User login
Survey Finds Mental Health Issues Increased After Cosmetic Procedure Complications
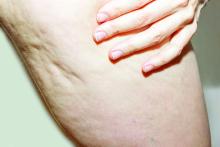
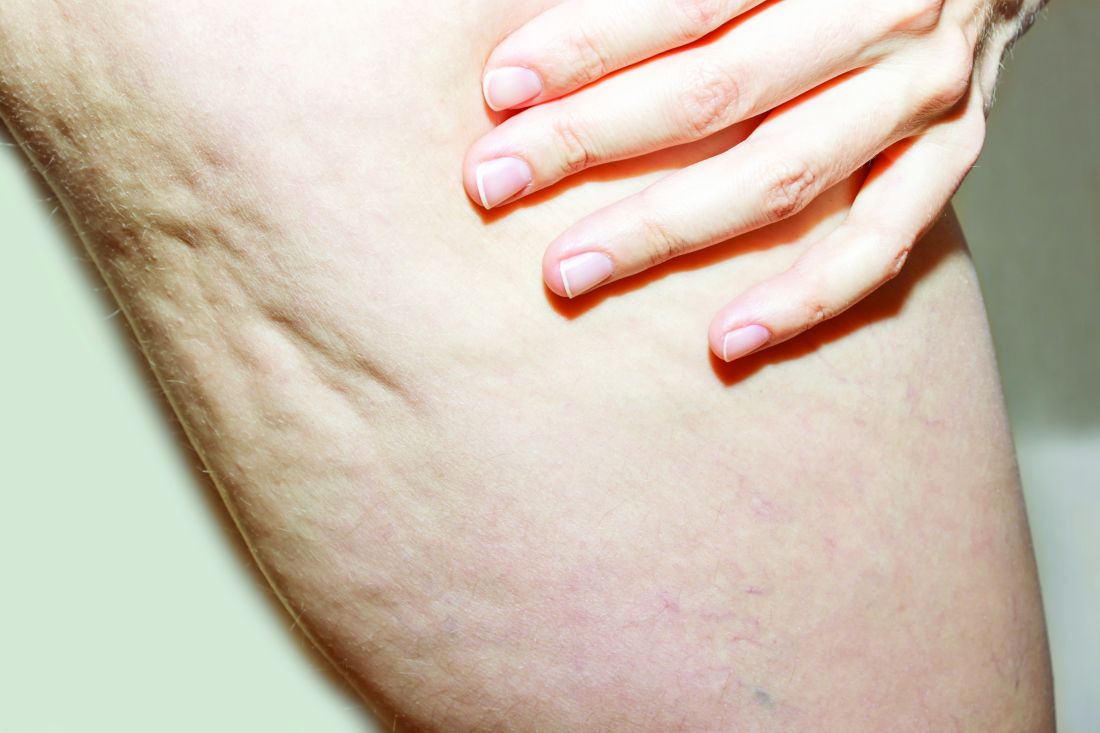
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
FROM ASLMS 2024
Association Calls For Increased Oversight in Response to Reports of Possibly Counterfeit Botulinum Toxin
, including medical spas.
In a press release issued on April 12, the ASDSA referenced investigations in Illinois and Tennessee in which suspected counterfeit neurotoxins were associated with individuals’ symptoms resembling botulism, including several that required hospitalization. These cases “emphasize the patient safety risks associated with receiving medical procedures in unlicensed, unapproved settings without proper oversight of medical care,” the release adds.
The cases also “highlight the need for increased public protection measures, like the recommendations in the ASDSA’s “Medical Spa Safety Act” to ensure patients’ safety,” according to the press release, which notes the increasing demand for facial fillers and neuromodulators in the United States.
Enforcement is needed to ensure that all patients receive US Food and Drug Administration (FDA)-approved products “and not counterfeit products or unsafe treatments,” ASDSA president Seth L. Matarasso, MD, who practices dermatology in San Francisco, said in the press release. “Lack of regulation and enforcement has enabled many to offer medical procedures for cosmetic purposes outside of their training and expertise,” he said.
Key Takeaways
All clinicians need to understand that aesthetic procedures are medical procedures and require a level of due diligence in patient evaluation and care before, during, and after the procedure, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview.
“FDA-approved medications should only be offered, and these should be obtained through well-defined sources to ensure their safety and purity,” she said.
However, some challenges to the enforcement of safety in medical spa settings persist, Dr. Sodha told this news organization. “To my knowledge, state and federal policies providing clear and up-to-date safety and legal guidelines for aesthetic procedures performed at medical spas by registered nurses, nurse practitioners, physician assistants, and physicians are limited, and in our current medical care structure, national oversight is challenging,” she said.
A pretreatment checklist assessment, she suggested, could be helpful “to ensure patient safety and help to standardize clinical practice in nonmedical settings.”
