User login
How you can aid your patient’s claim for long-term disability
Neuropsychiatric disorders are associated with high rates of impaired work capacity despite the best efforts of treating clinicians to help their patients stay employed or resume working after symptoms improve.1
In the past, a note from the psychiatrist stating that the patient was unable to work because of a neuropsychiatric condition often was sufficient to approve a disability claim. This is no longer the case in today’s more restrictive climate, and what constitutes prima facie evidence of a patient’s inability to sustain competitive employment secondary to neuropsychiatric illness has significantly changed.
The following practices can help facilitate approval of your patient’s disability claim.
Document as you go. Progress notes should include the type, frequency, context, duration, and severity of symptoms supporting ≥1 psychiatric diagnoses which prevent your patient from holding a job. It also is important to document the parameters of treatment and the patient’s response, including compliance with treatment recommendations. Preferably, progress notes should include quantitative ratings over time that pertain to everyday functioning, highlighting how your patient is coping with the psychosocial, cognitive, and executive functioning demands of his (her) job.
When documented over time, ratings based on the Global Assessment of Functioning scale or a comparable scale are useful in quantifying the nature and degree of impaired functioning related to work capacity. Consider administering rating scales at periodic intervals to show changes over time. When feasible, scales should be based on a patient’s and informant’s report of symptomatic status and everyday functioning, and could include use of instruments such as the World Health Organization’s Disability Assessment Schedule.2,3
Include documentation specific to work capacity. Disability claims often are denied, in part, because the treating psychiatrist’s judgment regarding work capacity seems to “come out of the blue,” appears premature, or lacks discussion of the functional implications of the patient’s clinical status in regards to recent or current job expectations. Therefore, progress notes should include reference to long-standing, emerging, or worsening behaviors or symptoms that have clear implications for your patient’s ability to work.
Outline the functional implications of the patient’s preserved and impaired abilities and skills as they relate to work capacity, vocational history, and recent or current job situation. For example, work requirements that are highly dependent on interaction with the public, supervisors, or coworkers would be significantly affected by recurrent or persistent psychosis, even if the patient adheres to treatment and symptoms are relatively mild. Problems with working memory or anterograde memory could impair work that routinely involves learning and retention of new instructions and procedures.
Provide psychoeducation and support. Educate your patient and their family about the disability claims process, including the high rate that claims are initially denied. Consider retaining an advocate—clinical case manager, family member, or non-family third party—to assist your patient in navigating the disability application process, such as help completing paperwork, setting up appointments, and providing transportation.
Remain responsive to inquiries from disability examiners. Return forms and phone calls from disability examiners, psychiatrists, and other health care professionals reviewing your patient’s claim for long-term disability in a timely manner. Failure to do so can be used to support denial of the claim.
Consider referral for consultations and diagnostics to support the claim of impaired work capacity. Depending on the nature of the case, this could involve additional medical workup (including neuroimaging), a consultation from a vocational rehabilitation specialist, or referral for psychological or neuropsychological testing.
Psychometric assessment is becoming the preferred method for garnering support for impaired work capacity caused by neuropsychiatric factors. Findings from psychometric assessment hold up to scrutiny better if the evaluation includes symptom validity testing to rule out factitious disorder, malingering, or somatization, and results from self-report and informant-based measures of adaptive behavior and functioning.4
1. Gold LH, Shuman DW. Evaluating mental health disability in the workplace: models, process and analysis. New York, NY: Springer; 2009.
2. Traxler J. Mental health disability: a resident’s perspective of problems and solutions. Psychiatric Times. http://www.psychiatrictimes.com/residents-corner/mental-health-disability-residents-perspective-problems-and-solutions. Published November 26, 2014. Accessed August 31, 2016.
3. Zimmerman M. The importance of measuring outcomes in clinical practice. Psychiatric Times. http://www.psychiatrictimes.com/uspc2014/importance-measuring-outcomes-clinical-practice. Published October 1, 2014. Accessed August 31, 2016.
4. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):34-39.
Neuropsychiatric disorders are associated with high rates of impaired work capacity despite the best efforts of treating clinicians to help their patients stay employed or resume working after symptoms improve.1
In the past, a note from the psychiatrist stating that the patient was unable to work because of a neuropsychiatric condition often was sufficient to approve a disability claim. This is no longer the case in today’s more restrictive climate, and what constitutes prima facie evidence of a patient’s inability to sustain competitive employment secondary to neuropsychiatric illness has significantly changed.
The following practices can help facilitate approval of your patient’s disability claim.
Document as you go. Progress notes should include the type, frequency, context, duration, and severity of symptoms supporting ≥1 psychiatric diagnoses which prevent your patient from holding a job. It also is important to document the parameters of treatment and the patient’s response, including compliance with treatment recommendations. Preferably, progress notes should include quantitative ratings over time that pertain to everyday functioning, highlighting how your patient is coping with the psychosocial, cognitive, and executive functioning demands of his (her) job.
When documented over time, ratings based on the Global Assessment of Functioning scale or a comparable scale are useful in quantifying the nature and degree of impaired functioning related to work capacity. Consider administering rating scales at periodic intervals to show changes over time. When feasible, scales should be based on a patient’s and informant’s report of symptomatic status and everyday functioning, and could include use of instruments such as the World Health Organization’s Disability Assessment Schedule.2,3
Include documentation specific to work capacity. Disability claims often are denied, in part, because the treating psychiatrist’s judgment regarding work capacity seems to “come out of the blue,” appears premature, or lacks discussion of the functional implications of the patient’s clinical status in regards to recent or current job expectations. Therefore, progress notes should include reference to long-standing, emerging, or worsening behaviors or symptoms that have clear implications for your patient’s ability to work.
Outline the functional implications of the patient’s preserved and impaired abilities and skills as they relate to work capacity, vocational history, and recent or current job situation. For example, work requirements that are highly dependent on interaction with the public, supervisors, or coworkers would be significantly affected by recurrent or persistent psychosis, even if the patient adheres to treatment and symptoms are relatively mild. Problems with working memory or anterograde memory could impair work that routinely involves learning and retention of new instructions and procedures.
Provide psychoeducation and support. Educate your patient and their family about the disability claims process, including the high rate that claims are initially denied. Consider retaining an advocate—clinical case manager, family member, or non-family third party—to assist your patient in navigating the disability application process, such as help completing paperwork, setting up appointments, and providing transportation.
Remain responsive to inquiries from disability examiners. Return forms and phone calls from disability examiners, psychiatrists, and other health care professionals reviewing your patient’s claim for long-term disability in a timely manner. Failure to do so can be used to support denial of the claim.
Consider referral for consultations and diagnostics to support the claim of impaired work capacity. Depending on the nature of the case, this could involve additional medical workup (including neuroimaging), a consultation from a vocational rehabilitation specialist, or referral for psychological or neuropsychological testing.
Psychometric assessment is becoming the preferred method for garnering support for impaired work capacity caused by neuropsychiatric factors. Findings from psychometric assessment hold up to scrutiny better if the evaluation includes symptom validity testing to rule out factitious disorder, malingering, or somatization, and results from self-report and informant-based measures of adaptive behavior and functioning.4
Neuropsychiatric disorders are associated with high rates of impaired work capacity despite the best efforts of treating clinicians to help their patients stay employed or resume working after symptoms improve.1
In the past, a note from the psychiatrist stating that the patient was unable to work because of a neuropsychiatric condition often was sufficient to approve a disability claim. This is no longer the case in today’s more restrictive climate, and what constitutes prima facie evidence of a patient’s inability to sustain competitive employment secondary to neuropsychiatric illness has significantly changed.
The following practices can help facilitate approval of your patient’s disability claim.
Document as you go. Progress notes should include the type, frequency, context, duration, and severity of symptoms supporting ≥1 psychiatric diagnoses which prevent your patient from holding a job. It also is important to document the parameters of treatment and the patient’s response, including compliance with treatment recommendations. Preferably, progress notes should include quantitative ratings over time that pertain to everyday functioning, highlighting how your patient is coping with the psychosocial, cognitive, and executive functioning demands of his (her) job.
When documented over time, ratings based on the Global Assessment of Functioning scale or a comparable scale are useful in quantifying the nature and degree of impaired functioning related to work capacity. Consider administering rating scales at periodic intervals to show changes over time. When feasible, scales should be based on a patient’s and informant’s report of symptomatic status and everyday functioning, and could include use of instruments such as the World Health Organization’s Disability Assessment Schedule.2,3
Include documentation specific to work capacity. Disability claims often are denied, in part, because the treating psychiatrist’s judgment regarding work capacity seems to “come out of the blue,” appears premature, or lacks discussion of the functional implications of the patient’s clinical status in regards to recent or current job expectations. Therefore, progress notes should include reference to long-standing, emerging, or worsening behaviors or symptoms that have clear implications for your patient’s ability to work.
Outline the functional implications of the patient’s preserved and impaired abilities and skills as they relate to work capacity, vocational history, and recent or current job situation. For example, work requirements that are highly dependent on interaction with the public, supervisors, or coworkers would be significantly affected by recurrent or persistent psychosis, even if the patient adheres to treatment and symptoms are relatively mild. Problems with working memory or anterograde memory could impair work that routinely involves learning and retention of new instructions and procedures.
Provide psychoeducation and support. Educate your patient and their family about the disability claims process, including the high rate that claims are initially denied. Consider retaining an advocate—clinical case manager, family member, or non-family third party—to assist your patient in navigating the disability application process, such as help completing paperwork, setting up appointments, and providing transportation.
Remain responsive to inquiries from disability examiners. Return forms and phone calls from disability examiners, psychiatrists, and other health care professionals reviewing your patient’s claim for long-term disability in a timely manner. Failure to do so can be used to support denial of the claim.
Consider referral for consultations and diagnostics to support the claim of impaired work capacity. Depending on the nature of the case, this could involve additional medical workup (including neuroimaging), a consultation from a vocational rehabilitation specialist, or referral for psychological or neuropsychological testing.
Psychometric assessment is becoming the preferred method for garnering support for impaired work capacity caused by neuropsychiatric factors. Findings from psychometric assessment hold up to scrutiny better if the evaluation includes symptom validity testing to rule out factitious disorder, malingering, or somatization, and results from self-report and informant-based measures of adaptive behavior and functioning.4
1. Gold LH, Shuman DW. Evaluating mental health disability in the workplace: models, process and analysis. New York, NY: Springer; 2009.
2. Traxler J. Mental health disability: a resident’s perspective of problems and solutions. Psychiatric Times. http://www.psychiatrictimes.com/residents-corner/mental-health-disability-residents-perspective-problems-and-solutions. Published November 26, 2014. Accessed August 31, 2016.
3. Zimmerman M. The importance of measuring outcomes in clinical practice. Psychiatric Times. http://www.psychiatrictimes.com/uspc2014/importance-measuring-outcomes-clinical-practice. Published October 1, 2014. Accessed August 31, 2016.
4. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):34-39.
1. Gold LH, Shuman DW. Evaluating mental health disability in the workplace: models, process and analysis. New York, NY: Springer; 2009.
2. Traxler J. Mental health disability: a resident’s perspective of problems and solutions. Psychiatric Times. http://www.psychiatrictimes.com/residents-corner/mental-health-disability-residents-perspective-problems-and-solutions. Published November 26, 2014. Accessed August 31, 2016.
3. Zimmerman M. The importance of measuring outcomes in clinical practice. Psychiatric Times. http://www.psychiatrictimes.com/uspc2014/importance-measuring-outcomes-clinical-practice. Published October 1, 2014. Accessed August 31, 2016.
4. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):34-39.
