User login
Opioid exposure in early pregnancy linked to congenital anomalies
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
Exposure to opioid analgesics during the first trimester of pregnancy appears to increase the risk of congenital anomalies diagnosed in the first year of life, researchers report.
While the absolute risk of congenital anomalies was low, these findings add to an increasing body of evidence suggesting that prenatal exposure to opioids may confer harm to infants post partum.
“We undertook a population-based cohort study to estimate associations between opioid analgesic exposure during the first trimester and congenital anomalies using health administrative data capturing all narcotic prescriptions during pregnancy,” lead author Alexa C. Bowie, MPH, of Queen’s University in Kingston, Ont., and colleagues reported in CMAJ.
The researchers retrospectively reviewed administrative health data in a single-payer health care system from 2013 to 2018. They identified parent-infant pair records for all live births and stillbirths that occurred at more than 20 weeks’ gestation.
The exposure of interest was a prescription for any opioid analgesic with a fill date between the estimated date of conception and less than 14 weeks’ gestation. The referent group included any infant not exposed to an opioid analgesic during the index pregnancy period.
Results
The study cohort included a total of 599,579 gestational parent-infant pairs. Of these, 11,903 (2.0%) were exposed to opioid analgesics, and most were exposed during the first trimester only (75.8%).
Overall, 2.0% of these infants developed a congenital anomaly during the first year of life; the prevalence of congenital anomalies was 2.0% in unexposed infants and 2.8% in exposed infants.
Relative to unexposed infants, the researchers observed greater risks among infants who were exposed for some anomaly groups, including many specific anomalies, such as ankyloglossia (any opioid: adjusted risk ratio, 1.88; 95% confidence interval, 1.30-2.72; codeine: aRR, 2.14; 95% CI, 1.35-3.40), as well as gastrointestinal anomalies (any opioid: aRR, 1.46; 95% CI, 1.15-1.85; codeine: aRR, 1.53; 95% CI, 1.12-2.09; tramadol: aRR, 2.69; 95% CI 1.34-5.38).
After sensitivity analyses, which included exposure 4 weeks before conception or excluded individuals with exposure to opioid analgesics before pregnancy, the findings remained unchanged.
“Although the overall risk was low, we observed an increased risk of any congenital anomaly with tramadol, and a previously unreported risk with morphine,” the researchers wrote.
“Previous studies reported elevated risks of heart anomalies with first-trimester exposure to any opioid analgesic, codeine, and tramadol, but others reported no association with any opioid analgesic or codeine,” they explained.
Interpreting the results
Study author Susan Brogly, PhD, of Queen’s University said “Our population-based study confirms evidence of a small increased risk of birth defects from opioid analgesic exposure in the first trimester that was observed in a recent study of private insurance and Medicaid beneficiaries in the U.S. We further show that this small increased risk is not due to other risk factors for fetal harm in women who may take these medications.”
“An opioid prescription dispensed in the first trimester would imply that there was an acute injury or chronic condition also present in the first trimester, which may also be associated with congenital abnormalities,” commented Elisabeth Poorman, MD, MPH, a clinical instructor and primary care physician at the University of Washington in Seattle.
“Opioid use disorder is often diagnosed incorrectly; since the researchers used diagnostic billing codes to exclude individuals with opioid use disorder, some women may have been missed,” Dr. Poorman explained.
Ms. Bowie and colleagues acknowledged that a key limitation of the study was the identification of cases using diagnostic billing codes. As a result, exposure-dependent recording bias could be present and limit the applicability of the findings.
“The diagnosis and documentation of minor anomalies and those with subtle medical significance could be vulnerable to exposure-dependent recording bias,” Ms. Bowie wrote.
Dr. Poorman recommended that these results should be interpreted with caution given these and other limitations. “Overall, results from this study may imply that there is limited evidence to suspect opioids are related to congenital abnormalities due to a very small difference observed in relatively unequal groups,” she concluded.
This study received funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development and was also supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health. One author reported receiving honoraria from the National Institutes of Health and a grant from the Canadian Institute of Health Research, outside the submitted work. No other competing interests were declared.
FROM CMAJ
Two emerging drugs exacerbating opioid crisis
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Marijuana use linked to nausea, vomiting of pregnancy
Use of marijuana during pregnancy was associated with symptoms of nausea and vomiting and with use of prescribed antiemetics, according to a study presented Feb. 3 at the meeting sponsored by the Society for Maternal-Fetal Medicine. It’s unclear, however, whether the association suggests that pregnant individuals are using marijuana in an attempt to treat their symptoms or whether the marijuana use is contributing to nausea and vomiting – or neither, Torri D. Metz, MD, of the University of Utah Health in Salt Lake City, told attendees.
“Cannabis use has been increasing among pregnant individuals,” Dr. Metz said. “Reported reasons for use range from habit to perceived benefit for treatment of medical conditions, including nausea and vomiting.” She noted a previous study that found that dispensary employees in Colorado recommended cannabis to pregnant callers for treating of nausea despite no clinical evidence of it being an effective treatment.
”Anecdotally, I can say that many patients have told me that marijuana is the only thing that makes them feel better in the first trimester, but that could also be closely tied to marijuana alleviating their other symptoms, such as anxiety or sleep disturbances,” Ilina Pluym, MD, of the department of maternal-fetal medicine at the University of California, Los Angeles, said in an interview. ”In the brain, marijuana acts to alleviate nausea and vomiting, and it has been used successfully to treat nausea [caused by] chemotherapy,” said Dr. Pluym, who attended the abstract presentation but was not involved in the research. “But in the gut, with long-term marijuana use, it can have the opposite effect, which is what is seen in cannabinoid hyperemesis syndrome.”
Past research that has identified a link between cannabis use and nausea in pregnancy has typically relied on administrative data or self-reporting that are subject to recall and social desirability bias instead of a biomarker to assess cannabis use. This study therefore assessed marijuana use based on the presence of THC-COOH in urine samples and added the element of investigating antiemetic use in the population.
The study enrolled 10,038 nulliparous pregnant patients from eight U.S. centers from 2010 to 2013 who were an average 11 weeks pregnant. All participants completed the Pregnancy-Unique Quantification of Emesis (PUQE) tool at their first study visit and consented to testing of their previously frozen urine samples. The PUQE tool asks participants how often they have experienced nausea, vomiting, or retching or dry heaves within the previous 12 hours. A score of 1-6 is mild, a score of 7-12 is moderate, and a score of 13 or higher is severe.
Overall, 15.8% of participants reported moderate to severe nausea and 38.2% reported mild nausea. A total of 5.8% of participants tested positive for marijuana use based on THC levels in urine. Those with incrementally higher levels of THC, at least 500 ng/mg of creatinine, were 1.6 times more likely to report moderate to severe nausea after accounting for maternal age, body mass index, antiemetic drug use, and gestational age (adjusted odds ratio, 1.6; P < .001). An association did not exist, however, with any level of nausea overall. Those with higher creatinine levels were also 1.9 times more likely to report vomiting and 1.6 times more likely to report dry heaves or retching (P < .001).
