User login
Study Provides Insight Into Alcohol’s Effects on the Brain
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
Survey: Americans support regulation of vaping products
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.
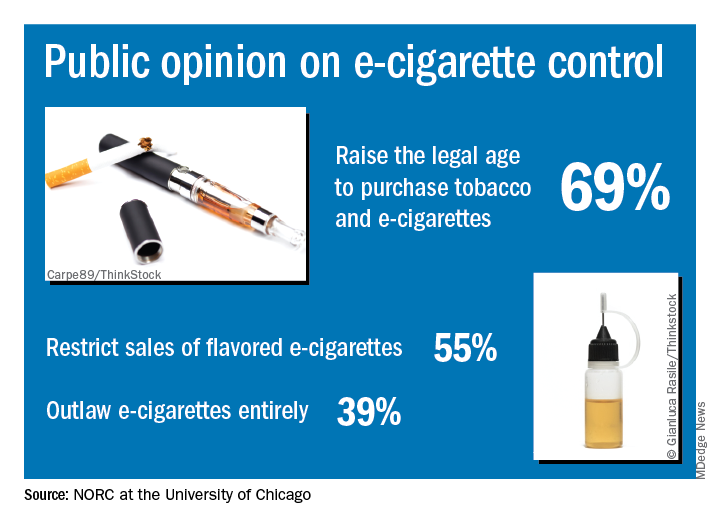
“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Integrating Care for Patients With Chronic Liver Disease and Mental Health and Substance Use Disorders (FULL)
Chronic liver disease (CLD) encompasses a spectrum of common diseases associated with high morbidity and mortality. In 2010, cirrhosis, or advanced-stage CLD, was the eighth leading cause of death in the U.S., accounting for about 49,500 deaths.1 The leading causes of CLD are hepatitis C virus (HCV), which affects about 3.6 million people in the US; nonalcoholic fatty liver disease (NAFLD), which has been increasing in prevalence in up to 75% of CLD cases; and alcohol misuse.2,3 Substance use disorders (SUDs) are a common cause of CLD. About one-third of cirrhosis cases can be attributed to alcohol use, and there is a strong association between IV drug use and HCV. Individual studies point to the high prevalence of mental health disorders (MHDs) among patients with CLD.4-19 It is clear that mental health disorders and SUDs impact outcomes for patients with CLD such that addressing these co-occurring disorders is critical to caring for this population.
An integrated or multidisciplinary approach to medical care attempts to coordinate the delivery of health and social care to patients with complex disease and comorbidities.20 Integrated care models have been shown to positively impact outcomes in many chronic diseases. For example, in patients with heart failure, multidisciplinary interventions such as home visits, remote physiologic monitoring, telehealth, telephone follow-up, or a hospital/clinic team-based intervention have been shown to reduce both hospital admissions and all-cause mortality.21 Similarly, there have been studies in patients with CLD exploring integrated care models. Although individual studies have assessed outcomes associated with various MHDs/SUDs among patients with different etiologies of liver disease, this review assesses the role of integrated care models for patients with CLD and MHDs/SUDs across etiologies.
Methods
A search of the PubMed database was conducted in November 2016 with the following keywords: “liver disease” and “mental health,” “liver disease” and “depression,” “liver disease” and “integrated care,” “substance use” and “liver disease,” “integrated care” and “hepatitis,” “integrated care” and “cirrhosis,” “integrated care” and “advanced liver disease,” and “integrated care” and “alcoholic liver disease” or “nonalcoholic fatty liver disease.” Articles covered a range of study types, including qualitative and quantitative analyses as well as other systematic reviews on focused topics within the area of interest. The authors reviewed the abstracts for eligibility criteria, which included topics focused on the study of mental health or substance use aspects and/or integrated mental health/substance use care for liver diseases (across etiologies and stages), published from January 2004 to November 2016, written in English, and focused on an adult population. Five members of the research team reviewed abstracts and eliminated any that did not meet the eligibility criteria.
A total of 636 records were screened and 378 were excluded based on abstract relevance to the stated topics as well as eligibility criteria. Following this review, full articles (N = 263) were reviewed by at least 2 members of the research team. For both levels of review, articles were removed for the criteria above and additional exclusion criteria: editorial style articles, duplicates, transplant focus, or primarily focused on health-related quality of life (QOL) not specific to MHDs. Although many articles fit more than one exclusion criteria, an article was removed once it met one exclusion criteria. After individual assessment by members of the research team, 71 articles were kept in the review. The team identified 14 additional articles that contributed to the topic but were not located through the original database search. The final analysis included 85 articles that fell into 3 key areas: (1) prevalence of comorbid MHD/SUD in liver disease; (2) associations between MHD/SUD and disease progression/management; and (3) the use of integrated care models in patients with CLD.
Results
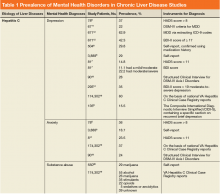
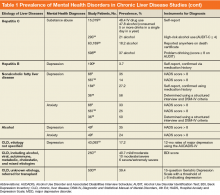
In general, depression and anxiety were common among patients with CLD regardless of etiology.5 Across VA and non-VA studies, depressive disorders were found in one-third to two-thirds of patients with CLD and anxiety disorders in about one-third of patients with CLD. 5,7,8,10,15,16, 22-25Results of the studies that assess the prevalence of MHDs in patients with CLD are shown in Table 1.
MHDs and SUDs in Patients With CLD
Mental health symptoms have been associated with the severity of liver disease in some but not all studies.17,18,26 Mental health disorders also may have more dire consequences in this population. In a national survey of adults, 1.6% of patients with depression were found to have liver disease. Among this group with depression, suicide attempts were 3-fold higher among patients with CLD vs patients without CLD.19
Substance use disorders (including alcohol) are common among patients with CLD. This has been best studied in the context of patients with HCV.22, 27-32 For example among patients with HCV, the prevalence of injection drug use (IDU) was 48% to 65%, and the prevalence of marijuana use was 29%.33-36 In a report of 174,302 veterans with HCV receiving VA care, the following SUDs were reported as diagnosis in this patient population: alcohol, 55%; cannabis, 26%; stimulants, 35%; opioids, 22%; sedatives or anxiolytics, 5%; and other drug use, 39%.10
Both Non-VA and VA studies have found overlap between HCV and alcohol-related liver disease with a number of patients with HCV using alcohol and a number of patients with alcohol-related liver disease having a past history of IDU and HCV.37,38 Across VA and non-VA studies, patients with HIV/HCV co-infection have been found to have particularly high rates of MHDs and SUDs. One VA retrospective cohort study of 18,349 HIV-infected patients noted 37% were seropositive for HCV as well.39-41 These patients with HIV/HCV infection when compared with patients with only HIV infection were more likely to have a diagnosis of mental health illness (76.1% vs 63.1%), depression (56.6% vs 45.6%), alcohol abuse (64.2% vs 30.1%), substance abuse (68.0% vs 25.7%), and hard drug use (62.9% vs 20.6%).42 Patients with CLD and ongoing alcohol use have been found to have increased mental health symptoms compared with patients without ongoing alcohol use.17 Thus MHDs and SUDs are common and often coexist among patients with CLD.
MHDs Impact Patient Outcomes
Mental health disorders can affect how providers care for patients. In the past, for example, in both VA and non-VA studies, patients were often excluded from interferon-based HCV treatments due to MHDs.22,35,43-45 These exclusions included psychiatric issues (35%), alcohol abuse (31%), drug abuse (9%), or > 1 of these reasons (26%).46 Depression also has been associated with decreased care seeking by patients. Patients with cirrhosis and depression often do not seek medical care due to perceived stigma.47 Nearly one-fifth of patients with HCV in one study reported that they did not share information about their disease with others to avoid being stigmatized.48 Other studies have noted similar difficulty with patients’ seeking HCV treatment, advances in medications notwithstanding.49-52
Depression among patients with cirrhosis has been associated with reduced QOL, worsened cognitive function, increased mortality, and frailty.18,53,54 Psychiatric symptoms have been associated with disability and pain among patients with cirrhosis and with weight gain among patients with NAFLD.5,55 Mental health symptoms also predicted lower work productivity in patients with HCV.8 Histologic changes in the liver have been described among patients with psychiatric disorders, although the mechanism is not well understood.15,16
Although not a focus of this review, it is well established that MHDs are associated with increased substance use. Since there is a well-established connection between alcohol and adverse liver-related outcomes regardless of etiology of liver disease, mental health is thus indirectly linked to poor liver outcomes through this mechanism.37,38,56-67
Integrated Care in Liver Disease
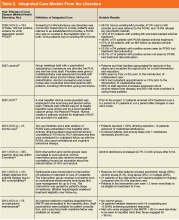
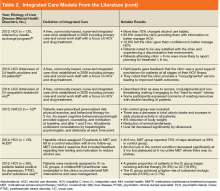
Although there are no set guidelines on how to approach patients with liver disease and MHD/SUD comorbidities, integrated care approaches that include attention to both CLD and psychiatric needs seem promising. Integrated care models have been recommended by several authors specifically for patients with HCV and co-occurring MHDs and SUDs.4,33,42,43,45,68-72 Various integrated care models for CLD and psychiatric comorbidities have been studied and are detailed in Table 2.
The most well described models of integrated care in CLD have been used for patients with HCV as noted in prior reviews.22,34,49,73 These studies included liver care integrated with substance abuse clinics/specialists, mental health professionals, and/or case managers. Outcomes that have been assessed include adherence, HCV treatment completion, HCV treatment eligibility/initiation, and reduction in alcohol use.31,46, 74-77 A large randomized controlled trial (RCT) comparing integrated care with usual care found that integrated care, including collaborative consultation with mental health providers and case managers, was associated with increased antiviral treatment and sustained virologic response (SVR).50,78 One study of integrated care in the era of direct-acting antiviral treatment for HCV found that twice as many veterans initiated treatment with integrated care (with case management and a mental health provider) as opposed to usual care. In this integrated care model, mental health providers provided ongoing brief psychological interventions designed to address the specific risk factors identified at screening, facilitated treatment, and served as a regular contact.79 Overall, integrating mental health care and HCV care has resulted in increased adherence, increased treatment eligibility/initiation, treatment completion, higher rates of SVR, and reduction in alcohol use.31,46,74-77
In addition to positive medical outcomes with integrated care models, patients and providers generally have favorable impressions of the clinics using an integrated care approach. For example, multiple qualitative studies of the Hepatitis C Community Clinic in New Zealand have described that patients and providers have positive feelings about integrated care models for HCV.80-82 Another study evaluating integrated care at 4 hepatitis clinics in British Columbia, Canada found that clients overall valued the clinic and viewed it favorably; however, they identified several areas for continued improvement, including communication and time spent with clients, follow-up and access to care, as well as education on coping and managing their disease.83
Beyond HCV, other patients with CLD could benefit from integrated care approaches. Given the association of psychiatric symptoms with weight outcomes among patients with NAFLD, integrating behavioral support has been recommended.55 Multidisciplinary care has been trialed in patients with NAFLD. One model included behavioral therapy with psychological counseling, motivation for lifestyle changes, and support by a trained expert cognitive behavioral psychologist. Although this study did not include a control group, the patients in the study experienced an 8% weight reduction, reduction in aminotransferases, and decreased hepatic steatosis by ultrasound.84
Integrated care also has been advocated for patients with alcohol-related liver disease. One study recommended creating a personalized framework to support self-management for this population.85 Another study assessed patients with alcohol-related cirrhosis and hepatic encephalopathy and recommended integrating individual coping strategies and support into liver care for this group of patients.86
A United Kingdom study of multidisciplinary care that included a team of gastroenterologists, psychiatrists, and a psychiatric liaison nurse, found improved accessibility to care and patient/family satisfaction using this model. Outpatient appointments were offered to 84% of patients after collaborative care was introduced as opposed to 12% previously. Patients and family members reported that this approach decreased the stigma of mental health care, allowing patients to be more open to intervention and education in this setting.87 A systematic review of patients with alcohol-related CLD found that among 5 RCTs with 1,945 cumulative patients, integrated care was associated with increased short-term abstinence but not sustained abstinence.88 Thus integrated care has been used most in patients with HCV-related CLD, but growing evidence supports its use for patients with other etiologies of CLD, including NAFLD and alcohol.
Discussion
This review found that MHDs are common among patients with CLD and that there is an association between the worsening of liver disease outcomes for patients with comorbid mental health and substance use diagnoses as well as an association of poor MHD/SUD outcomes among patients with CLD (eg, increased suicide attempts among those with comorbid CLD and depression). These data synthesis support screening for MHDs in patients with CLD and providing integrated or multidisciplinary care where possible. Integrated care provides both mental health and CLD care in a combined setting. Integrated care models have been associated with improved health outcomes in patients with CLD and psychiatric comorbidities, including increased adherence, increased HCV treatment eligibility; initiation, and completion; higher rates of HCV treatment cure; reduction in alcohol use; and increased weight loss among patients with NAFLD.
Integrated care is becoming the standard of care for patients with CLD in many countries with national medical care systems. Scotland, for example, initiated an HCV action plan that included mental health and social care. It reported a reduced incidence of HCV infection among patients with a history of IDU, increased treatment initiation, and increased HCV testing with this approach.89 Multidisciplinary care is a class 1 level B recommendation for HCV care in Canada, meaning that it is the highest class of evidence and is supported by at least 1 randomized or multiple nonrandomized studies.90 Similarly, the US Department of Health and Human Services has developed a “National Viral Hepatitis Action Plan” with more than 20 participating federal agencies. The plan highlights the importance of integrating public health and clinical services to successfully improve viral hepatitis care, prevention, and treatment across the US.
The content of the integrated care interventions has been variable. Models with the highest success of liver disease outcomes in this study seem to have screened patients for MHDs and/or SUDs and then used trained professionals to address these issues while also focusing on liver care. An approach that includes evidence-based treatments or intervention for MHDs/SUDs is likely preferable to nonspecific support or information giving. However, it is notable that even minimal interventions (eg, providing informational materials) have been associated with improved outcomes in CLD. The actual implementation of integrated care for MHDs/SUDs into liver care likely has to be tailored to the context and available resources.
One study proposed several models of integrated care that can be adapted to the available resources of a given clinical practice setting. These included fully integrated models where services are colocated, collaborative practice models in which there is a strong relationship between providers in hepatology and mental health and SUD clinics, and then hybrid models that integrate/colocate when possible and collaborate when colocation isn’t available. Although the fully integrated care model likely is the most ideal, any multidisciplinary approach has the potential to decrease barriers and increase access to treatment.91
Another study used modeling to develop an integrated care framework for vulnerable veterans with HCV that incorporated both implementation factors (eg, research evidence, clinical experience, facilitation, and leadership) based on the Promoting Action on Research Implementation in Health Services framework and patients’ factors from the Andersen Behavioral Model (eg, geography and finances) to form a hybrid framework for this population.92
Limitations
There are several notable limitations of this review. Although the review focused on depression, anxiety, and SUDs, given the high prevalence of these disorders, other MHDs are also common among patients with CLD and were not addressed. For example, veterans with HCV also commonly had posttraumatic stress disorder, bipolar disorder, and schizophrenia.10 Further investigation should focus on these disorders and their impacts. Additionally, the authors did not specifically search for alcohol-related care in the search terms. This review also did not address nonpsychiatric types of integrated care, which could be the focus of future reviews. Despite these limitations, this review provides support for the use of integrated care in the context of CLD and co-occurring MHDs and SUDs.
Conclusion
Several studies support integrated care for patients with liver disease and co-occurring psychiatric disorders. There are multiple integrated care models in place, although they have largely been used in patients with HCV. More studies are needed to assess the role of integrated mental health care in other populations of patients with CLD. There is an abundance of research supporting the role of integrated care in improving health outcomes across many chronic diseases, including implementation of mental health into primary care in large health care systems like the VA health care system.93 Health care systems should work toward alignment of resources to meet these needs in specialty care settings, such as liver disease care in order optimize both liver disease and MHD/SUD outcomes for these patients.
Click here to read the digital edition.
1. Murray CJ, Atkinson C, Bhalla K, et al; US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608.
2. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.e1-e6.
3. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524-530.e1; quiz e60.
4. Neuman MG, Monteiro M, Rehm J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Subst Use Misuse. 2006;41(10-12):1395-1463.
5. Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(5):1009-1016.
6. Weinstein AA, Kallman Price J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52(2):127-132.
7. Erim Y, Tagay S, Beckmann M, et al. Depression and protective factors of mental health in people with hepatitis C: a questionnaire survey. Int J Nurs Stud. 2010;47(3):342-349.
8. Younossi I, Weinstein A, Stepanova M, Hunt S, Younossi ZM. Mental and emotional impairment in patients with hepatitis C is related to lower work productivity. Psychosomatics. 2016;57(1):82-88.
9. Carta MG, Angst J, Moro MF, et al. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord. 2012;141(2-3):361-366.
10. Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37(1):131-143.
11. Birerdinc A, Afendy A, Stepanova M, Younossi I, Baranova A, Younossi ZM. Gene expression profiles associated with depression in patients with chronic hepatitis C (CH-C). Brain Behav. 2012;2(5):525-531.
12. Patterson AL, Morasco BJ, Fuller BE, Indest DW, Loftis JM, Hauser P. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
13. Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27(6):431-438.
14. Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40(suppl 5):S286-S291.
15. Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68(4):563-569.
16. Youssef NA, Abdelmalek MF, Binks M, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33(7):1062-1070.
17. Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593-600.
18. Cron DC, Friedman JF, Winder GS, et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transplant. 2016;16(6):1805-1811.
19. Le Strat Y, Le Foll B, Dubertret C. Major depression and suicide attempts in patients with liver disease in the United States. Liver Int. 2015;35(7):1910-1916.
20. Lemmens LC, Molema CC, Versnel N, Baan CA, de Bruin SR. Integrated care programs for patients with psychological comorbidity: a systematic review and meta-analysis. J Psychosom Res. 2015;79(6):580-594.
21. Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899-906.
22. Ho SB, Groessl E, Dollarhide A, Robinson S, Kravetz D, Dieperink E. Management of chronic hepatitis C in veterans: the potential of integrated care models. Am J Gastroenterol. 2008;103(7):1810-1823.
23. Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21(8):2269-2280.
24. Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. 2013;54(1):52-59.
25. Rosenthal E, Cacoub P. Extrahepatic manifestations in chronic hepatitis C virus carriers. Lupus. 2015;24(4-5):469-482.
26. Duan Z, Kong Y, Zhang J, Guo H. Psychological comorbidities in Chinese patients with acute-on-chronic liver failure. Gen Hosp Psychiatry. 2012;34(3):276-281.
27. Cariello R, Federico A, Sapone A, et al. Intestinal permeability in patients with chronic liver diseases: its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42(3):200-204.
28. Wise M, Finelli L, Sorvillo F. Prognostic factors associated with hepatitis C disease: a case-control study utilizing U.S. multiple-cause-of-death data. Public Health Rep. 2010;125(3):414-422.
29. Wurst FM, Dürsteler-MacFarland KM, Auwaerter V, et al. Assessment of alcohol use among methadone maintenance patients by direct ethanol metabolites and self-reports. Alcohol Clin Exp Res. 2008;32(9):1552-1557.
30. Campbell JV, Hagan H, Latka MH, et al; The STRIVE Project. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend. 2006;81(3):259-265.
31. Dieperink E, Fuller B, Isenhart C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction. 2014;109(11):1869-1877.
32. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705-714
33. Arain A, Robaeys G. Eligibility of persons who inject drugs for treatment of hepatitis C virus infection. World J Gastroenterol. 2014;20(36):12722-12733.
34. North CS, Hong BA, Kerr T. Hepatitis C and substance use: new treatments and novel approaches. Curr Opin Psychiatry. 2012;25(3):206-212.
35. Coffin PO, Reynolds A. Ending hepatitis C in the United States: the role of screening. Hepat Med. 2014;6:79-87.
36. Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28(7):381-384.
37. Kamal A, Cheung R. Positive CAGE screen correlates with cirrhosis in veterans with chronic hepatitis C. Dig Dis Sci. 2007;52(10):2564-2569.
38. Fuster D, Sanvisens A, Bolao F, et al. Impact of hepatitis C virus infection on the risk of death of alcohol-dependent patients. J Viral Hepat. 2015;22(1):18-24.
39. Klein MB, Rollet KC, Saeed S, et al; Canadian HIV-HCV Cohort Investigators. HIV and hepatitis C virus coinfection in Canada: challenges and opportunities for reducing preventable morbidity and mortality. HIV Med. 2013;14(1):10-20.
40. Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006;3(4):176-181.
41. Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(suppl 3):S99-S105.
42. Backus LI, Boothroyd D, Deyton LR. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19(suppl 3):S13-S19.
43. Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126-133.
44. Gidding HF, Law MG, Amin J, et al; ACHOS Investigator Team. Predictors of deferral of treatment for hepatitis C infection in Australian clinics. Med J Aust. 2011;194(8):398-402.
45. Chainuvati S, Khalid SK, Kancir S, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat. 2006;13(4):235-241.
46. Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011; 106(10):1777-1786.
47. Vaughn-Sandler V, Sherman C, Aronsohn A, Volk ML. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci. 2014;59(3):681-686.
48. Blasiole JA, Shinkunas L, Labrecque DR, Arnold RM, Zickmund SL. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World J Gastroenterol. 2006;12(29):4665-4672.
49. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(suppl 2):S56-S61.
50. Groessl EJ, Sklar M, Cheung RC, Bräu N, Ho SB. Increasing antiviral treatment through integrated hepatitis C care: a randomized multicenter trial. Contemp Clin Trials. 2013;35(2):97-107.
51. Alavi M, Grebely J, Micallef M, et al; Enhancing Treatment for Hepatitis C in Opioid Substitution Settings (ETHOS) Study Group. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis. 2013;57(suppl 2):S62-S69.
52. Evon DM, Golin CE, Fried MW, Keefe FJ. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? J Consult Clin Psychol. 2013;81(2):361-374.
53. Mullish BH, Kabir MS, Thursz MR, Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther. 2014;40(8):880-892.
54. Stewart CA, Enders FT, Mitchell MM, Felmlee-Devine D, Smith GE. The cognitive profile of depressed patients with cirrhosis. Prim Care Companion CNS Disord. 2011;13(3):pii. PCC.10m01090
55. Stewart KE, Haller DL, Sargeant C, Levenson JL, Puri P, Sanyal AJ. Readiness for behaviour change in non-alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015;35(3):936-943.
56. Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3(11):1150-1159.
57. Chaudhry AA, Sulkowski MS, Chander G, Moore RD. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10(3):133-142.
58. McMahon BJ, Bruden D, Bruce MG, et al. Adverse outcomes in Alaska natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010;138(3):922-931.e1.
59. Anand BS, Thornby J. Alcohol has no effect on hepatitis C virus replication: a meta-analysis. Gut. 2005;54(10):1468-1472.
60. Au DH, Kivlahan DR, Bryson CL, Blough D, Bradley KA. Alcohol screening scores and risk of hospitalizations for GI conditions in men. Alcohol Clin Exp Res. 2007;31(3):443-451.
61. Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(suppl 1):77-84.
62. Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31(9):1460-1466.
63. Barve S, Kapoor R, Moghe A, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33(3):229-236.
64. Trimble G, Zheng L, Mishra A, Kalwaney S, Mir HM, Younossi ZM. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther. 2013;38(6):596-602.
65. Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8(10):891-898.e1-e2.
66. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
67. Lim JK, Tate JP, Fultz SL, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis. 2014;58(10):1449-1458.
68. Kanwal F, White DL, Tavakoli-Tabasi S, et al. Many patients with interleukin 28B genotypes associated with response to therapy are ineligible for treatment because of comorbidities. Clin Gastroenterol Hepatol. 2014;12(2):327-333.e1.
69. Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19(suppl 3):S179-S189.
70. McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22(2):133-137.
71. Treloar C, Rance J, Dore GJ, Grebely J; ETHOS Study Group. Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ETHOS study. J Viral Hepat. 2014;21(8):560-567.
72. Treloar C, Rance J, Grebely J, Dore GJ. Client and staff experiences of a co-located service for hepatitis C care in opioid substitution treatment settings in New South Wales, Australia. Drug Alcohol Depend. 2013;133(2):529-534.
73. Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(suppl 5):S276-S285.
74. Martinez AD, Dimova R, Marks KM, et al. Integrated internist—addiction medicine— hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19(1):47-54.
75. Fahey S. Developing a nursing service for patients with hepatitis C. Nurs Stand. 2007;21(43):35-40.
76. Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101(10):2254-2262.
77. Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51(2):149-156.
78. Ho SB, Bräu N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol. 2015;13(11):2005-2014.e1-e3.
79. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
80. Treloar C, Gray R, Brener L. A piece of the jigsaw of primary care: health professional perceptions of an integrated care model of hepatitis C management in the community. J Prim Health Care. 2014;6(2):129-134.
81. Brener L, Gray R, Cama EJ, Treloar C. “Makes you wanna do treatment”: benefits of a hepatitis C specialist clinic to clients in Christchurch, New Zealand. Health Soc Care Community. 2013;21(2):216-223.
82. Horwitz R, Brener L, Treloar C. Evaluation of an integrated care service facility for people living with hepatitis C in New Zealand. Int J Integr Care. 2012;12(Spec Ed Integrated Care Pathways):e229.
83. Christianson TM, Moralejo D. Assessing the quality of care in a regional integrated viral hepatitis clinic in British Columbia: a cross-sectional study. Gastroenterol Nurs. 2009;32(5):315-324.
84. Scaglioni F, Marino M, Ciccia S, et al. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD). Clin Res Hepatol Gastroenterol. 2013;37(4):353-358.
85. Lau-Walker M, Presky J, Webzell I, Murrells T, Heaton N. Patients with alcohol-related liver disease—beliefs about their illness and factors that influence their self-management. J Adv Nurs. 2016;72(1):173-185.
86. Mikkelsen MR, Hendriksen C, Schiødt FV, Rydahl-Hansen S. Coping and rehabilitation in alcoholic liver disease patients after hepatic encephalopathy—in interaction with professionals and relatives. J Clin Nurs. 2015;24(23-24):3627-3637.
87. Moriarty KJ, Platt H, Crompton S, et al. Collaborative care for alcohol-related liver disease. Clin Med (Lond). 2007;7(2):125-128.
88. Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol. 2016;14(2):191-202.e1-e4;quiz e20.
89. Wylie L, Hutchinson S, Liddell D, Rowan N. The successful implementation of Scotland’s Hepatitis C Action Plan: what can other European stakeholders learn from the experience? A Scottish voluntary sector perspective. BMC Infect Dis. 2014;14(suppl 6):S7.
90. Hull M, Shafran S, Wong A, et al. CIHR Canadian HIV trials network coinfection and concurrent diseases core research group: 2016 updated Canadian HIV/hepatitis C adult guidelines for management and treatment. Can J Infect Dis Med Microbiol. 2016;2016:4385643.
