User login
Influenza: Still more important than Zika virus
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
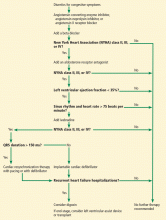
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
KEY POINTS
- Influenza vaccine remains the most effective way to prevent influenza. Healthcare providers should continue to vaccinate all people older than 6 months.
- For the 2016–2017 influenza season, only the inactivated influenza vaccine, not the live-attenuated vaccine, is recommended, regardless of age group or underlying disease.
- Early initiation of a neuraminidase inhibitor is advised for an influenza-like illness while awaiting a confirmatory diagnostic test.
Breaking the pain contract: A better controlled-substance agreement for patients on chronic opioid therapy
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
Regulatory bodies and professional societies have encouraged or mandated written pain treatment agreements for over a decade as a way to establish informed consent, improve adherence, and mitigate risk. Unfortunately, the content of these agreements varies, their efficacy is uncertain, and some are stigmatizing or coercive and jeopardize trust. Additionally, many are written at reading levels beyond most patients’ understanding. However, we believe a well-written agreement is still an important tool in chronic pain management.
In this article, we explore common limitations of current pain treatment “contracts” and propose strategies to improve their usefulness and acceptance.
PAIN AND ITS TREATMENT HAVE COSTS
Chronic pain affects 100 million US adults and is estimated to cost $635 billion each year in treatment, lost wages, and reduced productivity.1
Opioid therapy for chronic noncancer pain is being called into question,2–5 and a 2016 guideline from the US Centers for Disease Control and Prevention has called for more limited and judicious use of opioids in primary care.6 Nevertheless, long-term opioid therapy is probably helpful in some circumstances and will likely continue to have a role in chronic pain management for the foreseeable future.7
Concerns about opioids include risks of overdose and death. Unintentional drug overdoses, typically with opioids, exceeded motor vehicle accidents in 2009 as the leading cause of accidental death in the United States8; by 2014, nearly one and a half times as many people were dying of a drug overdose than of a car accident.9 Even when used appropriately, opioids are associated with sedation, falls, motor vehicle accidents, addiction, and unintended overdose.10
The potential harm extends beyond the patient to the community at large. Diversion of prescription drugs for nonmedical use is common11 and, after marijuana and alcohol abuse, is the most common form of drug abuse in the United States.12 Misuse of prescription drugs costs health insurers an estimated $72.5 billion each year—a cost largely passed on to consumers through higher premiums.13 Most individuals who abuse prescription opioids get them from friends and family, sometimes by stealing them.14
THE SPECIAL ROLE OF THE PRIMARY CARE PHYSICIAN
Chronic pain is extremely prevalent in general internal medicine and primary care practice.15,16 It has tremendous associated medical, social, and economic costs.1
In light of the risks and complexity of opioid use and the increasing regulatory requirements for safe prescribing, some primary care physicians have stopped prescribing opioids altogether and refer patients elsewhere for pain management.
This does a disservice to patients. Primary care physicians cannot entirely avoid chronic pain management or absolutely refuse to prescribe opioids in all circumstances and still provide quality care. And although some primary care physicians may need more training in prescribing opioids, their comprehensive understanding of the patient’s other health issues enables them to address the psychosocial generators and consequences of the patient’s chronic pain more fully than a specialist can.
Furthermore, access to board-certified pain specialists is limited. There are only four such specialists for every 100,000 patients with chronic pain,17 and those who are available often restrict the types of insurance they accept, disproportionately excluding Medicaid patients.
We encourage primary care physicians to undertake continuing medical education and professional development as needed to prescribe opioids as safely and effectively as possible.
A CONTROLLED-SUBSTANCE AGREEMENT INSTEAD OF A ‘NARCOTIC CONTRACT’
To address the challenges of long-term opioid therapy, many state officials, medical licensing boards, professional societies, and other regulatory bodies recommend proactive monitoring and management of prescribing risks. Often promoted and sometimes mandated is the use of a written pain treatment agreement, sometimes called a “pain contract” or “narcotic contract,” in which the patient and the physician ostensibly agree to various conditions under which opioids will be prescribed or discontinued. Although well-intentioned, these documents can cause several problems.
Contracts were being advocated in treating opiate addiction as early as 1981.18 Since then, the term “narcotic contract” has become widely used, even as most professional guidelines have now moved away from using it. A Google search for the term on November 27, 2015, yielded 2,000 results, with numerous examples of the documents in clinical use.
But the phrase is misleading, and we believe physicians should avoid using it. Clinically, the word “narcotic” is imprecise and can refer to substances other than opioids. For example, the US Controlled Substances Act lists cocaine as a narcotic.19 The word also carries a stigma, as law enforcement agencies and drug abuse programs commonly use phrases such as “narcotic task force” or “narcotic treatment program.” On the other hand, the more accurate term “opioid” may be unfamiliar to patients. We recommend using the term “controlled substance” instead.
Similarly, the word “contract” can be perceived as coercive, can erode physician-patient trust, and implies that failure to agree to it will result in loss of access to pain medications.20–23
For these reasons, we encourage physicians to adopt the phrase “controlled-substance agreement” or something similar. This label accurately reflects the specificity of the treatment and connotes a partnership between patient and physician. Furthermore, it allows the physician to use the agreement when prescribing other controlled substances such as benzodiazepines and stimulants that also carry a risk of addiction, misuse, and adverse effects.
STIGMATIZING THE PATIENT
Although no studies have systematically assessed the style and tone of available treatment agreements, many of the agreements seem to stigmatize the patient, using language that is mistrustful, accusatory, and even confrontational and that implies that the patient will misuse or abuse the medications.21,24 For example, “Failure to comply with the terms of the contract will risk loss of medication or discharge from the medical practice” is inflammatory and coercive, but variations of this phrase appear in many of the results of the aforementioned Google search.
Such language defeats attempts to communicate openly and implies a deprecatory attitude towards patients. Stigmatization may result in undertreatment of pain, physician refusal to prescribe opioids, and patient refusal to submit to the terms of a one-sided agreement perceived as unfair. Therefore, poorly written opioid agreements impair the trust necessary for a therapeutic physician-patient relationship and can interfere with optimal pain management.20–23
Some physicians stigmatize inadvertently. Believing that they can identify which patients will misuse their prescriptions, they use controlled-substance agreements only in this subgroup. But in fact, physicians are notoriously poor at predicting which patients will misuse prescription opioids or suffer adverse effects.25 Therefore, it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.26
Framing the controlled-substance agreement in terms of safety and using it universally can minimize miscommunication and unintentional stigmatization.
SHARED DECISION-MAKING AND CHRONIC OPIOID THERAPY
We recommend using controlled-substance agreements only in the context of personalized patient counseling and shared decision-making.
Shared decision-making promotes mutual respect between patients and physicians, is feasible to implement in primary care, and may improve health outcomes.27,28 A study found that physicians who received 2 hours of training in shared decision-making for chronic opioid therapy were more likely to complete treatment agreements and set mutually agreed-upon functional goals with patients, and they felt more confident, competent, and comfortable treating chronic pain.29 Additionally, after learning about the risks, some patients may choose to forgo opioid therapy.
To be consistent with shared decision-making, the controlled-substance agreement must:
- Engage the patient, emphasizing the shared, reciprocal obligations of physician and patient
- Address goals of treatment that are personalized and mutually agreed-upon and that incorporate the patient’s values and preferences
- Explain treatment options in a way that is understandable and informative for the patient.