About 1 in 10 participants (9.6%) overall had used a prescription antiemetic drug. Antiemetics were more common among those who had used marijuana: 18% of those with detectable THC had used antiemetics, compared with 12% of those without evidence of cannabis use (P < .001). However, most of those who used marijuana (83%) took only one antiemetic.
Among the study’s limitations were its lack of data on the reasons for cannabis use and the fact that it took place before widespread cannabidiol products became available, which meant most participants were using marijuana by smoking it.
Dr. Pluym also pointed out that the overall rate of marijuana use during pregnancy is likely higher today than it was in 2010-2013, before many states legalized its use. “But legalization shouldn’t equal normalization in pregnancy,” she added.
In addition, while the PUQE score assesses symptoms within the previous 12 hours, THC can remain in urine samples anywhere from several days to several weeks after marijuana is used.
”We’re unable to establish cause and effect,” Dr. Metz said, “but what we can conclude is that marijuana use was associated with early pregnancy nausea and vomiting.”
The findings emphasize the need for physicians to ask patients about their use of marijuana and seek to find out why they’re using it, Dr. Metz said. If it’s to treat nausea and vomiting of pregnancy, ob.gyns. should ensure patients are aware of the potential adverse effects of marijuana use in pregnancy and mention safe, effective alternatives. Research from the National Academy of Sciences has shown consistent evidence of decreased fetal growth with marijuana use in pregnancy, but there hasn’t been enough evidence to assess potential long-term neurological effects.
The research was funded by the National Institute on Drug Abuse and the National Institute of Child Health and Human Development. Dr. Metz and Dr. Pluym reported no disclosures.
Use of marijuana during pregnancy was associated with symptoms of nausea and vomiting and with use of prescribed antiemetics, according to a study presented Feb. 3 at the meeting sponsored by the Society for Maternal-Fetal Medicine. It’s unclear, however, whether the association suggests that pregnant individuals are using marijuana in an attempt to treat their symptoms or whether the marijuana use is contributing to nausea and vomiting – or neither, Torri D. Metz, MD, of the University of Utah Health in Salt Lake City, told attendees.
“Cannabis use has been increasing among pregnant individuals,” Dr. Metz said. “Reported reasons for use range from habit to perceived benefit for treatment of medical conditions, including nausea and vomiting.” She noted a previous study that found that dispensary employees in Colorado recommended cannabis to pregnant callers for treating of nausea despite no clinical evidence of it being an effective treatment.
”Anecdotally, I can say that many patients have told me that marijuana is the only thing that makes them feel better in the first trimester, but that could also be closely tied to marijuana alleviating their other symptoms, such as anxiety or sleep disturbances,” Ilina Pluym, MD, of the department of maternal-fetal medicine at the University of California, Los Angeles, said in an interview. ”In the brain, marijuana acts to alleviate nausea and vomiting, and it has been used successfully to treat nausea [caused by] chemotherapy,” said Dr. Pluym, who attended the abstract presentation but was not involved in the research. “But in the gut, with long-term marijuana use, it can have the opposite effect, which is what is seen in cannabinoid hyperemesis syndrome.”
Past research that has identified a link between cannabis use and nausea in pregnancy has typically relied on administrative data or self-reporting that are subject to recall and social desirability bias instead of a biomarker to assess cannabis use. This study therefore assessed marijuana use based on the presence of THC-COOH in urine samples and added the element of investigating antiemetic use in the population.
The study enrolled 10,038 nulliparous pregnant patients from eight U.S. centers from 2010 to 2013 who were an average 11 weeks pregnant. All participants completed the Pregnancy-Unique Quantification of Emesis (PUQE) tool at their first study visit and consented to testing of their previously frozen urine samples. The PUQE tool asks participants how often they have experienced nausea, vomiting, or retching or dry heaves within the previous 12 hours. A score of 1-6 is mild, a score of 7-12 is moderate, and a score of 13 or higher is severe.
Overall, 15.8% of participants reported moderate to severe nausea and 38.2% reported mild nausea. A total of 5.8% of participants tested positive for marijuana use based on THC levels in urine. Those with incrementally higher levels of THC, at least 500 ng/mg of creatinine, were 1.6 times more likely to report moderate to severe nausea after accounting for maternal age, body mass index, antiemetic drug use, and gestational age (adjusted odds ratio, 1.6; P < .001). An association did not exist, however, with any level of nausea overall. Those with higher creatinine levels were also 1.9 times more likely to report vomiting and 1.6 times more likely to report dry heaves or retching (P < .001).
About 1 in 10 participants (9.6%) overall had used a prescription antiemetic drug. Antiemetics were more common among those who had used marijuana: 18% of those with detectable THC had used antiemetics, compared with 12% of those without evidence of cannabis use (P < .001). However, most of those who used marijuana (83%) took only one antiemetic.
Among the study’s limitations were its lack of data on the reasons for cannabis use and the fact that it took place before widespread cannabidiol products became available, which meant most participants were using marijuana by smoking it.
Dr. Pluym also pointed out that the overall rate of marijuana use during pregnancy is likely higher today than it was in 2010-2013, before many states legalized its use. “But legalization shouldn’t equal normalization in pregnancy,” she added.
In addition, while the PUQE score assesses symptoms within the previous 12 hours, THC can remain in urine samples anywhere from several days to several weeks after marijuana is used.
”We’re unable to establish cause and effect,” Dr. Metz said, “but what we can conclude is that marijuana use was associated with early pregnancy nausea and vomiting.”
The findings emphasize the need for physicians to ask patients about their use of marijuana and seek to find out why they’re using it, Dr. Metz said. If it’s to treat nausea and vomiting of pregnancy, ob.gyns. should ensure patients are aware of the potential adverse effects of marijuana use in pregnancy and mention safe, effective alternatives. Research from the National Academy of Sciences has shown consistent evidence of decreased fetal growth with marijuana use in pregnancy, but there hasn’t been enough evidence to assess potential long-term neurological effects.
The research was funded by the National Institute on Drug Abuse and the National Institute of Child Health and Human Development. Dr. Metz and Dr. Pluym reported no disclosures.
Use of marijuana during pregnancy was associated with symptoms of nausea and vomiting and with use of prescribed antiemetics, according to a study presented Feb. 3 at the meeting sponsored by the Society for Maternal-Fetal Medicine. It’s unclear, however, whether the association suggests that pregnant individuals are using marijuana in an attempt to treat their symptoms or whether the marijuana use is contributing to nausea and vomiting – or neither, Torri D. Metz, MD, of the University of Utah Health in Salt Lake City, told attendees.
