User login
Four factors complicate treating OUD in primary care
Four themes in responses to a survey describe the multilevel barriers that make it difficult for primary care teams to incorporate medication for opioid use disorder (MOUD) in their practices, according to research published in JAMA Network Open.
Elizabeth J. Austin, PhD, MPH, with the department of health systems and population health at the University of Washington, Seattle, and colleagues describe the four major themes in the answers:
- Structural barriers delay or limit primary care team responsiveness to patients needing opioid-related care.
- Patient engagement was more challenging than expected.
- Prescribing physicians needed tools and to be able to see the patients on an ongoing basis.
- Teams had conflicting views on expanding MOUD care.
The survey
The researchers used a cohort of 12 clinics diverse in geography and structure and explored the experiences multidisciplinary primary care teams had in expanding MOUD services, such as use of buprenorphine and naltrexone.
A sample of 62 team members completed the survey for a response rate of 77%. Two-thirds (66%) identified as female and 46 (74%) identified as White. Evaluation of responses occurred between 2020 and 2022 in a sample of primary care clinics that agreed to participate in the Collaborating to Heal Addiction and Mental Health in Primary Care (CHAMP) study. The trial is ongoing.
Rigid scheduling a barrier
Some respondents said inflexible scheduling tied their hands.
One clinician responded, “[M]y practice has been really busy right now ... it’s been tough to find openings for my current patients as it is.”
Others described closed or limited patient panels, often set by their health systems. Twenty clinicians (32%) said they were worried their clinic couldn’t accommodate the volume of patients seeking OUD treatment.
Some reported productivity pressure from their health systems to keep the schedule full, which doesn’t allow for walk-in patients needing MOUD.
Frustration with no-shows
Some responses indicated frustration in locating patients and with no-shows.
One responded, “[W]e can’t find these people for months and months. [...] I’m spending 3 weeks, 4 weeks, trying to get them in.” Another said, “[I]t’s frustrating when patients don’t show up when they have been referred.”
Margret Chang, MD, a primary care doctor at Tri-River Family Health Center of Worcester, Mass., who was not part of the study, said the four categories the authors describe ring true.
Stigma for providers and patients
Dr. Chang said the biggest overarching part of those barriers comes down to stigma, but she says it’s not just a problem for patients, but for providers as well.
In fact, a responder in the Austin et al. survey wrote, “Our faculty group as a whole has expressed that that’s not the direction they want for our clinic; we already provide more psychiatric care and addiction medicine than other clinics, but we can’t be like the addiction medicine clinic in town either.”
Dr. Chang’s clinic, on the other hand, recruits addicted patients to their primary care practice by making a local drug court, addiction-support services in the community, and their colleagues in the UMass Health System aware that their services are available. Patients also refer their friends to the clinic and the clinic has a steady influx.
“I honestly feel that primary care is the discipline that really should be involved in substance disorder treatment,” says Dr. Chang, who is an assistant professor of medicine and the addiction curriculum director for internal medicine at UMass Chan Medical School, Worcester. “In medicine there’s a huge stigma around even being able to help these patients even though we have medications that are pretty effective.”
She runs a medication-assisted treatment program and said her semirural clinic and one other are the only two primary care clinics in the Worcester area with such a program.
Patients also have “huge inertia around taking a medication to recover from addiction or substance abuse,” she says.
Confidence lacking in treating patients
Dr. Chang said primary care residents in recent years are coming out of medical school with knowledge about treating OUD, but they often run into more experienced physicians who didn’t get training in the treatment so they feel intimidated about initiating the treatment.
At their clinic, Dr. Chang says, they have a nurse dedicated to OUD, which helps alleviate some of the barriers described in the survey. Patients know they can contact a particular person at the clinic who is dedicated to their needs. The nurse can track down patients who may miss appointments or be hard to locate so physicians don’t have to add that to their workload. They can collect fluid samples and connect patients to services.
Dr. Chang says a nurse might say, “I see we had you on (buprenorphine-naloxone) for opioid use disorder but I see you also have cocaine in your urine. How can we keep you safe?”
Having a health team member, whether a nurse or medical assistant or social worker, designated to help with people who need OUD treatment really makes a difference, she says.
People living with addiction “have a lot of needs,” she says, “and they are hard to address in the typical template a primary care provider might have.”
Family medicine, she says, has been more open to adding support staff for this population than other specialties.
Coauthor Andrew J. Saxon, MD, reported grants from the National Institute of Mental Health (NIMH) during the conduct of the study as well as personal fees from Indivior and royalties from UpToDate outside the submitted work. Coauthor John C. Fortney, PhD, reported grants from the Patient-Centered Outcomes Research Institute during the conduct of the study. Coauthor Anna D. Ratzliff, MD, PhD, reported grants from the University of Washington during the conduct of the study and royalties from Wiley outside the submitted work. No other disclosures were reported.
This story was updated on 8/15/2023.
Four themes in responses to a survey describe the multilevel barriers that make it difficult for primary care teams to incorporate medication for opioid use disorder (MOUD) in their practices, according to research published in JAMA Network Open.
Elizabeth J. Austin, PhD, MPH, with the department of health systems and population health at the University of Washington, Seattle, and colleagues describe the four major themes in the answers:
- Structural barriers delay or limit primary care team responsiveness to patients needing opioid-related care.
- Patient engagement was more challenging than expected.
- Prescribing physicians needed tools and to be able to see the patients on an ongoing basis.
- Teams had conflicting views on expanding MOUD care.
The survey
The researchers used a cohort of 12 clinics diverse in geography and structure and explored the experiences multidisciplinary primary care teams had in expanding MOUD services, such as use of buprenorphine and naltrexone.
A sample of 62 team members completed the survey for a response rate of 77%. Two-thirds (66%) identified as female and 46 (74%) identified as White. Evaluation of responses occurred between 2020 and 2022 in a sample of primary care clinics that agreed to participate in the Collaborating to Heal Addiction and Mental Health in Primary Care (CHAMP) study. The trial is ongoing.
Rigid scheduling a barrier
Some respondents said inflexible scheduling tied their hands.
One clinician responded, “[M]y practice has been really busy right now ... it’s been tough to find openings for my current patients as it is.”
Others described closed or limited patient panels, often set by their health systems. Twenty clinicians (32%) said they were worried their clinic couldn’t accommodate the volume of patients seeking OUD treatment.
Some reported productivity pressure from their health systems to keep the schedule full, which doesn’t allow for walk-in patients needing MOUD.
Frustration with no-shows
Some responses indicated frustration in locating patients and with no-shows.
One responded, “[W]e can’t find these people for months and months. [...] I’m spending 3 weeks, 4 weeks, trying to get them in.” Another said, “[I]t’s frustrating when patients don’t show up when they have been referred.”
Margret Chang, MD, a primary care doctor at Tri-River Family Health Center of Worcester, Mass., who was not part of the study, said the four categories the authors describe ring true.
Stigma for providers and patients
Dr. Chang said the biggest overarching part of those barriers comes down to stigma, but she says it’s not just a problem for patients, but for providers as well.
In fact, a responder in the Austin et al. survey wrote, “Our faculty group as a whole has expressed that that’s not the direction they want for our clinic; we already provide more psychiatric care and addiction medicine than other clinics, but we can’t be like the addiction medicine clinic in town either.”
Dr. Chang’s clinic, on the other hand, recruits addicted patients to their primary care practice by making a local drug court, addiction-support services in the community, and their colleagues in the UMass Health System aware that their services are available. Patients also refer their friends to the clinic and the clinic has a steady influx.
“I honestly feel that primary care is the discipline that really should be involved in substance disorder treatment,” says Dr. Chang, who is an assistant professor of medicine and the addiction curriculum director for internal medicine at UMass Chan Medical School, Worcester. “In medicine there’s a huge stigma around even being able to help these patients even though we have medications that are pretty effective.”
She runs a medication-assisted treatment program and said her semirural clinic and one other are the only two primary care clinics in the Worcester area with such a program.
Patients also have “huge inertia around taking a medication to recover from addiction or substance abuse,” she says.
Confidence lacking in treating patients
Dr. Chang said primary care residents in recent years are coming out of medical school with knowledge about treating OUD, but they often run into more experienced physicians who didn’t get training in the treatment so they feel intimidated about initiating the treatment.
At their clinic, Dr. Chang says, they have a nurse dedicated to OUD, which helps alleviate some of the barriers described in the survey. Patients know they can contact a particular person at the clinic who is dedicated to their needs. The nurse can track down patients who may miss appointments or be hard to locate so physicians don’t have to add that to their workload. They can collect fluid samples and connect patients to services.
Dr. Chang says a nurse might say, “I see we had you on (buprenorphine-naloxone) for opioid use disorder but I see you also have cocaine in your urine. How can we keep you safe?”
Having a health team member, whether a nurse or medical assistant or social worker, designated to help with people who need OUD treatment really makes a difference, she says.
People living with addiction “have a lot of needs,” she says, “and they are hard to address in the typical template a primary care provider might have.”
Family medicine, she says, has been more open to adding support staff for this population than other specialties.
Coauthor Andrew J. Saxon, MD, reported grants from the National Institute of Mental Health (NIMH) during the conduct of the study as well as personal fees from Indivior and royalties from UpToDate outside the submitted work. Coauthor John C. Fortney, PhD, reported grants from the Patient-Centered Outcomes Research Institute during the conduct of the study. Coauthor Anna D. Ratzliff, MD, PhD, reported grants from the University of Washington during the conduct of the study and royalties from Wiley outside the submitted work. No other disclosures were reported.
This story was updated on 8/15/2023.
Four themes in responses to a survey describe the multilevel barriers that make it difficult for primary care teams to incorporate medication for opioid use disorder (MOUD) in their practices, according to research published in JAMA Network Open.
Elizabeth J. Austin, PhD, MPH, with the department of health systems and population health at the University of Washington, Seattle, and colleagues describe the four major themes in the answers:
- Structural barriers delay or limit primary care team responsiveness to patients needing opioid-related care.
- Patient engagement was more challenging than expected.
- Prescribing physicians needed tools and to be able to see the patients on an ongoing basis.
- Teams had conflicting views on expanding MOUD care.
The survey
The researchers used a cohort of 12 clinics diverse in geography and structure and explored the experiences multidisciplinary primary care teams had in expanding MOUD services, such as use of buprenorphine and naltrexone.
A sample of 62 team members completed the survey for a response rate of 77%. Two-thirds (66%) identified as female and 46 (74%) identified as White. Evaluation of responses occurred between 2020 and 2022 in a sample of primary care clinics that agreed to participate in the Collaborating to Heal Addiction and Mental Health in Primary Care (CHAMP) study. The trial is ongoing.
Rigid scheduling a barrier
Some respondents said inflexible scheduling tied their hands.
One clinician responded, “[M]y practice has been really busy right now ... it’s been tough to find openings for my current patients as it is.”
Others described closed or limited patient panels, often set by their health systems. Twenty clinicians (32%) said they were worried their clinic couldn’t accommodate the volume of patients seeking OUD treatment.
Some reported productivity pressure from their health systems to keep the schedule full, which doesn’t allow for walk-in patients needing MOUD.
Frustration with no-shows
Some responses indicated frustration in locating patients and with no-shows.
One responded, “[W]e can’t find these people for months and months. [...] I’m spending 3 weeks, 4 weeks, trying to get them in.” Another said, “[I]t’s frustrating when patients don’t show up when they have been referred.”
Margret Chang, MD, a primary care doctor at Tri-River Family Health Center of Worcester, Mass., who was not part of the study, said the four categories the authors describe ring true.
Stigma for providers and patients
Dr. Chang said the biggest overarching part of those barriers comes down to stigma, but she says it’s not just a problem for patients, but for providers as well.
In fact, a responder in the Austin et al. survey wrote, “Our faculty group as a whole has expressed that that’s not the direction they want for our clinic; we already provide more psychiatric care and addiction medicine than other clinics, but we can’t be like the addiction medicine clinic in town either.”
Dr. Chang’s clinic, on the other hand, recruits addicted patients to their primary care practice by making a local drug court, addiction-support services in the community, and their colleagues in the UMass Health System aware that their services are available. Patients also refer their friends to the clinic and the clinic has a steady influx.
“I honestly feel that primary care is the discipline that really should be involved in substance disorder treatment,” says Dr. Chang, who is an assistant professor of medicine and the addiction curriculum director for internal medicine at UMass Chan Medical School, Worcester. “In medicine there’s a huge stigma around even being able to help these patients even though we have medications that are pretty effective.”
She runs a medication-assisted treatment program and said her semirural clinic and one other are the only two primary care clinics in the Worcester area with such a program.
Patients also have “huge inertia around taking a medication to recover from addiction or substance abuse,” she says.
Confidence lacking in treating patients
Dr. Chang said primary care residents in recent years are coming out of medical school with knowledge about treating OUD, but they often run into more experienced physicians who didn’t get training in the treatment so they feel intimidated about initiating the treatment.
At their clinic, Dr. Chang says, they have a nurse dedicated to OUD, which helps alleviate some of the barriers described in the survey. Patients know they can contact a particular person at the clinic who is dedicated to their needs. The nurse can track down patients who may miss appointments or be hard to locate so physicians don’t have to add that to their workload. They can collect fluid samples and connect patients to services.
Dr. Chang says a nurse might say, “I see we had you on (buprenorphine-naloxone) for opioid use disorder but I see you also have cocaine in your urine. How can we keep you safe?”
Having a health team member, whether a nurse or medical assistant or social worker, designated to help with people who need OUD treatment really makes a difference, she says.
People living with addiction “have a lot of needs,” she says, “and they are hard to address in the typical template a primary care provider might have.”
Family medicine, she says, has been more open to adding support staff for this population than other specialties.
Coauthor Andrew J. Saxon, MD, reported grants from the National Institute of Mental Health (NIMH) during the conduct of the study as well as personal fees from Indivior and royalties from UpToDate outside the submitted work. Coauthor John C. Fortney, PhD, reported grants from the Patient-Centered Outcomes Research Institute during the conduct of the study. Coauthor Anna D. Ratzliff, MD, PhD, reported grants from the University of Washington during the conduct of the study and royalties from Wiley outside the submitted work. No other disclosures were reported.
This story was updated on 8/15/2023.
FROM JAMA NETWORK OPEN
Medications for opioid addiction significantly underutilized
Using data from the 2021 National Survey on Drug Use and Health (NSDUH), investigators found that of the 2.5 million adults with OUD in that year, 35.6% received some kind of substance abuse treatment, but only 22.3% received recommended medications for the condition, such as methadone, buprenorphine, or extended-release naltrexone.
“More than 80,000 people are dying of a drug overdose involving an opioid every year, while safe and effective medicines to treat opioid use disorder are sitting on the shelf unused,” senior author Wilson Compton, MD, MPE, deputy director of the National Institute on Drug Abuse (NIDA), said in a statement. “This study adds to the growing evidence that telehealth services are an important strategy that could help us bridge this gap, supporting the delivery of safe, effective, and lifesaving care for people with opioid use disorder.”
The findings were published online as a research letter in JAMA Network Open.
The study included 47,291 adults aged 18 years or older in the 2021 NSDUH, which provides nationally representative data of the U.S. civilian, noninstitutionalized population based on past-year OUD.
Men, people aged 35 years or older, urban residents, and non-Hispanic Whites were the most likely to receive medication for opioid use disorder (MOUD). MOUD use was more common among those who received substance use treatment via telehealth, those with severe OUD, and people with annual incomes below $50,000.
Black people, women, unemployed individuals, those living in rural areas, and people with past-year cannabis use disorder were less likely to receive MOUD.
“It is not a matter of whether we should address health disparities and inequities that many racial/ethnic minority groups face when trying to access substance use treatment,” lead author Christopher M. Jones, PharmD, MPH, DrPH, director of the National Center for Injury Prevention and Control in the Centers for Disease Control and Prevention, said in a statement. “We must address these issues if we hope to reverse the trend of increasing drug overdose deaths.”
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Compton reported long-term stock holdings in General Electric, 3M Companies, and Pfizer outside the submitted work.
A version of this article first appeared on Medscape.com.
Using data from the 2021 National Survey on Drug Use and Health (NSDUH), investigators found that of the 2.5 million adults with OUD in that year, 35.6% received some kind of substance abuse treatment, but only 22.3% received recommended medications for the condition, such as methadone, buprenorphine, or extended-release naltrexone.
“More than 80,000 people are dying of a drug overdose involving an opioid every year, while safe and effective medicines to treat opioid use disorder are sitting on the shelf unused,” senior author Wilson Compton, MD, MPE, deputy director of the National Institute on Drug Abuse (NIDA), said in a statement. “This study adds to the growing evidence that telehealth services are an important strategy that could help us bridge this gap, supporting the delivery of safe, effective, and lifesaving care for people with opioid use disorder.”
The findings were published online as a research letter in JAMA Network Open.
The study included 47,291 adults aged 18 years or older in the 2021 NSDUH, which provides nationally representative data of the U.S. civilian, noninstitutionalized population based on past-year OUD.
Men, people aged 35 years or older, urban residents, and non-Hispanic Whites were the most likely to receive medication for opioid use disorder (MOUD). MOUD use was more common among those who received substance use treatment via telehealth, those with severe OUD, and people with annual incomes below $50,000.
Black people, women, unemployed individuals, those living in rural areas, and people with past-year cannabis use disorder were less likely to receive MOUD.
“It is not a matter of whether we should address health disparities and inequities that many racial/ethnic minority groups face when trying to access substance use treatment,” lead author Christopher M. Jones, PharmD, MPH, DrPH, director of the National Center for Injury Prevention and Control in the Centers for Disease Control and Prevention, said in a statement. “We must address these issues if we hope to reverse the trend of increasing drug overdose deaths.”
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Compton reported long-term stock holdings in General Electric, 3M Companies, and Pfizer outside the submitted work.
A version of this article first appeared on Medscape.com.
Using data from the 2021 National Survey on Drug Use and Health (NSDUH), investigators found that of the 2.5 million adults with OUD in that year, 35.6% received some kind of substance abuse treatment, but only 22.3% received recommended medications for the condition, such as methadone, buprenorphine, or extended-release naltrexone.
“More than 80,000 people are dying of a drug overdose involving an opioid every year, while safe and effective medicines to treat opioid use disorder are sitting on the shelf unused,” senior author Wilson Compton, MD, MPE, deputy director of the National Institute on Drug Abuse (NIDA), said in a statement. “This study adds to the growing evidence that telehealth services are an important strategy that could help us bridge this gap, supporting the delivery of safe, effective, and lifesaving care for people with opioid use disorder.”
The findings were published online as a research letter in JAMA Network Open.
The study included 47,291 adults aged 18 years or older in the 2021 NSDUH, which provides nationally representative data of the U.S. civilian, noninstitutionalized population based on past-year OUD.
Men, people aged 35 years or older, urban residents, and non-Hispanic Whites were the most likely to receive medication for opioid use disorder (MOUD). MOUD use was more common among those who received substance use treatment via telehealth, those with severe OUD, and people with annual incomes below $50,000.
Black people, women, unemployed individuals, those living in rural areas, and people with past-year cannabis use disorder were less likely to receive MOUD.
“It is not a matter of whether we should address health disparities and inequities that many racial/ethnic minority groups face when trying to access substance use treatment,” lead author Christopher M. Jones, PharmD, MPH, DrPH, director of the National Center for Injury Prevention and Control in the Centers for Disease Control and Prevention, said in a statement. “We must address these issues if we hope to reverse the trend of increasing drug overdose deaths.”
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Compton reported long-term stock holdings in General Electric, 3M Companies, and Pfizer outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
OxyContin marketing push still exacting a deadly toll, study says
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
The uptick in rates of infectious diseases, namely, hepatitis and infective endocarditis, occurred after 2010, when OxyContin maker Purdue Pharma reformulated OxyContin to make it harder to crush and snort. This led many people who were already addicted to the powerful pain pills to move on to injecting heroin or fentanyl, which fueled the spread of infectious disease.
“Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient more than 25 years later,” write Julia Dennett, PhD, and Gregg Gonsalves, PhD, with Yale University School of Public Health, New Haven, Conn.
Their study was published online in Health Affairs.
Long-term effects revealed
Until now, the long-term effects of widespread OxyContin marketing with regard to complications of injection drug use were unknown.
Dr. Dennett and Dr. Gonsalves evaluated the effects of OxyContin marketing on the long-term trajectories of various injection drug use–related outcomes. Using a difference-in-difference analysis, they compared states with high vs. low exposure to OxyContin marketing before and after the 2010 reformulation of the drug.
Before 2010, rates of infections associated with injection drug use and overdose deaths were similar in high- and low-marketing states, they found.
Those rates diverged after the 2010 reformulation, with more infections related to injection drug use in states exposed to more marketing.
Specifically, from 2010 until 2020, high-exposure states saw, on average, an additional 0.85 acute hepatitis B cases, 0.83 hepatitis C cases, and 0.62 cases of death from infective endocarditis per 100,000 residents.
High-exposure states also had 5.3 more deaths per 100,000 residents from synthetic opioid overdose.
“Prior to 2010, among these states, there were generally no statistically significant differences in these outcomes. After 2010, you saw them diverge dramatically,” Dr. Dennett said in a news release.
Dr. Dennett and Dr. Gonsalves say their findings support the view that the opioid epidemic is creating a converging public health crisis, as it is fueling a surge in infectious diseases, particularly hepatitis, infective endocarditis, and HIV.
“This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with injection drug use, both in the states that were subject to Purdue’s promotional campaign and across the U.S. more broadly,” they add.
Purdue Pharma did not provide a comment on the study.
Funding for the study was provided by the National Institute on Drug Abuse. Disclosures for Dr. Dennett and Dr. Gonsalves were not available.
A version of this article first appeared on Medscape.com.
FROM HEALTH AFFAIRS
A new and completely different pain medicine
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.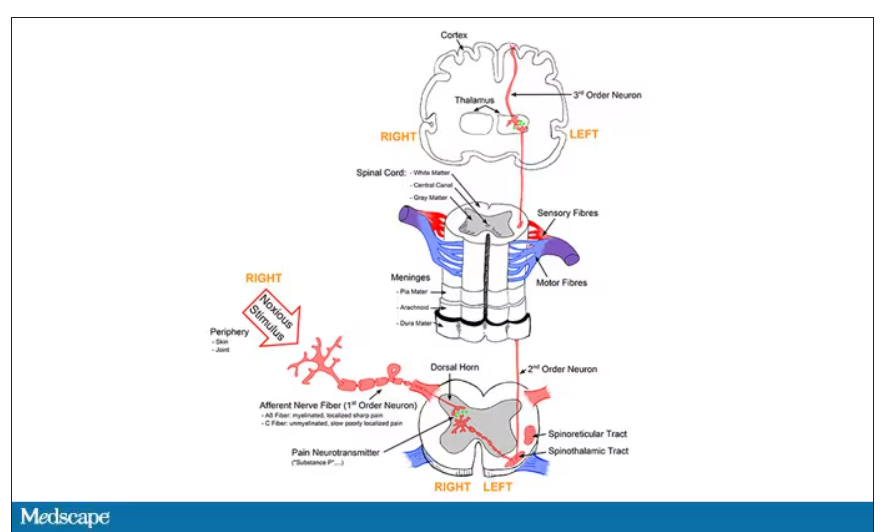
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.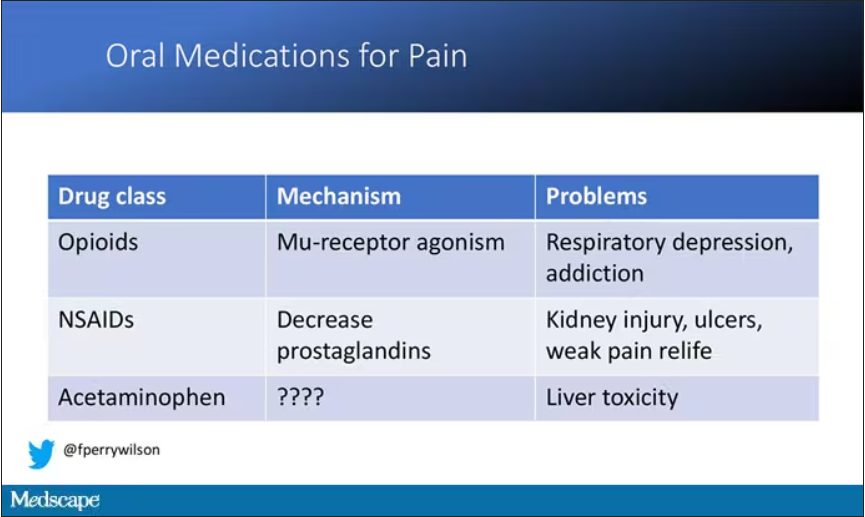
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.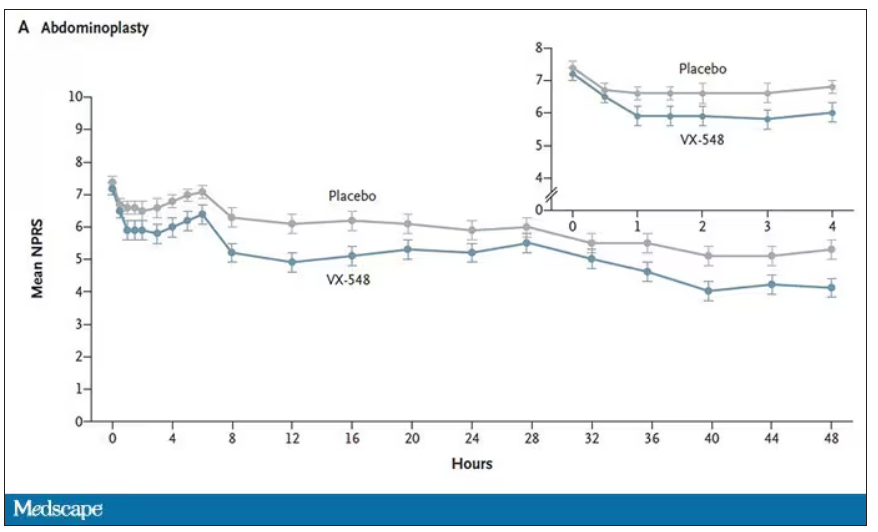
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.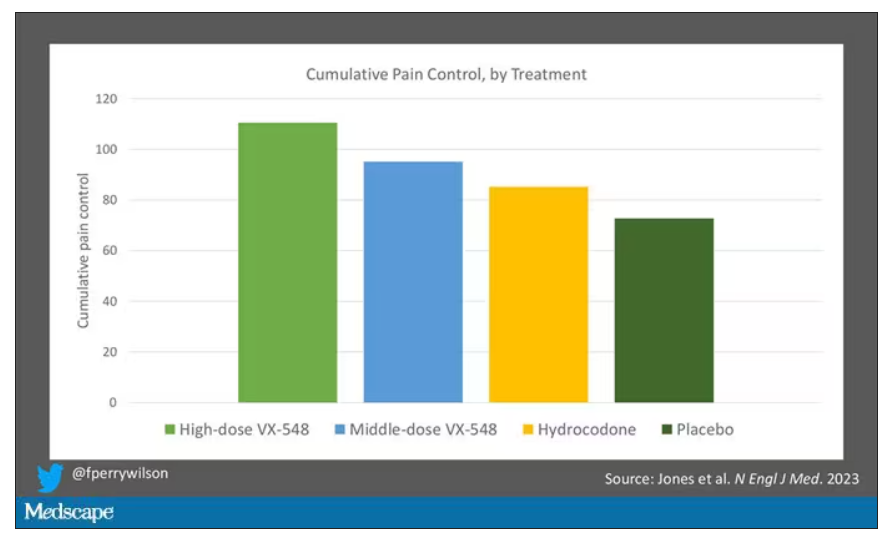
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.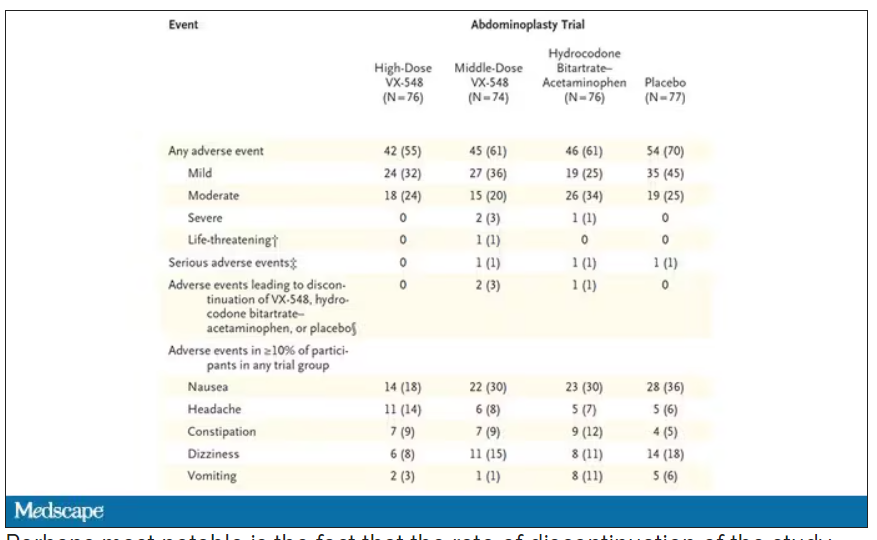
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prescribing lifestyle changes: When medicine isn’t enough
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
Nonalcohol substance use disorder tied to bariatric surgery
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM OBESITY
Oxycodone tied to persistent use only after vaginal delivery
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Off-label medications for addictive disorders
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52
The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Infested with worms, but are they really there?
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
Extended-release injectable naltrexone for opioid use disorder
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622