there was “little suggestion of publication bias was detected,” the authors noted.
“Although the extent of the associations was modest, the ubiquitous prevalence of the quiescent infection worldwide ... suggests that the consequences for cognitive function of the population as a whole may be substantial, although it is difficult to quantify the global impact,” they wrote.
They note that because the studies were cross-sectional in nature, causality cannot be established.
Nevertheless, Dr. Sutterland suggested several possible mechanisms through which T. gondii might affect neurocognition.
“We know the parasite forms cysts in the brain and can influence dopaminergic neurotransmission, which, in turn, affects neurocognition. Alternatively, it is also possible that the immune response to the infection in the brain causes cognitive impairment. This remains an important question to explore further,” he said.
He noted that clinicians can reassure patients who test positive for T. gondii that although the infection can have a negative impact on cognition, the effect is “small.”
Prevention programs warranted
Commenting on the study in an interview, Shawn D. Gale, PhD, associate professor, department of psychology and neuroscience center, Brigham Young University, Provo, Utah, called it a “great meta-analysis.” He noted that his group is researching the subject and has obtained similar findings. A big plus is that the researchers assessed several cognitive domains, not just one.
Although the data showed “mild effects,” the findings could be important on a population level. Because 30% of the world’s population are seropositive for T. gondii, a potentially large number of people are at risk for cognitive impairment, noted Dr. Gale, who was not involved with the study.
“If you look at the United States, perhaps 10%-15% of people might test positive [for T. gondii], but in Germany and France, the number comes closer to 50%, and in other places in the world – especially countries that have a harder time economically – the rates are even higher. So if it can affect cognition, even a small effect is a big deal,” Dr. Gale said.
“I think prevention will be the most important thing, and perhaps down the road, I hope that a vaccine will be considered,” Dr. Gale added.
“These findings indicate that primary prevention of the infection could have substantial global impact on mental health” and that public health programs to prevent T. gondii “are warranted.”
These programs might consist of hygienic measures, especially after human contact with contaminated sources, as well as research into vaccine development.
No source of funding for the study was listed. The authors and Dr. Gale reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatric genomics has a diversity problem
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.