“Cannabis use has been increasing among pregnant individuals,” Dr. Metz said. “Reported reasons for use range from habit to perceived benefit for treatment of medical conditions, including nausea and vomiting.” She noted a previous study that found that dispensary employees in Colorado recommended cannabis to pregnant callers for treating of nausea despite no clinical evidence of it being an effective treatment.
”Anecdotally, I can say that many patients have told me that marijuana is the only thing that makes them feel better in the first trimester, but that could also be closely tied to marijuana alleviating their other symptoms, such as anxiety or sleep disturbances,” Ilina Pluym, MD, of the department of maternal-fetal medicine at the University of California, Los Angeles, said in an interview. ”In the brain, marijuana acts to alleviate nausea and vomiting, and it has been used successfully to treat nausea [caused by] chemotherapy,” said Dr. Pluym, who attended the abstract presentation but was not involved in the research. “But in the gut, with long-term marijuana use, it can have the opposite effect, which is what is seen in cannabinoid hyperemesis syndrome.”
Past research that has identified a link between cannabis use and nausea in pregnancy has typically relied on administrative data or self-reporting that are subject to recall and social desirability bias instead of a biomarker to assess cannabis use. This study therefore assessed marijuana use based on the presence of THC-COOH in urine samples and added the element of investigating antiemetic use in the population.
The study enrolled 10,038 nulliparous pregnant patients from eight U.S. centers from 2010 to 2013 who were an average 11 weeks pregnant. All participants completed the Pregnancy-Unique Quantification of Emesis (PUQE) tool at their first study visit and consented to testing of their previously frozen urine samples. The PUQE tool asks participants how often they have experienced nausea, vomiting, or retching or dry heaves within the previous 12 hours. A score of 1-6 is mild, a score of 7-12 is moderate, and a score of 13 or higher is severe.
Overall, 15.8% of participants reported moderate to severe nausea and 38.2% reported mild nausea. A total of 5.8% of participants tested positive for marijuana use based on THC levels in urine. Those with incrementally higher levels of THC, at least 500 ng/mg of creatinine, were 1.6 times more likely to report moderate to severe nausea after accounting for maternal age, body mass index, antiemetic drug use, and gestational age (adjusted odds ratio, 1.6; P < .001). An association did not exist, however, with any level of nausea overall. Those with higher creatinine levels were also 1.9 times more likely to report vomiting and 1.6 times more likely to report dry heaves or retching (P < .001).
About 1 in 10 participants (9.6%) overall had used a prescription antiemetic drug. Antiemetics were more common among those who had used marijuana: 18% of those with detectable THC had used antiemetics, compared with 12% of those without evidence of cannabis use (P < .001). However, most of those who used marijuana (83%) took only one antiemetic.
Among the study’s limitations were its lack of data on the reasons for cannabis use and the fact that it took place before widespread cannabidiol products became available, which meant most participants were using marijuana by smoking it.
Dr. Pluym also pointed out that the overall rate of marijuana use during pregnancy is likely higher today than it was in 2010-2013, before many states legalized its use. “But legalization shouldn’t equal normalization in pregnancy,” she added.
In addition, while the PUQE score assesses symptoms within the previous 12 hours, THC can remain in urine samples anywhere from several days to several weeks after marijuana is used.
”We’re unable to establish cause and effect,” Dr. Metz said, “but what we can conclude is that marijuana use was associated with early pregnancy nausea and vomiting.”
The findings emphasize the need for physicians to ask patients about their use of marijuana and seek to find out why they’re using it, Dr. Metz said. If it’s to treat nausea and vomiting of pregnancy, ob.gyns. should ensure patients are aware of the potential adverse effects of marijuana use in pregnancy and mention safe, effective alternatives. Research from the National Academy of Sciences has shown consistent evidence of decreased fetal growth with marijuana use in pregnancy, but there hasn’t been enough evidence to assess potential long-term neurological effects.
The research was funded by the National Institute on Drug Abuse and the National Institute of Child Health and Human Development. Dr. Metz and Dr. Pluym reported no disclosures.
AT THE PREGNANCY MEETING
Intranasal oxytocin shows early promise for cocaine dependence
Intranasal oxytocin (INOT) is showing early promise as a treatment for cocaine dependence, new research suggests.
Results of a small 6-week randomized, placebo-controlled trial in patients with cocaine use disorder showed a high level of abstinence in those who received INOT beginning 2 weeks after treatment initiation.
“In this population of cocaine-dependent individuals in a community clinic setting, , compared to placebo,” lead author Wilfrid Noel Raby, PhD, MD, a Teaneck, N.J.–based psychiatrist, said in an interview.
On the other hand, “the findings were paradoxical because there was a greater dropout rate in the intranasal oxytocin group after week 1, suggesting that oxytocin might have a biphasic effect, which should be addressed in future studies,” added Dr. Raby, who was an adjunct clinical professor of psychiatry, division on substance abuse, Montefiore Medical Center, Albert Einstein College of Medicine, New York, when the trial was conducted.
The study was published in the March issue of Drug and Alcohol Dependence Reports.
‘Crying need’
“Focus on stress reactivity in addiction and on the loss of social norms among drug users has generated interest in oxytocin, due to its purported role in these traits and regulation of stress,” the authors wrote.
Oxytocin is a neuropeptide that regulates autonomic functions. Previous research in cannabis users suggests it may have a role in treating addiction by reportedly reducing cravings. In addition, earlier research also suggests it cuts stress reactivity and state anger in cocaine users.
A previous trial of INOT showed it decreased cocaine craving, and additional research has revealed recurrent cocaine use results in lower endogenous oxytocin levels and depleted oxytocin in the hypothalamus and amygdala.
“The bias of my work is to look for simple, nonaddictive medicinal approaches that can be used in the community settings, because that’s where the greatest crying need lies and where most problems from drug addiction occur,” said Dr. Raby.
“There has been long-standing interest in how the brain adaptive systems, or so-called ‘stress systems,’ adjust in the face of drug dependence in general, and the main focus of the study has been to understand this response and use the insight from these adaptations to develop medicinal treatments for drug abuse, particularly cocaine dependence,” he added.
To investigate the potential for INOT to promote abstinence from cocaine, the researchers randomized 26 patients with cocaine use disorder (73% male, mean [SD] age, 50.2 [5.4] years). Most participants had been using cocaine on a regular basis for about 25 years, and baseline average days of cocaine use was 11.1 (5.7) during the 30 days prior to study entry.
At a baseline, the researchers collected participants’ medical history and conducted a physical examination, urine toxicology, electrocardiogram, comprehensive metabolic panel, and complete blood count. They used the MINI International Neuropsychiatric Interview to confirm the diagnosis of cocaine dependence.
The study began with a 7-day inpatient abstinence induction stage, after which participants were randomized to receive either INOT 24 IU or intranasal placebo (n = 15 and n = 11, respectively).