91. Bonner JE, Barritt AS 4th, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci. 2012;57(6):1469-1474.
92. Rongey C, Asch S, Knight SJ. Access to care for vulnerable veterans with hepatitis C: a hybrid conceptual framework and a case study to guide translation. Transl Behav Med. 2011;1(4):644-651.
93. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs health care system. J Clin Psychol Med Settings. 2008;15(1):73-78.
94. Drumright LN, Hagan H, Thomas DL, et al. Predictors and effects of alcohol use on liver function among young HCV-infected injection drug users in a behavioral intervention. J Hepatol. 2011;55(1):45-52.
Chronic liver disease (CLD) encompasses a spectrum of common diseases associated with high morbidity and mortality. In 2010, cirrhosis, or advanced-stage CLD, was the eighth leading cause of death in the U.S., accounting for about 49,500 deaths.1 The leading causes of CLD are hepatitis C virus (HCV), which affects about 3.6 million people in the US; nonalcoholic fatty liver disease (NAFLD), which has been increasing in prevalence in up to 75% of CLD cases; and alcohol misuse.2,3 Substance use disorders (SUDs) are a common cause of CLD. About one-third of cirrhosis cases can be attributed to alcohol use, and there is a strong association between IV drug use and HCV. Individual studies point to the high prevalence of mental health disorders (MHDs) among patients with CLD.4-19 It is clear that mental health disorders and SUDs impact outcomes for patients with CLD such that addressing these co-occurring disorders is critical to caring for this population.
An integrated or multidisciplinary approach to medical care attempts to coordinate the delivery of health and social care to patients with complex disease and comorbidities.20 Integrated care models have been shown to positively impact outcomes in many chronic diseases. For example, in patients with heart failure, multidisciplinary interventions such as home visits, remote physiologic monitoring, telehealth, telephone follow-up, or a hospital/clinic team-based intervention have been shown to reduce both hospital admissions and all-cause mortality.21 Similarly, there have been studies in patients with CLD exploring integrated care models. Although individual studies have assessed outcomes associated with various MHDs/SUDs among patients with different etiologies of liver disease, this review assesses the role of integrated care models for patients with CLD and MHDs/SUDs across etiologies.
Methods
A search of the PubMed database was conducted in November 2016 with the following keywords: “liver disease” and “mental health,” “liver disease” and “depression,” “liver disease” and “integrated care,” “substance use” and “liver disease,” “integrated care” and “hepatitis,” “integrated care” and “cirrhosis,” “integrated care” and “advanced liver disease,” and “integrated care” and “alcoholic liver disease” or “nonalcoholic fatty liver disease.” Articles covered a range of study types, including qualitative and quantitative analyses as well as other systematic reviews on focused topics within the area of interest. The authors reviewed the abstracts for eligibility criteria, which included topics focused on the study of mental health or substance use aspects and/or integrated mental health/substance use care for liver diseases (across etiologies and stages), published from January 2004 to November 2016, written in English, and focused on an adult population. Five members of the research team reviewed abstracts and eliminated any that did not meet the eligibility criteria.
A total of 636 records were screened and 378 were excluded based on abstract relevance to the stated topics as well as eligibility criteria. Following this review, full articles (N = 263) were reviewed by at least 2 members of the research team. For both levels of review, articles were removed for the criteria above and additional exclusion criteria: editorial style articles, duplicates, transplant focus, or primarily focused on health-related quality of life (QOL) not specific to MHDs. Although many articles fit more than one exclusion criteria, an article was removed once it met one exclusion criteria. After individual assessment by members of the research team, 71 articles were kept in the review. The team identified 14 additional articles that contributed to the topic but were not located through the original database search. The final analysis included 85 articles that fell into 3 key areas: (1) prevalence of comorbid MHD/SUD in liver disease; (2) associations between MHD/SUD and disease progression/management; and (3) the use of integrated care models in patients with CLD.
Results
In general, depression and anxiety were common among patients with CLD regardless of etiology.5 Across VA and non-VA studies, depressive disorders were found in one-third to two-thirds of patients with CLD and anxiety disorders in about one-third of patients with CLD. 5,7,8,10,15,16, 22-25Results of the studies that assess the prevalence of MHDs in patients with CLD are shown in Table 1.
MHDs and SUDs in Patients With CLD
Mental health symptoms have been associated with the severity of liver disease in some but not all studies.17,18,26 Mental health disorders also may have more dire consequences in this population. In a national survey of adults, 1.6% of patients with depression were found to have liver disease. Among this group with depression, suicide attempts were 3-fold higher among patients with CLD vs patients without CLD.19
Substance use disorders (including alcohol) are common among patients with CLD. This has been best studied in the context of patients with HCV.22, 27-32 For example among patients with HCV, the prevalence of injection drug use (IDU) was 48% to 65%, and the prevalence of marijuana use was 29%.33-36 In a report of 174,302 veterans with HCV receiving VA care, the following SUDs were reported as diagnosis in this patient population: alcohol, 55%; cannabis, 26%; stimulants, 35%; opioids, 22%; sedatives or anxiolytics, 5%; and other drug use, 39%.10
Both Non-VA and VA studies have found overlap between HCV and alcohol-related liver disease with a number of patients with HCV using alcohol and a number of patients with alcohol-related liver disease having a past history of IDU and HCV.37,38 Across VA and non-VA studies, patients with HIV/HCV co-infection have been found to have particularly high rates of MHDs and SUDs. One VA retrospective cohort study of 18,349 HIV-infected patients noted 37% were seropositive for HCV as well.39-41 These patients with HIV/HCV infection when compared with patients with only HIV infection were more likely to have a diagnosis of mental health illness (76.1% vs 63.1%), depression (56.6% vs 45.6%), alcohol abuse (64.2% vs 30.1%), substance abuse (68.0% vs 25.7%), and hard drug use (62.9% vs 20.6%).42 Patients with CLD and ongoing alcohol use have been found to have increased mental health symptoms compared with patients without ongoing alcohol use.17 Thus MHDs and SUDs are common and often coexist among patients with CLD.
MHDs Impact Patient Outcomes
Mental health disorders can affect how providers care for patients. In the past, for example, in both VA and non-VA studies, patients were often excluded from interferon-based HCV treatments due to MHDs.22,35,43-45 These exclusions included psychiatric issues (35%), alcohol abuse (31%), drug abuse (9%), or > 1 of these reasons (26%).46 Depression also has been associated with decreased care seeking by patients. Patients with cirrhosis and depression often do not seek medical care due to perceived stigma.47 Nearly one-fifth of patients with HCV in one study reported that they did not share information about their disease with others to avoid being stigmatized.48 Other studies have noted similar difficulty with patients’ seeking HCV treatment, advances in medications notwithstanding.49-52
Depression among patients with cirrhosis has been associated with reduced QOL, worsened cognitive function, increased mortality, and frailty.18,53,54 Psychiatric symptoms have been associated with disability and pain among patients with cirrhosis and with weight gain among patients with NAFLD.5,55 Mental health symptoms also predicted lower work productivity in patients with HCV.8 Histologic changes in the liver have been described among patients with psychiatric disorders, although the mechanism is not well understood.15,16
Although not a focus of this review, it is well established that MHDs are associated with increased substance use. Since there is a well-established connection between alcohol and adverse liver-related outcomes regardless of etiology of liver disease, mental health is thus indirectly linked to poor liver outcomes through this mechanism.37,38,56-67
Integrated Care in Liver Disease
Although there are no set guidelines on how to approach patients with liver disease and MHD/SUD comorbidities, integrated care approaches that include attention to both CLD and psychiatric needs seem promising. Integrated care models have been recommended by several authors specifically for patients with HCV and co-occurring MHDs and SUDs.4,33,42,43,45,68-72 Various integrated care models for CLD and psychiatric comorbidities have been studied and are detailed in Table 2.
The most well described models of integrated care in CLD have been used for patients with HCV as noted in prior reviews.22,34,49,73 These studies included liver care integrated with substance abuse clinics/specialists, mental health professionals, and/or case managers. Outcomes that have been assessed include adherence, HCV treatment completion, HCV treatment eligibility/initiation, and reduction in alcohol use.31,46, 74-77 A large randomized controlled trial (RCT) comparing integrated care with usual care found that integrated care, including collaborative consultation with mental health providers and case managers, was associated with increased antiviral treatment and sustained virologic response (SVR).50,78 One study of integrated care in the era of direct-acting antiviral treatment for HCV found that twice as many veterans initiated treatment with integrated care (with case management and a mental health provider) as opposed to usual care. In this integrated care model, mental health providers provided ongoing brief psychological interventions designed to address the specific risk factors identified at screening, facilitated treatment, and served as a regular contact.79 Overall, integrating mental health care and HCV care has resulted in increased adherence, increased treatment eligibility/initiation, treatment completion, higher rates of SVR, and reduction in alcohol use.31,46,74-77
In addition to positive medical outcomes with integrated care models, patients and providers generally have favorable impressions of the clinics using an integrated care approach. For example, multiple qualitative studies of the Hepatitis C Community Clinic in New Zealand have described that patients and providers have positive feelings about integrated care models for HCV.80-82 Another study evaluating integrated care at 4 hepatitis clinics in British Columbia, Canada found that clients overall valued the clinic and viewed it favorably; however, they identified several areas for continued improvement, including communication and time spent with clients, follow-up and access to care, as well as education on coping and managing their disease.83
Beyond HCV, other patients with CLD could benefit from integrated care approaches. Given the association of psychiatric symptoms with weight outcomes among patients with NAFLD, integrating behavioral support has been recommended.55 Multidisciplinary care has been trialed in patients with NAFLD. One model included behavioral therapy with psychological counseling, motivation for lifestyle changes, and support by a trained expert cognitive behavioral psychologist. Although this study did not include a control group, the patients in the study experienced an 8% weight reduction, reduction in aminotransferases, and decreased hepatic steatosis by ultrasound.84
Integrated care also has been advocated for patients with alcohol-related liver disease. One study recommended creating a personalized framework to support self-management for this population.85 Another study assessed patients with alcohol-related cirrhosis and hepatic encephalopathy and recommended integrating individual coping strategies and support into liver care for this group of patients.86
A United Kingdom study of multidisciplinary care that included a team of gastroenterologists, psychiatrists, and a psychiatric liaison nurse, found improved accessibility to care and patient/family satisfaction using this model. Outpatient appointments were offered to 84% of patients after collaborative care was introduced as opposed to 12% previously. Patients and family members reported that this approach decreased the stigma of mental health care, allowing patients to be more open to intervention and education in this setting.87 A systematic review of patients with alcohol-related CLD found that among 5 RCTs with 1,945 cumulative patients, integrated care was associated with increased short-term abstinence but not sustained abstinence.88 Thus integrated care has been used most in patients with HCV-related CLD, but growing evidence supports its use for patients with other etiologies of CLD, including NAFLD and alcohol.
Discussion
This review found that MHDs are common among patients with CLD and that there is an association between the worsening of liver disease outcomes for patients with comorbid mental health and substance use diagnoses as well as an association of poor MHD/SUD outcomes among patients with CLD (eg, increased suicide attempts among those with comorbid CLD and depression). These data synthesis support screening for MHDs in patients with CLD and providing integrated or multidisciplinary care where possible. Integrated care provides both mental health and CLD care in a combined setting. Integrated care models have been associated with improved health outcomes in patients with CLD and psychiatric comorbidities, including increased adherence, increased HCV treatment eligibility; initiation, and completion; higher rates of HCV treatment cure; reduction in alcohol use; and increased weight loss among patients with NAFLD.
Integrated care is becoming the standard of care for patients with CLD in many countries with national medical care systems. Scotland, for example, initiated an HCV action plan that included mental health and social care. It reported a reduced incidence of HCV infection among patients with a history of IDU, increased treatment initiation, and increased HCV testing with this approach.89 Multidisciplinary care is a class 1 level B recommendation for HCV care in Canada, meaning that it is the highest class of evidence and is supported by at least 1 randomized or multiple nonrandomized studies.90 Similarly, the US Department of Health and Human Services has developed a “National Viral Hepatitis Action Plan” with more than 20 participating federal agencies. The plan highlights the importance of integrating public health and clinical services to successfully improve viral hepatitis care, prevention, and treatment across the US.
The content of the integrated care interventions has been variable. Models with the highest success of liver disease outcomes in this study seem to have screened patients for MHDs and/or SUDs and then used trained professionals to address these issues while also focusing on liver care. An approach that includes evidence-based treatments or intervention for MHDs/SUDs is likely preferable to nonspecific support or information giving. However, it is notable that even minimal interventions (eg, providing informational materials) have been associated with improved outcomes in CLD. The actual implementation of integrated care for MHDs/SUDs into liver care likely has to be tailored to the context and available resources.
One study proposed several models of integrated care that can be adapted to the available resources of a given clinical practice setting. These included fully integrated models where services are colocated, collaborative practice models in which there is a strong relationship between providers in hepatology and mental health and SUD clinics, and then hybrid models that integrate/colocate when possible and collaborate when colocation isn’t available. Although the fully integrated care model likely is the most ideal, any multidisciplinary approach has the potential to decrease barriers and increase access to treatment.91
Another study used modeling to develop an integrated care framework for vulnerable veterans with HCV that incorporated both implementation factors (eg, research evidence, clinical experience, facilitation, and leadership) based on the Promoting Action on Research Implementation in Health Services framework and patients’ factors from the Andersen Behavioral Model (eg, geography and finances) to form a hybrid framework for this population.92
Limitations
There are several notable limitations of this review. Although the review focused on depression, anxiety, and SUDs, given the high prevalence of these disorders, other MHDs are also common among patients with CLD and were not addressed. For example, veterans with HCV also commonly had posttraumatic stress disorder, bipolar disorder, and schizophrenia.10 Further investigation should focus on these disorders and their impacts. Additionally, the authors did not specifically search for alcohol-related care in the search terms. This review also did not address nonpsychiatric types of integrated care, which could be the focus of future reviews. Despite these limitations, this review provides support for the use of integrated care in the context of CLD and co-occurring MHDs and SUDs.
