User login
Reassuring data on stimulants for ADHD in kids and later substance abuse
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Women increasingly dying of alcohol-related causes
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Most Americans in favor of regulated therapeutic psychedelics
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
App cuts alcohol intake in risky drinkers
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ALCOHOL: CLINICAL & EXPERIMENTAL RESEARCH
How psychedelics can heal a broken mind
As children learn to walk and talk, their brains are remarkably open to new information. They gather knowledge from parents, their environment, and trial and error. Teenagers do too, as they adopt the emotional and intellectual skills needed to become adults.
In adulthood, however, our minds become relatively locked, closed to new information. This saves energy and lets us navigate the world more efficiently. But that also makes it harder to adapt, learn a new language or skill, or recover from psychological or physical trauma. For those who’ve dealt with abuse, abandonment, or physical violence, that lockdown can lead to a lifetime of suffering, substance abuse, and other maladaptive behaviors.
The study, published in Nature, reflects a renaissance of using and researching psychedelics to treat a range of mental health conditions.
Scientists at Johns Hopkins University in Baltimore were investigating the drugs’ effects on “critical periods” for social learning, times when the brain is more open to new information that diminish as we age. Success in mice suggests that psychedelics can start a fresh period of learning.
If the finding bears out in future studies, the therapeutic horizon for psychedelics could expand to other opportunities to retrain the brain, including recovery from a stroke, traumatic brain injury, and even hearing loss and paralysis.
The stakes are big, and the future is promising, said lead researcher Gul Dolen, MD, PhD, an associate professor of neuroscience at Johns Hopkins University. Psychedelics “could be the key that unlocks the brain and helps people after one dose, rather than subjecting them to a lifetime of drugs.”
The psychedelic advantage
Dr. Dolen, who launched her career in addiction studies, has long been fascinated by critical periods and their influence on adult behavior.
“There have been three Nobel Prizes awarded for work on critical periods,” she said. One study in mice, for instance, identified 15 periods of social learning that define their behaviors for a lifetime.
Prior research has found that MDMA (commonly known as ecstasy) can help soldiers reconsider traumatic events on the battlefield, learn from them, and move on. That phenomenon had all the earmarks of a critical period for social learning. Perhaps, Dr. Dolen said, psychedelics could open a critical period in a soldier’s life – or a drug-addicted person’s or rape survivor’s – and give them tools to process their trauma.
In the placebo-controlled experiment, she and her team gave mice psychedelic drugs and a behavioral test to gauge the rodents’ ability to learn from their environment.
“All of the psychedelics opened the critical period of social learning for varying lengths of time,” said Dr. Dolen.
Ketamine achieved that reopening for 2 days, while the other drugs – ibogaine, LSD, MDMA, and psilocybin – opened critical periods of between 2 and 4 weeks, long after the drugs’ acute effects had worn off.
In humans, Dr. Dolen stressed, opening a critical period would be a sensitive process.
“You wouldn’t achieve these results if you dropped ecstasy and attended a rave,” she said. “The key seems to be to establish an intention for the therapy: Discuss what you hope to get from the experience, be guided through it, and process it with the therapist after the fact.”
“You need to be careful with a patient once they’re off the psychedelic,” she said, “because they’re in a state of openness and vulnerability similar to a child.”
The push for psychedelic therapy
Another psychedelics researcher, Matthew Lowe, PhD, sees promise in the Johns Hopkins study. The drugs “place the brain in a more malleable and flexible state,” said Dr. Lowe, the executive director and chief science officer for Unlimited Sciences, a psychedelics research nonprofit.
He expects that psychedelics may help people break out of negative behavior patterns.
“These findings show significant promise for treating a wide range of neuropsychiatric diseases, including depression, PTSD, and addiction,” he said.
Dr. Dolen said using psychedelics in critical-period therapy “opens up all sorts of possibilities for the rest of the brain.” Future research may also lead to treatments for deafness, physical disabilities, and drug and alcohol addiction. She is currently raising funds for a clinical trial to see if psychedelics can improve motor impairment after a stroke.
“Growing legislative openness” to the use of psychedelics could open the door for millions to benefit from mental health treatment “through clinical trials and legal therapeutic pathways as they open up,” said Benjamin Lightburn, CEO and cofounder of Filament Health, a company based in British Columbia that provides naturally derived psilocybin for clinical trials.
Several states have made moves toward decriminalization or permitting the drugs’ use under medical supervision. In a scientific paper, Washington University researchers, using an analytic model based on marijuana legalization, projected that most states will legalize psychedelics in the next 10-15 years. And on July 1, Australia became the first country to allow psilocybin and MDMA to be prescribed by doctors to treat psychiatric conditions. The U.S. could potentially approve MDMA for therapy later in 2023.
A version of this article first appeared on WebMD.com.
As children learn to walk and talk, their brains are remarkably open to new information. They gather knowledge from parents, their environment, and trial and error. Teenagers do too, as they adopt the emotional and intellectual skills needed to become adults.
In adulthood, however, our minds become relatively locked, closed to new information. This saves energy and lets us navigate the world more efficiently. But that also makes it harder to adapt, learn a new language or skill, or recover from psychological or physical trauma. For those who’ve dealt with abuse, abandonment, or physical violence, that lockdown can lead to a lifetime of suffering, substance abuse, and other maladaptive behaviors.
The study, published in Nature, reflects a renaissance of using and researching psychedelics to treat a range of mental health conditions.
Scientists at Johns Hopkins University in Baltimore were investigating the drugs’ effects on “critical periods” for social learning, times when the brain is more open to new information that diminish as we age. Success in mice suggests that psychedelics can start a fresh period of learning.
If the finding bears out in future studies, the therapeutic horizon for psychedelics could expand to other opportunities to retrain the brain, including recovery from a stroke, traumatic brain injury, and even hearing loss and paralysis.
The stakes are big, and the future is promising, said lead researcher Gul Dolen, MD, PhD, an associate professor of neuroscience at Johns Hopkins University. Psychedelics “could be the key that unlocks the brain and helps people after one dose, rather than subjecting them to a lifetime of drugs.”
The psychedelic advantage
Dr. Dolen, who launched her career in addiction studies, has long been fascinated by critical periods and their influence on adult behavior.
“There have been three Nobel Prizes awarded for work on critical periods,” she said. One study in mice, for instance, identified 15 periods of social learning that define their behaviors for a lifetime.
Prior research has found that MDMA (commonly known as ecstasy) can help soldiers reconsider traumatic events on the battlefield, learn from them, and move on. That phenomenon had all the earmarks of a critical period for social learning. Perhaps, Dr. Dolen said, psychedelics could open a critical period in a soldier’s life – or a drug-addicted person’s or rape survivor’s – and give them tools to process their trauma.
In the placebo-controlled experiment, she and her team gave mice psychedelic drugs and a behavioral test to gauge the rodents’ ability to learn from their environment.
“All of the psychedelics opened the critical period of social learning for varying lengths of time,” said Dr. Dolen.
Ketamine achieved that reopening for 2 days, while the other drugs – ibogaine, LSD, MDMA, and psilocybin – opened critical periods of between 2 and 4 weeks, long after the drugs’ acute effects had worn off.
In humans, Dr. Dolen stressed, opening a critical period would be a sensitive process.
“You wouldn’t achieve these results if you dropped ecstasy and attended a rave,” she said. “The key seems to be to establish an intention for the therapy: Discuss what you hope to get from the experience, be guided through it, and process it with the therapist after the fact.”
“You need to be careful with a patient once they’re off the psychedelic,” she said, “because they’re in a state of openness and vulnerability similar to a child.”
The push for psychedelic therapy
Another psychedelics researcher, Matthew Lowe, PhD, sees promise in the Johns Hopkins study. The drugs “place the brain in a more malleable and flexible state,” said Dr. Lowe, the executive director and chief science officer for Unlimited Sciences, a psychedelics research nonprofit.
He expects that psychedelics may help people break out of negative behavior patterns.
“These findings show significant promise for treating a wide range of neuropsychiatric diseases, including depression, PTSD, and addiction,” he said.
Dr. Dolen said using psychedelics in critical-period therapy “opens up all sorts of possibilities for the rest of the brain.” Future research may also lead to treatments for deafness, physical disabilities, and drug and alcohol addiction. She is currently raising funds for a clinical trial to see if psychedelics can improve motor impairment after a stroke.
“Growing legislative openness” to the use of psychedelics could open the door for millions to benefit from mental health treatment “through clinical trials and legal therapeutic pathways as they open up,” said Benjamin Lightburn, CEO and cofounder of Filament Health, a company based in British Columbia that provides naturally derived psilocybin for clinical trials.
Several states have made moves toward decriminalization or permitting the drugs’ use under medical supervision. In a scientific paper, Washington University researchers, using an analytic model based on marijuana legalization, projected that most states will legalize psychedelics in the next 10-15 years. And on July 1, Australia became the first country to allow psilocybin and MDMA to be prescribed by doctors to treat psychiatric conditions. The U.S. could potentially approve MDMA for therapy later in 2023.
A version of this article first appeared on WebMD.com.
As children learn to walk and talk, their brains are remarkably open to new information. They gather knowledge from parents, their environment, and trial and error. Teenagers do too, as they adopt the emotional and intellectual skills needed to become adults.
In adulthood, however, our minds become relatively locked, closed to new information. This saves energy and lets us navigate the world more efficiently. But that also makes it harder to adapt, learn a new language or skill, or recover from psychological or physical trauma. For those who’ve dealt with abuse, abandonment, or physical violence, that lockdown can lead to a lifetime of suffering, substance abuse, and other maladaptive behaviors.
The study, published in Nature, reflects a renaissance of using and researching psychedelics to treat a range of mental health conditions.
Scientists at Johns Hopkins University in Baltimore were investigating the drugs’ effects on “critical periods” for social learning, times when the brain is more open to new information that diminish as we age. Success in mice suggests that psychedelics can start a fresh period of learning.
If the finding bears out in future studies, the therapeutic horizon for psychedelics could expand to other opportunities to retrain the brain, including recovery from a stroke, traumatic brain injury, and even hearing loss and paralysis.
The stakes are big, and the future is promising, said lead researcher Gul Dolen, MD, PhD, an associate professor of neuroscience at Johns Hopkins University. Psychedelics “could be the key that unlocks the brain and helps people after one dose, rather than subjecting them to a lifetime of drugs.”
The psychedelic advantage
Dr. Dolen, who launched her career in addiction studies, has long been fascinated by critical periods and their influence on adult behavior.
“There have been three Nobel Prizes awarded for work on critical periods,” she said. One study in mice, for instance, identified 15 periods of social learning that define their behaviors for a lifetime.
Prior research has found that MDMA (commonly known as ecstasy) can help soldiers reconsider traumatic events on the battlefield, learn from them, and move on. That phenomenon had all the earmarks of a critical period for social learning. Perhaps, Dr. Dolen said, psychedelics could open a critical period in a soldier’s life – or a drug-addicted person’s or rape survivor’s – and give them tools to process their trauma.
In the placebo-controlled experiment, she and her team gave mice psychedelic drugs and a behavioral test to gauge the rodents’ ability to learn from their environment.
“All of the psychedelics opened the critical period of social learning for varying lengths of time,” said Dr. Dolen.
Ketamine achieved that reopening for 2 days, while the other drugs – ibogaine, LSD, MDMA, and psilocybin – opened critical periods of between 2 and 4 weeks, long after the drugs’ acute effects had worn off.
In humans, Dr. Dolen stressed, opening a critical period would be a sensitive process.
“You wouldn’t achieve these results if you dropped ecstasy and attended a rave,” she said. “The key seems to be to establish an intention for the therapy: Discuss what you hope to get from the experience, be guided through it, and process it with the therapist after the fact.”
“You need to be careful with a patient once they’re off the psychedelic,” she said, “because they’re in a state of openness and vulnerability similar to a child.”
The push for psychedelic therapy
Another psychedelics researcher, Matthew Lowe, PhD, sees promise in the Johns Hopkins study. The drugs “place the brain in a more malleable and flexible state,” said Dr. Lowe, the executive director and chief science officer for Unlimited Sciences, a psychedelics research nonprofit.
He expects that psychedelics may help people break out of negative behavior patterns.
“These findings show significant promise for treating a wide range of neuropsychiatric diseases, including depression, PTSD, and addiction,” he said.
Dr. Dolen said using psychedelics in critical-period therapy “opens up all sorts of possibilities for the rest of the brain.” Future research may also lead to treatments for deafness, physical disabilities, and drug and alcohol addiction. She is currently raising funds for a clinical trial to see if psychedelics can improve motor impairment after a stroke.
“Growing legislative openness” to the use of psychedelics could open the door for millions to benefit from mental health treatment “through clinical trials and legal therapeutic pathways as they open up,” said Benjamin Lightburn, CEO and cofounder of Filament Health, a company based in British Columbia that provides naturally derived psilocybin for clinical trials.
Several states have made moves toward decriminalization or permitting the drugs’ use under medical supervision. In a scientific paper, Washington University researchers, using an analytic model based on marijuana legalization, projected that most states will legalize psychedelics in the next 10-15 years. And on July 1, Australia became the first country to allow psilocybin and MDMA to be prescribed by doctors to treat psychiatric conditions. The U.S. could potentially approve MDMA for therapy later in 2023.
A version of this article first appeared on WebMD.com.
FROM NATURE
‘Body size is not a choice’ and deserves legal protections
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
Medical cannabis does not reduce use of prescription meds
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
‘Landmark’ trial shows opioids for back, neck pain no better than placebo
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
New international guidelines on opioid deprescribing
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.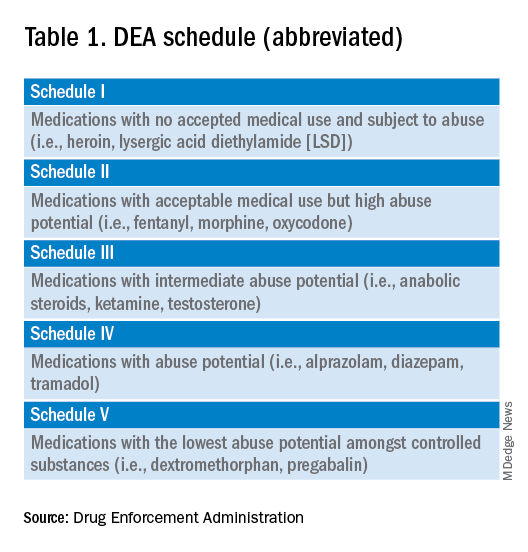
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 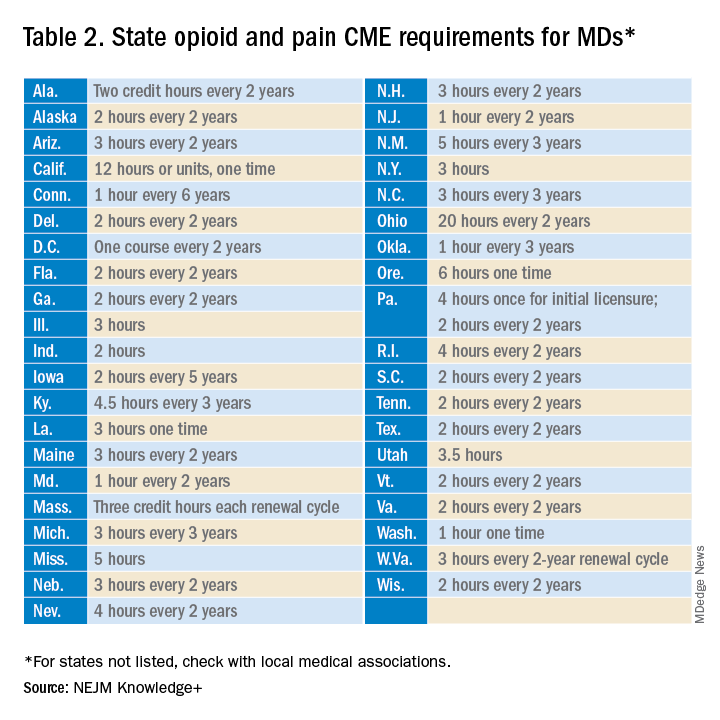
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
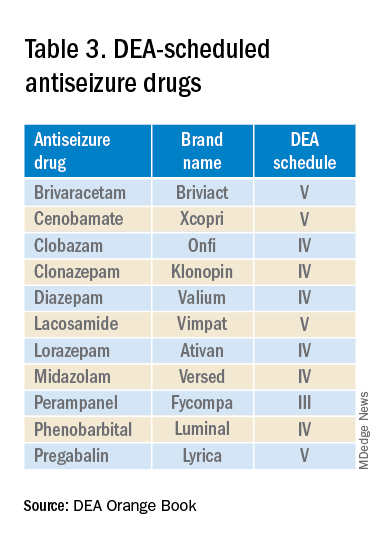
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.