Patients attended the clinic three times per week. At each visit, they completed the cocaine craving scale, the Perceived Stress Scale, and the Clinician Global Inventory (all self-reports), as well as the Time Line Follow Back (TLFB) to document cocaine use.
Participants were trained to self-administer an intranasal solution at home, with compliance monitored in two ways – staff observed self-administration of the randomized medication at the time of clinic visits and weighed the “at home bottle.”
Cocaine use was determined via urine toxicology and TLFB self-report.
Threshold period
INOT did not induce ≥ 3 weeks of continuous abstinence. However, beginning with week 3, the odds of weekly abstinence increased dramatically in the INOT group, from 4.61 (95% confidence interval,1.05, 20.3) to 15.0 (1.18, 190.2) by week 6 (t = 2.12, P = .037).
The overall medication group by time interaction across all 6 weeks was not significant (F1,69 = 1.73, P = .19); but when the interaction was removed, the difference between the overall effect of medication (INOT vs. placebo) over all 6 weeks “reached trend-level significance” (F1,70) = 3.42, P = .07).
The subjective rating outcomes (cravings, perceived stress, cocaine dependence, and depression) “did not show a significant medication group by time interaction effect,” the authors reported, although stress-induced cravings did tend toward a significant difference between the groups.
Half of the patients did not complete the full 6 weeks. Of those who discontinued, 85% came from the INOT group and 15% from the placebo group. Of the 11 who dropped out from the treatment group, seven were abstinent at the time of discontinuation for ≥ 1 week.
There were no significant differences in rates of reported side effects between the two groups.
“This study highlights some promise that perhaps there is a threshold period of time you need to cross, after which time oxytocin could really be really helpful as acute or maintenance medication,” said Dr. Raby. The short study duration might have been a disadvantage. “We might have seen better results if the study had been 8 or 12 weeks in duration.”
Using motivational approaches during the early phase – e.g., psychotherapy or a voucher system – might increase adherence, and then “after this initial lag, we might see a more therapeutic effect,” he suggested.
Dr. Raby noted that his group studied stress hormone secretions in the cocaine-dependent study participants during the 7-day induction period and that the findings, when published, could shed light on this latency period. “Cocaine dependence creates adaptations in the stress system,” he said.
‘Nice first step’
Commenting on the study, Jane Joseph, PhD, professor in the department of neurosciences and director of the neuroimaging division at Medical University of South Carolina, Charleston, said it is “nice to see a clinical trial using oxytocin in cocaine dependence [because] preclinical research has shown fairly convincing effects of oxytocin in reducing craving or stress in the context of cocaine seeking, but findings are rather mixed in human studies.”
Dr. Joseph, who was not involved with the study, said her group’s research showed oxytocin to be the most helpful for men with cocaine use disorder who reported childhood trauma, while for women, oxytocin “seemed to worsen their reactivity to cocaine cues.”
She said the current study is a “nice first step” and suggested that future research should include larger sample sizes to “address some of the individual variability in the response to oxytocin by examining sex differences or trauma history.”
The study was supported by an award from the National Institute of Drug Abuse. Dr. Raby and coauthors and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intranasal oxytocin (INOT) is showing early promise as a treatment for cocaine dependence, new research suggests.
Results of a small 6-week randomized, placebo-controlled trial in patients with cocaine use disorder showed a high level of abstinence in those who received INOT beginning 2 weeks after treatment initiation.
“In this population of cocaine-dependent individuals in a community clinic setting, , compared to placebo,” lead author Wilfrid Noel Raby, PhD, MD, a Teaneck, N.J.–based psychiatrist, said in an interview.
On the other hand, “the findings were paradoxical because there was a greater dropout rate in the intranasal oxytocin group after week 1, suggesting that oxytocin might have a biphasic effect, which should be addressed in future studies,” added Dr. Raby, who was an adjunct clinical professor of psychiatry, division on substance abuse, Montefiore Medical Center, Albert Einstein College of Medicine, New York, when the trial was conducted.
The study was published in the March issue of Drug and Alcohol Dependence Reports.
‘Crying need’
“Focus on stress reactivity in addiction and on the loss of social norms among drug users has generated interest in oxytocin, due to its purported role in these traits and regulation of stress,” the authors wrote.
Oxytocin is a neuropeptide that regulates autonomic functions. Previous research in cannabis users suggests it may have a role in treating addiction by reportedly reducing cravings. In addition, earlier research also suggests it cuts stress reactivity and state anger in cocaine users.
A previous trial of INOT showed it decreased cocaine craving, and additional research has revealed recurrent cocaine use results in lower endogenous oxytocin levels and depleted oxytocin in the hypothalamus and amygdala.
“The bias of my work is to look for simple, nonaddictive medicinal approaches that can be used in the community settings, because that’s where the greatest crying need lies and where most problems from drug addiction occur,” said Dr. Raby.
“There has been long-standing interest in how the brain adaptive systems, or so-called ‘stress systems,’ adjust in the face of drug dependence in general, and the main focus of the study has been to understand this response and use the insight from these adaptations to develop medicinal treatments for drug abuse, particularly cocaine dependence,” he added.
To investigate the potential for INOT to promote abstinence from cocaine, the researchers randomized 26 patients with cocaine use disorder (73% male, mean [SD] age, 50.2 [5.4] years). Most participants had been using cocaine on a regular basis for about 25 years, and baseline average days of cocaine use was 11.1 (5.7) during the 30 days prior to study entry.
At a baseline, the researchers collected participants’ medical history and conducted a physical examination, urine toxicology, electrocardiogram, comprehensive metabolic panel, and complete blood count. They used the MINI International Neuropsychiatric Interview to confirm the diagnosis of cocaine dependence.
The study began with a 7-day inpatient abstinence induction stage, after which participants were randomized to receive either INOT 24 IU or intranasal placebo (n = 15 and n = 11, respectively).
Patients attended the clinic three times per week. At each visit, they completed the cocaine craving scale, the Perceived Stress Scale, and the Clinician Global Inventory (all self-reports), as well as the Time Line Follow Back (TLFB) to document cocaine use.
Participants were trained to self-administer an intranasal solution at home, with compliance monitored in two ways – staff observed self-administration of the randomized medication at the time of clinic visits and weighed the “at home bottle.”
Cocaine use was determined via urine toxicology and TLFB self-report.
Threshold period
INOT did not induce ≥ 3 weeks of continuous abstinence. However, beginning with week 3, the odds of weekly abstinence increased dramatically in the INOT group, from 4.61 (95% confidence interval,1.05, 20.3) to 15.0 (1.18, 190.2) by week 6 (t = 2.12, P = .037).
The overall medication group by time interaction across all 6 weeks was not significant (F1,69 = 1.73, P = .19); but when the interaction was removed, the difference between the overall effect of medication (INOT vs. placebo) over all 6 weeks “reached trend-level significance” (F1,70) = 3.42, P = .07).
The subjective rating outcomes (cravings, perceived stress, cocaine dependence, and depression) “did not show a significant medication group by time interaction effect,” the authors reported, although stress-induced cravings did tend toward a significant difference between the groups.
Half of the patients did not complete the full 6 weeks. Of those who discontinued, 85% came from the INOT group and 15% from the placebo group. Of the 11 who dropped out from the treatment group, seven were abstinent at the time of discontinuation for ≥ 1 week.
There were no significant differences in rates of reported side effects between the two groups.
“This study highlights some promise that perhaps there is a threshold period of time you need to cross, after which time oxytocin could really be really helpful as acute or maintenance medication,” said Dr. Raby. The short study duration might have been a disadvantage. “We might have seen better results if the study had been 8 or 12 weeks in duration.”
Using motivational approaches during the early phase – e.g., psychotherapy or a voucher system – might increase adherence, and then “after this initial lag, we might see a more therapeutic effect,” he suggested.
Dr. Raby noted that his group studied stress hormone secretions in the cocaine-dependent study participants during the 7-day induction period and that the findings, when published, could shed light on this latency period. “Cocaine dependence creates adaptations in the stress system,” he said.
‘Nice first step’
Commenting on the study, Jane Joseph, PhD, professor in the department of neurosciences and director of the neuroimaging division at Medical University of South Carolina, Charleston, said it is “nice to see a clinical trial using oxytocin in cocaine dependence [because] preclinical research has shown fairly convincing effects of oxytocin in reducing craving or stress in the context of cocaine seeking, but findings are rather mixed in human studies.”
Dr. Joseph, who was not involved with the study, said her group’s research showed oxytocin to be the most helpful for men with cocaine use disorder who reported childhood trauma, while for women, oxytocin “seemed to worsen their reactivity to cocaine cues.”
She said the current study is a “nice first step” and suggested that future research should include larger sample sizes to “address some of the individual variability in the response to oxytocin by examining sex differences or trauma history.”
The study was supported by an award from the National Institute of Drug Abuse. Dr. Raby and coauthors and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intranasal oxytocin (INOT) is showing early promise as a treatment for cocaine dependence, new research suggests.
Results of a small 6-week randomized, placebo-controlled trial in patients with cocaine use disorder showed a high level of abstinence in those who received INOT beginning 2 weeks after treatment initiation.
“In this population of cocaine-dependent individuals in a community clinic setting, , compared to placebo,” lead author Wilfrid Noel Raby, PhD, MD, a Teaneck, N.J.–based psychiatrist, said in an interview.
On the other hand, “the findings were paradoxical because there was a greater dropout rate in the intranasal oxytocin group after week 1, suggesting that oxytocin might have a biphasic effect, which should be addressed in future studies,” added Dr. Raby, who was an adjunct clinical professor of psychiatry, division on substance abuse, Montefiore Medical Center, Albert Einstein College of Medicine, New York, when the trial was conducted.
The study was published in the March issue of Drug and Alcohol Dependence Reports.
‘Crying need’
“Focus on stress reactivity in addiction and on the loss of social norms among drug users has generated interest in oxytocin, due to its purported role in these traits and regulation of stress,” the authors wrote.
Oxytocin is a neuropeptide that regulates autonomic functions. Previous research in cannabis users suggests it may have a role in treating addiction by reportedly reducing cravings. In addition, earlier research also suggests it cuts stress reactivity and state anger in cocaine users.
A previous trial of INOT showed it decreased cocaine craving, and additional research has revealed recurrent cocaine use results in lower endogenous oxytocin levels and depleted oxytocin in the hypothalamus and amygdala.
“The bias of my work is to look for simple, nonaddictive medicinal approaches that can be used in the community settings, because that’s where the greatest crying need lies and where most problems from drug addiction occur,” said Dr. Raby.
“There has been long-standing interest in how the brain adaptive systems, or so-called ‘stress systems,’ adjust in the face of drug dependence in general, and the main focus of the study has been to understand this response and use the insight from these adaptations to develop medicinal treatments for drug abuse, particularly cocaine dependence,” he added.
To investigate the potential for INOT to promote abstinence from cocaine, the researchers randomized 26 patients with cocaine use disorder (73% male, mean [SD] age, 50.2 [5.4] years). Most participants had been using cocaine on a regular basis for about 25 years, and baseline average days of cocaine use was 11.1 (5.7) during the 30 days prior to study entry.
At a baseline, the researchers collected participants’ medical history and conducted a physical examination, urine toxicology, electrocardiogram, comprehensive metabolic panel, and complete blood count. They used the MINI International Neuropsychiatric Interview to confirm the diagnosis of cocaine dependence.
The study began with a 7-day inpatient abstinence induction stage, after which participants were randomized to receive either INOT 24 IU or intranasal placebo (n = 15 and n = 11, respectively).
Patients attended the clinic three times per week. At each visit, they completed the cocaine craving scale, the Perceived Stress Scale, and the Clinician Global Inventory (all self-reports), as well as the Time Line Follow Back (TLFB) to document cocaine use.
Participants were trained to self-administer an intranasal solution at home, with compliance monitored in two ways – staff observed self-administration of the randomized medication at the time of clinic visits and weighed the “at home bottle.”
Cocaine use was determined via urine toxicology and TLFB self-report.
Threshold period
INOT did not induce ≥ 3 weeks of continuous abstinence. However, beginning with week 3, the odds of weekly abstinence increased dramatically in the INOT group, from 4.61 (95% confidence interval,1.05, 20.3) to 15.0 (1.18, 190.2) by week 6 (t = 2.12, P = .037).
The overall medication group by time interaction across all 6 weeks was not significant (F1,69 = 1.73, P = .19); but when the interaction was removed, the difference between the overall effect of medication (INOT vs. placebo) over all 6 weeks “reached trend-level significance” (F1,70) = 3.42, P = .07).
The subjective rating outcomes (cravings, perceived stress, cocaine dependence, and depression) “did not show a significant medication group by time interaction effect,” the authors reported, although stress-induced cravings did tend toward a significant difference between the groups.
Half of the patients did not complete the full 6 weeks. Of those who discontinued, 85% came from the INOT group and 15% from the placebo group. Of the 11 who dropped out from the treatment group, seven were abstinent at the time of discontinuation for ≥ 1 week.
There were no significant differences in rates of reported side effects between the two groups.
“This study highlights some promise that perhaps there is a threshold period of time you need to cross, after which time oxytocin could really be really helpful as acute or maintenance medication,” said Dr. Raby. The short study duration might have been a disadvantage. “We might have seen better results if the study had been 8 or 12 weeks in duration.”
Using motivational approaches during the early phase – e.g., psychotherapy or a voucher system – might increase adherence, and then “after this initial lag, we might see a more therapeutic effect,” he suggested.
Dr. Raby noted that his group studied stress hormone secretions in the cocaine-dependent study participants during the 7-day induction period and that the findings, when published, could shed light on this latency period. “Cocaine dependence creates adaptations in the stress system,” he said.
‘Nice first step’
Commenting on the study, Jane Joseph, PhD, professor in the department of neurosciences and director of the neuroimaging division at Medical University of South Carolina, Charleston, said it is “nice to see a clinical trial using oxytocin in cocaine dependence [because] preclinical research has shown fairly convincing effects of oxytocin in reducing craving or stress in the context of cocaine seeking, but findings are rather mixed in human studies.”
Dr. Joseph, who was not involved with the study, said her group’s research showed oxytocin to be the most helpful for men with cocaine use disorder who reported childhood trauma, while for women, oxytocin “seemed to worsen their reactivity to cocaine cues.”
She said the current study is a “nice first step” and suggested that future research should include larger sample sizes to “address some of the individual variability in the response to oxytocin by examining sex differences or trauma history.”
The study was supported by an award from the National Institute of Drug Abuse. Dr. Raby and coauthors and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DRUG AND ALCOHOL DEPENDENCE REPORTS
Naloxone Dispensing in Patients at Risk for Opioid Overdose After Total Knee Arthroplasty Within the Veterans Health Administration
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
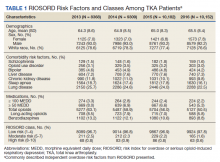
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
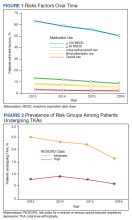
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
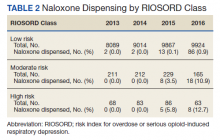
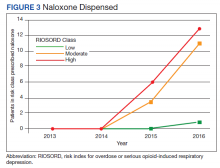
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
A test for cannabis-caused impairment
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you give a mouse a genetically engineered bitcoin wallet
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
The world’s most valuable mouse
You’ve heard of Mighty Mouse. Now say hello to the world’s newest mouse superhero, Crypto-Mouse! After being bitten by a radioactive cryptocurrency investor, Crypto-Mouse can tap directly into the power of the blockchain itself, allowing it to perform incredible, death-defying feats of strength!
We’re going to stop right there before Crypto-Mouse gains entry into the Marvel cinematic universe. Let’s rewind to the beginning, because that’s precisely where this crazy scheme is at. In late January, a new decentralized autonomous organization, BitMouseDAO, launched to enormous … -ly little fanfare, according to Vice. Two investors as of Jan. 31. But what they lack in money they make up for in sheer ambition.
BitMouseDAO’s $100 million dollar idea is to genetically engineer mice to carry bitcoin, the first cryptocurrency and one of the most valuable. This isn’t as crazy an idea as it sounds since DNA can be modified to store information, potentially even bitcoin information. Their plan is to create a private bitcoin wallet, which will be stored in the mouse DNA, and purchase online bitcoin to store in this wallet.
BitMouseDAO, being a “collection of artists,” plans to partner with a lab to translate its private key into a specific DNA sequence to be encoded into the mice during fertilization; or, if that doesn’t work, inject them with a harmless virus that carries the key.
Since these are artists, their ultimate plan is to use their bitcoin mice to make NFTs (scratch that off your cryptocurrency bingo card) and auction them off to people. Or, as Vice put it, BitMouseDAO essentially plans to send preserved dead mice to people. Artistic dead mice! Artistic dead mice worth millions! Maybe. Even BitMouseDAO admits bitcoin could be worthless by the time the project gets off the ground.
If this all sounds completely insane, that’s because it is. But it also sounds crazy enough to work. Now, if you’ll excuse us, we’re off to write a screenplay about a scrappy group of high-tech thieves who steal a group of genetically altered bitcoin mice to sell for millions, only to keep them as their adorable pets. Trust us Hollywood, it’ll make millions!
Alcoholic monkeys vs. the future of feces
Which is more important, the journey or the destination? Science is all about the destination, yes? Solving the problem, saving a life, expanding horizons. That’s science. Or is it? The scientific method is a process, so does that make it a journey?
For us, today’s journey begins at the University of Iowa, where investigators are trying to reduce alcohol consumption. A worthy goal, and they seem to have made some progress by targeting a liver hormone called fibroblast growth factor 21 (FGF21). But we’re more interested in the process right now, so bring on the alcoholic monkeys. And no, that’s not a death metal/reggae fusion band. Should be, though.
“The vervet monkey population is [composed] of alcohol avoiders, moderate alcohol drinkers, and a group of heavy drinkers,” Matthew Potthoff, PhD, and associates wrote in Cell Metabolism. When this particular bunch of heavy-drinking vervets were given FGF21, they consumed 50% less alcohol than did vehicle-treated controls, so mission accomplished.
Maybe it could be a breakfast cereal. Who wouldn’t enjoy a bowl of alcoholic monkeys in the morning?
And after breakfast, you might be ready for a digitized bowel movement, courtesy of researchers at University of California, San Diego. They’re studying ulcerative colitis (UC) by examining the gut microbiome, and their “most useful biological sample is patient stool,” according to a written statement from the university.
“Once we had all the technology to digitize the stool, the question was, is this going to tell us what’s happening in these patients? The answer turned out to be yes,” co-senior author Rob Knight, PhD, said in the statement. “Digitizing fecal material is the future.” The road to UC treatment, in other words, is paved with digital stool.
About 40% of the UC patients had elevated protease levels, and their high-protease feces were then transplanted into germ-free mice, which subsequently developed colitis and were successfully treated with protease inhibitors. And that is our final destination.
As our revered founder and mentor, Josephine Lotmevich, used to say, an alcoholic monkey in the hand is worth a number 2 in the bush.
Raise a glass to delinquency
You wouldn’t think that a glass of water could lead to a life of crime, but a recent study suggests just that.
Children exposed to lead in their drinking water during their early years had a 21% higher risk of delinquency after the age of 14 years and a 38% higher risk of having a record for a serious complaint, Jackie MacDonald Gibson and associates said in a statement on Eurekalert.
Data for the study came from Wake County, N.C., which includes rural areas, wealthy exurban developments, and predominantly Black communities. The investigators compared the blood lead levels for children tested between 1998 and 2011 with juvenile delinquency reports of the same children from the N.C. Department of Public Safety.
The main culprit, they found, was well water. Blood lead levels were 11% higher in the children whose water came from private wells, compared with children using community water. About 13% of U.S. households rely on private wells, which are not regulated under the Safe Drinking Water Act, for their water supply.
The researchers said there is an urgent need for better drinking-water solutions in communities that rely on well water, whether it be through subsidized home filtration or infrastructure redevelopment.
An earlier study had estimated that preventing just one child from entering the adult criminal justice system would save $1.3 to $1.5 million in 1997 dollars. That’s about $2.2 to $2.5 million dollars today!
If you do the math, it’s not hard to see what’s cheaper (and healthier) in the long run.
A ‘dirty’ scam
Another one? This is just getting sad. You’ve probably heard of muds and clays being good for the skin and maybe you’ve gone to a spa and sat in a mud bath, but would you believe it if someone told you that mud can cure all your ailments? No? Neither would we. Senatorial candidate Beto O’Rourke was definitely someone who brought this strange treatment to light, but it seems like this is something that has been going on for years, even before the pandemic.
A company called Black Oxygen Organics (BOO) was selling “magic dirt” for $110 per 4-ounce package. It claimed the dirt was high in fulvic acid and humic acid, which are good for many things. They were, however, literally getting this mud from bogs with landfills nearby, Mel magazine reported.
That doesn’t sound appealing at all, but wait, there’s more. People were eating, drinking, bathing, and feeding their families this sludge in hopes that they would be cured of their ailments. A lot of people jumped aboard the magic dirt train when the pandemic arose, but it quickly became clear that this mud was not as helpful as BOO claimed it to be.
“We began to receive inquiries and calls on our website with people having problems and issues. Ultimately, we sent the products out for independent testing, and then when that came back and showed that there were toxic heavy metals [lead, arsenic, and cadmium among them] at an unsafe level, that’s when we knew we had to act,” Atlanta-based attorney Matt Wetherington, who filed a federal lawsuit against BOO, told Mel.
After a very complicated series of events involving an expose by NBC, product recalls, extortion claims, and grassroots activism, BOO was shut down by both the Canadian and U.S. governments.
As always, please listen only to health care professionals when you wish to use natural remedies for illnesses and ailments.
Buprenorphine may curb opioid-induced respiratory depression
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
High plasma concentrations of buprenorphine may reduce fentanyl-induced respiratory depression, new research suggests.
The primary endpoint measure in a small “proof of principal” pharmacology study was effect of escalating fentanyl dosing on respiratory depression by way of decreased isohypercapnic minute ventilation (VE) – or volume of gas inhaled or exhaled per minute from the lungs.
Results showed the maximum decrease in highest-dose fentanyl-induced VE was almost 50% less for opioid-tolerant patients receiving a 2.0 ng/mL concentration of steady-state plasma buprenorphine than when receiving matching placebo.
Risk for apnea requiring stimulation after fentanyl dosing was also significantly lower with buprenorphine.
“Even though the study is small, a lot of data were collected which will allow us to very accurately predict which plasma concentrations, and therefore drug doses, are needed to protect people adequately in practice,” study coinvestigator Geert Jan Groeneveld, MD, PhD, neurologist and clinical pharmacologist at the Centre for Human Drug Research, Leiden, the Netherlands, and professor of clinical neuropharmacology at Leiden University Medical Center, told this news organization.
He added the “beautiful results” were in line with what the researchers expected and although further research is needed, the study provides a lot of useful information for clinicians.
“I think this is an approach that works, and this study makes that clear,” Dr. Groeneveld added.
The findings were published online Jan. 27, 2022, in PLoS One.
High death rate from synthetic opioids
A recent report from the Centers for Disease Control and Prevention noted that, between June 2020 and June 2021, there were more than 100,000 drug overdose deaths in the United States. Of these, more than 73,000 were attributed to opioids and more than 60,000 to synthetic opioids such as fentanyl.
Most opioid-related overdose deaths in the United States are attributable to synthetic opioids “that can unexpectedly cause respiratory depression by being ingested as a substitute for heroin or with [other] drugs,” Indivior noted in a press release.
Buprenorphine is a partial agonist that “binds with high affinity to mu-opioid receptors but displays partial respiratory depression effects,” the investigators wrote.
As reported by this news organization, the Food and Drug Administration approved buprenorphine extended release (Sublocade, Indivior) in 2017 as the first once-monthly injection for the treatment of opioid use disorder.
In the current study, which was conducted in Leiden, the Netherlands, the investigators used continuous intravenous buprenorphine in order to “mimic” the sustained plasma concentrations of the drug that can be delivered with the long-acting injectable, noted Christian Heidbreder, PhD, chief scientific officer at Indivior.
“This was an experimental medicine study, whereby we used intravenous buprenorphine to really understand the interaction with escalating doses of fentanyl” on respiratory depression, he told this news organization.
Two-part, two-period study
In part A, period one of the two-period crossover study, 14 healthy volunteers were randomly assigned to receive for 360 minutes continuous infusion of 0.02 or 0.05 mg/70 kg per hour of buprenorphine to target plasma concentrations of 0.2 or 0.5 ng/mL, respectively, or matching placebo. In the second period, participants received the alternative infusion – either placebo or the active drug.
In part B, eight opioid-tolerant patients who had used high-dose opioids for at least 3 months prior received a higher infusion rate of 0.1, 0.2, or 0.5 mg/70 kg per hour to target plasma concentrations of 1, 2, or 5 ng/mL, respectively.
The 2 ng/mL “is a very important threshold for us” and the result from several previous experiments, Dr. Heidbreder noted. So the investigators targeted that concentration as well as one below and one “much higher” in the current study.
“Because tolerance to opioid effects is poorly characterized in patients receiving long-term opioids, opioid-tolerant participants in part B had a fixed treatment sequence, receiving placebo infusion plus fentanyl challenges in period 1 to optimize the fentanyl dose escalation before buprenorphine and fentanyl were coadministered in period 2,” the investigators reported.
All participants received up to four escalating doses of intravenous fentanyl after reaching target buprenorphine plasma concentrations.
For healthy volunteers, the planned fentanyl doses were 0.075, 0.15, 0.25, and 0.35 mg/70 kg. For the opioid-tolerant patients, the doses were 0.25, 0.35, 0.5, and 0.7 mg/70 kg.
The infusions began after baseline VE had stabilized at 20 plus or minus 2 L/min, which is about four times above normal resting VE.
First clinical evidence?
Results showed fentanyl-induced adverse changes in VE were less at higher concentrations of buprenorphine plasma.
Opioid-tolerant patients receiving the 2.0 ng/mL concentration of buprenorphine had a 33.7% decrease in highest dose fentanyl-induced VE versus an 82.3% decrease when receiving placebo.
In addition, fentanyl reduced VE up to 49% (95% confidence interval, 21%-76%) in opioid-tolerant patients in all buprenorphine concentration groups combined versus reducing VE up to 100% (95% CI, 68%-132%) during placebo infusion (P = .006).
In addition, buprenorphine was associated with a lower risk versus placebo for apnea requiring verbal stimulation after fentanyl dosing (odds ratio, 0.07; P = .001).
For the healthy volunteers, the first fentanyl bolus reduced VE by 26% for those at target buprenorphine concentration of 0.5 ng/mL versus 51% when receiving placebo (P = .001). The second bolus reduced VE by 47% versus 79%, respectively (P < .001).
“Discontinuations for apnea limited treatment comparisons beyond the second fentanyl injection,” the investigators reported.
Overall, the findings “provide the first clinical evidence that high sustained plasma concentrations of buprenorphine may protect against respiratory depression induced by potent opioids,” they added.
Additional research is now “warranted to assess the competitive interaction of buprenorphine and fentanyl (as well as other illicitly manufactured fentanyl analogs) as we continue to deepen our understanding of buprenorphine as an evidence-based treatment for patients struggling with opioid use disorder,” Dr. Heidbreder said in a press release.
It’s unclear whether the study’s findings are generalizable to other populations, said Dr. Heidbreder.
“So and for that we’ll be using [the injectable] Sublocade as the medication of choice,” said Dr. Heidbreder.
“Conceptually, we feel confident about these data, but now we need to demonstrate what is happening in the real world,” he added.
The study was funded by Indivior. Dr. Groeneveld has reported no relevant financial relationships. Dr. Heidbreder is an employee of Indivior.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Marijuana use during pregnancy raised risk of adverse neonatal outcomes
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Doctor’s illegal opioid prescriptions lead to five deaths
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.
According to court documents, between January 2014 and October 2019, family physician David Chisholm, MD, 64, of Wasilla, Alaska, wrote more than 20,000 prescriptions to approximately 350 patients for oxycodone, methadone, and hydrocodone, often prescribing the pills using variations of patients’ names in an attempt to avoid being red-flagged by payers.
When Walmart refused to continue filling the prescriptions, Dr. Chisholm told his staff to advise the patients to use other pharmacies. In addition, he often prescribed combinations of medications, such as concurrent opioids, benzodiazepines, sedatives, and carisoprodol, thus increasing the chances that his patients would become addicted to or overdose on the drugs. Chisholm, who pleaded guilty in June, acknowledged to federal officials that his prescriptions were a significant contributing factor to the overdose deaths of five of his patients during this time, according to a statement by the U.S. Attorney’s Office for the District of Alaska.
According to the Anchorage Daily News, Dr. Chisholm, who was not board certified in pain medicine, said his reason for prescribing the drugs was not to make money but to help patients suffering from chronic pain and because he enjoyed the challenge.
Dr. Chisholm’s attorney, Nick Oberheiden, told CNN his client “sacrificed his reputation as a patient advocate and his years of service to the Alaskan community” in overprescribing opioids. “He expressed his sincere remorse in open court and he accepts the consequences of his misconduct. He hopes that his case serves as a warning to other physicians facing the same dilemma when treating chronic pain.”
He surrendered his medical license in November 2020 before being formally charged in April 2021.
Texas hospital CEO, seven doctors settle kickback
A hospital executive and seven physicians have agreed to pay a total of $1.1 million to settle allegations that they violated the Anti-Kickback Statute and Stark Law. The eight have also agreed to cooperate in investigations and litigation involving other parties.
The Texas physicians involved in the settlement are internist Jaspaul Bhangoo, MD, of Denton; family physician Robert Megna, DO, of Ferris; cardiologist Baxter Montgomery, MD, of Houston; internist Murtaza Mussaji, DO, of Houston; family physician David Sneed, DO, of Austin; family physician Kevin Lewis, DO, of Houston; and family physician Angela Mosley-Nunnery, MD, of Kingwood.
Also settling was Richard DeFoore, former CEO of Jones County Regional Healthcare (dba Stamford Memorial Hospital).
The physicians were accused of accepting payments from organizations in exchange for ordering lab tests from True Health Diagnostics, Little River Healthcare, and Boston Heart. The payments to the physicians were disguised as investment returns but, according to the allegations, were in fact offered in exchange for the doctors’ referrals. Mr. DeFoore, the hospital executive involved in the settlement, allegedly oversaw a similar scheme that benefited the now-defunct Stamford Memorial.
“Paying kickbacks to physicians distorts the medical decision-making process, corrupts our health care system, and increases the cost of healthcare funded by the taxpayer,” Brit Featherston, U.S. attorney for the Eastern District of Texas, said in a statement announcing the agreement. “Laboratories, marketers, and physicians cannot immunize their conduct by attempting to disguise the kickbacks as some sort of investment arrangement.”
Practice administrative assistant sentenced for fraudulent prescriptions
An administrative assistant at an Illinois orthopedics office was sentenced to a year and a day in federal prison for writing fraudulent prescriptions for opioids.
Amanda Biesiada, 39, of Alsip, Ill., who worked as an administrative assistant at Hinsdale Orthopaedics in Westmont, Ill., was not a licensed physician and could not legally write the prescriptions unless instructed to do so and supervised by licensed doctors.
According to a statement by the U.S. Attorney’s Office for the Northern District of Illinois, the prescriptions for hydrocodone, oxycodone, and other controlled substances – 85 prescriptions in all from 2017 to 2019 – were made out to an acquaintance of Biesiada’s and written without the knowledge or approval of the providers in whose names she wrote them.
Federal officials said Ms. Biesiada attempted to conceal the fraudulent prescriptions by marking them “filed in error” in the practice’s prescription system.
Lab owner pleads guilty to $6.9 million testing fraud scheme
A Florida lab owner has pleaded guilty to conspiracy to defraud Medicare through false and fraudulent claims totaling more than $6.9 million.
According to court documents, Christopher Licata, 45, of Delray Beach, Fla., admitted to bribing patient brokers to refer orders for medically unnecessary genetic testing to his lab. The tests were then billed to Medicare.
Mr. Licata and the patient brokers entered sham agreements meant to disguise the true purpose of the payments, according to a statement from the Department of Justice. The 45-year-old owner of Boca Toxicology (dba Lab Dynamics) pleaded guilty to one count of conspiring to commit health care fraud.
The scheme began in 2018; however, once the pandemic began, Mr. Licata shifted strategies, playing on patients’ fears of COVID-19 to bundle inexpensive COVID tests with more expensive medically unnecessary tests. These tests included respiratory pathogen panels and genetic testing for cardiovascular disease, cancer, diabetes, Alzheimer’s disease, and other illnesses. In all, Mr. Licata’s laboratory submitted over $6.9 million in false and fraudulent claims to Medicare for these unnecessary tests, according to the DOJ statement.
The case is a part of the U.S. Attorney General’s COVID-19 Fraud Enforcement Task Force that was established to enhance the efforts of agencies and governments across the country to combat and prevent pandemic-related fraud.
Mr. Licata faces a maximum penalty of 10 years in prison. His sentencing is scheduled for March 24.
A version of this article first appeared on Medscape.com.