Conclusion
Several studies support integrated care for patients with liver disease and co-occurring psychiatric disorders. There are multiple integrated care models in place, although they have largely been used in patients with HCV. More studies are needed to assess the role of integrated mental health care in other populations of patients with CLD. There is an abundance of research supporting the role of integrated care in improving health outcomes across many chronic diseases, including implementation of mental health into primary care in large health care systems like the VA health care system.93 Health care systems should work toward alignment of resources to meet these needs in specialty care settings, such as liver disease care in order optimize both liver disease and MHD/SUD outcomes for these patients.
Click here to read the digital edition.
Chronic liver disease (CLD) encompasses a spectrum of common diseases associated with high morbidity and mortality. In 2010, cirrhosis, or advanced-stage CLD, was the eighth leading cause of death in the U.S., accounting for about 49,500 deaths.1 The leading causes of CLD are hepatitis C virus (HCV), which affects about 3.6 million people in the US; nonalcoholic fatty liver disease (NAFLD), which has been increasing in prevalence in up to 75% of CLD cases; and alcohol misuse.2,3 Substance use disorders (SUDs) are a common cause of CLD. About one-third of cirrhosis cases can be attributed to alcohol use, and there is a strong association between IV drug use and HCV. Individual studies point to the high prevalence of mental health disorders (MHDs) among patients with CLD.4-19 It is clear that mental health disorders and SUDs impact outcomes for patients with CLD such that addressing these co-occurring disorders is critical to caring for this population.
An integrated or multidisciplinary approach to medical care attempts to coordinate the delivery of health and social care to patients with complex disease and comorbidities.20 Integrated care models have been shown to positively impact outcomes in many chronic diseases. For example, in patients with heart failure, multidisciplinary interventions such as home visits, remote physiologic monitoring, telehealth, telephone follow-up, or a hospital/clinic team-based intervention have been shown to reduce both hospital admissions and all-cause mortality.21 Similarly, there have been studies in patients with CLD exploring integrated care models. Although individual studies have assessed outcomes associated with various MHDs/SUDs among patients with different etiologies of liver disease, this review assesses the role of integrated care models for patients with CLD and MHDs/SUDs across etiologies.
Methods
A search of the PubMed database was conducted in November 2016 with the following keywords: “liver disease” and “mental health,” “liver disease” and “depression,” “liver disease” and “integrated care,” “substance use” and “liver disease,” “integrated care” and “hepatitis,” “integrated care” and “cirrhosis,” “integrated care” and “advanced liver disease,” and “integrated care” and “alcoholic liver disease” or “nonalcoholic fatty liver disease.” Articles covered a range of study types, including qualitative and quantitative analyses as well as other systematic reviews on focused topics within the area of interest. The authors reviewed the abstracts for eligibility criteria, which included topics focused on the study of mental health or substance use aspects and/or integrated mental health/substance use care for liver diseases (across etiologies and stages), published from January 2004 to November 2016, written in English, and focused on an adult population. Five members of the research team reviewed abstracts and eliminated any that did not meet the eligibility criteria.
A total of 636 records were screened and 378 were excluded based on abstract relevance to the stated topics as well as eligibility criteria. Following this review, full articles (N = 263) were reviewed by at least 2 members of the research team. For both levels of review, articles were removed for the criteria above and additional exclusion criteria: editorial style articles, duplicates, transplant focus, or primarily focused on health-related quality of life (QOL) not specific to MHDs. Although many articles fit more than one exclusion criteria, an article was removed once it met one exclusion criteria. After individual assessment by members of the research team, 71 articles were kept in the review. The team identified 14 additional articles that contributed to the topic but were not located through the original database search. The final analysis included 85 articles that fell into 3 key areas: (1) prevalence of comorbid MHD/SUD in liver disease; (2) associations between MHD/SUD and disease progression/management; and (3) the use of integrated care models in patients with CLD.
Results
In general, depression and anxiety were common among patients with CLD regardless of etiology.5 Across VA and non-VA studies, depressive disorders were found in one-third to two-thirds of patients with CLD and anxiety disorders in about one-third of patients with CLD. 5,7,8,10,15,16, 22-25Results of the studies that assess the prevalence of MHDs in patients with CLD are shown in Table 1.
MHDs and SUDs in Patients With CLD
Mental health symptoms have been associated with the severity of liver disease in some but not all studies.17,18,26 Mental health disorders also may have more dire consequences in this population. In a national survey of adults, 1.6% of patients with depression were found to have liver disease. Among this group with depression, suicide attempts were 3-fold higher among patients with CLD vs patients without CLD.19
Substance use disorders (including alcohol) are common among patients with CLD. This has been best studied in the context of patients with HCV.22, 27-32 For example among patients with HCV, the prevalence of injection drug use (IDU) was 48% to 65%, and the prevalence of marijuana use was 29%.33-36 In a report of 174,302 veterans with HCV receiving VA care, the following SUDs were reported as diagnosis in this patient population: alcohol, 55%; cannabis, 26%; stimulants, 35%; opioids, 22%; sedatives or anxiolytics, 5%; and other drug use, 39%.10
Both Non-VA and VA studies have found overlap between HCV and alcohol-related liver disease with a number of patients with HCV using alcohol and a number of patients with alcohol-related liver disease having a past history of IDU and HCV.37,38 Across VA and non-VA studies, patients with HIV/HCV co-infection have been found to have particularly high rates of MHDs and SUDs. One VA retrospective cohort study of 18,349 HIV-infected patients noted 37% were seropositive for HCV as well.39-41 These patients with HIV/HCV infection when compared with patients with only HIV infection were more likely to have a diagnosis of mental health illness (76.1% vs 63.1%), depression (56.6% vs 45.6%), alcohol abuse (64.2% vs 30.1%), substance abuse (68.0% vs 25.7%), and hard drug use (62.9% vs 20.6%).42 Patients with CLD and ongoing alcohol use have been found to have increased mental health symptoms compared with patients without ongoing alcohol use.17 Thus MHDs and SUDs are common and often coexist among patients with CLD.
MHDs Impact Patient Outcomes
Mental health disorders can affect how providers care for patients. In the past, for example, in both VA and non-VA studies, patients were often excluded from interferon-based HCV treatments due to MHDs.22,35,43-45 These exclusions included psychiatric issues (35%), alcohol abuse (31%), drug abuse (9%), or > 1 of these reasons (26%).46 Depression also has been associated with decreased care seeking by patients. Patients with cirrhosis and depression often do not seek medical care due to perceived stigma.47 Nearly one-fifth of patients with HCV in one study reported that they did not share information about their disease with others to avoid being stigmatized.48 Other studies have noted similar difficulty with patients’ seeking HCV treatment, advances in medications notwithstanding.49-52
Depression among patients with cirrhosis has been associated with reduced QOL, worsened cognitive function, increased mortality, and frailty.18,53,54 Psychiatric symptoms have been associated with disability and pain among patients with cirrhosis and with weight gain among patients with NAFLD.5,55 Mental health symptoms also predicted lower work productivity in patients with HCV.8 Histologic changes in the liver have been described among patients with psychiatric disorders, although the mechanism is not well understood.15,16
Although not a focus of this review, it is well established that MHDs are associated with increased substance use. Since there is a well-established connection between alcohol and adverse liver-related outcomes regardless of etiology of liver disease, mental health is thus indirectly linked to poor liver outcomes through this mechanism.37,38,56-67
Integrated Care in Liver Disease
Although there are no set guidelines on how to approach patients with liver disease and MHD/SUD comorbidities, integrated care approaches that include attention to both CLD and psychiatric needs seem promising. Integrated care models have been recommended by several authors specifically for patients with HCV and co-occurring MHDs and SUDs.4,33,42,43,45,68-72 Various integrated care models for CLD and psychiatric comorbidities have been studied and are detailed in Table 2.
The most well described models of integrated care in CLD have been used for patients with HCV as noted in prior reviews.22,34,49,73 These studies included liver care integrated with substance abuse clinics/specialists, mental health professionals, and/or case managers. Outcomes that have been assessed include adherence, HCV treatment completion, HCV treatment eligibility/initiation, and reduction in alcohol use.31,46, 74-77 A large randomized controlled trial (RCT) comparing integrated care with usual care found that integrated care, including collaborative consultation with mental health providers and case managers, was associated with increased antiviral treatment and sustained virologic response (SVR).50,78 One study of integrated care in the era of direct-acting antiviral treatment for HCV found that twice as many veterans initiated treatment with integrated care (with case management and a mental health provider) as opposed to usual care. In this integrated care model, mental health providers provided ongoing brief psychological interventions designed to address the specific risk factors identified at screening, facilitated treatment, and served as a regular contact.79 Overall, integrating mental health care and HCV care has resulted in increased adherence, increased treatment eligibility/initiation, treatment completion, higher rates of SVR, and reduction in alcohol use.31,46,74-77
In addition to positive medical outcomes with integrated care models, patients and providers generally have favorable impressions of the clinics using an integrated care approach. For example, multiple qualitative studies of the Hepatitis C Community Clinic in New Zealand have described that patients and providers have positive feelings about integrated care models for HCV.80-82 Another study evaluating integrated care at 4 hepatitis clinics in British Columbia, Canada found that clients overall valued the clinic and viewed it favorably; however, they identified several areas for continued improvement, including communication and time spent with clients, follow-up and access to care, as well as education on coping and managing their disease.83
Beyond HCV, other patients with CLD could benefit from integrated care approaches. Given the association of psychiatric symptoms with weight outcomes among patients with NAFLD, integrating behavioral support has been recommended.55 Multidisciplinary care has been trialed in patients with NAFLD. One model included behavioral therapy with psychological counseling, motivation for lifestyle changes, and support by a trained expert cognitive behavioral psychologist. Although this study did not include a control group, the patients in the study experienced an 8% weight reduction, reduction in aminotransferases, and decreased hepatic steatosis by ultrasound.84
Integrated care also has been advocated for patients with alcohol-related liver disease. One study recommended creating a personalized framework to support self-management for this population.85 Another study assessed patients with alcohol-related cirrhosis and hepatic encephalopathy and recommended integrating individual coping strategies and support into liver care for this group of patients.86
A United Kingdom study of multidisciplinary care that included a team of gastroenterologists, psychiatrists, and a psychiatric liaison nurse, found improved accessibility to care and patient/family satisfaction using this model. Outpatient appointments were offered to 84% of patients after collaborative care was introduced as opposed to 12% previously. Patients and family members reported that this approach decreased the stigma of mental health care, allowing patients to be more open to intervention and education in this setting.87 A systematic review of patients with alcohol-related CLD found that among 5 RCTs with 1,945 cumulative patients, integrated care was associated with increased short-term abstinence but not sustained abstinence.88 Thus integrated care has been used most in patients with HCV-related CLD, but growing evidence supports its use for patients with other etiologies of CLD, including NAFLD and alcohol.
Discussion
This review found that MHDs are common among patients with CLD and that there is an association between the worsening of liver disease outcomes for patients with comorbid mental health and substance use diagnoses as well as an association of poor MHD/SUD outcomes among patients with CLD (eg, increased suicide attempts among those with comorbid CLD and depression). These data synthesis support screening for MHDs in patients with CLD and providing integrated or multidisciplinary care where possible. Integrated care provides both mental health and CLD care in a combined setting. Integrated care models have been associated with improved health outcomes in patients with CLD and psychiatric comorbidities, including increased adherence, increased HCV treatment eligibility; initiation, and completion; higher rates of HCV treatment cure; reduction in alcohol use; and increased weight loss among patients with NAFLD.
Integrated care is becoming the standard of care for patients with CLD in many countries with national medical care systems. Scotland, for example, initiated an HCV action plan that included mental health and social care. It reported a reduced incidence of HCV infection among patients with a history of IDU, increased treatment initiation, and increased HCV testing with this approach.89 Multidisciplinary care is a class 1 level B recommendation for HCV care in Canada, meaning that it is the highest class of evidence and is supported by at least 1 randomized or multiple nonrandomized studies.90 Similarly, the US Department of Health and Human Services has developed a “National Viral Hepatitis Action Plan” with more than 20 participating federal agencies. The plan highlights the importance of integrating public health and clinical services to successfully improve viral hepatitis care, prevention, and treatment across the US.
The content of the integrated care interventions has been variable. Models with the highest success of liver disease outcomes in this study seem to have screened patients for MHDs and/or SUDs and then used trained professionals to address these issues while also focusing on liver care. An approach that includes evidence-based treatments or intervention for MHDs/SUDs is likely preferable to nonspecific support or information giving. However, it is notable that even minimal interventions (eg, providing informational materials) have been associated with improved outcomes in CLD. The actual implementation of integrated care for MHDs/SUDs into liver care likely has to be tailored to the context and available resources.