Table 1 outlines other key elements in detail.27,30,31
Shared decision-making is especially useful when the balance between the risks and benefits of a treatment plan is uncertain. It is not a substitute for medical expertise, and a patient’s preferences do not override the physician’s clinical judgment. A physician should not offer or implement chronic opioid therapy if he or she believes it is not indicated or is contraindicated, or that the risks for that patient clearly outweigh the benefits.32
THE CONTROLLED-SUBSTANCE AGREEMENT: FOUR OBJECTIVES
Stigmatizing language in the controlled-substance agreement may result from physician ambivalence regarding its intent and objectives. For example, some may perceive the agreement as a way to facilitate communication, while others may use it in a possibly unethical manner to control patient behavior with the threat of cutting off access to pain medication.33
Controlled-substance agreements have four commonly identified objectives,34 explored further below:
- To improve adherence with the safe use of controlled substances while reducing aberrant behaviors
- To obtain informed consent
- To outline the prescribing policies of the practice
- To mitigate the prescriber’s legal risk.
Improving adherence
Many authors say that the primary goal of the controlled-substance agreement is to promote the use of the medication as prescribed, without variance, and from one physician only.35–38 This goal seems reasonable. However, many other classes of medications are also risky when used aberrantly, and we do not ask the patient to sign an agreement when we prescribe them. This double standard may reflect both the inherently higher risks associated with controlled substances and physician ambivalence regarding their use.
Regardless, the efficacy of controlledsubstance agreements in improving safe-use adherence and reducing aberrant medication-taking behaviors is uncertain. A 2010 systematic review based on observational and largely poor-quality studies concluded that using treatment agreements along with urine drug testing modestly reduced opioid misuse,39 while other reports have called their efficacy into question.40 We remain optimistic that well-written controlled-substance agreements can advance this objective, and that absence of evidence is not evidence of absence—ie, lack of efficacy. However, the data are not yet clear.
Interestingly, a 2014 survey found that most primary care physicians thought that controlled-substance agreements do not meaningfully reduce opioid misuse but do give a sense of protection against liability.41 Additionally, these documents are associated with a greater sense of physician satisfaction and mastery,42 and for some physicians these reasons may be enough to justify their use.
Somewhat alarmingly though, one study suggests that many patients do not even know that they signed a treatment agreement.43 Using a controlled-substance agreement without the full awareness and engagement of the patient cannot promote adherence and is likely counterproductive.
Obtaining informed consent
It is essential to discuss possible benefits and risks so that informed and shared decision-making can occur.
Controlled-substance agreements may advance this aim if carefully written, although medical practices often design them for use across a spectrum of patients with varying indications, contraindications, and risks, making these documents inherently inflexible. A one-size-fits-all document does not allow for meaningful personalization and is insufficient without patient-centered counseling.
We strongly recommend that treatment agreements complement but not replace personalized patient-centered counseling about individual risks and benefits. Well-written controlled-substance agreements may reduce the chance of overlooking key risks and launch further customized discussion. Additionally, they can be written in a manner that allows patients and physicians to agree on and document personalized goals (Table 2).
Furthermore, when crafted within a risk-benefit framework, a controlled-substance agreement can help to clarify an ethically important concept, ie, that the physician is judging the safety and appropriateness of the treatment, not the character of the patient.44 The prescriber can focus on evaluating the risks and benefits of treatment choices, not being a police officer or a judge of how “deserving” of opioid therapy the patient is.
Importantly, for patients to provide meaningful informed consent, the agreement must be understandable. A study of 162 opioid treatment agreements found that on average, they were written at a 14th grade level, which is beyond the reading comprehension of most patients.45 Another study evaluated patients’ ability to understand and follow instructions on labels for common prescriptions; even though 70% of the patients could read the labels, only 34.7% could demonstrate the instructions “take two tablets by mouth twice daily.”46
We recommend analyzing all controlled- substance agreements for readability by assessing their Flesch-Kincaid grade level or a similar literacy assessment, using readily available computer apps. The average education level of the patients cared for in each practice will vary based on the demographic served, and the controlled-substance agreement can be modified accordingly, but typically writing the document at the 6th- to 7th-grade reading level is suggested.
Outlining practice policies
Opioids are federally controlled substances with prescribing restrictions that vary based on the drug’s Drug Enforcement Agency schedule. State laws and regulations also govern opioid prescribing and are constantly evolving.47
Refilling opioid prescriptions should be a deliberate process during which the prescriber reviews the appropriateness of the medication and issues the prescription as safely as possible.
To promote practice consistency and to share expectations transparently with patients, we recommend spelling out in the agreement your policies on:
- Who can manage this patient’s opioid therapy
- How to handle refill requests after hours and on weekends
- When and how patients should request opioid refills
- Which pharmacies patients will use
- Whether the practice allows others to pick up refills for the patient.
This not only serves as a reference for patients, who keep a copy for their records, it also reduces the risk of inconsistent processes within the office, which will quickly lead to chaos and confusion among patients and physicians alike. Inconsistent prescription and refill practices can give the impression that a double standard exists and that some patients get more leeway than others, without apparent justification.
There is little evidence that this approach truly improves practice efficiency,34,48 but we believe that it may avert future confusion and conflict.
Mitigating the prescriber’s risk
Most licensing boards and clinical guidelines recommend controlled-substance agreements as part of opioid risk mitigation. These documents are now the standard of care, with many bodies recommending or mandating them, including the Federation of State Medical Boards,49 many states,50 Physicians for Responsible Opioid Prescribing,51 the American Academy of Pain Management,52 and the American Pain Society along with the American Academy of Pain Medicine.53
Historically, primary care physicians have used controlled-substance agreements inconsistently and primarily for patients believed to be at high risk of misuse.54 However, because physicians cannot accurately predict who will misuse or divert medications,25 controlled-substance agreements should be used universally, ie, for all patients prescribed controlled substances.
A controlled-substance agreement can serve as documentation. The patient can keep a copy for future reference, and a cosigned document is evidence that a discussion took place and may lower the risk of malpractice litigation.55 Further, if a state requires physicians to check their prescription monitoring database before prescribing opioids, the controlled-substance agreement can serve to both inform patients about this obligation and to obtain their consent when required.
At a minimum, we recommend that prescribers learn about the regulatory framework in their state and use controlled-substance agreements as legislatively mandated.
A CHECKLIST FOR THE PHYSICIAN AND PATIENT
To facilitate the development and use of ethically appropriate controlled-substance agreements with a focus on shared decision-making, we offer a sample tool in the form of a checklist (Table 2). It can be modified and implemented instead of a traditional controlled-substance agreement or can be used alongside other more comprehensive documents to facilitate discussion.
The model presents critical information for the patient and physician to discuss and acknowledge (initial) in writing. It is divided into three sections: shared responsibilities, patient responsibilities, and physician responsibilities. Each contains an approximately equal number of items; this is deliberate and visually conveys the notion of equivalent and shared responsibilities for patient and physician. The patient, physician, or both should initial each item to indicate their agreement.
The document is customizable for the specific treatment prescribed. It is written at a Flesch-Kincaid grade level of 6.8, consistent with current health literacy recommendations, and avoids medical jargon and complex compound sentences as much as possible.
We indicate key elements of shared decision-making27,30,31 in parentheses in Table 2 and cross-reference them with Table 1, which describes them more fully.
A BETTER TOOL
Both chronic pain and prescription drug abuse are highly prevalent and carry serious consequences. These overlapping epidemics put the prescriber in the difficult position of trying to prevent misuse, abuse, and diversion while simultaneously adequately treating pain.
Physicians and policy makers look to controlled-substance agreements as tools to help them balance the benefits and risks, but frequently at the expense of eroding trust between the patient and physician, stigmatizing the patient, using pejorative and coercive language, not adhering to health literacy guidelines, and failing to share decisions.
We believe a better tool is possible and suggest that controlled-substance agreements be universally applied, use deliberate and understandable language, be framed in terms of safety, and be implemented according to the principles of shared decision-making.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
- Committee on Advancing Pain Research Care, Institute of Medicine. Relieving Pain In America: A Blueprint For Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. 030921484X.
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155:325–328.
- Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015; 162:276–286.
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician 2011; 14:E133–E156.
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician 2008; 11(suppl):S181–S200.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016; 65(1):1–49.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am 2016; 100:81–102.
- Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Injury Prev 2012; 18:70.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016; 64(50-51):1378–1382.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156:569–576.
- Cicero TJ, Kurtz SP, Surratt HL, et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 2011; 41:283–304.
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014: www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm. Accessed October 10, 2015.
- National Drug Intelligence Center, Drug Enforcement Administration. National Prescription Drug Threat Assessment. 2009.
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med 2014; 174:802–803.
- Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage 2002; 23:131–137.
- American Academy of Family Physicians. Pain management and opioid abuse: a public health concern. Position paper, executive summary. 2012; www.aafp.org/content/dam/AAFP/documents/patient_care/pain_management/opioid-abuse-position-paper.pdf. Accessed October 10, 2015.
- Breuer B, Pappagallo M, Tai JY, Portenoy RK. U.S. board-certified pain physician practices: uniformity and census data of their locations. J Pain 2007; 8:244–250.
- Rush AJ, Shaw BF. Psychotherapeutic treatment of opiate addiction. Am J Psychother 1981; 35:61–75.
- U.S. Department of Justice, Office of Diversion Control, Title 21 Code of Federal Regulations - Part 1300 - Definitions. 2015; www.deadiversion.usdoj.gov/21cfr/cfr/1300/1300_01.htm. Accessed October 10, 2016.
- McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med 2015; 16:25–29.
- Buchman DZ, Ho A. What’s trust got to do with it? Revisiting opioid contracts. J Med Ethics 2014; 40:673–677.
- Deep K. Use of narcotics contracts. Virtual Mentor 2013; 15:416–420.
- Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioethics 2010; 10:5–12.
- Goldberg DSDS. Job and the stigmatization of chronic pain. Perspect Biol Med 2010; 53:425–438.
- Bronstein K PS, Munitz L, Leider H. Can clinicians accurately predict which patients are misusing their medications? American Pain Society 30th Annual Scientific Meeting; May 18–21, 2011, 2011; Austin, TX.
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6:107–112.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44:681–692.
- Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al model to fit the context of general practice. Patient Educ Couns 2006; 62:205–211.
- Sullivan MD, Leigh J, Gaster B. Brief report: training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med 2006; 21:360–362.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49:651–661.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006; 60:301–312.
- Savage S. The patient-centered opioid treatment agreement. Am J Bioethics 2010; 10:18–19.
- Crowley-Matoka M. How to parse the protective, the punitive and the prejudicial in chronic opioid therapy? Pain 2013; 154:5–6.
- Arnold RM, Han PK, Seltzer D. Opioid contracts in chronic nonmalignant pain management: objectives and uncertainties. Am J Med 2006; 119:292–296.
- Kirkpatrick AF, Derasari M, Kovacs PL, Lamb BD, Miller R, Reading A. A protocol-contract for opioid use in patients with chronic pain not due to malignancy. J Clin Anesth 1998; 10:435–443.
- Fishman SM, Bandman TB, Edwards A, Borsook D. The opioid contract in the management of chronic pain. J Pain Symptom Manage 1999; 18:27–37.
- Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22:485–490.
- Fishman SM, Wilsey B, Yang J, Reisfield GM, Bandman TB, Borsook D. Adherence monitoring and drug surveillance in chronic opioid therapy. J Pain Symptom Manage 2000; 20:293–307.
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010; 152:712–720.
- King S. How useful are patient opioid agreements and urine drug testing? Psychiatric Times March 2, 2011; www.psychiatrictimes.com/how-useful-are-patient-opioid-agreements-and-urine-drug-testing. Accessed August 2, 2015.
- Starrels JL, Wu B, Peyser D, et al. It made my life a little easier: primary care providers’ beliefs and attitudes about using opioid treatment agreements. J Opioid Manag 2014; 10:95–102.
- Touchet BK, Yates WR, Coon KA. Opioid contract use is associated with physician training level and practice specialty. J Opioid Manage 2005; 1:195–200.
- Penko J, Mattson J, Miaskowski C, Kushel M. Do patients know they are on pain medication agreements? Results from a sample of high-risk patients on chronic opioid therapy. Pain Med 2012; 13:1174–1180.
- Nicolaidis C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med 2011; 12:890–897.
- Roskos SE, Keenum AJ, Newman LM, Wallace LS. Literacy demands and formatting characteristics of opioid contracts in chronic nonmalignant pain management. J Pain 2007; 8:753–758.
- Davis TC, Wolf MS, Bass PF 3rd, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med 2006; 21:847–851.
- American Academy of Pain Medicine. State legislative updates. www.painmed.org/advocacy/state-updates/. Accessed August 5, 2016.
- Burchman SL, Pagel PS. Implementation of a formal treatment agreement for outpatient management of chronic nonmalignant pain with opioid analgesics. J Pain Symptom Manage 1995; 10:556–563.
- Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. 2013; www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed August 2, 2016.
- University of Wisconsin-Madison. Pain & Policy Studies Group. Database of statutes, regulations, & other policies for pain management. www.painpolicy.wisc.edu/database-statutes-regulations-other-policies-pain-management. Accessed August 3, 2016.
- Cameron KA, Rintamaki LS, Kamanda-Kosseh M, Noskin GA, Baker DW, Makoul G. Using theoretical constructs to identify key issues for targeted message design: African American seniors’ perceptions about influenza and influenza vaccination. Health Commun 2009; 24:316–326.
- Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns 2009; 75:321–327.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage 2001; 22:791–796.
- Richeimer SH. Opioids for pain: risk management. Semin Anesthesia Periop Med Pain 2005; 24:165–169.
KEY POINTS
- Both chronic pain and opioid therapy impose costs and risks. Though controversial, long-term opioid therapy will probably have a role for the foreseeable future.
- The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract.”
- Controlled-substance agreements should be used only in the context of personalized patient counseling and shared decision-making.
- Objectives of controlled-substance agreements are to improve adherence, obtain informed consent, outline the prescribing policies of the practice, and mitigate risk.