Rediscovering clozapine: Clinically relevant off-label uses
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).
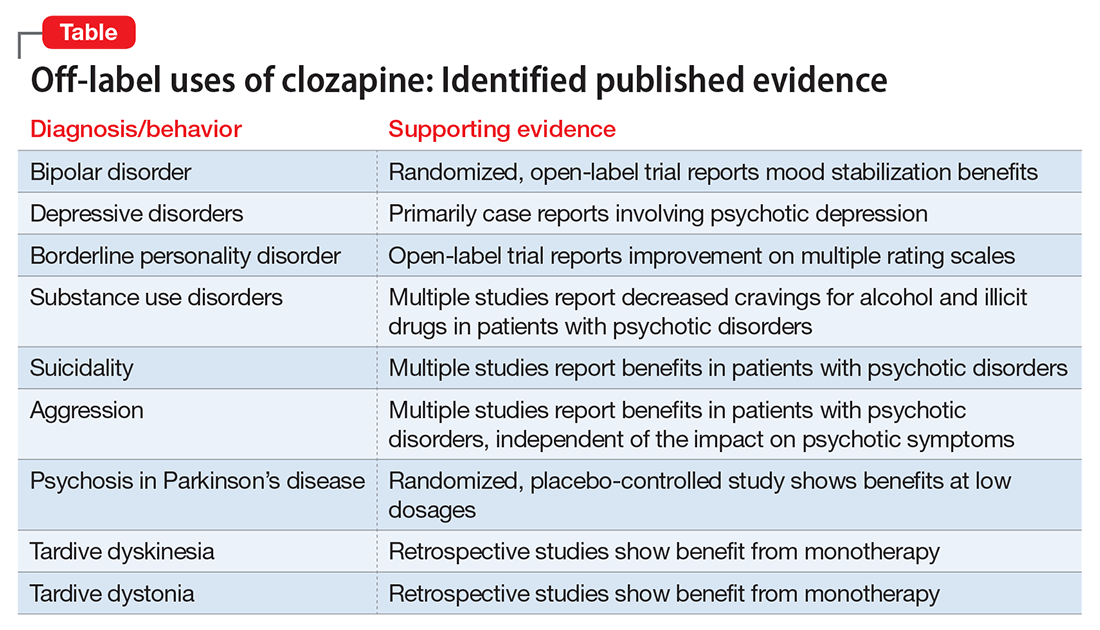
At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
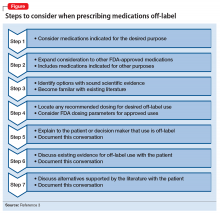
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
Where do you draw the line? Caveats for after-hours call coverage
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Rule out these causes of inattention before diagnosing ADHD
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
‘They’re out to get me!’: Evaluating rational fears and bizarre delusions in paranoia
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
An irritable, inattentive, and disruptive child: Is it ADHD or bipolar disorder?
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Skin findings associated with nutritional deficiencies
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
KEY POINTS
- Although nutritional deficiencies are relatively uncommon in the general population, certain groups have a higher risk, including infants, pregnant women, alcoholics, vegetarians, persons of poor socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery.
- Often, patients present with more than one deficiency.
- Zinc deficiency can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
- The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
- Manifestations of vitamin A deficiency include night-blindness, dry eyes, and phrynoderma (“toad skin”).
- The B-complex vitamins are linked. Vitamin B2 (riboflavin) deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12.
Nonatherosclerotic limb ischemia: Prompt evaluation and diagnosis
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
KEY POINTS
- A high index of suspicion should be maintained to recognize symptoms consistent with limb ischemia in a younger patient in the absence of the usual atherosclerosis risk factors.
- A workup for most conditions includes noninvasive vascular ultrasonography to detect and quantify limb ischemia.
- Prompt referral for surgical or endovascular treatment is necessary for optimal limb salvage.