One study proposed several models of integrated care that can be adapted to the available resources of a given clinical practice setting. These included fully integrated models where services are colocated, collaborative practice models in which there is a strong relationship between providers in hepatology and mental health and SUD clinics, and then hybrid models that integrate/colocate when possible and collaborate when colocation isn’t available. Although the fully integrated care model likely is the most ideal, any multidisciplinary approach has the potential to decrease barriers and increase access to treatment.91
Another study used modeling to develop an integrated care framework for vulnerable veterans with HCV that incorporated both implementation factors (eg, research evidence, clinical experience, facilitation, and leadership) based on the Promoting Action on Research Implementation in Health Services framework and patients’ factors from the Andersen Behavioral Model (eg, geography and finances) to form a hybrid framework for this population.92
Limitations
There are several notable limitations of this review. Although the review focused on depression, anxiety, and SUDs, given the high prevalence of these disorders, other MHDs are also common among patients with CLD and were not addressed. For example, veterans with HCV also commonly had posttraumatic stress disorder, bipolar disorder, and schizophrenia.10 Further investigation should focus on these disorders and their impacts. Additionally, the authors did not specifically search for alcohol-related care in the search terms. This review also did not address nonpsychiatric types of integrated care, which could be the focus of future reviews. Despite these limitations, this review provides support for the use of integrated care in the context of CLD and co-occurring MHDs and SUDs.
Conclusion
Several studies support integrated care for patients with liver disease and co-occurring psychiatric disorders. There are multiple integrated care models in place, although they have largely been used in patients with HCV. More studies are needed to assess the role of integrated mental health care in other populations of patients with CLD. There is an abundance of research supporting the role of integrated care in improving health outcomes across many chronic diseases, including implementation of mental health into primary care in large health care systems like the VA health care system.93 Health care systems should work toward alignment of resources to meet these needs in specialty care settings, such as liver disease care in order optimize both liver disease and MHD/SUD outcomes for these patients.
Click here to read the digital edition.
1. Murray CJ, Atkinson C, Bhalla K, et al; US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608.
2. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.e1-e6.
3. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524-530.e1; quiz e60.
4. Neuman MG, Monteiro M, Rehm J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Subst Use Misuse. 2006;41(10-12):1395-1463.
5. Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(5):1009-1016.
6. Weinstein AA, Kallman Price J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52(2):127-132.
7. Erim Y, Tagay S, Beckmann M, et al. Depression and protective factors of mental health in people with hepatitis C: a questionnaire survey. Int J Nurs Stud. 2010;47(3):342-349.
8. Younossi I, Weinstein A, Stepanova M, Hunt S, Younossi ZM. Mental and emotional impairment in patients with hepatitis C is related to lower work productivity. Psychosomatics. 2016;57(1):82-88.
9. Carta MG, Angst J, Moro MF, et al. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord. 2012;141(2-3):361-366.
10. Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37(1):131-143.
11. Birerdinc A, Afendy A, Stepanova M, Younossi I, Baranova A, Younossi ZM. Gene expression profiles associated with depression in patients with chronic hepatitis C (CH-C). Brain Behav. 2012;2(5):525-531.
12. Patterson AL, Morasco BJ, Fuller BE, Indest DW, Loftis JM, Hauser P. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
13. Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27(6):431-438.
14. Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40(suppl 5):S286-S291.
15. Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68(4):563-569.
16. Youssef NA, Abdelmalek MF, Binks M, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33(7):1062-1070.
17. Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593-600.
18. Cron DC, Friedman JF, Winder GS, et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transplant. 2016;16(6):1805-1811.
19. Le Strat Y, Le Foll B, Dubertret C. Major depression and suicide attempts in patients with liver disease in the United States. Liver Int. 2015;35(7):1910-1916.
20. Lemmens LC, Molema CC, Versnel N, Baan CA, de Bruin SR. Integrated care programs for patients with psychological comorbidity: a systematic review and meta-analysis. J Psychosom Res. 2015;79(6):580-594.
21. Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899-906.
22. Ho SB, Groessl E, Dollarhide A, Robinson S, Kravetz D, Dieperink E. Management of chronic hepatitis C in veterans: the potential of integrated care models. Am J Gastroenterol. 2008;103(7):1810-1823.
23. Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21(8):2269-2280.
24. Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. 2013;54(1):52-59.
25. Rosenthal E, Cacoub P. Extrahepatic manifestations in chronic hepatitis C virus carriers. Lupus. 2015;24(4-5):469-482.
26. Duan Z, Kong Y, Zhang J, Guo H. Psychological comorbidities in Chinese patients with acute-on-chronic liver failure. Gen Hosp Psychiatry. 2012;34(3):276-281.
27. Cariello R, Federico A, Sapone A, et al. Intestinal permeability in patients with chronic liver diseases: its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42(3):200-204.
28. Wise M, Finelli L, Sorvillo F. Prognostic factors associated with hepatitis C disease: a case-control study utilizing U.S. multiple-cause-of-death data. Public Health Rep. 2010;125(3):414-422.
29. Wurst FM, Dürsteler-MacFarland KM, Auwaerter V, et al. Assessment of alcohol use among methadone maintenance patients by direct ethanol metabolites and self-reports. Alcohol Clin Exp Res. 2008;32(9):1552-1557.
30. Campbell JV, Hagan H, Latka MH, et al; The STRIVE Project. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend. 2006;81(3):259-265.
31. Dieperink E, Fuller B, Isenhart C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction. 2014;109(11):1869-1877.
32. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705-714
33. Arain A, Robaeys G. Eligibility of persons who inject drugs for treatment of hepatitis C virus infection. World J Gastroenterol. 2014;20(36):12722-12733.
34. North CS, Hong BA, Kerr T. Hepatitis C and substance use: new treatments and novel approaches. Curr Opin Psychiatry. 2012;25(3):206-212.
35. Coffin PO, Reynolds A. Ending hepatitis C in the United States: the role of screening. Hepat Med. 2014;6:79-87.
36. Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28(7):381-384.
37. Kamal A, Cheung R. Positive CAGE screen correlates with cirrhosis in veterans with chronic hepatitis C. Dig Dis Sci. 2007;52(10):2564-2569.
38. Fuster D, Sanvisens A, Bolao F, et al. Impact of hepatitis C virus infection on the risk of death of alcohol-dependent patients. J Viral Hepat. 2015;22(1):18-24.
39. Klein MB, Rollet KC, Saeed S, et al; Canadian HIV-HCV Cohort Investigators. HIV and hepatitis C virus coinfection in Canada: challenges and opportunities for reducing preventable morbidity and mortality. HIV Med. 2013;14(1):10-20.
40. Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006;3(4):176-181.
41. Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(suppl 3):S99-S105.
42. Backus LI, Boothroyd D, Deyton LR. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19(suppl 3):S13-S19.
43. Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126-133.
44. Gidding HF, Law MG, Amin J, et al; ACHOS Investigator Team. Predictors of deferral of treatment for hepatitis C infection in Australian clinics. Med J Aust. 2011;194(8):398-402.
45. Chainuvati S, Khalid SK, Kancir S, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat. 2006;13(4):235-241.
46. Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011; 106(10):1777-1786.
47. Vaughn-Sandler V, Sherman C, Aronsohn A, Volk ML. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci. 2014;59(3):681-686.
48. Blasiole JA, Shinkunas L, Labrecque DR, Arnold RM, Zickmund SL. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World J Gastroenterol. 2006;12(29):4665-4672.
49. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(suppl 2):S56-S61.
50. Groessl EJ, Sklar M, Cheung RC, Bräu N, Ho SB. Increasing antiviral treatment through integrated hepatitis C care: a randomized multicenter trial. Contemp Clin Trials. 2013;35(2):97-107.
51. Alavi M, Grebely J, Micallef M, et al; Enhancing Treatment for Hepatitis C in Opioid Substitution Settings (ETHOS) Study Group. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis. 2013;57(suppl 2):S62-S69.
52. Evon DM, Golin CE, Fried MW, Keefe FJ. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? J Consult Clin Psychol. 2013;81(2):361-374.
53. Mullish BH, Kabir MS, Thursz MR, Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther. 2014;40(8):880-892.
54. Stewart CA, Enders FT, Mitchell MM, Felmlee-Devine D, Smith GE. The cognitive profile of depressed patients with cirrhosis. Prim Care Companion CNS Disord. 2011;13(3):pii. PCC.10m01090
55. Stewart KE, Haller DL, Sargeant C, Levenson JL, Puri P, Sanyal AJ. Readiness for behaviour change in non-alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015;35(3):936-943.
56. Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3(11):1150-1159.
57. Chaudhry AA, Sulkowski MS, Chander G, Moore RD. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10(3):133-142.
58. McMahon BJ, Bruden D, Bruce MG, et al. Adverse outcomes in Alaska natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010;138(3):922-931.e1.
59. Anand BS, Thornby J. Alcohol has no effect on hepatitis C virus replication: a meta-analysis. Gut. 2005;54(10):1468-1472.
60. Au DH, Kivlahan DR, Bryson CL, Blough D, Bradley KA. Alcohol screening scores and risk of hospitalizations for GI conditions in men. Alcohol Clin Exp Res. 2007;31(3):443-451.
61. Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(suppl 1):77-84.
62. Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31(9):1460-1466.
63. Barve S, Kapoor R, Moghe A, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33(3):229-236.
64. Trimble G, Zheng L, Mishra A, Kalwaney S, Mir HM, Younossi ZM. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther. 2013;38(6):596-602.
65. Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8(10):891-898.e1-e2.
66. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
67. Lim JK, Tate JP, Fultz SL, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis. 2014;58(10):1449-1458.
68. Kanwal F, White DL, Tavakoli-Tabasi S, et al. Many patients with interleukin 28B genotypes associated with response to therapy are ineligible for treatment because of comorbidities. Clin Gastroenterol Hepatol. 2014;12(2):327-333.e1.
69. Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19(suppl 3):S179-S189.
70. McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22(2):133-137.
71. Treloar C, Rance J, Dore GJ, Grebely J; ETHOS Study Group. Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ETHOS study. J Viral Hepat. 2014;21(8):560-567.
72. Treloar C, Rance J, Grebely J, Dore GJ. Client and staff experiences of a co-located service for hepatitis C care in opioid substitution treatment settings in New South Wales, Australia. Drug Alcohol Depend. 2013;133(2):529-534.
73. Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(suppl 5):S276-S285.
74. Martinez AD, Dimova R, Marks KM, et al. Integrated internist—addiction medicine— hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19(1):47-54.
75. Fahey S. Developing a nursing service for patients with hepatitis C. Nurs Stand. 2007;21(43):35-40.
76. Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101(10):2254-2262.
77. Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51(2):149-156.
78. Ho SB, Bräu N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol. 2015;13(11):2005-2014.e1-e3.
79. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
80. Treloar C, Gray R, Brener L. A piece of the jigsaw of primary care: health professional perceptions of an integrated care model of hepatitis C management in the community. J Prim Health Care. 2014;6(2):129-134.
81. Brener L, Gray R, Cama EJ, Treloar C. “Makes you wanna do treatment”: benefits of a hepatitis C specialist clinic to clients in Christchurch, New Zealand. Health Soc Care Community. 2013;21(2):216-223.
82. Horwitz R, Brener L, Treloar C. Evaluation of an integrated care service facility for people living with hepatitis C in New Zealand. Int J Integr Care. 2012;12(Spec Ed Integrated Care Pathways):e229.
83. Christianson TM, Moralejo D. Assessing the quality of care in a regional integrated viral hepatitis clinic in British Columbia: a cross-sectional study. Gastroenterol Nurs. 2009;32(5):315-324.
84. Scaglioni F, Marino M, Ciccia S, et al. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD). Clin Res Hepatol Gastroenterol. 2013;37(4):353-358.
85. Lau-Walker M, Presky J, Webzell I, Murrells T, Heaton N. Patients with alcohol-related liver disease—beliefs about their illness and factors that influence their self-management. J Adv Nurs. 2016;72(1):173-185.
86. Mikkelsen MR, Hendriksen C, Schiødt FV, Rydahl-Hansen S. Coping and rehabilitation in alcoholic liver disease patients after hepatic encephalopathy—in interaction with professionals and relatives. J Clin Nurs. 2015;24(23-24):3627-3637.
87. Moriarty KJ, Platt H, Crompton S, et al. Collaborative care for alcohol-related liver disease. Clin Med (Lond). 2007;7(2):125-128.
88. Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol. 2016;14(2):191-202.e1-e4;quiz e20.
89. Wylie L, Hutchinson S, Liddell D, Rowan N. The successful implementation of Scotland’s Hepatitis C Action Plan: what can other European stakeholders learn from the experience? A Scottish voluntary sector perspective. BMC Infect Dis. 2014;14(suppl 6):S7.
90. Hull M, Shafran S, Wong A, et al. CIHR Canadian HIV trials network coinfection and concurrent diseases core research group: 2016 updated Canadian HIV/hepatitis C adult guidelines for management and treatment. Can J Infect Dis Med Microbiol. 2016;2016:4385643.
91. Bonner JE, Barritt AS 4th, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci. 2012;57(6):1469-1474.
92. Rongey C, Asch S, Knight SJ. Access to care for vulnerable veterans with hepatitis C: a hybrid conceptual framework and a case study to guide translation. Transl Behav Med. 2011;1(4):644-651.
93. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs health care system. J Clin Psychol Med Settings. 2008;15(1):73-78.
94. Drumright LN, Hagan H, Thomas DL, et al. Predictors and effects of alcohol use on liver function among young HCV-infected injection drug users in a behavioral intervention. J Hepatol. 2011;55(1):45-52.
1. Murray CJ, Atkinson C, Bhalla K, et al; US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608.
2. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.e1-e6.
3. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524-530.e1; quiz e60.
4. Neuman MG, Monteiro M, Rehm J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Subst Use Misuse. 2006;41(10-12):1395-1463.
5. Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(5):1009-1016.
6. Weinstein AA, Kallman Price J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52(2):127-132.
7. Erim Y, Tagay S, Beckmann M, et al. Depression and protective factors of mental health in people with hepatitis C: a questionnaire survey. Int J Nurs Stud. 2010;47(3):342-349.
8. Younossi I, Weinstein A, Stepanova M, Hunt S, Younossi ZM. Mental and emotional impairment in patients with hepatitis C is related to lower work productivity. Psychosomatics. 2016;57(1):82-88.
9. Carta MG, Angst J, Moro MF, et al. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord. 2012;141(2-3):361-366.
10. Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37(1):131-143.
11. Birerdinc A, Afendy A, Stepanova M, Younossi I, Baranova A, Younossi ZM. Gene expression profiles associated with depression in patients with chronic hepatitis C (CH-C). Brain Behav. 2012;2(5):525-531.
12. Patterson AL, Morasco BJ, Fuller BE, Indest DW, Loftis JM, Hauser P. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
13. Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27(6):431-438.
14. Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40(suppl 5):S286-S291.
15. Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68(4):563-569.
16. Youssef NA, Abdelmalek MF, Binks M, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33(7):1062-1070.
17. Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593-600.
18. Cron DC, Friedman JF, Winder GS, et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transplant. 2016;16(6):1805-1811.
19. Le Strat Y, Le Foll B, Dubertret C. Major depression and suicide attempts in patients with liver disease in the United States. Liver Int. 2015;35(7):1910-1916.
20. Lemmens LC, Molema CC, Versnel N, Baan CA, de Bruin SR. Integrated care programs for patients with psychological comorbidity: a systematic review and meta-analysis. J Psychosom Res. 2015;79(6):580-594.
21. Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899-906.
22. Ho SB, Groessl E, Dollarhide A, Robinson S, Kravetz D, Dieperink E. Management of chronic hepatitis C in veterans: the potential of integrated care models. Am J Gastroenterol. 2008;103(7):1810-1823.
23. Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21(8):2269-2280.
24. Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. 2013;54(1):52-59.
25. Rosenthal E, Cacoub P. Extrahepatic manifestations in chronic hepatitis C virus carriers. Lupus. 2015;24(4-5):469-482.
26. Duan Z, Kong Y, Zhang J, Guo H. Psychological comorbidities in Chinese patients with acute-on-chronic liver failure. Gen Hosp Psychiatry. 2012;34(3):276-281.
27. Cariello R, Federico A, Sapone A, et al. Intestinal permeability in patients with chronic liver diseases: its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42(3):200-204.
28. Wise M, Finelli L, Sorvillo F. Prognostic factors associated with hepatitis C disease: a case-control study utilizing U.S. multiple-cause-of-death data. Public Health Rep. 2010;125(3):414-422.
29. Wurst FM, Dürsteler-MacFarland KM, Auwaerter V, et al. Assessment of alcohol use among methadone maintenance patients by direct ethanol metabolites and self-reports. Alcohol Clin Exp Res. 2008;32(9):1552-1557.
30. Campbell JV, Hagan H, Latka MH, et al; The STRIVE Project. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend. 2006;81(3):259-265.
31. Dieperink E, Fuller B, Isenhart C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction. 2014;109(11):1869-1877.
32. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705-714
33. Arain A, Robaeys G. Eligibility of persons who inject drugs for treatment of hepatitis C virus infection. World J Gastroenterol. 2014;20(36):12722-12733.
34. North CS, Hong BA, Kerr T. Hepatitis C and substance use: new treatments and novel approaches. Curr Opin Psychiatry. 2012;25(3):206-212.
35. Coffin PO, Reynolds A. Ending hepatitis C in the United States: the role of screening. Hepat Med. 2014;6:79-87.
36. Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28(7):381-384.
37. Kamal A, Cheung R. Positive CAGE screen correlates with cirrhosis in veterans with chronic hepatitis C. Dig Dis Sci. 2007;52(10):2564-2569.
38. Fuster D, Sanvisens A, Bolao F, et al. Impact of hepatitis C virus infection on the risk of death of alcohol-dependent patients. J Viral Hepat. 2015;22(1):18-24.
39. Klein MB, Rollet KC, Saeed S, et al; Canadian HIV-HCV Cohort Investigators. HIV and hepatitis C virus coinfection in Canada: challenges and opportunities for reducing preventable morbidity and mortality. HIV Med. 2013;14(1):10-20.
40. Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006;3(4):176-181.
41. Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(suppl 3):S99-S105.
42. Backus LI, Boothroyd D, Deyton LR. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19(suppl 3):S13-S19.
43. Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126-133.
44. Gidding HF, Law MG, Amin J, et al; ACHOS Investigator Team. Predictors of deferral of treatment for hepatitis C infection in Australian clinics. Med J Aust. 2011;194(8):398-402.
45. Chainuvati S, Khalid SK, Kancir S, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat. 2006;13(4):235-241.
46. Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011; 106(10):1777-1786.
47. Vaughn-Sandler V, Sherman C, Aronsohn A, Volk ML. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci. 2014;59(3):681-686.
48. Blasiole JA, Shinkunas L, Labrecque DR, Arnold RM, Zickmund SL. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World J Gastroenterol. 2006;12(29):4665-4672.
49. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(suppl 2):S56-S61.
50. Groessl EJ, Sklar M, Cheung RC, Bräu N, Ho SB. Increasing antiviral treatment through integrated hepatitis C care: a randomized multicenter trial. Contemp Clin Trials. 2013;35(2):97-107.
51. Alavi M, Grebely J, Micallef M, et al; Enhancing Treatment for Hepatitis C in Opioid Substitution Settings (ETHOS) Study Group. Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis. 2013;57(suppl 2):S62-S69.
52. Evon DM, Golin CE, Fried MW, Keefe FJ. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? J Consult Clin Psychol. 2013;81(2):361-374.
53. Mullish BH, Kabir MS, Thursz MR, Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther. 2014;40(8):880-892.
54. Stewart CA, Enders FT, Mitchell MM, Felmlee-Devine D, Smith GE. The cognitive profile of depressed patients with cirrhosis. Prim Care Companion CNS Disord. 2011;13(3):pii. PCC.10m01090
55. Stewart KE, Haller DL, Sargeant C, Levenson JL, Puri P, Sanyal AJ. Readiness for behaviour change in non-alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015;35(3):936-943.
56. Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3(11):1150-1159.
57. Chaudhry AA, Sulkowski MS, Chander G, Moore RD. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10(3):133-142.
58. McMahon BJ, Bruden D, Bruce MG, et al. Adverse outcomes in Alaska natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010;138(3):922-931.e1.
59. Anand BS, Thornby J. Alcohol has no effect on hepatitis C virus replication: a meta-analysis. Gut. 2005;54(10):1468-1472.
60. Au DH, Kivlahan DR, Bryson CL, Blough D, Bradley KA. Alcohol screening scores and risk of hospitalizations for GI conditions in men. Alcohol Clin Exp Res. 2007;31(3):443-451.
61. Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(suppl 1):77-84.
62. Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31(9):1460-1466.
63. Barve S, Kapoor R, Moghe A, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33(3):229-236.
64. Trimble G, Zheng L, Mishra A, Kalwaney S, Mir HM, Younossi ZM. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther. 2013;38(6):596-602.
65. Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol. 2010;8(10):891-898.e1-e2.
66. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
67. Lim JK, Tate JP, Fultz SL, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis. 2014;58(10):1449-1458.
68. Kanwal F, White DL, Tavakoli-Tabasi S, et al. Many patients with interleukin 28B genotypes associated with response to therapy are ineligible for treatment because of comorbidities. Clin Gastroenterol Hepatol. 2014;12(2):327-333.e1.
69. Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19(suppl 3):S179-S189.
70. McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22(2):133-137.
71. Treloar C, Rance J, Dore GJ, Grebely J; ETHOS Study Group. Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ETHOS study. J Viral Hepat. 2014;21(8):560-567.
72. Treloar C, Rance J, Grebely J, Dore GJ. Client and staff experiences of a co-located service for hepatitis C care in opioid substitution treatment settings in New South Wales, Australia. Drug Alcohol Depend. 2013;133(2):529-534.
73. Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(suppl 5):S276-S285.
74. Martinez AD, Dimova R, Marks KM, et al. Integrated internist—addiction medicine— hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19(1):47-54.
75. Fahey S. Developing a nursing service for patients with hepatitis C. Nurs Stand. 2007;21(43):35-40.
76. Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101(10):2254-2262.
77. Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51(2):149-156.
78. Ho SB, Bräu N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol. 2015;13(11):2005-2014.e1-e3.
79. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
80. Treloar C, Gray R, Brener L. A piece of the jigsaw of primary care: health professional perceptions of an integrated care model of hepatitis C management in the community. J Prim Health Care. 2014;6(2):129-134.
81. Brener L, Gray R, Cama EJ, Treloar C. “Makes you wanna do treatment”: benefits of a hepatitis C specialist clinic to clients in Christchurch, New Zealand. Health Soc Care Community. 2013;21(2):216-223.
82. Horwitz R, Brener L, Treloar C. Evaluation of an integrated care service facility for people living with hepatitis C in New Zealand. Int J Integr Care. 2012;12(Spec Ed Integrated Care Pathways):e229.
83. Christianson TM, Moralejo D. Assessing the quality of care in a regional integrated viral hepatitis clinic in British Columbia: a cross-sectional study. Gastroenterol Nurs. 2009;32(5):315-324.
84. Scaglioni F, Marino M, Ciccia S, et al. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD). Clin Res Hepatol Gastroenterol. 2013;37(4):353-358.
85. Lau-Walker M, Presky J, Webzell I, Murrells T, Heaton N. Patients with alcohol-related liver disease—beliefs about their illness and factors that influence their self-management. J Adv Nurs. 2016;72(1):173-185.
86. Mikkelsen MR, Hendriksen C, Schiødt FV, Rydahl-Hansen S. Coping and rehabilitation in alcoholic liver disease patients after hepatic encephalopathy—in interaction with professionals and relatives. J Clin Nurs. 2015;24(23-24):3627-3637.
87. Moriarty KJ, Platt H, Crompton S, et al. Collaborative care for alcohol-related liver disease. Clin Med (Lond). 2007;7(2):125-128.
88. Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol. 2016;14(2):191-202.e1-e4;quiz e20.
89. Wylie L, Hutchinson S, Liddell D, Rowan N. The successful implementation of Scotland’s Hepatitis C Action Plan: what can other European stakeholders learn from the experience? A Scottish voluntary sector perspective. BMC Infect Dis. 2014;14(suppl 6):S7.
90. Hull M, Shafran S, Wong A, et al. CIHR Canadian HIV trials network coinfection and concurrent diseases core research group: 2016 updated Canadian HIV/hepatitis C adult guidelines for management and treatment. Can J Infect Dis Med Microbiol. 2016;2016:4385643.
91. Bonner JE, Barritt AS 4th, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci. 2012;57(6):1469-1474.
92. Rongey C, Asch S, Knight SJ. Access to care for vulnerable veterans with hepatitis C: a hybrid conceptual framework and a case study to guide translation. Transl Behav Med. 2011;1(4):644-651.
93. Zeiss AM, Karlin BE. Integrating mental health and primary care services in the Department of Veterans Affairs health care system. J Clin Psychol Med Settings. 2008;15(1):73-78.
94. Drumright LN, Hagan H, Thomas DL, et al. Predictors and effects of alcohol use on liver function among young HCV-infected injection drug users in a behavioral intervention. J Hepatol. 2011;55(1):45-52.
Another practice’s experiences in “dialing back opioids”
It is with much enthusiasm that we read the article “Dialing back opioids for chronic pain one conversation at a time” (J Fam Pract. 2018;67:753-757) about the author’s approach to opioid tapering. We have implemented a similar process in our own medical home practice, based on the continuity relationship and the Ecological Systems Theory.
The use of the human resources within the medical home—care coordinator, pharmacist, community health worker, etc— distributes the responsibility and lessens the burden of care for the family physician. The Ecological Systems Theory provides a structure for understanding the interaction between proximal influencers (eg, the team) and more distal influences (eg, national guidelines and institutional mandates).
Recently, we presented our findings at the 2018 North American Primary Care Research Group (NAPCRG) Annual Meeting. Our results showed a 50% decline in per capita medication use over an almost 14-month period.
We feel that opioid tapering provides both a counterpoint and a complementary method to medication-assisted therapies (MAT). A counterpoint, because MAT involves the diagnosis and treatment of opioid misuse disorder. At the core of that diagnosis is the question of whether all chronic opioid use should be labelled as “misuse.” Tapering involves no such diagnosis and focuses on the safety of minimal opioid use, which, when MAT is used appropriately, is also a primary concern.
We appreciate the approach that the authors took in their project and look forward to seeing further iterations.
Bharat Gopal, MD
Cristina Capannolo, DO
Corvallis, Ore
It is with much enthusiasm that we read the article “Dialing back opioids for chronic pain one conversation at a time” (J Fam Pract. 2018;67:753-757) about the author’s approach to opioid tapering. We have implemented a similar process in our own medical home practice, based on the continuity relationship and the Ecological Systems Theory.
The use of the human resources within the medical home—care coordinator, pharmacist, community health worker, etc— distributes the responsibility and lessens the burden of care for the family physician. The Ecological Systems Theory provides a structure for understanding the interaction between proximal influencers (eg, the team) and more distal influences (eg, national guidelines and institutional mandates).
Recently, we presented our findings at the 2018 North American Primary Care Research Group (NAPCRG) Annual Meeting. Our results showed a 50% decline in per capita medication use over an almost 14-month period.
We feel that opioid tapering provides both a counterpoint and a complementary method to medication-assisted therapies (MAT). A counterpoint, because MAT involves the diagnosis and treatment of opioid misuse disorder. At the core of that diagnosis is the question of whether all chronic opioid use should be labelled as “misuse.” Tapering involves no such diagnosis and focuses on the safety of minimal opioid use, which, when MAT is used appropriately, is also a primary concern.
We appreciate the approach that the authors took in their project and look forward to seeing further iterations.
Bharat Gopal, MD
Cristina Capannolo, DO
Corvallis, Ore
It is with much enthusiasm that we read the article “Dialing back opioids for chronic pain one conversation at a time” (J Fam Pract. 2018;67:753-757) about the author’s approach to opioid tapering. We have implemented a similar process in our own medical home practice, based on the continuity relationship and the Ecological Systems Theory.
The use of the human resources within the medical home—care coordinator, pharmacist, community health worker, etc— distributes the responsibility and lessens the burden of care for the family physician. The Ecological Systems Theory provides a structure for understanding the interaction between proximal influencers (eg, the team) and more distal influences (eg, national guidelines and institutional mandates).
Recently, we presented our findings at the 2018 North American Primary Care Research Group (NAPCRG) Annual Meeting. Our results showed a 50% decline in per capita medication use over an almost 14-month period.
We feel that opioid tapering provides both a counterpoint and a complementary method to medication-assisted therapies (MAT). A counterpoint, because MAT involves the diagnosis and treatment of opioid misuse disorder. At the core of that diagnosis is the question of whether all chronic opioid use should be labelled as “misuse.” Tapering involves no such diagnosis and focuses on the safety of minimal opioid use, which, when MAT is used appropriately, is also a primary concern.
We appreciate the approach that the authors took in their project and look forward to seeing further iterations.
Bharat Gopal, MD
Cristina Capannolo, DO
Corvallis, Ore
Opioid overdose risk greater among HIV patients
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
REPORTING FROM CROI 2019
Achieving recovery not a one-size-fits-all proposition
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
The experience of recovering from addiction can look different in different people, according to a Washington Post article. Some patients hit “rock bottom” and are able to climb back after connecting with a therapist. Others maintain sobriety by working with sponsors through 12-step programs. Still others are able to attain sobriety and maintain it by carefully vetting social invitations and bypassing situations in which drugs or lots of alcohol are involved. Medications that manage cravings are another intervention used by some of the 22 million Americans reportedly in recovery from drugs and alcohol. A major milestone for those seeking recovery is reaching the 3- to 5-year mark, said Robert D. Ashford, MSW, of the Substance Use Disorders Institute at the University of Pennsylvania, Philadelphia. “That benchmark can signal a reduced risk of returning to substance use because The Washington Post.
University life can be rewarding, but stress is a reality – and for some, that stress can either exacerbate or trigger mental health challenges. More universities have recognized the mental toll that campus life can exact and have put supports in place. At the University of California, Los Angeles, Internet-based screenings and online mental health treatment are offered with one-on-one personal contact with “resilience peers.” The latter are not licensed counselors, but they are trained to listen and provide an outlet for stressed students. The online help teaches skills that are useful in combating anxiety and depression. The goal is to help as many students as fast as possible. “This program fundamentally changed who I am and how I approach my life,” said UCLA student Nivi Ahlawat. “I may not remember the structures of all the intermediates of the glycolysis pathway I learned in biochemistry class. But I’ll remember what I’ve learned about active listening, motivational interviewing, and mindfulness intervention for the rest of my life.” Meanwhile, Kent (Ohio) State University has provided mental health training to more than 700 students, faculty, and staff. And at Jefferson Community College in Watertown, N.Y., mental health help includes a “wraparound” model that provides aid to economically disadvantaged students whose stress includes putting food on the table for their children. The New York Times.
Sen. Richard Briggs, MD, has proposed a resolution that seeks to loosen the purses of insurance companies in Tennessee, with the aim of better coverage for those with mental health or substance use issues who are seeking treatment. In introducing the resolution, Dr. Briggs noted that, despite the opioid crisis in his state, there is an “undeniable difference in coverage for mental health and substance abuse services for Tennesseans suffering from substance use disorder or opioid use disorder,” compared with the way other traditional diseases are covered and insured. “Mental illness is an illness just like any other medical illness, and should be treated and reimbursed to physicians in the same manner,” said Dr. Briggs, a heart and lung surgeon who served combat tours in Iraq and Afghanistan. NewsChannel 5 in Nashville.
“When I tell you I moved down to Miami for the weather, I really mean I moved to South Florida to escape my depression,” wrote Minhae Shim Roth. But for some, other factors get in the way. “The problem is that the heat and humidity can be so oppressive that people are forced indoors, negating the positive benefits of the sunshine,” said Daniel E. Jimenez, PhD, an assistant professor of psychiatry and behavioral sciences at the University of Miami. Last year, more than 560,000 Floridians – or 3.5% of the state’s adults – reportedly contemplated suicide, statistics show. Those stats are comparable with those of New York state. One difference, however, is that people in Miami are less willing to talk about mental health challenges, Ms. Roth suggested. “It’s easy to believe living in the Magic City is like a booze-, drug-, and fun-filled party that never stops. This pervasive hedonistic reputation makes it unpopular and shameful to admit you’re depressed. Everyone’s having fun, so why aren’t you?” Ms. Roth wrote. Those who seek help face an understaffed and underfunded system where an appointment with a psychiatrist can take months to secure. Help needs to come in other forms, according to Ms. Roth, and include “compassion and empathy, public initiatives aimed at combating the stigma of mental illness, greater accessibility to mental health services, and readily available intervention tools.” Miami New Times.
Seven in 10 U.S. teens see anxiety and depression as major problems among their peers. The concerns cut across gender, racial and socioeconomic lines, according to a survey of 920 teens aged 13-17 years. The major reason for the anxiety and depression is school, with 61% of the respondents feeling pressure to excel academically. Girls were far more likely than boys to say they planned to attend a 4-year college (68% vs. 51%). About half of the teens surveyed viewed drug addiction and alcohol consumption as major problems among people their age. Pew Research Center.
Addressing substance use in patients with intellectual disability: 5 Steps
Approximately 5% of patients with intellectual disability (ID) have a comorbid substance use disorder (SUD).1 These patients frequently abuse alcohol, tobacco, and cannabis, but are largely underdiagnosed and undertreated for SUDs. Treatment for SUDs in these patients is critical because substance abuse among patients with ID is associated with developing mood disorders, long-term health consequences, incarceration, and interpersonal instability.1 To ensure that these often-marginalized patients are adequately assessed and treated for SUDs, consider the following 5 steps.
1. Perform screening tests. Unfortunately, no substance use screening tests are validated specifically for patients with ID. When presented with mainstream screening tools, patients with ID could produce false positives or false negatives for 2 reasons:
- Patients with ID are more likely to respond in the affirmative to screening questions that they do not understand.
- Many screening questionnaires assume that patients possess an amount of knowledge and cognitive ability to abstract information that patients with ID may lack.
Clinicians should therefore adapt screening questions to better match the cognitive and communicative abilities of their patients with ID by simplifying sentences, using graphics, and avoiding negative phrases and confrontation. For example, while all-encompassing, the term “alcohol” may be confusing for some patients. Instead of broadly asking a patient, “Do you drink alcoholic beverages?” it may be necessary to specifically ask, “Do you drink wine?” or “Do you drink beer?” Similarly, it may be insufficient to ask a patient, “Do you smoke marijuana?” Instead, use colloquial terms (ie, weed, reefer) to ensure that the patient knows which substance you mean. Screening questions can be complemented by ordering urine drug testing and obtaining collateral information from caregivers.2
2. Use approved medications to treat SUDs. Medication-assisted treatment (MAT) is underprescribed for patients with ID. Medication compliance in patients with ID may be a concern; however, many of these patients are compliant with treatment because they often live with family members, in group homes, or in other settings where their medications are administered to them.
Also, be mindful of whether your patient has epilepsy. This condition is common among patients with ID,3 and some MAT can lower the seizure threshold. When starting and titrating MAT, always monitor patients carefully for benefits and adverse effects.4
3. Make a thorough assessment before recommending Alcoholics Anonymous or Narcotics Anonymous meetings. While the 12-step recovery model has proven benefits, the typical structure of 12-step meetings is not conducive to all patients with ID. Only recommend such meetings to patients who have 60- to 90-minute attention spans and demonstrate the cognitive, communicative, literacy, and social skills to fully engage during the meetings.5
4. Employ motivational interviewing. Many patients with ID have cursory knowledge of the health risks associated with substance abuse, particularly those with mild ID. Motivational interviewing techniques that include health education may help produce favorable outcomes in these patients.6
Continue to: Provide ongoing support
5. Provide ongoing support. Remember that addiction is a chronic disease with a risk of relapse. Provide continuous support for patients with ID and comorbid SUDs throughout all phases of their recovery, and refer them to addiction specialists, pain specialists, or psychotherapists as appropriate.
1. Chapman SL, Wu L. Substance abuse among individuals with intellectual disabilities. Res Dev Disabil. 2012;33(4):1147-1156.
2. Kiewik M, Vandernagel J, Engles, R, et al. Intellectually disabled and addicted: a call for evidence based tailor-made interventions. Addiction. 2017;112(45):20 67-2068.
3. Mcgrother C, Bhaumik S, Thorp C, et al. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15(6):376-386.
4. Connery H. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
5. Slayter E. Disparities in access to substance abuse treatment among people with intellectual disabilities and serious mental illness. Health Soc Work. 2010;35(1):49-59.
6. Frielink N, Schuengel C, Kroon A, et al. Pretreatment for substance-abusing people with intellectual disabilities: intervening on autonomous motivation for treatment entry. J Intellect Disabil Res. 2015;59(12):1168-1182.
Approximately 5% of patients with intellectual disability (ID) have a comorbid substance use disorder (SUD).1 These patients frequently abuse alcohol, tobacco, and cannabis, but are largely underdiagnosed and undertreated for SUDs. Treatment for SUDs in these patients is critical because substance abuse among patients with ID is associated with developing mood disorders, long-term health consequences, incarceration, and interpersonal instability.1 To ensure that these often-marginalized patients are adequately assessed and treated for SUDs, consider the following 5 steps.
1. Perform screening tests. Unfortunately, no substance use screening tests are validated specifically for patients with ID. When presented with mainstream screening tools, patients with ID could produce false positives or false negatives for 2 reasons:
- Patients with ID are more likely to respond in the affirmative to screening questions that they do not understand.
- Many screening questionnaires assume that patients possess an amount of knowledge and cognitive ability to abstract information that patients with ID may lack.
Clinicians should therefore adapt screening questions to better match the cognitive and communicative abilities of their patients with ID by simplifying sentences, using graphics, and avoiding negative phrases and confrontation. For example, while all-encompassing, the term “alcohol” may be confusing for some patients. Instead of broadly asking a patient, “Do you drink alcoholic beverages?” it may be necessary to specifically ask, “Do you drink wine?” or “Do you drink beer?” Similarly, it may be insufficient to ask a patient, “Do you smoke marijuana?” Instead, use colloquial terms (ie, weed, reefer) to ensure that the patient knows which substance you mean. Screening questions can be complemented by ordering urine drug testing and obtaining collateral information from caregivers.2
2. Use approved medications to treat SUDs. Medication-assisted treatment (MAT) is underprescribed for patients with ID. Medication compliance in patients with ID may be a concern; however, many of these patients are compliant with treatment because they often live with family members, in group homes, or in other settings where their medications are administered to them.
Also, be mindful of whether your patient has epilepsy. This condition is common among patients with ID,3 and some MAT can lower the seizure threshold. When starting and titrating MAT, always monitor patients carefully for benefits and adverse effects.4
3. Make a thorough assessment before recommending Alcoholics Anonymous or Narcotics Anonymous meetings. While the 12-step recovery model has proven benefits, the typical structure of 12-step meetings is not conducive to all patients with ID. Only recommend such meetings to patients who have 60- to 90-minute attention spans and demonstrate the cognitive, communicative, literacy, and social skills to fully engage during the meetings.5
4. Employ motivational interviewing. Many patients with ID have cursory knowledge of the health risks associated with substance abuse, particularly those with mild ID. Motivational interviewing techniques that include health education may help produce favorable outcomes in these patients.6
Continue to: Provide ongoing support
5. Provide ongoing support. Remember that addiction is a chronic disease with a risk of relapse. Provide continuous support for patients with ID and comorbid SUDs throughout all phases of their recovery, and refer them to addiction specialists, pain specialists, or psychotherapists as appropriate.
Approximately 5% of patients with intellectual disability (ID) have a comorbid substance use disorder (SUD).1 These patients frequently abuse alcohol, tobacco, and cannabis, but are largely underdiagnosed and undertreated for SUDs. Treatment for SUDs in these patients is critical because substance abuse among patients with ID is associated with developing mood disorders, long-term health consequences, incarceration, and interpersonal instability.1 To ensure that these often-marginalized patients are adequately assessed and treated for SUDs, consider the following 5 steps.
1. Perform screening tests. Unfortunately, no substance use screening tests are validated specifically for patients with ID. When presented with mainstream screening tools, patients with ID could produce false positives or false negatives for 2 reasons:
- Patients with ID are more likely to respond in the affirmative to screening questions that they do not understand.
- Many screening questionnaires assume that patients possess an amount of knowledge and cognitive ability to abstract information that patients with ID may lack.
Clinicians should therefore adapt screening questions to better match the cognitive and communicative abilities of their patients with ID by simplifying sentences, using graphics, and avoiding negative phrases and confrontation. For example, while all-encompassing, the term “alcohol” may be confusing for some patients. Instead of broadly asking a patient, “Do you drink alcoholic beverages?” it may be necessary to specifically ask, “Do you drink wine?” or “Do you drink beer?” Similarly, it may be insufficient to ask a patient, “Do you smoke marijuana?” Instead, use colloquial terms (ie, weed, reefer) to ensure that the patient knows which substance you mean. Screening questions can be complemented by ordering urine drug testing and obtaining collateral information from caregivers.2
2. Use approved medications to treat SUDs. Medication-assisted treatment (MAT) is underprescribed for patients with ID. Medication compliance in patients with ID may be a concern; however, many of these patients are compliant with treatment because they often live with family members, in group homes, or in other settings where their medications are administered to them.
Also, be mindful of whether your patient has epilepsy. This condition is common among patients with ID,3 and some MAT can lower the seizure threshold. When starting and titrating MAT, always monitor patients carefully for benefits and adverse effects.4
3. Make a thorough assessment before recommending Alcoholics Anonymous or Narcotics Anonymous meetings. While the 12-step recovery model has proven benefits, the typical structure of 12-step meetings is not conducive to all patients with ID. Only recommend such meetings to patients who have 60- to 90-minute attention spans and demonstrate the cognitive, communicative, literacy, and social skills to fully engage during the meetings.5
4. Employ motivational interviewing. Many patients with ID have cursory knowledge of the health risks associated with substance abuse, particularly those with mild ID. Motivational interviewing techniques that include health education may help produce favorable outcomes in these patients.6
Continue to: Provide ongoing support
5. Provide ongoing support. Remember that addiction is a chronic disease with a risk of relapse. Provide continuous support for patients with ID and comorbid SUDs throughout all phases of their recovery, and refer them to addiction specialists, pain specialists, or psychotherapists as appropriate.
1. Chapman SL, Wu L. Substance abuse among individuals with intellectual disabilities. Res Dev Disabil. 2012;33(4):1147-1156.
2. Kiewik M, Vandernagel J, Engles, R, et al. Intellectually disabled and addicted: a call for evidence based tailor-made interventions. Addiction. 2017;112(45):20 67-2068.
3. Mcgrother C, Bhaumik S, Thorp C, et al. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15(6):376-386.
4. Connery H. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
5. Slayter E. Disparities in access to substance abuse treatment among people with intellectual disabilities and serious mental illness. Health Soc Work. 2010;35(1):49-59.
6. Frielink N, Schuengel C, Kroon A, et al. Pretreatment for substance-abusing people with intellectual disabilities: intervening on autonomous motivation for treatment entry. J Intellect Disabil Res. 2015;59(12):1168-1182.
1. Chapman SL, Wu L. Substance abuse among individuals with intellectual disabilities. Res Dev Disabil. 2012;33(4):1147-1156.
2. Kiewik M, Vandernagel J, Engles, R, et al. Intellectually disabled and addicted: a call for evidence based tailor-made interventions. Addiction. 2017;112(45):20 67-2068.
3. Mcgrother C, Bhaumik S, Thorp C, et al. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15(6):376-386.
4. Connery H. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75.
5. Slayter E. Disparities in access to substance abuse treatment among people with intellectual disabilities and serious mental illness. Health Soc Work. 2010;35(1):49-59.
6. Frielink N, Schuengel C, Kroon A, et al. Pretreatment for substance-abusing people with intellectual disabilities: intervening on autonomous motivation for treatment entry. J Intellect Disabil Res. 2015;59(12):1168-1182.
Career Choices: Addiction psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Teens likely to mimic parents’ opioid use
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
FROM PEDIATRICS
Alcohol abstinence questioned as addiction treatment goal
BONITA SPRINGS, FLA. – Reductions in alcohol use bring about significant improvement in adverse consequences, mental health status, and quality of life, even if the reductions do not reach the level of abstinence, according to recent research presented at the annual meeting of the American Academy of Addiction Psychiatry.
The findings, experts say, demonstrate how a fixation on abstinence or elimination of all heavy drinking – the endpoints the Food and Drug Administration now require in pivotal clinical trials on alcohol use disorder (AUD) treatments – is shortsighted and can unnecessarily discourage people with alcohol use disorder from pursuing treatment.
“We need to think a little differently, or a little out of the box, from the way we’ve been thinking in the past,” said Raymond F. Anton, MD, professor of psychiatry at the Medical University of South Carolina in Charleston and chair of the Alcohol Clinical Trials Initiative (ACTIVE), a group advocating for a new endpoint that recognizes the benefits of lesser reductions in alcohol intake.
In a new analysis using data from the COMBINE study, researchers looked at the associations between reduction in World Health Organization drinking risk levels of alcohol consumption and clinically meaningful outcomes among people in treatment.
There are four levels: very high (more than 7.1 14-g drinks a day for men and more than 4.3 for women); high (4.3-7.1 or 2.9-4.3, respectively); moderate (2.9-4.3 or 1.4-2.9); and low (less than 2.9 or less than 1.4).
Researchers assessed responses to treatment with acamprosate, naltrexone, and psychological intervention in patients an average of 44 years old with 71% of their days being heavy drinking days. They found significant improvement in consequences tied to drinking, according to the Drinker Inventory of Consequences, and in mental health according to the Short Form Health Survey and the WHO Quality of Life assessment – even when the improvement was only by one WHO risk level – or just a few drinks a day – and did not involve abstaining (P less than .001 for all improvement categories).
“Reductions in WHO drinking risk levels are predictive of clinical benefit” or how the patient feels and functions, said Daniel Falk, PhD, health scientist administrator at the National Institute on Alcohol Abuse and Alcoholism.
The ACTIVE group he chairs, Dr. Anton said, is proposing that the FDA add a new endpoint for phase 3 trials: percentage of subjects who attain a 1- or 2-level reduction in WHO drinking risk levels.
Stephanie O’Malley, PhD, professor of psychiatry at Yale University in New Haven, Conn., said a 30-year-old with a moderate drinking problem – occasionally missing a family function, but never letting it interfere with work, say – might be turned off by a treatment plan that preaches abstinence, she said. Varenicline, for example, results in abstinence just 7% of the time, with some risk of suicidality and nausea. That person might not want to stop drinking entirely for the rest of his or her life, but could benefit by reducing the drinking, she said.
But 55% of the time, varenicline produces a reduction of one level of risk, which is associated with clinically meaningful results. That will sound much more appealing to a prospective patient, she said.
“From a clinical perspective, these WHO outcomes have some advantages,” she said. “I think you’re going to, one, encourage more people to accept treatment, and … be more optimistic about the outcomes.”
Dr. Anton reported consulting and/or funding from Alkermes, Allergan, Indivior, Insys Life Epigenetics, and Laboratori Farmaceutico CT. Dr. O’Malley reported consulting and/or funding from Alkermes, Amygdala, Indivior, Mistubishi Tanabe, Opiant, and other sources. Dr. Falk reported no disclosures.
BONITA SPRINGS, FLA. – Reductions in alcohol use bring about significant improvement in adverse consequences, mental health status, and quality of life, even if the reductions do not reach the level of abstinence, according to recent research presented at the annual meeting of the American Academy of Addiction Psychiatry.
The findings, experts say, demonstrate how a fixation on abstinence or elimination of all heavy drinking – the endpoints the Food and Drug Administration now require in pivotal clinical trials on alcohol use disorder (AUD) treatments – is shortsighted and can unnecessarily discourage people with alcohol use disorder from pursuing treatment.
“We need to think a little differently, or a little out of the box, from the way we’ve been thinking in the past,” said Raymond F. Anton, MD, professor of psychiatry at the Medical University of South Carolina in Charleston and chair of the Alcohol Clinical Trials Initiative (ACTIVE), a group advocating for a new endpoint that recognizes the benefits of lesser reductions in alcohol intake.
In a new analysis using data from the COMBINE study, researchers looked at the associations between reduction in World Health Organization drinking risk levels of alcohol consumption and clinically meaningful outcomes among people in treatment.
There are four levels: very high (more than 7.1 14-g drinks a day for men and more than 4.3 for women); high (4.3-7.1 or 2.9-4.3, respectively); moderate (2.9-4.3 or 1.4-2.9); and low (less than 2.9 or less than 1.4).
Researchers assessed responses to treatment with acamprosate, naltrexone, and psychological intervention in patients an average of 44 years old with 71% of their days being heavy drinking days. They found significant improvement in consequences tied to drinking, according to the Drinker Inventory of Consequences, and in mental health according to the Short Form Health Survey and the WHO Quality of Life assessment – even when the improvement was only by one WHO risk level – or just a few drinks a day – and did not involve abstaining (P less than .001 for all improvement categories).
“Reductions in WHO drinking risk levels are predictive of clinical benefit” or how the patient feels and functions, said Daniel Falk, PhD, health scientist administrator at the National Institute on Alcohol Abuse and Alcoholism.
The ACTIVE group he chairs, Dr. Anton said, is proposing that the FDA add a new endpoint for phase 3 trials: percentage of subjects who attain a 1- or 2-level reduction in WHO drinking risk levels.
Stephanie O’Malley, PhD, professor of psychiatry at Yale University in New Haven, Conn., said a 30-year-old with a moderate drinking problem – occasionally missing a family function, but never letting it interfere with work, say – might be turned off by a treatment plan that preaches abstinence, she said. Varenicline, for example, results in abstinence just 7% of the time, with some risk of suicidality and nausea. That person might not want to stop drinking entirely for the rest of his or her life, but could benefit by reducing the drinking, she said.
But 55% of the time, varenicline produces a reduction of one level of risk, which is associated with clinically meaningful results. That will sound much more appealing to a prospective patient, she said.
“From a clinical perspective, these WHO outcomes have some advantages,” she said. “I think you’re going to, one, encourage more people to accept treatment, and … be more optimistic about the outcomes.”
Dr. Anton reported consulting and/or funding from Alkermes, Allergan, Indivior, Insys Life Epigenetics, and Laboratori Farmaceutico CT. Dr. O’Malley reported consulting and/or funding from Alkermes, Amygdala, Indivior, Mistubishi Tanabe, Opiant, and other sources. Dr. Falk reported no disclosures.
BONITA SPRINGS, FLA. – Reductions in alcohol use bring about significant improvement in adverse consequences, mental health status, and quality of life, even if the reductions do not reach the level of abstinence, according to recent research presented at the annual meeting of the American Academy of Addiction Psychiatry.
The findings, experts say, demonstrate how a fixation on abstinence or elimination of all heavy drinking – the endpoints the Food and Drug Administration now require in pivotal clinical trials on alcohol use disorder (AUD) treatments – is shortsighted and can unnecessarily discourage people with alcohol use disorder from pursuing treatment.
“We need to think a little differently, or a little out of the box, from the way we’ve been thinking in the past,” said Raymond F. Anton, MD, professor of psychiatry at the Medical University of South Carolina in Charleston and chair of the Alcohol Clinical Trials Initiative (ACTIVE), a group advocating for a new endpoint that recognizes the benefits of lesser reductions in alcohol intake.
In a new analysis using data from the COMBINE study, researchers looked at the associations between reduction in World Health Organization drinking risk levels of alcohol consumption and clinically meaningful outcomes among people in treatment.
There are four levels: very high (more than 7.1 14-g drinks a day for men and more than 4.3 for women); high (4.3-7.1 or 2.9-4.3, respectively); moderate (2.9-4.3 or 1.4-2.9); and low (less than 2.9 or less than 1.4).
Researchers assessed responses to treatment with acamprosate, naltrexone, and psychological intervention in patients an average of 44 years old with 71% of their days being heavy drinking days. They found significant improvement in consequences tied to drinking, according to the Drinker Inventory of Consequences, and in mental health according to the Short Form Health Survey and the WHO Quality of Life assessment – even when the improvement was only by one WHO risk level – or just a few drinks a day – and did not involve abstaining (P less than .001 for all improvement categories).
“Reductions in WHO drinking risk levels are predictive of clinical benefit” or how the patient feels and functions, said Daniel Falk, PhD, health scientist administrator at the National Institute on Alcohol Abuse and Alcoholism.
The ACTIVE group he chairs, Dr. Anton said, is proposing that the FDA add a new endpoint for phase 3 trials: percentage of subjects who attain a 1- or 2-level reduction in WHO drinking risk levels.
Stephanie O’Malley, PhD, professor of psychiatry at Yale University in New Haven, Conn., said a 30-year-old with a moderate drinking problem – occasionally missing a family function, but never letting it interfere with work, say – might be turned off by a treatment plan that preaches abstinence, she said. Varenicline, for example, results in abstinence just 7% of the time, with some risk of suicidality and nausea. That person might not want to stop drinking entirely for the rest of his or her life, but could benefit by reducing the drinking, she said.
But 55% of the time, varenicline produces a reduction of one level of risk, which is associated with clinically meaningful results. That will sound much more appealing to a prospective patient, she said.
“From a clinical perspective, these WHO outcomes have some advantages,” she said. “I think you’re going to, one, encourage more people to accept treatment, and … be more optimistic about the outcomes.”
Dr. Anton reported consulting and/or funding from Alkermes, Allergan, Indivior, Insys Life Epigenetics, and Laboratori Farmaceutico CT. Dr. O’Malley reported consulting and/or funding from Alkermes, Amygdala, Indivior, Mistubishi Tanabe, Opiant, and other sources. Dr. Falk reported no disclosures.
REPORTING FROM AAAP 2018