Psychiatric consultations in long-term care: An evidence-based practical guide
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
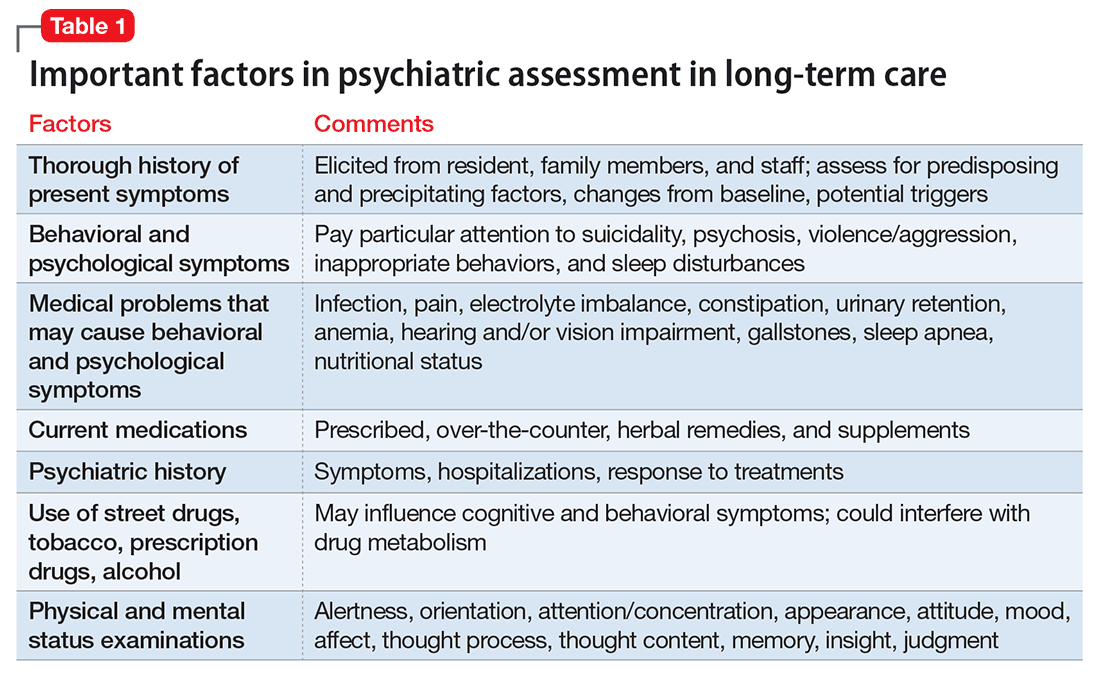
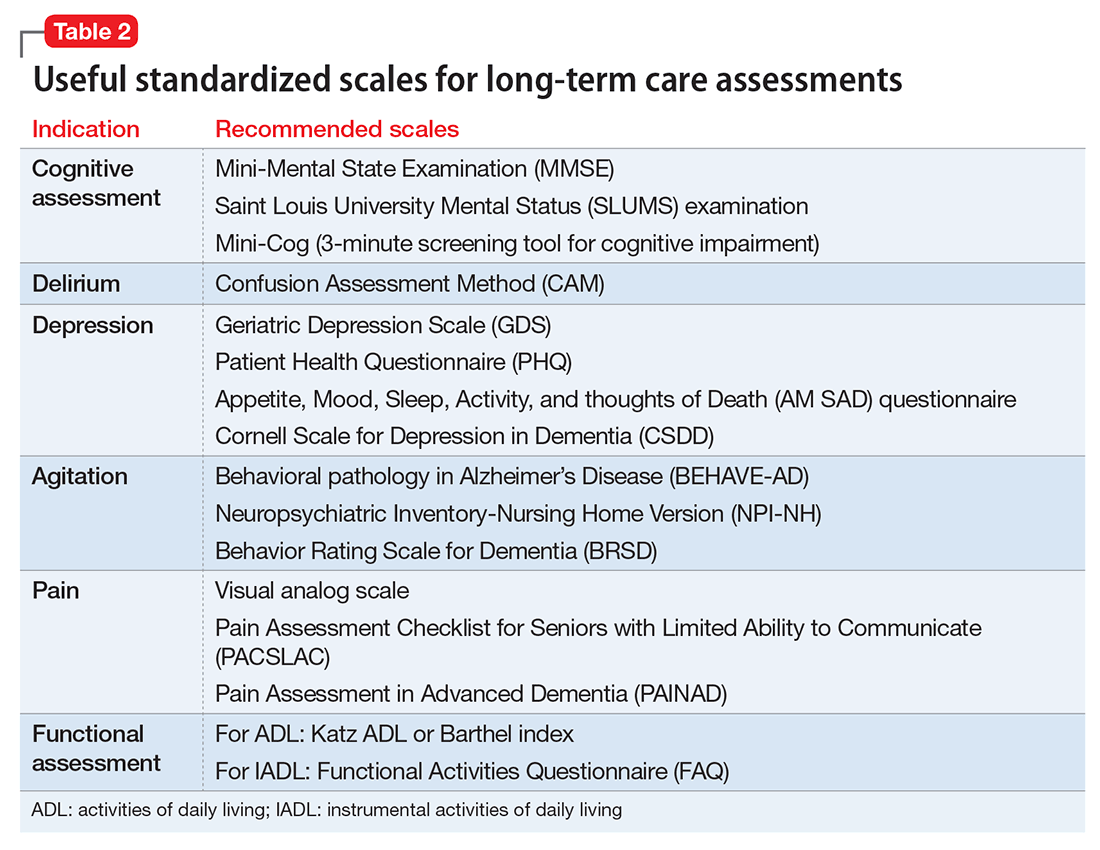
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
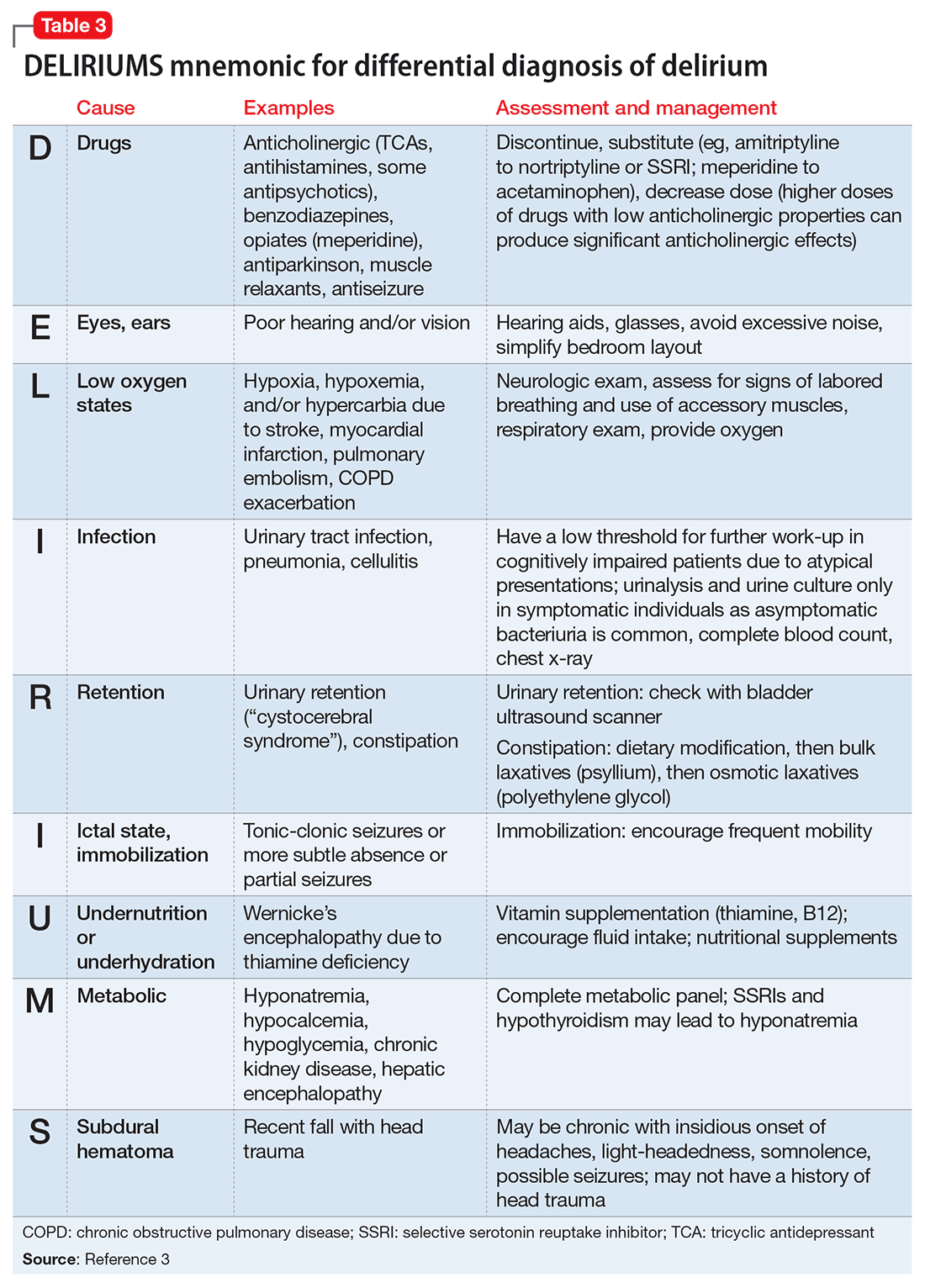
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14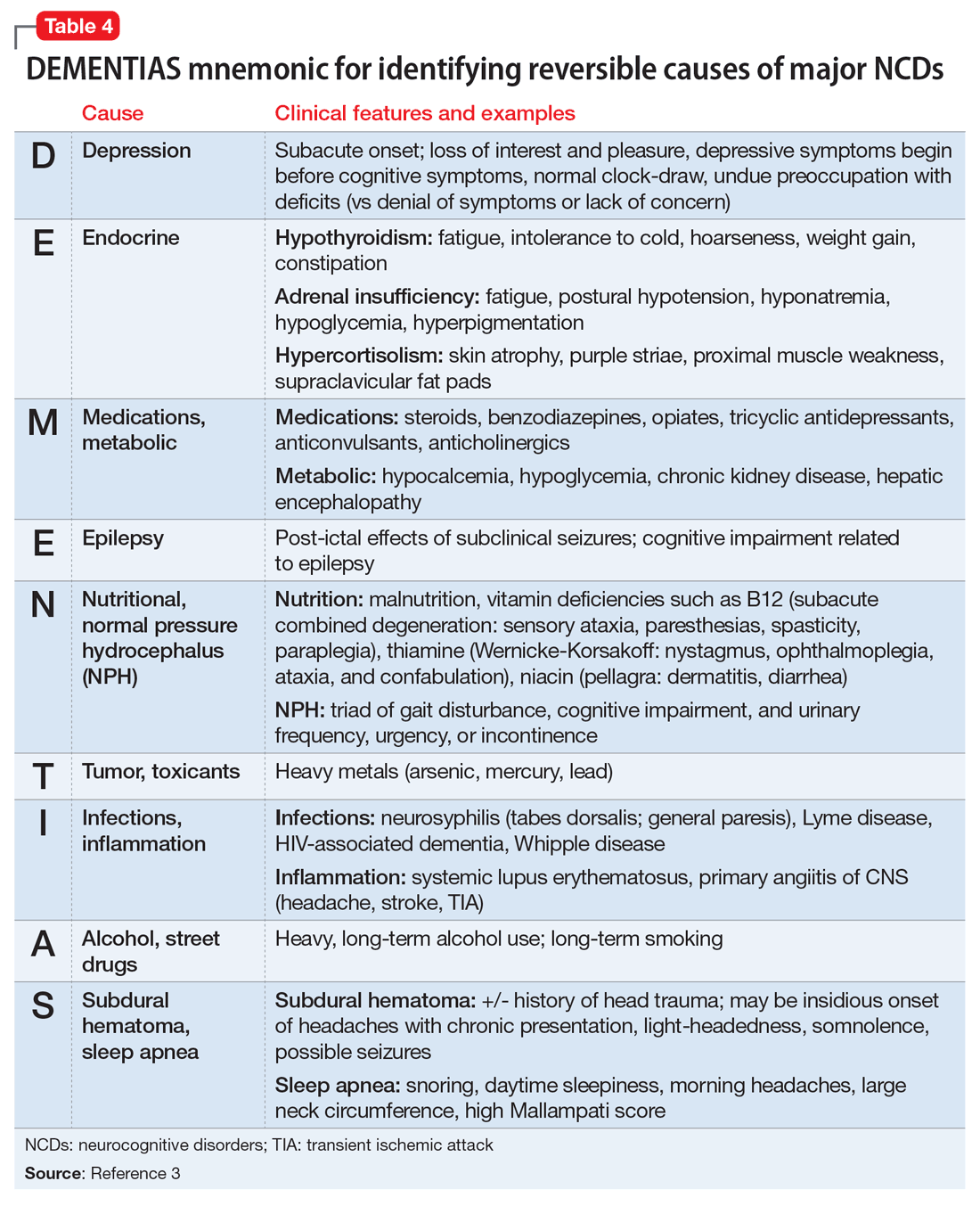
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
How to assess and manage high cholesterol in patients with mental illness
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16
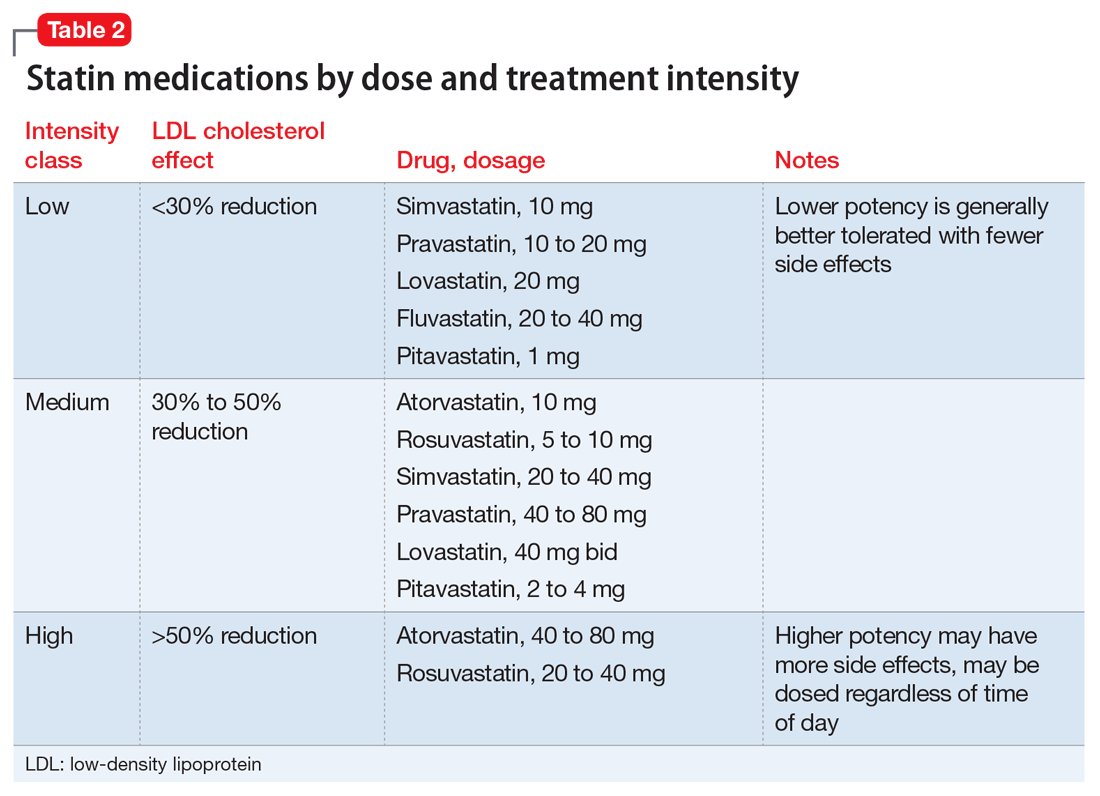
Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18
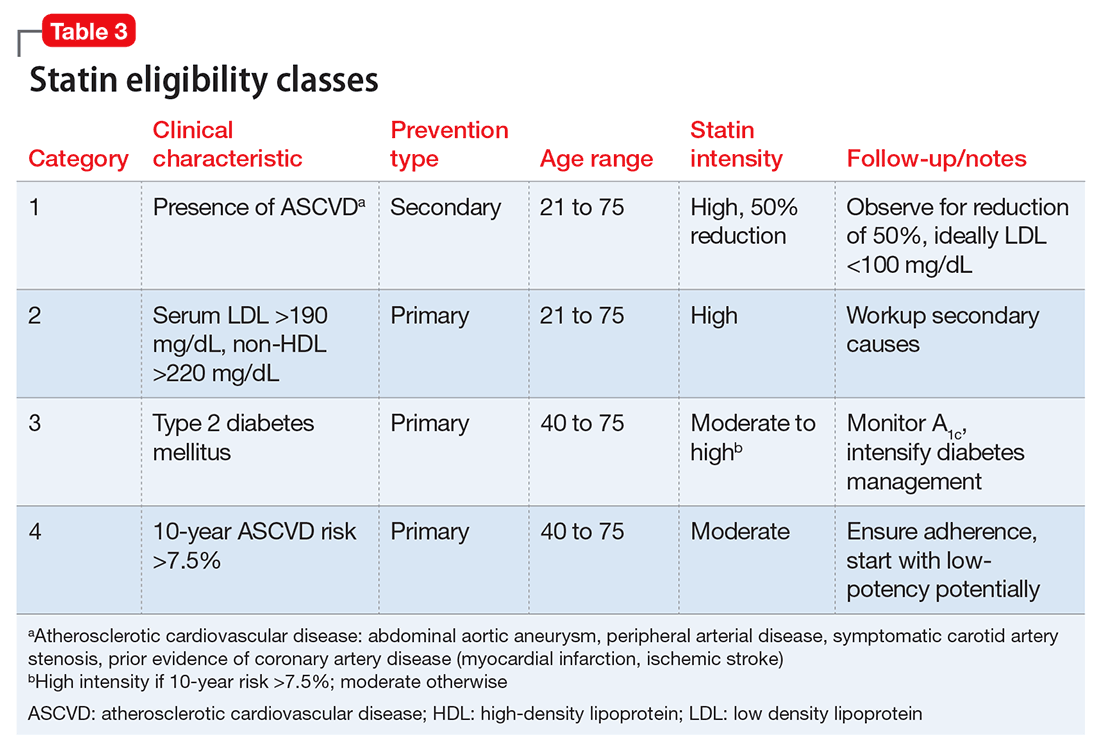
Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).
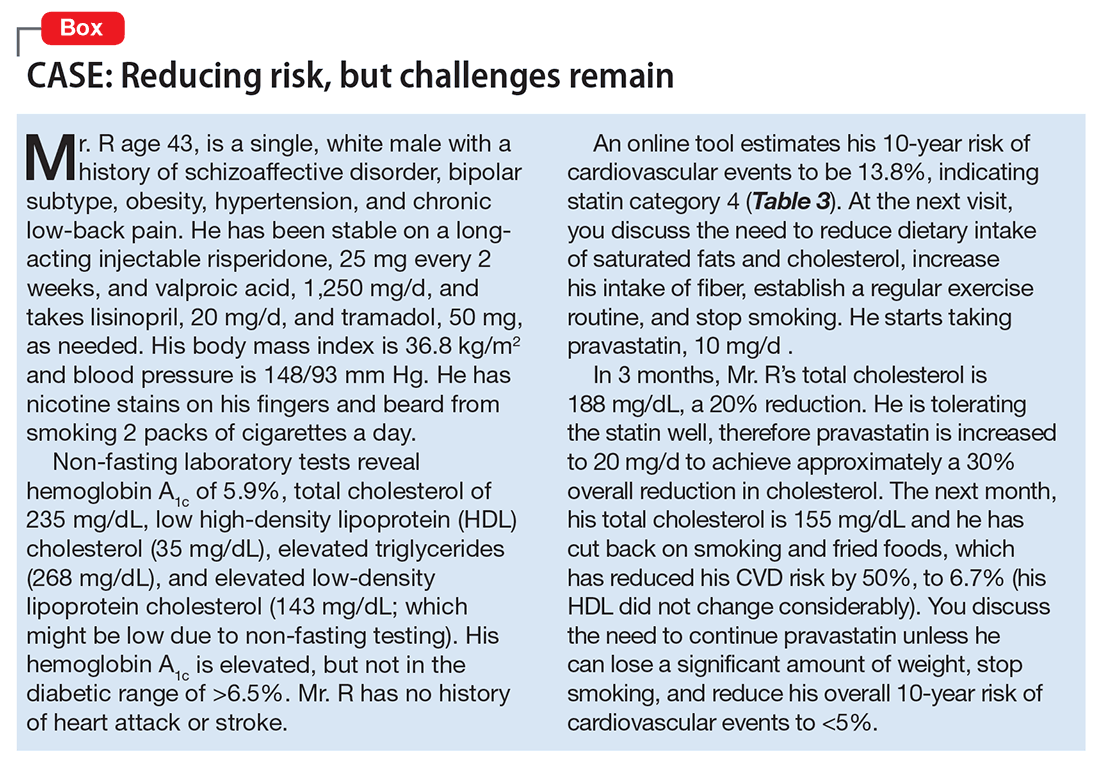
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16

Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18

Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).

High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16

Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18

Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).

1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
Assess and treat catatonia using this systematic approach
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
Catatonia is a neuropsychiatric condition with varying presentations that involve behavioral, motoric, cognitive, affective, and, occasionally, autonomic disturbances. Underlying causes of the syndrome include:
- mood disorders
- psychotic disorders
- neurologic disease
- general medical conditions
- metabolic abnormalities
- drug intoxication or withdrawal.
- deep vein thrombosis and pulmonary embolism
- pressure sores or ulcers
- muscle contractures
- nutritional deficiencies and dehydration from decreased oral intake.1
Prompt recognition, assessment, and treatment are vital.
We recommend the following systematic approach to evaluate and treat catatonia (Table).
Assess
Appropriate assessment of catatonia requires recognition of the array of potential underlying causes of the syndrome.
Obtain a complete history, including:
- recent changes in behavior
- past psychiatric illness and hospitalization
- past or current neurologic or medical disease
- prescription and illicit drug use.
Collateral informants, such as family members and caregivers, could provide valuable information. This history could reveal causative factors and identify appropriate targets for treatment.
Physical and mental status examinations can help characterize the type and severity of motoric and behavioral symptoms, such as rigidity, waxy flexibility, negativism, automatic obedience, ambitendency, and perseveration. Monitoring vital signs is crucial because of the risk of medical complications and malignant catatonia, which can be lethal if not treated.
Laboratory testing and imaging might be indicated to rule out medical causes, such as infection, metabolic disturbances, drug intoxication and withdrawal, and acute neurologic etiologies.
Rate
Identify and rate symptom severity. After determining that a patient has catatonia, consider using a standardized instrument, such as the Bush Francis Catatonia Rating Scale (BFCRS),2 to assess the patient’s type of symptoms and degree of impairment. Scores obtained on such instruments can be tracked as the patient receives treatment. Although the BFCRS is imperfect because of ambiguous symptom descriptions and because symptoms can remain after effective treatment, it is the most widely researched catatonia scale.
Treat and monitor
Although there are no published data from large-scale, randomized, controlled trials, clinical experience shows that the mainstays of treatment still are benzodiazepines and electroconvulsive therapy (ECT). A benzodiazepine challenge of IV lorazepam, 2 mg, can lead to rapid, substantial symptomatic relief with relatively low risk of harm. An estimated 50% to 70% of patients with catatonia respond within 5 days to IV lorazepam, 2 mg, every 3 to 8 hours.3
When patients do not respond to benzodiazepines, consider ECT. For patients with medical, neurologic, and toxic metabolic causes of catatonia, treat the underlying disturbance first.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
1. Clinebell K, Azzam PN, Gopalan P, et al. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644-651.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Fink M. Catatonia: syndrome or schizophrenia subtype? Recognition and treatment. J Neural Transmission (Vienna). 2001;108(6):637-644.
Your patients are talking: Isn’t it time you take responsibility for your online reputation?
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In a web-focused world, it should not take much convincing that monitoring your online reputation is time well spent. For some of us, it may be hard to believe that online reviews have evolved beyond restaurants and plumbers, but today your patients are flocking to the Internet to read and leave reviews about you, your staff, and your services. What can you do to protect your online reputation?
We first addressed this topic in December 2014 (“Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews”1). Have you implemented any of the tactics we offered then? We hope that you do take proactive steps to protect your online image.
What is a physician’s most precious asset?
You might answer this question with, “my patients” or “the training and education that I have obtained to practice my craft.” But the real answer is that your most precious asset is your reputation.
Physicians live and die by their reputations. We spend our entire medical careers polishing and protecting this status. The Internet dramatically has altered the way people gather information. It is sad but true that a single comment that only takes a few seconds and a single mouse-click to post can be seen by thousands and ruin that life-long effort.
How are physicians rated on the web?
Online physician reviews are positive 70% to 90% of the time.2 Most physicians have 5 or fewer reviews on any one site.3 Of the approximately 30 sites that monitor physicians and hospitals online, one of the most popular is AngiesList.com. This site requires registration and a fee; a member can review a physician every 6 months. On free websites such as Yelp.com and doctorsscorecard.com the reviewer can comment once. Other sites such as vitals.com or DrScore.com limit the reviews from 1 source, which prevents an angry patient from stuffing the ballot box.2
Pay attention
At a minimum, physicians should be monitoring their reputation by conducting periodic searches—“Googling” their name and practice name—to identify what information is already online. You may find that 3, 4, or even 10 reviews appear on various sites. If you are lucky, these reviews will be positive. Don’t be surprised, however, if 1 or 2 are not. Let’s face it: even the most accredited and experienced physician cannot possibly satisfy every patient who walks through the door.
Neil Baum, MD, and Ron Romano have offered tips on ways to manage online reputations in the past,1 and they urge Ob-Gyns to take an active role in this process in order to increase positive exposure to patients and maintain an active practice. Is active reputation management something that ObGyns are spending their valuable time on? To find out, OBG Management reached out to its Virtual Editorial Board. We found that many readers are paying attention to patient satisfaction. Some are soliciting online reviews and maintaining active upkeep on their online reputation. Here are a few responses we received from practicing ObGyns across the United States.
William E. McGrath Jr, MD, of Fernandina Beach, Florida, says that his office provides patients with a list of 5 popular review websites during their visits, and that approximately 1 in 10 will follow up with a review. Patient reviews are also prominently posted on his practice’s website. The large, private, single-specialty group to which his practice belongs requires patient satisfaction surveys for quality assurance review and insurance contract negotiations. “It is all about physician-patient communication,” he says.
Keith S. Merlin, MD, of Brockton, Massachusetts, says that he has checked online reviews to ensure their accuracy. His practice uses surveys, a suggestion box, and a mystery shopper to measure patient satisfaction, a worthwhile effort he says to understand where the practice is doing well and what needs to be done better.
Wesley Hambright, MD, of Jacksonville, North Carolina, reports that he has established a Google Alert to monitor for new content relating to his practice.
Patrick Pevoto, MD, MBA, of Austin, Texas, informs us that he has just started to think about ways to manage his online reputation. He has created a website and is writing a monthly blog, which he posts on his site. He acknowledges the importance of assessing patient satisfaction in his practice but is not applying large-scale measurement techniques yet. To keep his patients happy, he handles concerns that arise on a personal, case-by-case basis.
John Armstrong, MD, MS, of Napa, California, also reports that management of his online reputation is in the beginning stages. He uses focus groups and feels that listening to his patients when they do comment on their experience is important to his overall practice. Listening helps to “identify areas to improve and reaffirms when we are doing well,” he says. To keep his patients happy, he strives to “give extraordinary care and simply be nice to people.” When issues arise, making it right and being polite are important elements, he asserts.
Delos J. Clow, DO, MS, of Chillicothe, Missouri, does measure patient satisfaction, and feels this is very important to his practice in order to identify and correct any negative trends. He does not actively monitor his practice reputation online.
Robert del Rosario, MD, of Camp Hill, Pennsylvania, similarly does not actively manage an online reputation, but does focus on patient satisfaction. To enhance satisfaction, he tries to de-emphasize the electronic medical record to “make visits more personal and less interrogative.” Additionally, his practice objectively gauges aspects of care that might be able to be improved upon.
Reference
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
Tell your own story
As physicians, you may not have control over what others say about you, but you can take ownership of your online presence by establishing a website, blog, and social media platforms, ensuring your story is being properly communicated. Without an online presence, you are entirely at the mercy of directory and review sites.
Optimize your website
A site that successfully uses search engine optimization (SEO) will have the upper hand when patients hunt for a physician in your area because the information will be posted at the top of the search page, well above the reviews and listings left by patients and other third-party sources. This is a critical step for your online brand because it will be difficult for other sites to mask your credibility. This should motivate you to develop an online presence, regularly update information, and participate in Internet dialog with other sites.
Generate quality, natural reviews
If your site is in good standing in search results, the next step is to implement a patient reviews strategy to start acquiring positive online reviews. A third-party provider can work with you to launch a local search engine optimization strategy and a natural reviews management program tailored for your practice’s needs. For the most part, however, we do not recommend using an online reputation management company. It is far better and more economical to ask satisfied patients to provide reviews.
At first, you may be tempted to actively petition or solicit reviews through survey software, but this method is manipulative and can lead to reputation problems for your practice. Google actively tracks where reviews originate and uses advanced algorithms to determine the review’s integrity. A petitioned review is classified as less valid, and therefore Google will assume it was not written under the same pretense as a natural, unsolicited review.
Quality customer service and outstanding patient care are often what achieve the organic reviews you are striving for. To encourage a steady flow, administer a process that encourages your most satisfied, loyal patients to review your practice.
Keep the process simple. Capture positive compliments at the point of service. Before a patient leaves your office, hand her a card (FIGURE) with easy steps for posting an online review, or offer her a tablet that links directly to your website review section. If your patient is not computer savvy, ask her to complete a 4- to 5-question survey and give her a clipboard and a pen. Then have a staff member post it on your website.

In Dr. Baum’s practice, there is a poster in every exam room and in the reception area where patients can scan the quick response (QR) code and immediately submit a testimonial. Using this system, the practice is able to collect 3 to 5 positive reviews every day.
A patient pleased with your staff’s service will happily take 5 minutes to submit a review. Acquire 5 to 10 reviews monthly and within a year’s time you will have generated enough positive reviews to negate any damaging comments that inevitably will emerge from time to time.
CASE Patient criticizes physician in a review forum
A physician with a robust Internet presence will have his or her name and the practice appear at the top of search engine results pages (as is the case with Dr. Neil Baum when “urologist” plus “New Orleans” is typed into the Google search engine window). By far most of Dr. Baum’s reviews are positive. In one instance, however, a patient on a physician review website referred to Dr. Baum as “technologically advanced but more motivated to increase his income by performing too many diagnostic tests.”
If you find a negative comment in an online directory or review website, what should you do?
The Office for Civil Rights (OCR) within the US Department of Health and Human Services (HHS) is responsible for handling Health Insurance Portability and Accountability Act of 1996 (HIPAA)1 complaints. Deven McGraw, OCR’s deputy director of health information privacy, states that “just because patients have rated their health provider publicly doesn’t give their health provider permission to rate them in return.”2,3 In fact, some health care providers who responded to poor online reviews ran into trouble with privacy rules established by HIPAA.2,3
Mr. McGraw notes that, when responding to online reviews, health professionals should speak generally about the way they treat patients while complying with HIPAA regulations. He suggests, “If the complaint is about poor patient care … say, ‘I provide all of my patients with good patient care’ and ‘I’ve been reviewed in other contexts and have good reviews.’”2,3
According to Yelp’s senior director of litigation, Aaron Schur, most patient complaints center on practice-based concerns such as wait times, office staff, and billing, not about the medical service delivered. Although most physicians do not respond, says Mr. Schur, those who do, tend to ask patients to discuss the matter in private or to apologize.2,3
What are the consequences of a HIPAA violation?
OCR Director Jocelyn Samuels says that the office’s primary role is to help health providers follow HIPAA regulations.2,5 The OCR can resolve HIPPA violations privately and informally, impose fines of up to $50,000 per violation, or it can file criminal charges against violators.2,4
The majority of the office’s investigation and enforcement of HIPAA has been against large medical data breaches.2,5 Small privacy breaches by large health care providers (eg, CVS, Walmart, Lab Corp, Quest Diagnostics, and others) generally do not result in legal consequences; the providers are privately warned. According to ProPublica, even repeated HIPAA violations tend not to be fined.2,4
Small-scale infractions can be more damaging on a personal level to both patients and physicians. However, the OCR does not typically become involved in privacy breaches that include only a few individuals. Health care providers are rarely punished for small HIPPA breaches; instead, the OCR typically settles for pledges to fix any problems and issues reminders of HIPPA requirements.2,5
Although the OCR is often the only place patients can go to seek vindication, HIPAA does not support the right to sue for violation of personal privacy. People who seek a legal remedy must find another means, which is easier in some states than in others.2,5
Health care providers have tried myriad ways to attempt to combat negative reviews. Some have sued patients, attracting a flood of attention but achieving little legal success. Others have asked patients to remove their complaints.2,3
Best practices
Create and circulate a policy. Medical privacy breaches involving sensitive health details can occur when office or hospital staff share patient information due to personal hostility or lack of understanding of HIPPA policy.2,5 Have a practice policy for responding to online reviews by patients, and make sure the staff members who have access to the practice’s online accounts understand your policy and the possible repercussions of not following it. Teach and continue to remind your staff about HIPPA regulations and hold them to a high ethical level of privacy.
Solicit reviews on an ongoing basis. Jeffrey Segal, a review site critic, says that all reviews are valuable. Physicians should respond carefully to negative comments and encourage satisfied patients to post positive reviews. “’For doctors who get bent out of shape to get rid of negative reviews, it’s a denominator problem,’ he said. ‘If they only have three reviews and two are negative, the denominator is the problem. … If you can figure out a way to cultivate reviews from hundreds of patients rather than a few patients, the problem is solved.’”2,3
CASE Resolved
Dr. Baum never responded directly to the negative patient review, and others he has received. He balances the rare negative response with numerous and plentiful positive responses by making it a practice to encourage reviews from all of his patients.
References
- HIPAA for Professionals. US Department of Health & Human Services. http://www.hhs.gov/hipaa/for-professionals/index.html. Accessed October 11, 2016.
- Hall SD. Providers responding to Yelp reviews must be mindful of HIPAA. FierceHealthcare. http://www.fiercehealthcare.com/it/providers-responding-to-yelp-reviews-must-be-mindful-hipaa. Published May 31, 2016. Accessed October 7, 2016.
- Ornstein C. Stung by Yelp reviews, health providers spill patient secrets. ProPublica. https://www.propublica.org/article/stung-by-yelp-reviews-health -providers-spill-patient-secrets. Published May 27, 2016. Accessed October 11, 2016.
- Ornstein C, Waldman A. Few consequences for health privacy law’s repeat offenders. ProPublica. https://www.propublica.org/article/few-consequences-for-health-privacy-law-repeat-offenders. Published December 29, 2015. Accessed October 11, 2016.
- Ornstein C. Small-scale violations of medical privacy often cause the most harm. ProPublica. https://www.propublica.org/article/small-scale-violations-of-medical-privacy-often-cause-the-most-harm. Published December 10, 2015. Accessed October 11, 2016.
The bottom line
Patients are seeking and leaving reviews about you and your practice online and you need to actively manage your online reputation. Do not let one disgruntled patient ruin your reputation. Our advice: Do not wait for a negative review to begin your reputation management. Take an active role and generate positive reviews to drown out negative remarks made by an occasional patient. This is an inexpensive process that does work.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
- Romano R, Baum NH. Using the Internet in your practice. Part 4: Reputation management-how to gather kudos and combat negative online reviews. OBG Manag. 2014;26(12):23,24,26,28.
- Lagu T, Hannon NS, Rothberg MB, Lindenauer PK. Patients' evaluations of health care providers in the era of social networking: an analysis of physician rating websites. J Gen Intern Med. 2010;25(9):942-946.
- Gunter J. For better or maybe, worse, patients are judging your care online. OBG Manag. 2011;23(3):47-51.
In this Article
- How your peers manage online reputation
- Should you respond to a negative review?
- Generate quality, natural reviews
Consider Rx metformin to prevent metabolic syndrome
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
Public speaking fundamentals. Presentation follow-up: What to do after the last slide is shown

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

This third article in a series on public speaking describes steps you can take immediately following the program to strengthen the impact of your diligent preparation (“Preparation: Tips that lead to a solid, engaging presentation.” OBG Manag. 2016;28[7]:31–36) and honed presentation (“The program: Key elements in capturing and holding audience attention.” OBG Manag. 2016;28[9]:46–50). Don’t overlook these details.
Find ways to stay in touch
You have concluded your talk. The audience response was enthusiastic and the brief Q&A session productive. But it is not over yet. Postpone putting away your computer and disconnecting the audiovisual equipment. Instead, mingle with the attendees—as you also did, we hope, before the program began. There is always more you can learn about your listeners. And, importantly, a few of them would undoubtedly like to ask you one-on-one about a case related to the topic you covered or about another problem in your area of expertise.
We suggest that, as part of your follow-up, you take the names of attendees you speak with. Make a note relevant to each one and plan to send a personal letter that perhaps includes an article you wrote or one published by a credible source. For example, if one of us (MK) gives a talk for a physician audience on a clinical topic, I will send the inquiring physician a note and an article on the topic, with the key sentences related to his or her question highlighted. A sticky note on the article’s front page directs the physician’s attention to the page containing the answer to his or her question (FIGURE). Using this simple technique can make you a value-added resource long after your presentation.

Alternatively, you could e-mail the article to a representative for the organization that you were speaking for. This makes you an asset to the representative, who will likely tell colleagues about your assistance, which could earn you a return speaking engagement.
Make sure, too, that you have an ample supply of business cards—the quickest and easiest way to give out your contact information.
You may also want to distribute a handout of your presentation. This could of course be a printout of your slide show presentation (assuming there are no copyright concerns). But we think it is better to distribute a single page with salient points you would like the audience to take away from your program. However you prepare your handout, be certain each page displays your name, address, phone numbers, and e-mail and website addresses.
Another suggestion: An unobtrusive way to obtain the names of those who attend your program is to collect their business cards in a container before the presentation and hold a drawing for a prize at the end of the program. We often give away a copy of one of our books, but any small gift would work.
Ask for feedback
If you are speaking on behalf of an organization or another sponsoring entity, it is helpful to ask the meeting planner what they thought of the program. Ask for constructive criticism and input on how you might improve the program. Also ask if you were able to get across your most important points.
Finally, send a note to the meeting planner or representative expressing your thanks for the invitation and offering to provide any additional information they might need or want.
Extend the reach of your message
If your talk would be appropriate for a broader audience, you could consider adapting it for publication. Be sure to understand the audience of the publication and review the selected journal’s guidance for authors.
If you do write an article, share it with your colleagues and perhaps your patients. You might also consider posting the article on your website. Yet another option would be to videotape your presentation, keeping it under 10 minutes, and upload it to the video-sharing website YouTube.
Bottom line. The payoff for your research and preparation need not end with the speaking engagement.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Update in perioperative cardiac medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
KEY POINTS
- Outcomes are worse in patients with poor functional capacity or stable angina, and these factors should be considered in preoperative risk assessment.
- Perioperative use of beta-blockers may benefit only patients at highest risk and may harm other patients.
- Statins seem to provide perioperative protection.
- If an ARB is withheld for surgery, it should be restarted soon after.
- For patients with a coronary stent, the type of stent and duration of dual antiplatelet therapy need to be considered before noncardiac surgery.
- Bridging anticoagulant therapy should not be used in patients at intermediate or low risk of thromboembolism.
The ABCs of managing systolic heart failure: Past, present, and future
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
KEY POINTS
- Most patients with systolic heart failure (also called heart failure with reduced ejection fraction) should receive either an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. Most should also receive a beta-blocker (carvedilol, metoprolol succinate, or bisoprolol).
- If symptoms persist or progress despite these treatments, an aldosterone receptor antagonist (spironolactone or eplerenone) is recommended.
- Since the publication of the ACC/AHA guidelines in 2013, the combination of sacubitril and valsartan has been approved, as has ivabradine.
- Patients with advanced heart failure should be identified early for consideration of resynchronization therapy, an implantable cardiac defibrillator, digoxin, a left ventricular assist device, or heart transplant.
- B-type natriuretic peptide levels can be used to guide therapy.