Take steps to relieve ataxia in patients with alcohol use disorder
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
Ataxia is a well-known complication of chronic alcohol abuse, which is attributed to degeneration of the cerebellar vermis. However, effective treatment approaches, as well as the timing and level of recovery, remain unclear. One cross-sectional study found that long-term abstainers from alcohol had less severe ataxia than short-term abstainers,1 suggesting that improvement is possible with continued sobriety. However, a recent longitudinal study contradicts this finding, reporting no improvement in ataxia in patients abstinent for 10 weeks to 1 year.2
CASE REPORT
Unable to walk, heavy alcohol use
Mr. G, a 59-year-old white male with a history of daily, heavy alcohol use, presents to the emergency room reporting that he has “not been able to walk right” for 3 weeks. He is in a wheelchair because of ataxia and difficulty balancing. He denies headaches, visual changes, weakness, numbness, and difficulty speaking or swallowing.
Mr. G reports drinking one 40-oz bottle of malt liquor and 2 pints of vodka per day for more than 40 years. His alcohol abuse led to homelessness, unemployment, and divorce. Despite heavy drinking, he denies signs of withdrawal, including shaking, sweating, seizures, and delirium.
Mr. G has no other medical conditions. He denies a family history of neurologic disorders or substance abuse.
His pulse is 100 beats per minute, respirations of 16 breaths per minute, temperature of 37°C, and blood pressure of 143/89 mm Hg. Physical examination reveals a wide-based gait.
Mr. G is admitted to the inpatient psychiatric unit to monitor and treat his alcohol withdrawal and to undergo further workup of the gait disturbance.
A head CT scan shows non-specific changes; an EEG also is within normal limits. Complete blood count, basic metabolic panel, liver function test, HIV test, acute hepatitis panel, thyroid function test, erythrocyte sedimentation rate, and vitamin B12 tests are within normal ranges.
A full neurologic exam reveals a wide-based gait, impaired heel-shin test, and dysmetria on finger-nose-finger test. Mr. G is given a diagnosis of ataxia due to alcoholic cerebellar degeneration. Thiamine repletion is suggested.
Treatment and outcome
Mr. G continues on thiamine, 100 mg, twice daily, and oxazepam, 15 mg, as needed, to manage withdrawal symptoms. He receives gait training 3 times per week.
Approximately 10 days after admission, Mr. G is able to ambulate with a walker. Three weeks after admission, his gait has improved and he walks with a cane. (See the video at CurrentPsychiatry.com for an illustration of this progressive recovery.)
After discharge, Mr. G is referred to an addiction psychiatrist and addiction psychotherapist for ongoing treatment of alcohol use disorder.
Making the diagnosis
In a patient complaining of balance difficulties, consider ataxia secondary to cerebellar degeneration.
- Take a complete history. Ask about the onset and progression of ataxia.
- Obtain a family history. Some types of ataxia are genetic.
- Perform a neurologic examination, which may reveal signs of cerebellar deficits, particularly characteristic wide-based gait. These patients will have difficulty when walking in tandem. Other impairments on the neurologic exam that may raise suspicion for a cerebellar disorder include: impaired heel-shin test, impaired finger-nose-finger test (dysmetria), impaired rapid alternating movements (dysdiadochokinesia), nystagmus, impaired smooth pursuits, intention tremor, or speech abnormalities.
- Perform head imaging, such as a CT scan or MRI. In patients with ataxia secondary to alcohol abuse, imaging might reveal degeneration of the cerebellar vermis.
- Perform laboratory tests, such as inflammatory markers, vitamin levels, and thyroid function testing to detect possible toxic-metabolic or inflammatory causes.
Alcohol-induced ataxia can be diagnosed in patients with a history of heavy drinking if the workup does not reveal another possible cause for the gait disturbance. Other less common deficits associated with alcohol-induced cerebellar injury include:
- dysarthria
- abnormal rate and force of movement
- limb ataxia.3
Recommendations
- Be able to recognize the characteristic gait of patients with alcohol-induced ataxia.
- Provide thiamine supplementation.
- Refer patients to physical therapy.
- Educate your patients that their gait will not improve and may worsen if they continue to drink.
- Refer patients for ongoing treatment for alcohol use disorder, including medication management and psychotherapy.
Our experience suggests that patients with alcohol use disorder with cerebellar ataxia could have a good prognosis for ambulation. Improvement could occur over several weeks; it is unclear whether further gains can be expected with months or years of abstinence.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
1. Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35(12):2184-2192.
2. Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37(1):86-95.
3. Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36(11):1942-1951.
No more 'stickies'!: Help your patients bring their ‘to-do’ list into the 21st century
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,
Difficulty with time management and organization is one of the most common complaints of patients with attention-deficit/hyperactivity disorder (ADHD). Being unproductive and inefficient also is anxiety-producing and depressing, leaving patients with additional comorbidity.
Although medication can help improve a person’s focus, if the patient is focusing on a set of poorly designed systems, he (she) will see little improvement. A comprehensive approach to improving day-to-day task management, similar to the one I describe here and use with my patients, is therefore as important as medication.
Needed: An ‘organizing principle’
Imagine that supermarkets displayed food in the order it arrives from the food distributors and producers. You’d walk in to the store and see a display of food that lacks hierarchy—1 random item placed next to another. The experience would be jarring, and shopping would be a much slower chore. Furthermore, what if you had to go to 5 stores to cover all your needs?
Yet, that is how most “to-do” lists are executed: A thought comes in, a thought goes down on paper. Or on a sticky note. Or in an app. Or in a calendar. Or all of the above! Often, there is neither an organizing principle (other than perhaps chronological order) or a central repository. No wonder it’s hard to feel present and clear-minded. Add to this disorganization the volume of information coming in from the environment—e-mails, voice mails, texts, notifications, dings, beeps, buzzes, and maybe even snail mail—and the feeling of being overwhelmed grows.
Unconscious motives for maintaining poor systems also might play a role. People with a “need to please” personality type or who are more passive-aggressive in their communication are more likely to overcommit, and then forget or be late completing their tasks, rather than saying “No” from the outset or delegating the work.
Survival basics for time management
Assuming there is simply a skills deficit, you can teach basic time and project management skills to patients with ADHD (and to any patient with suboptimal executive functioning). Here are basic principles to adopt:
- If you can forget it, you will, so all tasks should go onto the to-do list.
- You should keep only 1 list. Adding on “stickies” is not allowed.
- Your list is like an extra lobe of your brain: It should be present at all times, whether you keep it in “the Cloud,” on your desktop, or on paper.
- Review your list and clean it up at least daily. This takes time, but it also saves time—in spades—when you can call upon the right task, at the right time, with energy and drive.
- The first action you should take in the daily review is to weed out or delegate tasks.
- Next, categorize remaining tasks. (Note: The free smartphone app Evernote allows you to do this with “tags.”) Categorizing allows you to process sets of tasks in buckets that can be tackled as a bundle and, therefore, more efficiently. For example, having all of your errands, items to research, and telephone calls that need to be returned in separate buckets allows for speedier processing—as opposed to veering back and forth between line items.
- Then, move remaining high-priority items to the top of the list. However, remember that, if everything is urgent, nothing is. Items that are low-hanging fruit that you can cross off the list in a matter of minutes can be prioritized even if they are not as urgent. By doing that, your list becomes more manageable and your brain can dive deeper into more complex tasks.
- Block out calendar time for each of your buckets with this formula: (1) Estimate how much time you’ll need to complete the tasks in each bucket, then add 50% for each bucket. (2) Add in commuting, set-up, or wind-down time, if you need it, to the grand total for all buckets, and then add 50% more than you’ve estimated
Set the brain free!
This process will seem like a burden at the beginning, when the synapses underneath it still need to get stronger (much like how the body responds to exercise). However, as long as these principles are put into action daily, they will become a trusted, second-nature system that frees the brain from distraction and anxiety—and, ultimately,