Other challenges include a lack of clear guidelines for aesthetic providers regarding initial assessment examinations, postprocedure follow-up, and evaluation for any future medical treatment, as well as “continued ambiguity on the exact meaning of physician oversight for those sites that delegate aesthetic services and appropriate and clear guidelines on what procedures require a licensed provider to perform versus oversee the treatment,” she said.
Additional Guidance, Actions Needed
As for additional guidance or actions, “we may be migrating towards a system that designates and assigns clearer licenses and authorizations to perform these services and care for patients,” said Dr. Sodha. A licensing process would entail academic understanding of anatomy, pharmacology, and tissue interactions, as well as practical hands-on training that emphasizes the importance of the preprocedure consultation and postprocedure follow-up and care, she said. “Experience in caring for the unintended outcomes is vital to delivering the best care we can,” she added.
D. Sodha had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, including medical spas.
In a press release issued on April 12, the ASDSA referenced investigations in Illinois and Tennessee in which suspected counterfeit neurotoxins were associated with individuals’ symptoms resembling botulism, including several that required hospitalization. These cases “emphasize the patient safety risks associated with receiving medical procedures in unlicensed, unapproved settings without proper oversight of medical care,” the release adds.
The cases also “highlight the need for increased public protection measures, like the recommendations in the ASDSA’s “Medical Spa Safety Act” to ensure patients’ safety,” according to the press release, which notes the increasing demand for facial fillers and neuromodulators in the United States.
Enforcement is needed to ensure that all patients receive US Food and Drug Administration (FDA)-approved products “and not counterfeit products or unsafe treatments,” ASDSA president Seth L. Matarasso, MD, who practices dermatology in San Francisco, said in the press release. “Lack of regulation and enforcement has enabled many to offer medical procedures for cosmetic purposes outside of their training and expertise,” he said.
Key Takeaways
All clinicians need to understand that aesthetic procedures are medical procedures and require a level of due diligence in patient evaluation and care before, during, and after the procedure, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview.
“FDA-approved medications should only be offered, and these should be obtained through well-defined sources to ensure their safety and purity,” she said.
However, some challenges to the enforcement of safety in medical spa settings persist, Dr. Sodha told this news organization. “To my knowledge, state and federal policies providing clear and up-to-date safety and legal guidelines for aesthetic procedures performed at medical spas by registered nurses, nurse practitioners, physician assistants, and physicians are limited, and in our current medical care structure, national oversight is challenging,” she said.
A pretreatment checklist assessment, she suggested, could be helpful “to ensure patient safety and help to standardize clinical practice in nonmedical settings.”
Other challenges include a lack of clear guidelines for aesthetic providers regarding initial assessment examinations, postprocedure follow-up, and evaluation for any future medical treatment, as well as “continued ambiguity on the exact meaning of physician oversight for those sites that delegate aesthetic services and appropriate and clear guidelines on what procedures require a licensed provider to perform versus oversee the treatment,” she said.
Additional Guidance, Actions Needed
As for additional guidance or actions, “we may be migrating towards a system that designates and assigns clearer licenses and authorizations to perform these services and care for patients,” said Dr. Sodha. A licensing process would entail academic understanding of anatomy, pharmacology, and tissue interactions, as well as practical hands-on training that emphasizes the importance of the preprocedure consultation and postprocedure follow-up and care, she said. “Experience in caring for the unintended outcomes is vital to delivering the best care we can,” she added.
D. Sodha had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, including medical spas.
In a press release issued on April 12, the ASDSA referenced investigations in Illinois and Tennessee in which suspected counterfeit neurotoxins were associated with individuals’ symptoms resembling botulism, including several that required hospitalization. These cases “emphasize the patient safety risks associated with receiving medical procedures in unlicensed, unapproved settings without proper oversight of medical care,” the release adds.
The cases also “highlight the need for increased public protection measures, like the recommendations in the ASDSA’s “Medical Spa Safety Act” to ensure patients’ safety,” according to the press release, which notes the increasing demand for facial fillers and neuromodulators in the United States.
Enforcement is needed to ensure that all patients receive US Food and Drug Administration (FDA)-approved products “and not counterfeit products or unsafe treatments,” ASDSA president Seth L. Matarasso, MD, who practices dermatology in San Francisco, said in the press release. “Lack of regulation and enforcement has enabled many to offer medical procedures for cosmetic purposes outside of their training and expertise,” he said.
Key Takeaways
All clinicians need to understand that aesthetic procedures are medical procedures and require a level of due diligence in patient evaluation and care before, during, and after the procedure, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview.
“FDA-approved medications should only be offered, and these should be obtained through well-defined sources to ensure their safety and purity,” she said.
However, some challenges to the enforcement of safety in medical spa settings persist, Dr. Sodha told this news organization. “To my knowledge, state and federal policies providing clear and up-to-date safety and legal guidelines for aesthetic procedures performed at medical spas by registered nurses, nurse practitioners, physician assistants, and physicians are limited, and in our current medical care structure, national oversight is challenging,” she said.
A pretreatment checklist assessment, she suggested, could be helpful “to ensure patient safety and help to standardize clinical practice in nonmedical settings.”
Other challenges include a lack of clear guidelines for aesthetic providers regarding initial assessment examinations, postprocedure follow-up, and evaluation for any future medical treatment, as well as “continued ambiguity on the exact meaning of physician oversight for those sites that delegate aesthetic services and appropriate and clear guidelines on what procedures require a licensed provider to perform versus oversee the treatment,” she said.
Additional Guidance, Actions Needed
As for additional guidance or actions, “we may be migrating towards a system that designates and assigns clearer licenses and authorizations to perform these services and care for patients,” said Dr. Sodha. A licensing process would entail academic understanding of anatomy, pharmacology, and tissue interactions, as well as practical hands-on training that emphasizes the importance of the preprocedure consultation and postprocedure follow-up and care, she said. “Experience in caring for the unintended outcomes is vital to delivering the best care we can,” she added.
D. Sodha had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
CDC Investigating Adverse Events Related to Counterfeit, Mishandled Botulinum Toxin
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
, such as homes and spas, according to an announcement of an investigation into these reports from the Centers for Disease Control and Prevention posted online April 15.
Reactions have included blurry vision, double vision, drooping eyelids, difficult swallowing or breathing, and other symptoms of botulism.
Of the 19 individuals — all of whom identified as female and had a mean age of 39 years — 9 (60%) were hospitalized and 4 (21%) were treated with botulism antitoxin because of concerns that the botulinum toxin could have spread beyond the injection site. Also, five were tested for botulism and their results were negative.
The CDC, several state and local health departments, and the US Food and Drug Administration (FDA) are investigating these reports, according to the announcement.
States reporting these cases include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. According to the CDC summary, some of the individuals “received injections with counterfeit products or products with unverified sources. Investigation into the sources of these products is ongoing.” All but one report involved receiving botulinum toxin injections for cosmetic purposes.
Recent cases of botulism-like illnesses possibly related to counterfeit botulinum toxin reported in Illinois and Tennessee, prompted the American Society for Dermatologic Surgery Association (ASDSA) to call on states to increase oversight of medical care in all settings, including medical spas, the ASDSA announced on April 12.
The CDC summary advises clinicians to consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism near the injection site. Symptoms of botulism include blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, and muscle weakness.
For people who are considering botulinum toxin for cosmetic or medical reasons, recommendations from the CDC include asking the provider and setting, such as a clinic or spa, if they are licensed and trained to provide these injections, and to ask if the product is approved by the FDA and from a reliable source, and, “if in doubt, don’t get the injection.”
This ‘Should Never Happen’
“The report of people getting botulism from botulinum toxin injections is frightening, and should never happen,” Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, told this news organization.
These reports show “how important it is to receive botulinum toxin injections only in a medical office, and from or under the direction of a qualified, trained, and licensed individual, like a board certified dermatologist,” added Dr. Green, who practices in Rockville, Maryland. “Other types of practitioners may not adhere to the same standards of professionalism, especially not always putting patient safety first.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For cases of suspected systemic botulism, the CDC recommends calling the local or state health department for consultation and antitoxin release (as well as information on reporting adverse events). Alternatively, the 24/7 phone number for the CDC clinical botulism service is 770-488-7100.
New Tool Helps Clinicians Detect Zoom Dysmorphia in Virtual Settings
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
FROM AAD 2024
Expert Highlights Emerging Trends in Neuromodulators
SAN DIEGO — .
“This technique is more popular in Asia than it is here in the US,” Dr. Green, who practices dermatology in Coral Gables, Florida, said at the annual meeting of the American Academy of Dermatology. As opposed to intramuscular injections, “it’s an intradermal delivery, so you use numbing cream prior, and you’re injecting botulinum toxin A nearly parallel to the skin surface with the bevel of the needle up,” he said. “You want to use a precise product. It’s uncomfortable delivering volume so superficially due to the tissue distention, so I also use a massager. I inject approximately 0.05 mL to 0.1 mL per point. This does really work.”
This mode of delivery was evaluated in a prospective, double-blind, split-face study in South Korea, which enrolled 18 volunteers who received an intradermal injection of botulinum toxin A into one cheek and normal saline into the contralateral side as a control. Participants were between 30 and 54 years of age and were seen at the clinic 2, 4, 8, and 12 weeks after the injection. At each visit, investigators took photographs, used a facial analyzer to evaluate the pores and wrinkles of the infraorbital area, and used a Sebumeter to evaluate sebum secretions from both cheeks. Improvement or aggravation in skin texture was evaluated by both volunteers and clinicians on a numeric scale from –4 (severe aggravation) to +4 (marked improvement) at each visit, and following photographic review, the wrinkle score of the nasolabial fold was graded on a 5-point scale.
The researchers observed no significant effects on the wrinkles of the infraorbital area and on sebum secretion. However, on the side where botulinum toxin A was injected, there were significant improvements in the wrinkles of the nasolabial fold and skin texture, they reported. The effects on nasolabial fold wrinkles lasted 12 weeks, effects on skin texture lasted 8 weeks, and improvement in pore size was only observed at week 2, they wrote. One serious adverse event occurred: a case of facial palsy after the injection of 30 units of botulinum toxin A in one cheek. However, injection of 20 units of botulinum toxin A in one cheek was not associated with any adverse events.
“The duration of these treatments is yet to be determined, but I think this is definitely going to gain popularity in the US,” said Dr. Green, clinical assistant professor of dermatology at the University of Miami Department of Dermatology and Cutaneous Surgery.
Recently Approved Neurotoxin
He also discussed letibotulinumtoxinA-wlbg (Letybo), an injectable neurotoxin long used in South Korea, which the US Food and Drug Administration (FDA) approved for the temporary improvement in the appearance of moderate to severe glabellar (frown) lines in adults on March 4, 2024. Approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1,000 individuals in the United States and Europe.
“This is the sixth approved neurotoxin in the US,” Dr. Green said. “It is derived from the CBFC26 strain of Clostridium botulinum, and it’s a purified 900 kDa type A toxin complex with human serum albumin and sodium chloride as its excipients.” It comes in a 50-unit or 100-unit vial and requires refrigeration. “To me, the most fascinating thing about this product is that it has been the number-one selling botulinum toxin on the South Korea market for the last 5 years,” he said. “But what do we know about its characteristics?”
In a non-inferiority trial, Chinese researchers enrolled 500 patients with moderate to severe glabellar wrinkles to investigate the efficacy and safety of letibotulinumtoxinA-wlbg and onabotulinumtoxinA. Participants were randomized 3:1 to receive 20 U of letibotulinumtoxinA-wlbg or onabotulinumtoxinA and then observed them for 16 weeks. The primary endpoint was noninferiority in the proportion of study participants who received a score of 0 or 1 for glabellar wrinkles on a four-point photographic evaluation scale, as assessed by an evaluator at maximum frown at 4 weeks.
At week 4, 88.49% of participants in the letibotulinumtoxinA-wlbg arm achieved a score of 0 or 1 for glabellar wrinkles, compared with 87.39% of those in the onabotulinumtoxinA arm (P = .7469). No significant differences were observed for secondary efficacy or safety endpoints between the two treatments. “It will be interesting to see how this product does when it’s available to us,” Dr. Green said.
Another potential newcomer is ready-to-use liquid botulinum neurotoxin. RelabotulinumtoxinA is a complex, protein-free, ready-to-use liquid botulinum toxin A designed to avoid the traditional requirement to reconstitute it from powder, according to Galderma. It features a saline phosphate buffer solution, so it contains no human or animal-derived excipients, Dr. Green pointed out, and it eliminates the variability, errors, and risks associated with reconstitution.
“There was a report in the neurology literature of botulinum toxin being reconstituted with sterile water for cervical dystonia,” he noted. “When this was injected, it was excruciatingly painful, because it created an osmotic gradient within the muscle. So, if we can take a step away from human error, that would be a good thing.”
To date, Dr. Green said, four phase 3 trials of relabotulinumtoxinA involving more than 1,900 patients have been conducted in the United States and Canada evaluating its use for glabellar frown lines and lateral canthal lines, “and the data is impressive,” he said. This product is still investigational, said Dr. Green, who has not had experience injecting it in the clinical trial program.
The idea of a rapid onset botulinum toxin is also emerging. TrenibotulinumtoxinE, which is being developed by Allergan, “is similar to a type A neurotoxin,” Dr. Green said. “It inhibits neuromuscular transmission via presynaptic vesicular protein synaptosomal-associated protein (SNAP)-25 but at a different cleavage site. It has a faster onset — within one day — but a shorter duration — 3-4 weeks.”
In a dose escalation study of its use for glabellar frown lines, 80% of participants achieved a two-grade investigator-rated improvement in glabellar frown line severity at maximum frown at the highest dose. The maximum clinical effect of trenibotulinumtoxinE was seen within 24 hours and lasted between 14 and 30 days.
“The question is, if it is approved by the FDA, where would this product fit in our practices?” Dr. Green asked. “The effect is gone in 3 weeks as opposed to 4 months,” so this may be an option to recommend for someone who is reticent to try neurotoxins, he said, “or a patient who comes to you on a Friday and says, ‘I have a gala tomorrow night.’ ”
Dr. Green disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including Allergan and Galderma.
SAN DIEGO — .
“This technique is more popular in Asia than it is here in the US,” Dr. Green, who practices dermatology in Coral Gables, Florida, said at the annual meeting of the American Academy of Dermatology. As opposed to intramuscular injections, “it’s an intradermal delivery, so you use numbing cream prior, and you’re injecting botulinum toxin A nearly parallel to the skin surface with the bevel of the needle up,” he said. “You want to use a precise product. It’s uncomfortable delivering volume so superficially due to the tissue distention, so I also use a massager. I inject approximately 0.05 mL to 0.1 mL per point. This does really work.”
This mode of delivery was evaluated in a prospective, double-blind, split-face study in South Korea, which enrolled 18 volunteers who received an intradermal injection of botulinum toxin A into one cheek and normal saline into the contralateral side as a control. Participants were between 30 and 54 years of age and were seen at the clinic 2, 4, 8, and 12 weeks after the injection. At each visit, investigators took photographs, used a facial analyzer to evaluate the pores and wrinkles of the infraorbital area, and used a Sebumeter to evaluate sebum secretions from both cheeks. Improvement or aggravation in skin texture was evaluated by both volunteers and clinicians on a numeric scale from –4 (severe aggravation) to +4 (marked improvement) at each visit, and following photographic review, the wrinkle score of the nasolabial fold was graded on a 5-point scale.
The researchers observed no significant effects on the wrinkles of the infraorbital area and on sebum secretion. However, on the side where botulinum toxin A was injected, there were significant improvements in the wrinkles of the nasolabial fold and skin texture, they reported. The effects on nasolabial fold wrinkles lasted 12 weeks, effects on skin texture lasted 8 weeks, and improvement in pore size was only observed at week 2, they wrote. One serious adverse event occurred: a case of facial palsy after the injection of 30 units of botulinum toxin A in one cheek. However, injection of 20 units of botulinum toxin A in one cheek was not associated with any adverse events.
“The duration of these treatments is yet to be determined, but I think this is definitely going to gain popularity in the US,” said Dr. Green, clinical assistant professor of dermatology at the University of Miami Department of Dermatology and Cutaneous Surgery.
Recently Approved Neurotoxin
He also discussed letibotulinumtoxinA-wlbg (Letybo), an injectable neurotoxin long used in South Korea, which the US Food and Drug Administration (FDA) approved for the temporary improvement in the appearance of moderate to severe glabellar (frown) lines in adults on March 4, 2024. Approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1,000 individuals in the United States and Europe.
“This is the sixth approved neurotoxin in the US,” Dr. Green said. “It is derived from the CBFC26 strain of Clostridium botulinum, and it’s a purified 900 kDa type A toxin complex with human serum albumin and sodium chloride as its excipients.” It comes in a 50-unit or 100-unit vial and requires refrigeration. “To me, the most fascinating thing about this product is that it has been the number-one selling botulinum toxin on the South Korea market for the last 5 years,” he said. “But what do we know about its characteristics?”
In a non-inferiority trial, Chinese researchers enrolled 500 patients with moderate to severe glabellar wrinkles to investigate the efficacy and safety of letibotulinumtoxinA-wlbg and onabotulinumtoxinA. Participants were randomized 3:1 to receive 20 U of letibotulinumtoxinA-wlbg or onabotulinumtoxinA and then observed them for 16 weeks. The primary endpoint was noninferiority in the proportion of study participants who received a score of 0 or 1 for glabellar wrinkles on a four-point photographic evaluation scale, as assessed by an evaluator at maximum frown at 4 weeks.
At week 4, 88.49% of participants in the letibotulinumtoxinA-wlbg arm achieved a score of 0 or 1 for glabellar wrinkles, compared with 87.39% of those in the onabotulinumtoxinA arm (P = .7469). No significant differences were observed for secondary efficacy or safety endpoints between the two treatments. “It will be interesting to see how this product does when it’s available to us,” Dr. Green said.
Another potential newcomer is ready-to-use liquid botulinum neurotoxin. RelabotulinumtoxinA is a complex, protein-free, ready-to-use liquid botulinum toxin A designed to avoid the traditional requirement to reconstitute it from powder, according to Galderma. It features a saline phosphate buffer solution, so it contains no human or animal-derived excipients, Dr. Green pointed out, and it eliminates the variability, errors, and risks associated with reconstitution.
“There was a report in the neurology literature of botulinum toxin being reconstituted with sterile water for cervical dystonia,” he noted. “When this was injected, it was excruciatingly painful, because it created an osmotic gradient within the muscle. So, if we can take a step away from human error, that would be a good thing.”
To date, Dr. Green said, four phase 3 trials of relabotulinumtoxinA involving more than 1,900 patients have been conducted in the United States and Canada evaluating its use for glabellar frown lines and lateral canthal lines, “and the data is impressive,” he said. This product is still investigational, said Dr. Green, who has not had experience injecting it in the clinical trial program.
The idea of a rapid onset botulinum toxin is also emerging. TrenibotulinumtoxinE, which is being developed by Allergan, “is similar to a type A neurotoxin,” Dr. Green said. “It inhibits neuromuscular transmission via presynaptic vesicular protein synaptosomal-associated protein (SNAP)-25 but at a different cleavage site. It has a faster onset — within one day — but a shorter duration — 3-4 weeks.”
In a dose escalation study of its use for glabellar frown lines, 80% of participants achieved a two-grade investigator-rated improvement in glabellar frown line severity at maximum frown at the highest dose. The maximum clinical effect of trenibotulinumtoxinE was seen within 24 hours and lasted between 14 and 30 days.
“The question is, if it is approved by the FDA, where would this product fit in our practices?” Dr. Green asked. “The effect is gone in 3 weeks as opposed to 4 months,” so this may be an option to recommend for someone who is reticent to try neurotoxins, he said, “or a patient who comes to you on a Friday and says, ‘I have a gala tomorrow night.’ ”
Dr. Green disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including Allergan and Galderma.
SAN DIEGO — .
“This technique is more popular in Asia than it is here in the US,” Dr. Green, who practices dermatology in Coral Gables, Florida, said at the annual meeting of the American Academy of Dermatology. As opposed to intramuscular injections, “it’s an intradermal delivery, so you use numbing cream prior, and you’re injecting botulinum toxin A nearly parallel to the skin surface with the bevel of the needle up,” he said. “You want to use a precise product. It’s uncomfortable delivering volume so superficially due to the tissue distention, so I also use a massager. I inject approximately 0.05 mL to 0.1 mL per point. This does really work.”
This mode of delivery was evaluated in a prospective, double-blind, split-face study in South Korea, which enrolled 18 volunteers who received an intradermal injection of botulinum toxin A into one cheek and normal saline into the contralateral side as a control. Participants were between 30 and 54 years of age and were seen at the clinic 2, 4, 8, and 12 weeks after the injection. At each visit, investigators took photographs, used a facial analyzer to evaluate the pores and wrinkles of the infraorbital area, and used a Sebumeter to evaluate sebum secretions from both cheeks. Improvement or aggravation in skin texture was evaluated by both volunteers and clinicians on a numeric scale from –4 (severe aggravation) to +4 (marked improvement) at each visit, and following photographic review, the wrinkle score of the nasolabial fold was graded on a 5-point scale.
The researchers observed no significant effects on the wrinkles of the infraorbital area and on sebum secretion. However, on the side where botulinum toxin A was injected, there were significant improvements in the wrinkles of the nasolabial fold and skin texture, they reported. The effects on nasolabial fold wrinkles lasted 12 weeks, effects on skin texture lasted 8 weeks, and improvement in pore size was only observed at week 2, they wrote. One serious adverse event occurred: a case of facial palsy after the injection of 30 units of botulinum toxin A in one cheek. However, injection of 20 units of botulinum toxin A in one cheek was not associated with any adverse events.
“The duration of these treatments is yet to be determined, but I think this is definitely going to gain popularity in the US,” said Dr. Green, clinical assistant professor of dermatology at the University of Miami Department of Dermatology and Cutaneous Surgery.
Recently Approved Neurotoxin
He also discussed letibotulinumtoxinA-wlbg (Letybo), an injectable neurotoxin long used in South Korea, which the US Food and Drug Administration (FDA) approved for the temporary improvement in the appearance of moderate to severe glabellar (frown) lines in adults on March 4, 2024. Approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1,000 individuals in the United States and Europe.
“This is the sixth approved neurotoxin in the US,” Dr. Green said. “It is derived from the CBFC26 strain of Clostridium botulinum, and it’s a purified 900 kDa type A toxin complex with human serum albumin and sodium chloride as its excipients.” It comes in a 50-unit or 100-unit vial and requires refrigeration. “To me, the most fascinating thing about this product is that it has been the number-one selling botulinum toxin on the South Korea market for the last 5 years,” he said. “But what do we know about its characteristics?”
In a non-inferiority trial, Chinese researchers enrolled 500 patients with moderate to severe glabellar wrinkles to investigate the efficacy and safety of letibotulinumtoxinA-wlbg and onabotulinumtoxinA. Participants were randomized 3:1 to receive 20 U of letibotulinumtoxinA-wlbg or onabotulinumtoxinA and then observed them for 16 weeks. The primary endpoint was noninferiority in the proportion of study participants who received a score of 0 or 1 for glabellar wrinkles on a four-point photographic evaluation scale, as assessed by an evaluator at maximum frown at 4 weeks.
At week 4, 88.49% of participants in the letibotulinumtoxinA-wlbg arm achieved a score of 0 or 1 for glabellar wrinkles, compared with 87.39% of those in the onabotulinumtoxinA arm (P = .7469). No significant differences were observed for secondary efficacy or safety endpoints between the two treatments. “It will be interesting to see how this product does when it’s available to us,” Dr. Green said.
Another potential newcomer is ready-to-use liquid botulinum neurotoxin. RelabotulinumtoxinA is a complex, protein-free, ready-to-use liquid botulinum toxin A designed to avoid the traditional requirement to reconstitute it from powder, according to Galderma. It features a saline phosphate buffer solution, so it contains no human or animal-derived excipients, Dr. Green pointed out, and it eliminates the variability, errors, and risks associated with reconstitution.
“There was a report in the neurology literature of botulinum toxin being reconstituted with sterile water for cervical dystonia,” he noted. “When this was injected, it was excruciatingly painful, because it created an osmotic gradient within the muscle. So, if we can take a step away from human error, that would be a good thing.”
To date, Dr. Green said, four phase 3 trials of relabotulinumtoxinA involving more than 1,900 patients have been conducted in the United States and Canada evaluating its use for glabellar frown lines and lateral canthal lines, “and the data is impressive,” he said. This product is still investigational, said Dr. Green, who has not had experience injecting it in the clinical trial program.
The idea of a rapid onset botulinum toxin is also emerging. TrenibotulinumtoxinE, which is being developed by Allergan, “is similar to a type A neurotoxin,” Dr. Green said. “It inhibits neuromuscular transmission via presynaptic vesicular protein synaptosomal-associated protein (SNAP)-25 but at a different cleavage site. It has a faster onset — within one day — but a shorter duration — 3-4 weeks.”
In a dose escalation study of its use for glabellar frown lines, 80% of participants achieved a two-grade investigator-rated improvement in glabellar frown line severity at maximum frown at the highest dose. The maximum clinical effect of trenibotulinumtoxinE was seen within 24 hours and lasted between 14 and 30 days.
“The question is, if it is approved by the FDA, where would this product fit in our practices?” Dr. Green asked. “The effect is gone in 3 weeks as opposed to 4 months,” so this may be an option to recommend for someone who is reticent to try neurotoxins, he said, “or a patient who comes to you on a Friday and says, ‘I have a gala tomorrow night.’ ”
Dr. Green disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including Allergan and Galderma.
FROM AAD 2024
Formulation of Sclerotherapy Agent Found Useful for Submental Fat Reduction
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
FROM AAD 2024
Rosemary, Part 2
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
Used as a spice in various, particularly Mediterranean, cuisines and in traditional medicine for hundreds of years, this aromatic shrub has been the focus of substantial research this century to clarify its roles in skin care. It is used broadly in cosmetic formulations, particularly to preserve the product, and acts as a skin conditioner and fragrance in safe concentrations.1 Rosemary essential oil is also a popular choice frequently used in aromatherapy.2,3 This column focuses on recent promising results supporting the antioxidant and anti-photoaging activities, especially, of rosemary.
UV Protection and Rosemary in Combination
A 2021 study in mice authored by Auh and Madhavan showed that a mixture of marigold and rosemary extracts yielded anti-photoaging effects, with the botanical formula suppressing UV-induced damage.4
Seven years earlier, Pérez-Sánchez et al. combined rosemary and citrus extracts and found that they exerted protective effects against UV damage in human HaCaT keratinocytes as well as human volunteers after oral consumption. Significant increases in minimal erythema dose (MED) were seen in participants, with daily intake of 250 mg of botanical combination, at 8 weeks (34%) and 12 weeks (56%). The investigators attributed the photoprotective effects of the formula to rosemary polyphenols and diterpenes as well as citrus flavonoids.5
Evaluation of a human skin cell model by Sánchez-Marzo et al. in 2020 revealed that rosemary diterpenes were instrumental in an herbal extract that combined citrus, olive, and rosemary in conferring genoprotection against UV-induced DNA damage. The authors note that human trials are needed to overcome the limitations of the cellular model in ascertaining whether the tested herbal formulations can yield oral and/or topical photoprotection.6
Anti-Photoaging and Anti-Pollution
In 2022, Ibrahim et al. assessed a hexane extract of rosemary leaves for anti-photoaging activity. Their evaluation showed an abundance of triterpenoids, monoterpenoids, and phenolic diterpenes in rosemary, with in vitro assays verifying the anti-aging, antioxidant, and wound healing functions of the extract. Further, topical rosemary hexane extract–loaded lipid nanocapsules protected rat skin from UV radiation, as epidermal and dermal histological parameters improved, antioxidant biochemical balance was restored, and inflammatory markers and wrinkling were diminished. The researchers concluded that the use of rosemary hexane extract represents a safe, efficient, and cost-effective way to deliver anti-aging, photoprotective functions to cosmeceutical formulations.7
In March 2021, Nobile et al. published a report on their randomized, double-blind, placebo-controlled parallel group study to investigate the efficacy of a marketed polyphenol-enriched dietary supplement (Zeropollution, which contains four standardized herbal extracts: Olea europaea leaf, Lippia citriodora, S. rosmarinus, and Sophora japonica) in diminishing pollution-induced oxidative stress and in improving skin aging in 100 White and Asian women who were outdoor workers living in a polluted environment (Milan, Italy). Statistically significant improvements in reducing wrinkle depth and hyperpigmentation, enhancing elasticity and firmness, as well as promoting skin moisturization and diminishing transepidermal water loss were noted as early as 2 weeks after product consumption began, with inter-group and intra-group analysis verifying that all skin parameters were ameliorated in Asian and White subjects.8
Previously, Nobile et al. conducted a randomized, parallel-group study on 90 subjects to evaluate the photoprotective effects of a combination of rosemary and grapefruit (Citrus paradisi) extracts (Nutroxsun). The investigators also performed a pilot, randomized crossover study on five participants. Both studies included only females with Fitzpatrick skin phototypes I-III who manifested mild to moderate chronological aging or photoaging. Within as little as 2 weeks, treated individuals exhibited reductions in UVA- and UVB-induced skin changes. Skin elasticity improved in this group, with wrinkles diminishing along with skin redness and lipoperoxides. The investigators concluded that the oral blend of rosemary and grapefruit consumed long term merits consideration as an adjuvant approach to preventing the deleterious effects of solar exposure.9
In 2021, Hoskin et al. used ex vivo human biopsies exposed to diesel engine exhaust to study the impact of spray-dried algae-rosemary particles against pollution-induced damage. The spirulina-rosemary gel that was developed lowered levels of 4-hydroxynonenal protein adducts (4HNE-PA) as well as matrix metalloproteinase-9 (MMP-9) and reduced the loss of filaggrin. The researchers concluded that their topically applied spirulina-rosemary gel was effective in mitigating or preventing skin aging and cutaneous damage caused by diesel air pollution.10
Antioxidant, Antibacterial, and Anti-Inflammatory Activity
Based on a 2023 literature search by Li Pomi et al. of in vitro as well as in vivo animal and human studies involving S. rosmarinus and the skin, researchers reported on substantial evidence buttressing the antioxidant role of the botanical agent. They cautioned that, while data support the harnessing of the bioactive constituents of rosemary to address inflammatory and infectious skin conditions, large controlled trials remain necessary to establish its potential functions in dermatologic clinical practice.11
Ten years earlier, Park et al. determined that a phenolic diterpene from rosemary (carnosic acid) prevented UV-induced expression of MMP-1, MMP-3, and MMP-9 in human skin fibroblasts and keratinocytes in a concentration-dependent manner by suppressing reactive oxygen species and blocking through the inhibition of ROS and the suppression of extracellular signal-regulated kinase (ERK)-mediated AP-1 activation.12
Around the same time, Sienkiewicz et al. showed that rosemary essential oil exhibits antibacterial activity against the standard strain Escherichia coli ATCC 25922 and 60 other clinical strains of the bacteria.13
Further, anti-inflammatory properties have been attributed to rosemary essential oil, which are thought to be due to its suppression of nuclear factor kappa B transcription and inhibition of the arachidonic acid cascade.14
Other Functions of Rosemary
In 2022, Sutkowska-Skolimowska et al. demonstrated that rosemary extract in concentrations of 50 and 100mcg/mL significantly diminished accumulated collagen in the fibroblasts of four patients with severe and fatal osteogenesis imperfecta, suggesting that the botanical agent may have a role targeting cellular stress and inducing autophagy in therapy for this condition.15
In 2015, Akbari et al. established that 0.5% and 1% concentrations of rosemary essential oil were effective in facilitating the percutaneous absorption of diclofenac sodium topical gel.16
Conclusion
In Western culture, rosemary is thought of more as a spice to add flavor to food. However, there appears to be an emerging body of evidence suggesting various possible functions for rosemary in the dermatologic armamentarium. Much more research is necessary, though, to ascertain the most appropriate and optimal roles for this popular herb in skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami, Florida. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
2. Sayorwan W et al. Sci Pharm. 2013 Apr-Jun;81(2):531-42.
3. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
4. Auh JH and Madhavan J Biomed Pharmacother. 2021 Mar;135:111178.
5. Pérez-Sánchez A et al. J Photochem Photobiol B. 2014 Jul 5;136:12-8.
6. Sánchez-Marzo N et al. Antioxidants (Basel). 2020 Mar 20;9(3):255.
7. Ibrahim N et al. Sci Rep. 2022 Jul 30;12(1):13102.
8. Nobile V et al. Food Nutr Res. 2021 Mar 29:65.
9. Nobile V et al. Food Nutr Res. 2016 Jul 1;60:31871.
10. Hoskin R et al. Molecules. 2021 Jun 22;26(13):3781.
11. Li Pomi F et al. Antioxidants (Basel). 2023 Mar 9;12(3):680.
12. Park M et al. Exp Dermatol. 2013 May;22(5):336-41.
13. Sienkiewicz M et al. Molecules. 2013 Aug 5;18(8):9334-51.
14. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
15. Sutkowska-Skolimowska. Int J Mol Sci. 2022 Sep 7;23(18):10341.
16. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
What Do Results from Acoustic Subcision for Cellulite Look Like at One Year?
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
FROM DERMATOLOGIC SURGERY
Study Evaluates Aesthetic Concerns in Asian Women
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
AT ASDS 2023
Smoking Associated With Increased Risk for Hair Loss Among Men
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY