User login
Simultaneous marijuana, alcohol use linked to worse outcomes
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. counties hit hard by a lack of psychiatric care
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Supplements Are Not a Synonym for Safe: Suspected Liver Injury From Ashwagandha
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
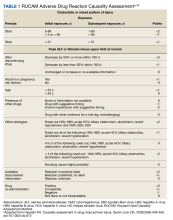
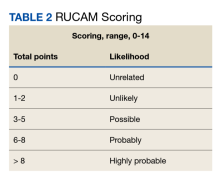
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Nurses maintain more stigma toward pregnant women with OUD
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL ON ADDICTIONS
Growing public perception that cannabis is safer than tobacco
TOPLINE:
METHODOLOGY:
- While aggressive campaigns have led to a dramatic reduction in the prevalence of cigarette smoking and created safer smoke-free environments, regulation governing cannabis – which is associated with some health benefits but also many negative health outcomes – has been less restrictive.
- The study included a nationally representative sample of 5,035 mostly White U.S. adults, mean age 53.4 years, who completed three online surveys between 2017 and 2021 on the safety of tobacco and cannabis.
- In all three waves of the survey, respondents were asked to rate the safety of smoking one marijuana joint a day to smoking one cigarette a day, and of secondhand smoke from marijuana to that from tobacco.
- Respondents also expressed views on the safety of secondhand smoke exposure (of both marijuana and tobacco) on specific populations, including children, pregnant women, and adults (ratings were from “completely unsafe” to “completely safe”).
- Independent variables included age, sex, race, ethnicity, education level, annual income, employment status, marital status, and state of residence.
TAKEAWAY:
- There was a significant shift over time toward an increasingly favorable perception of cannabis; more respondents reported cannabis was “somewhat safer” or “much safer” than tobacco in 2021 than 2017 (44.3% vs. 36.7%; P < .001), and more believed secondhand smoke was somewhat or much safer for cannabis vs. tobacco in 2021 than in 2017 (40.2% vs. 35.1%; P < .001).
- More people endorsed the greater safety of secondhand smoke from cannabis vs. tobacco for children and pregnant women, and these perceptions remained similar over the study period.
- Younger and unmarried individuals were significantly more likely to move toward viewing smoking cannabis as safer than cigarettes, but legality of cannabis in respondents’ state of residence was not associated with change over time, suggesting the increasing perception of cannabis safety may be a national trend rather than a trend seen only in states with legalized cannabis.
IN PRACTICE:
“Understanding changing views on tobacco and cannabis risk is important given that increases in social acceptance and decreases in risk perception may be directly associated with public health and policies,” the investigators write.
SOURCE:
The study was conducted by Julia Chambers, MD, department of medicine, University of California, San Francisco, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The generalizability of the study may be limited by nonresponse and loss to follow-up over time. The wording of survey questions may have introduced bias in respondents. Participants were asked about safety of smoking cannabis joints vs. tobacco cigarettes and not to compare safety of other forms of smoked and vaped cannabis, tobacco, and nicotine.
DISCLOSURES:
The study received support from the California Tobacco-Related Disease Research Program. Dr. Chambers has no relevant conflicts of interest; author Katherine J. Hoggatt, PhD, MPH, department of medicine, UCSF, reported receiving grants from the Veterans Health Administration during the conduct of the study and grants from the National Institutes of Health, Rubin Family Foundation, and Veterans Health Administration outside the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While aggressive campaigns have led to a dramatic reduction in the prevalence of cigarette smoking and created safer smoke-free environments, regulation governing cannabis – which is associated with some health benefits but also many negative health outcomes – has been less restrictive.
- The study included a nationally representative sample of 5,035 mostly White U.S. adults, mean age 53.4 years, who completed three online surveys between 2017 and 2021 on the safety of tobacco and cannabis.
- In all three waves of the survey, respondents were asked to rate the safety of smoking one marijuana joint a day to smoking one cigarette a day, and of secondhand smoke from marijuana to that from tobacco.
- Respondents also expressed views on the safety of secondhand smoke exposure (of both marijuana and tobacco) on specific populations, including children, pregnant women, and adults (ratings were from “completely unsafe” to “completely safe”).
- Independent variables included age, sex, race, ethnicity, education level, annual income, employment status, marital status, and state of residence.
TAKEAWAY:
- There was a significant shift over time toward an increasingly favorable perception of cannabis; more respondents reported cannabis was “somewhat safer” or “much safer” than tobacco in 2021 than 2017 (44.3% vs. 36.7%; P < .001), and more believed secondhand smoke was somewhat or much safer for cannabis vs. tobacco in 2021 than in 2017 (40.2% vs. 35.1%; P < .001).
- More people endorsed the greater safety of secondhand smoke from cannabis vs. tobacco for children and pregnant women, and these perceptions remained similar over the study period.
- Younger and unmarried individuals were significantly more likely to move toward viewing smoking cannabis as safer than cigarettes, but legality of cannabis in respondents’ state of residence was not associated with change over time, suggesting the increasing perception of cannabis safety may be a national trend rather than a trend seen only in states with legalized cannabis.
IN PRACTICE:
“Understanding changing views on tobacco and cannabis risk is important given that increases in social acceptance and decreases in risk perception may be directly associated with public health and policies,” the investigators write.
SOURCE:
The study was conducted by Julia Chambers, MD, department of medicine, University of California, San Francisco, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The generalizability of the study may be limited by nonresponse and loss to follow-up over time. The wording of survey questions may have introduced bias in respondents. Participants were asked about safety of smoking cannabis joints vs. tobacco cigarettes and not to compare safety of other forms of smoked and vaped cannabis, tobacco, and nicotine.
DISCLOSURES:
The study received support from the California Tobacco-Related Disease Research Program. Dr. Chambers has no relevant conflicts of interest; author Katherine J. Hoggatt, PhD, MPH, department of medicine, UCSF, reported receiving grants from the Veterans Health Administration during the conduct of the study and grants from the National Institutes of Health, Rubin Family Foundation, and Veterans Health Administration outside the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While aggressive campaigns have led to a dramatic reduction in the prevalence of cigarette smoking and created safer smoke-free environments, regulation governing cannabis – which is associated with some health benefits but also many negative health outcomes – has been less restrictive.
- The study included a nationally representative sample of 5,035 mostly White U.S. adults, mean age 53.4 years, who completed three online surveys between 2017 and 2021 on the safety of tobacco and cannabis.
- In all three waves of the survey, respondents were asked to rate the safety of smoking one marijuana joint a day to smoking one cigarette a day, and of secondhand smoke from marijuana to that from tobacco.
- Respondents also expressed views on the safety of secondhand smoke exposure (of both marijuana and tobacco) on specific populations, including children, pregnant women, and adults (ratings were from “completely unsafe” to “completely safe”).
- Independent variables included age, sex, race, ethnicity, education level, annual income, employment status, marital status, and state of residence.
TAKEAWAY:
- There was a significant shift over time toward an increasingly favorable perception of cannabis; more respondents reported cannabis was “somewhat safer” or “much safer” than tobacco in 2021 than 2017 (44.3% vs. 36.7%; P < .001), and more believed secondhand smoke was somewhat or much safer for cannabis vs. tobacco in 2021 than in 2017 (40.2% vs. 35.1%; P < .001).
- More people endorsed the greater safety of secondhand smoke from cannabis vs. tobacco for children and pregnant women, and these perceptions remained similar over the study period.
- Younger and unmarried individuals were significantly more likely to move toward viewing smoking cannabis as safer than cigarettes, but legality of cannabis in respondents’ state of residence was not associated with change over time, suggesting the increasing perception of cannabis safety may be a national trend rather than a trend seen only in states with legalized cannabis.
IN PRACTICE:
“Understanding changing views on tobacco and cannabis risk is important given that increases in social acceptance and decreases in risk perception may be directly associated with public health and policies,” the investigators write.
SOURCE:
The study was conducted by Julia Chambers, MD, department of medicine, University of California, San Francisco, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The generalizability of the study may be limited by nonresponse and loss to follow-up over time. The wording of survey questions may have introduced bias in respondents. Participants were asked about safety of smoking cannabis joints vs. tobacco cigarettes and not to compare safety of other forms of smoked and vaped cannabis, tobacco, and nicotine.
DISCLOSURES:
The study received support from the California Tobacco-Related Disease Research Program. Dr. Chambers has no relevant conflicts of interest; author Katherine J. Hoggatt, PhD, MPH, department of medicine, UCSF, reported receiving grants from the Veterans Health Administration during the conduct of the study and grants from the National Institutes of Health, Rubin Family Foundation, and Veterans Health Administration outside the submitted work.
A version of this article first appeared on Medscape.com.
Adaptive treatment aids smoking cessation
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Marijuana, hallucinogen use, binge drinking at all-time high
The latest results of the Monitoring the Future (MTF) longitudinal survey show that American adults are consuming marijuana and hallucinogens, vaping, and binge drinking at historic levels.
“In 2022, we are seeing that marijuana and hallucinogen use, and vaping of nicotine and marijuana, are higher than ever among young adults ages 19-30,” said Megan Patrick, research professor and principal investigator of the MTF study. “In addition, midlife adults ages 35-50 have the highest level of binge drinking we have ever seen in that age group,” she said in a statement.
The survey, conducted annually since 1975 by the University of Michigan’s Institute for Social Research, Ann Arbor, queries nationally representative samples of 8th, 10th, and 12th graders and then follows a subset through adulthood to come up with longitudinal data. It is funded by the National Institute on Drug Abuse.
The adult data for 2022 were gathered by online and paper surveys from April to October 2022 and included responses from some 10,000 individuals. Participants were divided into two cohorts: those aged 19-30 years and those aged 35-50 years.
About a third of the older age group reported using marijuana in the past year, an all-time high, up from 25% in 2021 and more than double the users in 2012 (13%). Of this group, 4% reported past-year hallucinogen use, also a record high and double the reported use in 2021.
Alcohol use among adults aged 35-50 has gradually increased over the past decade. Of this group, 85% reported past-year drinking in 2022, up from 83% in 2012.
Binge drinking – defined as having five or more drinks in a row in the past 2 weeks – has also been on the rise in the past decade. One-third of older adults reported binge drinking in 2022. Binge drinking was highest among White (31.4%) and Hispanic (30.6%) midlife adults and was lower among Black (17.1%) midlife adults.
Vaping among the older age cohort has remained at similar levels since first measured in 2019; 9% vaped marijuana in the past year, while 7% vaped nicotine.
Marijuana popular among younger Americans
“In 2022, marijuana use among young adults reached the highest levels ever recorded since the indices were first available in 1988,” the study authors write. Both past-year and daily use hit record levels for the cohort of those aged 19-30.
Forty-four percent reported past-year marijuana use, up from 28% in 2012. The highest levels of use were in those aged 27-28. One in five reported daily use, up from 6% a decade ago; almost 14% of 23- to 24-year-olds reported daily use.
Past-year use of hallucinogens – including LSD, MDMA, mescaline, peyote, mushrooms or psilocybin, and PCP – was reported by 8% of this age group. Most of the increase was driven by use of hallucinogens other than LSD, which accounted for 7% of the reported use.
Young adults also reported record levels of vaping marijuana, with 21% reporting past-year use and 14% reporting past-month use. Vaping of nicotine has doubled in prevalence since the survey started asking about it, from 14% for past-year use in 2017 to 24% in 2022.
NIDA Director Nora Volkow, MD, noted in a statement that the survey results show that “substance use is not limited to teens and young adults,” adding that “these data help us understand how people use drugs across the lifespan.”
A version of this article first appeared on Medscape.com.
The latest results of the Monitoring the Future (MTF) longitudinal survey show that American adults are consuming marijuana and hallucinogens, vaping, and binge drinking at historic levels.
“In 2022, we are seeing that marijuana and hallucinogen use, and vaping of nicotine and marijuana, are higher than ever among young adults ages 19-30,” said Megan Patrick, research professor and principal investigator of the MTF study. “In addition, midlife adults ages 35-50 have the highest level of binge drinking we have ever seen in that age group,” she said in a statement.
The survey, conducted annually since 1975 by the University of Michigan’s Institute for Social Research, Ann Arbor, queries nationally representative samples of 8th, 10th, and 12th graders and then follows a subset through adulthood to come up with longitudinal data. It is funded by the National Institute on Drug Abuse.
The adult data for 2022 were gathered by online and paper surveys from April to October 2022 and included responses from some 10,000 individuals. Participants were divided into two cohorts: those aged 19-30 years and those aged 35-50 years.
About a third of the older age group reported using marijuana in the past year, an all-time high, up from 25% in 2021 and more than double the users in 2012 (13%). Of this group, 4% reported past-year hallucinogen use, also a record high and double the reported use in 2021.
Alcohol use among adults aged 35-50 has gradually increased over the past decade. Of this group, 85% reported past-year drinking in 2022, up from 83% in 2012.
Binge drinking – defined as having five or more drinks in a row in the past 2 weeks – has also been on the rise in the past decade. One-third of older adults reported binge drinking in 2022. Binge drinking was highest among White (31.4%) and Hispanic (30.6%) midlife adults and was lower among Black (17.1%) midlife adults.
Vaping among the older age cohort has remained at similar levels since first measured in 2019; 9% vaped marijuana in the past year, while 7% vaped nicotine.
Marijuana popular among younger Americans
“In 2022, marijuana use among young adults reached the highest levels ever recorded since the indices were first available in 1988,” the study authors write. Both past-year and daily use hit record levels for the cohort of those aged 19-30.
Forty-four percent reported past-year marijuana use, up from 28% in 2012. The highest levels of use were in those aged 27-28. One in five reported daily use, up from 6% a decade ago; almost 14% of 23- to 24-year-olds reported daily use.
Past-year use of hallucinogens – including LSD, MDMA, mescaline, peyote, mushrooms or psilocybin, and PCP – was reported by 8% of this age group. Most of the increase was driven by use of hallucinogens other than LSD, which accounted for 7% of the reported use.
Young adults also reported record levels of vaping marijuana, with 21% reporting past-year use and 14% reporting past-month use. Vaping of nicotine has doubled in prevalence since the survey started asking about it, from 14% for past-year use in 2017 to 24% in 2022.
NIDA Director Nora Volkow, MD, noted in a statement that the survey results show that “substance use is not limited to teens and young adults,” adding that “these data help us understand how people use drugs across the lifespan.”
A version of this article first appeared on Medscape.com.
The latest results of the Monitoring the Future (MTF) longitudinal survey show that American adults are consuming marijuana and hallucinogens, vaping, and binge drinking at historic levels.
“In 2022, we are seeing that marijuana and hallucinogen use, and vaping of nicotine and marijuana, are higher than ever among young adults ages 19-30,” said Megan Patrick, research professor and principal investigator of the MTF study. “In addition, midlife adults ages 35-50 have the highest level of binge drinking we have ever seen in that age group,” she said in a statement.
The survey, conducted annually since 1975 by the University of Michigan’s Institute for Social Research, Ann Arbor, queries nationally representative samples of 8th, 10th, and 12th graders and then follows a subset through adulthood to come up with longitudinal data. It is funded by the National Institute on Drug Abuse.
The adult data for 2022 were gathered by online and paper surveys from April to October 2022 and included responses from some 10,000 individuals. Participants were divided into two cohorts: those aged 19-30 years and those aged 35-50 years.
About a third of the older age group reported using marijuana in the past year, an all-time high, up from 25% in 2021 and more than double the users in 2012 (13%). Of this group, 4% reported past-year hallucinogen use, also a record high and double the reported use in 2021.
Alcohol use among adults aged 35-50 has gradually increased over the past decade. Of this group, 85% reported past-year drinking in 2022, up from 83% in 2012.
Binge drinking – defined as having five or more drinks in a row in the past 2 weeks – has also been on the rise in the past decade. One-third of older adults reported binge drinking in 2022. Binge drinking was highest among White (31.4%) and Hispanic (30.6%) midlife adults and was lower among Black (17.1%) midlife adults.
Vaping among the older age cohort has remained at similar levels since first measured in 2019; 9% vaped marijuana in the past year, while 7% vaped nicotine.
Marijuana popular among younger Americans
“In 2022, marijuana use among young adults reached the highest levels ever recorded since the indices were first available in 1988,” the study authors write. Both past-year and daily use hit record levels for the cohort of those aged 19-30.
Forty-four percent reported past-year marijuana use, up from 28% in 2012. The highest levels of use were in those aged 27-28. One in five reported daily use, up from 6% a decade ago; almost 14% of 23- to 24-year-olds reported daily use.
Past-year use of hallucinogens – including LSD, MDMA, mescaline, peyote, mushrooms or psilocybin, and PCP – was reported by 8% of this age group. Most of the increase was driven by use of hallucinogens other than LSD, which accounted for 7% of the reported use.
Young adults also reported record levels of vaping marijuana, with 21% reporting past-year use and 14% reporting past-month use. Vaping of nicotine has doubled in prevalence since the survey started asking about it, from 14% for past-year use in 2017 to 24% in 2022.
NIDA Director Nora Volkow, MD, noted in a statement that the survey results show that “substance use is not limited to teens and young adults,” adding that “these data help us understand how people use drugs across the lifespan.”
A version of this article first appeared on Medscape.com.
Gene therapy offers new way to fight alcohol use disorder
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Do the data support psychedelics in addiction therapy?
PARIS – “We need to develop new therapies to treat addiction because of the related cost to society, which is extremely high,” said Bruno Roméo, MD, psychiatrist and addiction specialist at Paul Brousse Hospital in Villejuif, France, at the Paris-based Neuroscience, Psychiatry and Neurology Conference. Dr. Roméo spoke about the current place of psychedelics in the treatment of addiction.
“Smoking and alcohol consumption are the two main preventable causes of death in France,” he said. “Current management strategies for these addictions rarely involve pharmacological therapies, which are not very effective, in any case. We have massive relapse rates, signaling the need to develop other treatments, like psychedelic drugs.”
But what data are available on the efficacy of psychedelics in treating addiction?
Alcohol use disorder
There are few data concerning the role of psychedelics in the treatment of alcohol use disorder, but one controlled, randomized trial evaluated the efficacy of psilocybin. That trial was published in JAMA Psychiatry in 2022.
That study included 95 patients with alcohol use disorder; 49 were treated with psilocybin, and 46 were treated with diphenhydramine.
An initial medication session of psilocybin was given in week 4, then another in week 8 at a higher dose. The number of drinking days, the number of heavy drinking days, and the number of drinks consumed between weeks 32 and 36 were assessed.
The investigators showed that, after two sessions with psilocybin, there was a significant reduction in the number of heavy drinking days. In the control group, between weeks 5 and 36, 20% of days involved heavy drinking, whereas in the psilocybin group, 10% of days involved heavy drinking.
There was also a significant and rapid reduction in the number of drinking days, and this was maintained over time. Between weeks 5 and 36, just over 40% of days were reported as drinking days in the control group versus slightly more than 30% in the psilocybin group.
Similarly, the number of glasses per day was drastically reduced after taking psilocybin, and the effect occurred extremely quickly. Consumption went from six drinks to less than one drink between weeks 5 and 8. Overall, between weeks 5 and 36, the number of drinks consumed per day was more than two in the placebo group and more than one in the psilocybin group.
“Psilocybin was seen as having potential efficacy in treating alcohol use disorder. But we must tread carefully with these results; the profile of the patients enrolled in this study is different to that of the patients we regularly see in our addiction clinics. The patients enrolled in the study reported less than 60% of days as heavy drinking days,” said Dr. Romeo.
Candidates for psilocybin
According to a retrospective survey of 160 respondents that was conducted online at Paul Brousse Hospital, patients with the most severe cases of alcohol use disorder who have the most mystical psychedelic experiences seem to respond best to psilocybin and to reduce their alcohol use. It also appears that patients whose alcohol use decreased the most had lower psychological flexibility on enrollment in the study. (Psychological flexibility is the ability to adapt to change and to cope with positive and negative experiences in real time without being fazed or trying to flee from the situation.) “It’s as if they had a broader capacity for change, and psychedelics helped them more,” said Dr. Roméo.
Smoking cessation
“There are even fewer studies for smoking,” said Dr. Roméo. In a pilot study with 15 patients, the researchers gave two or three doses of psilocybin at 20-30 mg in combination with cognitive-behavioral therapy one session per week for 10 weeks. Thereafter, patients were assessed three times: after 6 months, 12 months, and 30 months.
The results showed a significant reduction in smoking. Patients went from smoking more than 15 cigarettes per day to smoking one to two cigarettes per day before going back up to six cigarettes daily.
Regarding abstinence rates, 12 of 15 patients had stopped smoking after 6 months, 8 of 15 after 1 year, and 7 of 15 after 30 months. “This study produced some interesting results, although caution must obviously be taken due to the very low number of patients enrolled,” said Dr. Roméo.
As is the case for alcohol, a retrospective survey conducted via questionnaire at Paul Brousse Hospital showed that the patients who smoked the most and who had the most mystical psychedelic experiences seemed to respond best to psilocybin and therefore to reduce their tobacco use. It also seemed to be the case that patients who reduced tobacco use the most had lower psychological flexibility on enrollment in the study.
Constraints on psychedelics
“Psychedelics are somewhat effective in treating addiction, but there are various limitations to their use,” said Dr. Roméo.
One of those limitations is societal. Laurence Bézo, MD, of the addiction services clinic at Paul Brousse Hospital, asked doctors to respond to a questionnaire to determine what they thought about psychedelics. To date, 407 have responded, including 280 general practitioners, 50 addiction specialists, and 50 specialist physicians. Overall, 50% think that psychedelics have no therapeutic potential. Three of five doctors also said that psychedelics are dangerous. Just over half thought that their use is associated with a severe risk of aggression aimed at oneself and toward others. Likewise, half think that the risk of dependency is very high and that there is a risk of co-occurring psychiatric disorders. “From the pool of physicians queried, the consensus is that psychedelics are pretty dangerous. People also seem to frown upon prescribing psychedelics in France,” said Dr. Roméo.
Participants went as far as to classify psychedelics as some of the most dangerous drugs out there.
Using a 7-point scale, they classified psychedelics below heroin and cocaine in terms of dangerousness. They are deemed much more harmful than alcohol, tobacco, and cannabis.
“A survey of the public carried out several years ago by leading French market research group IFOP had the exact same findings. Nevertheless, a number of studies have set out to determine how dangerous psychedelics are, and their findings point to this class of drugs as being among the least harmful for the individual patient and those around them. On the contrary, alcohol, heroin, crack cocaine (or even cocaine),methamphetamine, and tobacco were shown to be the most harmful. Additionally, psychedelics have a very low risk of dependency and the lowest risk of lethality. There is complete dissonance between what recent studies show us and what society, and some doctors, think,” said Dr. Roméo.
Besides these assumptions, another constraint to the use of psychedelics relates to methods adopted in related clinical studies. “Due to the effect psychedelics have, in the trials conducted, 9 participants and 9 doctors out of 10 are aware of what they have taken or given, respectively. This is a very important limitation. Nowadays, researchers don’t know how to conduct accurate double-blind studies,” said Dr. Roméo.
In sum, for psychiatrists, psychedelics are promising in addiction therapy, but health care professionals, public authorities, and society as a whole must be better informed about their use, and received ideas must be dispelled.
“The findings need to be replicated, but overall, psychedelics are really quite promising in treating both alcohol and tobacco use disorder. They are generally well tolerated with few serious side effects. There is no deterioration in patients with psychiatric conditions while they are taking psychedelics. And if persistent symptoms of psychosis do occur, which is extremely rare, it’s probably because there are preexisting underlying issues at play. We also don’t see increased blood pressure or any other serious physical anomalies. In a supervised setting, as is the case with studies involving psychotherapeutic support, we can no longer say, in this day and age, that psychedelics are harmful,” said Dr. Roméo.
Dr. Roméo reported no conflicts of interest regarding the content of this article.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
PARIS – “We need to develop new therapies to treat addiction because of the related cost to society, which is extremely high,” said Bruno Roméo, MD, psychiatrist and addiction specialist at Paul Brousse Hospital in Villejuif, France, at the Paris-based Neuroscience, Psychiatry and Neurology Conference. Dr. Roméo spoke about the current place of psychedelics in the treatment of addiction.
“Smoking and alcohol consumption are the two main preventable causes of death in France,” he said. “Current management strategies for these addictions rarely involve pharmacological therapies, which are not very effective, in any case. We have massive relapse rates, signaling the need to develop other treatments, like psychedelic drugs.”
But what data are available on the efficacy of psychedelics in treating addiction?
Alcohol use disorder
There are few data concerning the role of psychedelics in the treatment of alcohol use disorder, but one controlled, randomized trial evaluated the efficacy of psilocybin. That trial was published in JAMA Psychiatry in 2022.
That study included 95 patients with alcohol use disorder; 49 were treated with psilocybin, and 46 were treated with diphenhydramine.
An initial medication session of psilocybin was given in week 4, then another in week 8 at a higher dose. The number of drinking days, the number of heavy drinking days, and the number of drinks consumed between weeks 32 and 36 were assessed.
The investigators showed that, after two sessions with psilocybin, there was a significant reduction in the number of heavy drinking days. In the control group, between weeks 5 and 36, 20% of days involved heavy drinking, whereas in the psilocybin group, 10% of days involved heavy drinking.
There was also a significant and rapid reduction in the number of drinking days, and this was maintained over time. Between weeks 5 and 36, just over 40% of days were reported as drinking days in the control group versus slightly more than 30% in the psilocybin group.
Similarly, the number of glasses per day was drastically reduced after taking psilocybin, and the effect occurred extremely quickly. Consumption went from six drinks to less than one drink between weeks 5 and 8. Overall, between weeks 5 and 36, the number of drinks consumed per day was more than two in the placebo group and more than one in the psilocybin group.
“Psilocybin was seen as having potential efficacy in treating alcohol use disorder. But we must tread carefully with these results; the profile of the patients enrolled in this study is different to that of the patients we regularly see in our addiction clinics. The patients enrolled in the study reported less than 60% of days as heavy drinking days,” said Dr. Romeo.
Candidates for psilocybin
According to a retrospective survey of 160 respondents that was conducted online at Paul Brousse Hospital, patients with the most severe cases of alcohol use disorder who have the most mystical psychedelic experiences seem to respond best to psilocybin and to reduce their alcohol use. It also appears that patients whose alcohol use decreased the most had lower psychological flexibility on enrollment in the study. (Psychological flexibility is the ability to adapt to change and to cope with positive and negative experiences in real time without being fazed or trying to flee from the situation.) “It’s as if they had a broader capacity for change, and psychedelics helped them more,” said Dr. Roméo.
Smoking cessation
“There are even fewer studies for smoking,” said Dr. Roméo. In a pilot study with 15 patients, the researchers gave two or three doses of psilocybin at 20-30 mg in combination with cognitive-behavioral therapy one session per week for 10 weeks. Thereafter, patients were assessed three times: after 6 months, 12 months, and 30 months.
The results showed a significant reduction in smoking. Patients went from smoking more than 15 cigarettes per day to smoking one to two cigarettes per day before going back up to six cigarettes daily.
Regarding abstinence rates, 12 of 15 patients had stopped smoking after 6 months, 8 of 15 after 1 year, and 7 of 15 after 30 months. “This study produced some interesting results, although caution must obviously be taken due to the very low number of patients enrolled,” said Dr. Roméo.
As is the case for alcohol, a retrospective survey conducted via questionnaire at Paul Brousse Hospital showed that the patients who smoked the most and who had the most mystical psychedelic experiences seemed to respond best to psilocybin and therefore to reduce their tobacco use. It also seemed to be the case that patients who reduced tobacco use the most had lower psychological flexibility on enrollment in the study.
Constraints on psychedelics
“Psychedelics are somewhat effective in treating addiction, but there are various limitations to their use,” said Dr. Roméo.
One of those limitations is societal. Laurence Bézo, MD, of the addiction services clinic at Paul Brousse Hospital, asked doctors to respond to a questionnaire to determine what they thought about psychedelics. To date, 407 have responded, including 280 general practitioners, 50 addiction specialists, and 50 specialist physicians. Overall, 50% think that psychedelics have no therapeutic potential. Three of five doctors also said that psychedelics are dangerous. Just over half thought that their use is associated with a severe risk of aggression aimed at oneself and toward others. Likewise, half think that the risk of dependency is very high and that there is a risk of co-occurring psychiatric disorders. “From the pool of physicians queried, the consensus is that psychedelics are pretty dangerous. People also seem to frown upon prescribing psychedelics in France,” said Dr. Roméo.
Participants went as far as to classify psychedelics as some of the most dangerous drugs out there.
Using a 7-point scale, they classified psychedelics below heroin and cocaine in terms of dangerousness. They are deemed much more harmful than alcohol, tobacco, and cannabis.
“A survey of the public carried out several years ago by leading French market research group IFOP had the exact same findings. Nevertheless, a number of studies have set out to determine how dangerous psychedelics are, and their findings point to this class of drugs as being among the least harmful for the individual patient and those around them. On the contrary, alcohol, heroin, crack cocaine (or even cocaine),methamphetamine, and tobacco were shown to be the most harmful. Additionally, psychedelics have a very low risk of dependency and the lowest risk of lethality. There is complete dissonance between what recent studies show us and what society, and some doctors, think,” said Dr. Roméo.
Besides these assumptions, another constraint to the use of psychedelics relates to methods adopted in related clinical studies. “Due to the effect psychedelics have, in the trials conducted, 9 participants and 9 doctors out of 10 are aware of what they have taken or given, respectively. This is a very important limitation. Nowadays, researchers don’t know how to conduct accurate double-blind studies,” said Dr. Roméo.
In sum, for psychiatrists, psychedelics are promising in addiction therapy, but health care professionals, public authorities, and society as a whole must be better informed about their use, and received ideas must be dispelled.
“The findings need to be replicated, but overall, psychedelics are really quite promising in treating both alcohol and tobacco use disorder. They are generally well tolerated with few serious side effects. There is no deterioration in patients with psychiatric conditions while they are taking psychedelics. And if persistent symptoms of psychosis do occur, which is extremely rare, it’s probably because there are preexisting underlying issues at play. We also don’t see increased blood pressure or any other serious physical anomalies. In a supervised setting, as is the case with studies involving psychotherapeutic support, we can no longer say, in this day and age, that psychedelics are harmful,” said Dr. Roméo.
Dr. Roméo reported no conflicts of interest regarding the content of this article.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
PARIS – “We need to develop new therapies to treat addiction because of the related cost to society, which is extremely high,” said Bruno Roméo, MD, psychiatrist and addiction specialist at Paul Brousse Hospital in Villejuif, France, at the Paris-based Neuroscience, Psychiatry and Neurology Conference. Dr. Roméo spoke about the current place of psychedelics in the treatment of addiction.
“Smoking and alcohol consumption are the two main preventable causes of death in France,” he said. “Current management strategies for these addictions rarely involve pharmacological therapies, which are not very effective, in any case. We have massive relapse rates, signaling the need to develop other treatments, like psychedelic drugs.”
But what data are available on the efficacy of psychedelics in treating addiction?
Alcohol use disorder
There are few data concerning the role of psychedelics in the treatment of alcohol use disorder, but one controlled, randomized trial evaluated the efficacy of psilocybin. That trial was published in JAMA Psychiatry in 2022.
That study included 95 patients with alcohol use disorder; 49 were treated with psilocybin, and 46 were treated with diphenhydramine.
An initial medication session of psilocybin was given in week 4, then another in week 8 at a higher dose. The number of drinking days, the number of heavy drinking days, and the number of drinks consumed between weeks 32 and 36 were assessed.
The investigators showed that, after two sessions with psilocybin, there was a significant reduction in the number of heavy drinking days. In the control group, between weeks 5 and 36, 20% of days involved heavy drinking, whereas in the psilocybin group, 10% of days involved heavy drinking.
There was also a significant and rapid reduction in the number of drinking days, and this was maintained over time. Between weeks 5 and 36, just over 40% of days were reported as drinking days in the control group versus slightly more than 30% in the psilocybin group.
Similarly, the number of glasses per day was drastically reduced after taking psilocybin, and the effect occurred extremely quickly. Consumption went from six drinks to less than one drink between weeks 5 and 8. Overall, between weeks 5 and 36, the number of drinks consumed per day was more than two in the placebo group and more than one in the psilocybin group.
“Psilocybin was seen as having potential efficacy in treating alcohol use disorder. But we must tread carefully with these results; the profile of the patients enrolled in this study is different to that of the patients we regularly see in our addiction clinics. The patients enrolled in the study reported less than 60% of days as heavy drinking days,” said Dr. Romeo.
Candidates for psilocybin
According to a retrospective survey of 160 respondents that was conducted online at Paul Brousse Hospital, patients with the most severe cases of alcohol use disorder who have the most mystical psychedelic experiences seem to respond best to psilocybin and to reduce their alcohol use. It also appears that patients whose alcohol use decreased the most had lower psychological flexibility on enrollment in the study. (Psychological flexibility is the ability to adapt to change and to cope with positive and negative experiences in real time without being fazed or trying to flee from the situation.) “It’s as if they had a broader capacity for change, and psychedelics helped them more,” said Dr. Roméo.
Smoking cessation
“There are even fewer studies for smoking,” said Dr. Roméo. In a pilot study with 15 patients, the researchers gave two or three doses of psilocybin at 20-30 mg in combination with cognitive-behavioral therapy one session per week for 10 weeks. Thereafter, patients were assessed three times: after 6 months, 12 months, and 30 months.
The results showed a significant reduction in smoking. Patients went from smoking more than 15 cigarettes per day to smoking one to two cigarettes per day before going back up to six cigarettes daily.
Regarding abstinence rates, 12 of 15 patients had stopped smoking after 6 months, 8 of 15 after 1 year, and 7 of 15 after 30 months. “This study produced some interesting results, although caution must obviously be taken due to the very low number of patients enrolled,” said Dr. Roméo.
As is the case for alcohol, a retrospective survey conducted via questionnaire at Paul Brousse Hospital showed that the patients who smoked the most and who had the most mystical psychedelic experiences seemed to respond best to psilocybin and therefore to reduce their tobacco use. It also seemed to be the case that patients who reduced tobacco use the most had lower psychological flexibility on enrollment in the study.
Constraints on psychedelics
“Psychedelics are somewhat effective in treating addiction, but there are various limitations to their use,” said Dr. Roméo.
One of those limitations is societal. Laurence Bézo, MD, of the addiction services clinic at Paul Brousse Hospital, asked doctors to respond to a questionnaire to determine what they thought about psychedelics. To date, 407 have responded, including 280 general practitioners, 50 addiction specialists, and 50 specialist physicians. Overall, 50% think that psychedelics have no therapeutic potential. Three of five doctors also said that psychedelics are dangerous. Just over half thought that their use is associated with a severe risk of aggression aimed at oneself and toward others. Likewise, half think that the risk of dependency is very high and that there is a risk of co-occurring psychiatric disorders. “From the pool of physicians queried, the consensus is that psychedelics are pretty dangerous. People also seem to frown upon prescribing psychedelics in France,” said Dr. Roméo.
Participants went as far as to classify psychedelics as some of the most dangerous drugs out there.
Using a 7-point scale, they classified psychedelics below heroin and cocaine in terms of dangerousness. They are deemed much more harmful than alcohol, tobacco, and cannabis.
“A survey of the public carried out several years ago by leading French market research group IFOP had the exact same findings. Nevertheless, a number of studies have set out to determine how dangerous psychedelics are, and their findings point to this class of drugs as being among the least harmful for the individual patient and those around them. On the contrary, alcohol, heroin, crack cocaine (or even cocaine),methamphetamine, and tobacco were shown to be the most harmful. Additionally, psychedelics have a very low risk of dependency and the lowest risk of lethality. There is complete dissonance between what recent studies show us and what society, and some doctors, think,” said Dr. Roméo.
Besides these assumptions, another constraint to the use of psychedelics relates to methods adopted in related clinical studies. “Due to the effect psychedelics have, in the trials conducted, 9 participants and 9 doctors out of 10 are aware of what they have taken or given, respectively. This is a very important limitation. Nowadays, researchers don’t know how to conduct accurate double-blind studies,” said Dr. Roméo.
In sum, for psychiatrists, psychedelics are promising in addiction therapy, but health care professionals, public authorities, and society as a whole must be better informed about their use, and received ideas must be dispelled.
“The findings need to be replicated, but overall, psychedelics are really quite promising in treating both alcohol and tobacco use disorder. They are generally well tolerated with few serious side effects. There is no deterioration in patients with psychiatric conditions while they are taking psychedelics. And if persistent symptoms of psychosis do occur, which is extremely rare, it’s probably because there are preexisting underlying issues at play. We also don’t see increased blood pressure or any other serious physical anomalies. In a supervised setting, as is the case with studies involving psychotherapeutic support, we can no longer say, in this day and age, that psychedelics are harmful,” said Dr. Roméo.
Dr. Roméo reported no conflicts of interest regarding the content of this article.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
Implementing Smoking Cessation Telehealth Technologies Within the VHA: Lessons Learned
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp