User login
Pills to powder: An updated clinician’s reference for crushable psychotropics
Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
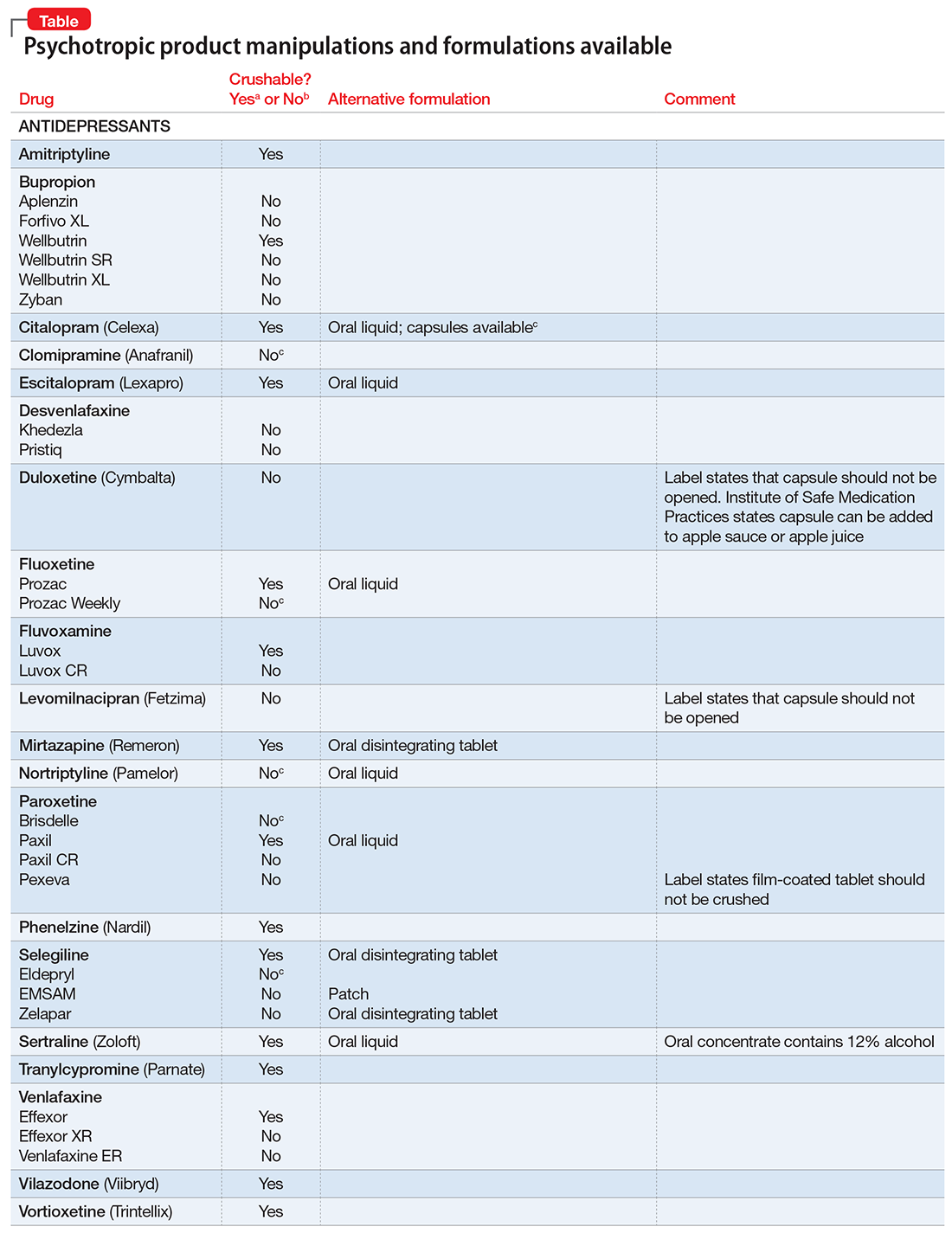
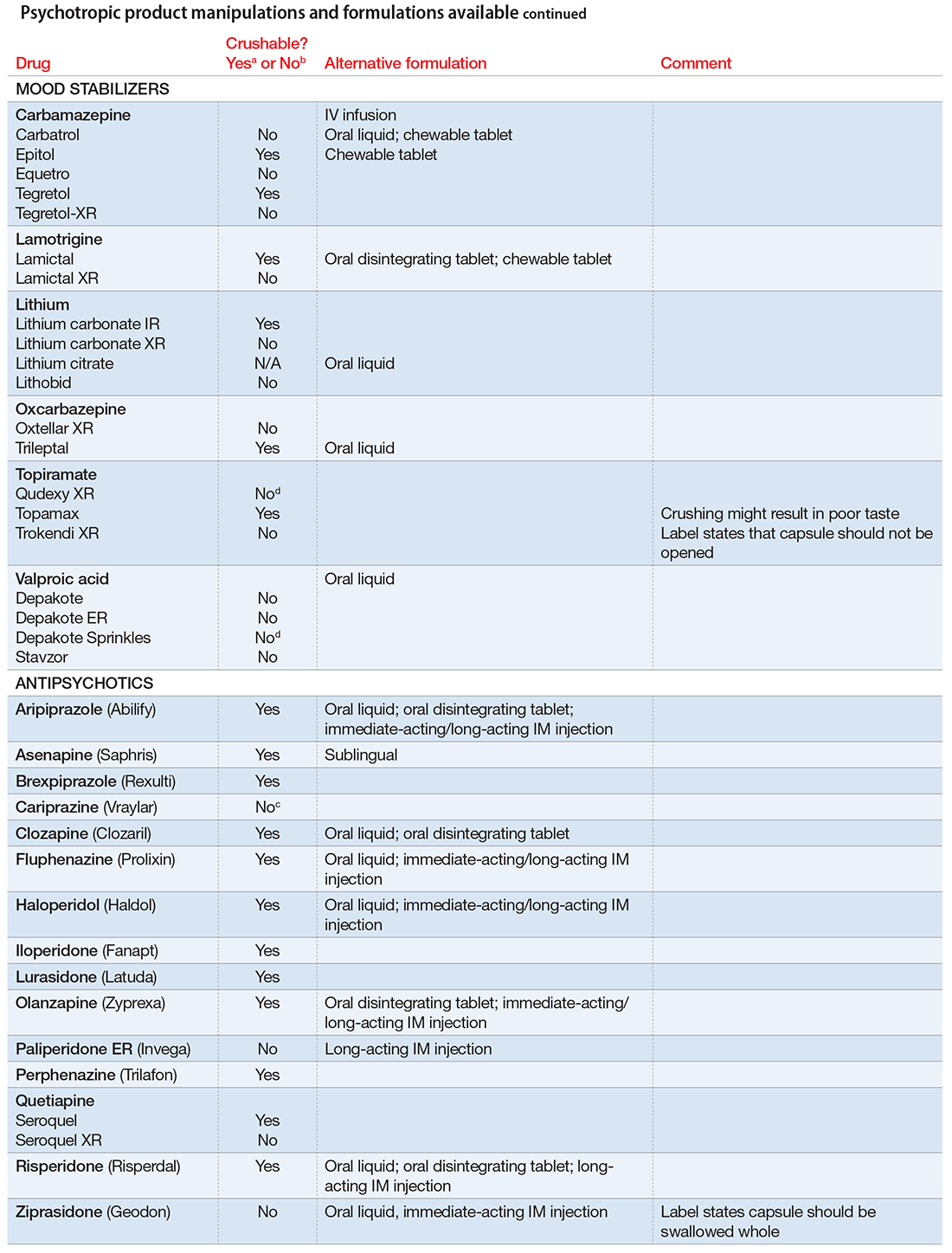
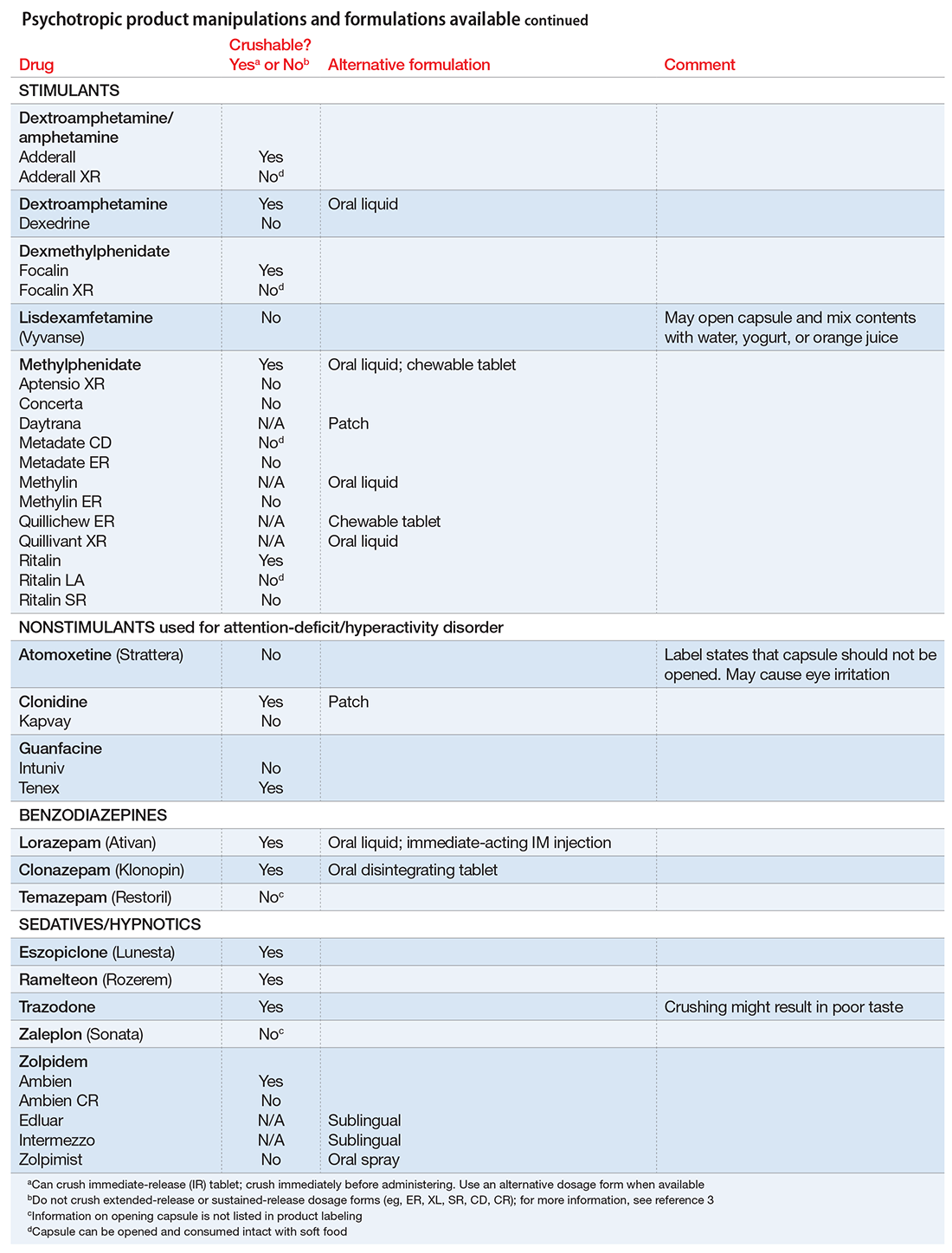
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.
1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.



Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.



1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
TRUST: How to build a support net for ObGyns affected by a medical error
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An estimated 98,000 Americans die each year due to medical errors. This is an attention-grabbing statistic—from the year 2000.1 A recent study (published in 2016) reported that medical errors are the third leading cause of death in the United States, ranking just behind heart disease and cancer.2
As expected, much has been done to reduce medical errors and improve patient safety as a result of these publications. Quality, safety, and outcomes are paramount, as evidenced by the Institute of Health Care Improvement’s “triple aim”: reduce cost of care, improve quality of care, and improve patient outcomes.3
While these 3 aims are of paramount importance, this article seeks to portray the “quadruple aim,” with an additional focus on physician well-being. Patients and their families (first victims) are not the only ones affected by medical errors. Clinicians are, too, and these effects can be devastating. Here I offer concrete strategies to support providers involved in medical errors, including tips on developing a formal support program. First, however, I describe the devastating effects medical errors can have on providers and the signs of a second victim.
Related article:
Medical errors: Caring for the second victim (you)
The scope of the problem
In 2000, it was Dr. Albert Wu’s publication in The British Medical Journal titled “Medical Error: The Second Victim” (the doctor who makes mistakes needs help too), that first addressed this important topic.4 In his article he shared a case of another house officer who missed signs of a pericardial tamponade and was judged incompetent by peers due to his mistake.
As physicians, we do not intrinsically support colleagues who have experienced a medical error. We all have taken, with pride and commitment, our Hippocratic Oath of “do no harm,” yet we are often held to standards of perfection by society, peers, and, above all, ourselves. Have technologic wonders and precise laboratory tests supplanted the adage “doctors are only human”? Dr. Wu also points out in this landmark essay his observation and dismay at the lack of empathy, sympathy, and compassion shown by peers when medical errors occur. All of these elements are needed for the healing of those involved to take place. If they are not provided, dysfunctional coping mechanisms ensue.4
Incidence of medical errors
Despite the Institute of Medicine report from 20001 and the recent study from Johns Hopkins,2 determining the exact number of errors and incidents is not easy. Most data reporting is sparse. A prospective longitudinal study of perceived medical errors and resident distress estimated medical errors to be between 5% and 10% in hospitalized patients, but that it could be up to 50%.5 According to a 2005 study, approximately one-third of internal medicine residents report at least 1 major medical error during their 3 years of training, while 18% of multidisciplinary residents report an adverse event under their care in the previous week.6
Related article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Who is at risk of becoming a second victim?
Any and all clinicians can become a second victim, and the state can be realized at varying points in the process of an experienced medical error. The circumstances of the initial error and the severity of the effect on the patient and/or the damaged physician−patient relationship can affect whether or not there is a second victim. A second victim also can emerge as a result of peers’ or colleagues’ comments and lack of empathy or support. Certainly a lawsuit can produce a second victim.7
How often do physicians become second victims?
The prevalence of second victims has a large variation in estimates. A 2006 study estimates a prevalence of 10.4%.8 In 2010, the estimate was 30%, and a prevalence of 43.3% was reported in 2000.9,10 Regarding emotional distress within a year of a major adverse event, 30% of almost 900 providers reported these feelings.11 Other studies note 50% of health care workers reported feelings consistent with those of a second victim.7
Next: What are the symptoms of a second victim?
The signs of, and long-lasting risks for, a second victim
Second victims are at risk for several well-documented symptoms, regardless of their stage of training, including6:
- depression (in fact, they have a 3-fold risk)
- decrease in overall quality of life
- increase in burnout
- increase in feelings of distress, guilt, and shame, which may be long lasting.
Health care providers as second victims also may experience shock and hopelessness, sleep disturbance, social avoidance, intrusive thoughts and nightmares, and poor memory and concentration. Interestingly, these emotions and reactions are indistinguishable from posttraumatic stress disorder. These continued symptoms can have short- and long-term implications for physicians, patients, and the health care organization.12
Next: How to support those affected by a medical error
How to support all of those affected by a medical error
Over the past decade or so, much attention has been paid to creating safer health systems, improving outcomes and patient satisfaction, and recognizing the needs of patients and families of first victims when medical errors occur. Much less has been done to acknowledge and address the needs of struggling clinicians.
Provide nurturing discussions and sympathy
Hospital systems do have embedded processes to review outcomes and medical errors, including, among others, peer review, quality improvement, morbidity and mortality review, and root cause analysis. Unfortunately, often a “name, blame, shame game” can result from the overall process, with certain individuals or groups of individuals singled out, and only worsen the incidence and effects of the second victim. Ideally, system processes for addressing medical errors should allow for an environment more focused on nurturing discussions to prevent error and recognize all the factors contributing to an error.
Of course in any outcome or error investigation, the goal is to identify what happened, what factors contributed to the incident, and what can be done to prevent future occurrences. The concern for the family as priority is understandable, as is the desire to prevent a lawsuit. The lack of attention and sympathy to the health care provider involved contributes to the second victim.7
It is all too easy to blame, even in a Just Culture. Deficiencies in sympathy and attention can occur without a system whose culture is focused on “name, blame, shame.” A Just Culture, as defined by the Institute for Healthcare Improvement, is one in which individuals come forward with a mistake without fear of punishment. Such a culture balances the need to learn from our mistakes and the need to have disciplinary action.13
David Marx, an outcomes engineer and author of “Whack a Mole: The Price We Pay for Expecting Perfection,” touts a Just Culture as one having the following sets of beliefs:
- recognition that professionals will make mistakes
- recognition that even professionals will develop unhealthy norms
- a fierce intolerance for reckless conduct.
He strongly asserts that human error be consoled while reckless behavior be punished.14 Punishing human error is a setup for the second victim.
Read on for tips to develop a coping program
Tips for developing a coping program
In 2009, Scott and colleagues described 6 stages of a second victim. These are:
- Stage 1: Chaos and event repair
- Stage 2: Intrusive thoughts, “what if”
- Stage 3: Restoring personal identity
- Stage 4: Enduring the inquisition
- Stage 5: Obtaining emotional first aid
- Stage 6: Moving on or dropping out; surviving and/or thriving
Throughout the stages, second victims look for support and share their experience of the medical error event, as well as their personal and professional impact of the error.15
A 2007 study that examined the emotional impact of medical errors on physicians revealed some startling data. A full 82% of physicians expressed interest in counseling to help cope with their distress. And 90% felt there was inadequate support at their hospitals or health care organizations for this distress.16
Use The Joint Commission’s toolkit
Unfortunately, there are only a few well-documented second-victim support programs in the United States, despite the growing evidence of the emotional distress that second victims experience. Many hospitals do not know how to develop or implement such a support system. Recognizing this challenge, The Joint Commission developed a toolkit to assist health care organizations in developing a second-victim program. The toolkit consists of 10 modules (TABLE) designed to assist organizations not only to implement a second-victim support process but also to customize it to their specific institutional culture. This toolkit can be downloaded for free or used online. Within the first year of its availability, over 6,000 people visited the website and there were more than 700 requests for a download.17

Follow forYOU’s example
An example and well-recognized second-victim support program is the “forYOU” team at the University of Missouri. The program is free to employees, confidential, and available 24-7. Its purpose is “providing care and support to our staff,” by helping members understand the phenomenon of the second victim and quickly returning members to a satisfying professional practice.18
The “forYOU” team was created in 2007 under the direction of the University of Missouri Health Care’s Office of Clinical Effectiveness with the goals of increasing institutional awareness, providing a second victim with a “safe zone,” and allowing for the expression of emotions and reactions in a confidential setting. Team members are multidisciplinary and include physicians, nurses, respiratory therapists, social workers, and chaplains. They strive to normalize the feelings and thoughts second victims experience after a stressful outcome or event. Team members are highly trained in second-victim responses and the stages of coping. The program has established institutional actions to each of the 6 stages (FIGURE).19

Read on to learn how peer mentors are crucial to a support program
Establish TRUST
At the Carilion Clinic in Roanoke, Virginia, we too have developed a second-victim support program for all of our employees: TRUST. In the beginning stages, we quickly reaffirmed the challenges in developing such a program.
Initial challenges you will face. First, education on what a second victim is needs to be recognized. The fact that not everyone experiences second-victim emotions needs to be validated. Administrators and staff must be convinced that needing support is not a sign of weakness. And the program must ensure confidentiality and recruit mentors. These are just a few of the obstacles we faced on our path to program realization. Our journey to develop our second-victim program was approximately 5 years and required participation, affirmation, and support from all levels of the organization.
Our program name embodies its inherent purpose and goals. TRUST stands for:
- Treatment that is just. Second victims deserve the right of a presumption that their intentions were good, and should be able to depend on organizational leaders for integrity, fairness, just treatment, and shared accountability for outcomes.
- Respect. Second victims deserve respect and common decency and should not be blamed and shamed for human fallibility.
- Understanding and compassion. Second victims need compassionate help to grieve and heal.
- Supportive care. Second victims are entitled to psychological and support services that are delivered in a professional and organized way.
- Transparency and opportunity to contribute. Second victims have a right to participate in the learning gathered from the event, to share important causal information with the organization, and to be provided with an opportunity to heal by contributing to the prevention of future events.
Employ peer mentors, who serve a vital role
We have identified the need to develop a more direct and active approach to the TRUST program’s recruitment and established a subcommittee to begin this process. We began by asking leaders to nominate potential peer mentors and spoke about the program and asked for volunteers at various hospital committees. Once we had most disciplines represented, leaders were asked to take an assessment for emotional intelligence.
Other than the initial training for the TRUST program, the time requirement for participation for peer mentors is likely less than an hour per month. The dedicated time certainly is dependent on how much support the second victim is requiring, however, and varies. We encourage the peer supporters to be aware of their time constraints and establish parameters for the relationship in a direct but supportive way.
Since the inception of the TRUST Team in September 2014, we have trained 12 peer mentors, 10 of whom currently still serve in that capacity. We have 3 additional peers awaiting training. To date, The TRUST team has supported 19 clinicians/staff, including 3 ACPs, 9 nurses, 6 physicians, and 1 other (pharmacist). Of those 10, 3 are still actively receiving support so closing data have yet to be collected. Of the 16 who have been closed, 6 were referred for ongoing support and 10 were able to return to baseline with TRUST Team Supports.
Related article:
Who is liable when a surgical error occurs?
Just surviving the medical error is not the goal
Medical errors are inevitable, and the effects on providers can be devastating. It is important that physicians and institutions are aware of the signs and symptoms of a second victim as well as provide support to them. Institutions must have a just culture in which all members of the health care team can come forward with medical errors without the fear of punishment. Ideally, these institutions also have a second-victim support system that identifies those who need assistance and assist all health care clinicians not only to survive the effects of medical errors but also to thrive after receiving the necessary support.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed December 18, 2016.
- Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Affairs (Millwood). 2008;27(3):759−769. http://www.ihi.org/resources/Pages/Publications/TripleAimCareHealthandCost.aspx. Accessed December 18, 2016.
- Wu AW. Medical error: The second victim. The doctor who makes the mistake needs help too. BMJ . 2000;320(7237):726−727.
- West CP, Huschka MM, Novotny PJ, et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. JAMA. 2006;296(9):1071−1078.
- Jagsi R, Kitch BT, Weinstein DF, Campbell EG, Hetter M, Weissman JS. Residents report on adverse events and their causes. Arch Intern Med. 2005;165(22):2607−2613.
- Wu AW, Steckelberg RC. Medical error, incident investigation, and the second victim: doing better but feeling worse? BMJ Qual Saf. 2012;21(4):267−270.
- Lander LI, Connor JA, Shah RK, Kentala E, Healy, GB, Roberson DW. Otolaryngologists’ responses to errors and adverse events. Laryngoscope. 2006;116(7):1114−1120.
- Scott SD, Hirschinger LE, Cox KR. Sharing the load. Rescuing the healer after trauma. RN. 2008;71(12):38−40,42−43.
- Wolf ZR. Stress management in response to practice errors: critical events in professional practice. PA-PSRS Patient Safety Advisory. 2005;2:1−2.
- Scott SD, Hirschinger LE, Cox KR, et al. Caring for our own: deploying a systemwide second victim rapid response team. Jt Comm J Qual Patient Saf. 2010;36(5):233−240.
- Edrees HH, Paine LA, Feroli ER, Wu AW. Health care workers as second victims of medical errors. Pol Arch Med Wewn. 2011;121(4):101−108.
- Leonard M. Organizational fairness/Just Culture. Cambridge, MA: Institute for Healthcare Improvement; 2012. http://app.ihi.org/extranetng/content/58886256-47d8-4f9c-bf7b-0afc352f013a/0efbd6cd-d0a3-4353-ad84-c86d07f499e1/4_5_Just%20Culture_ML.pdf. Accessed December 18, 2016.
- Marx D. Whack-a-Mole: The Price We Pay for Expecting Perfection. Plano, TX: By Your Side Studios; 2009.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. Qual Saf Health Care. 2009;18(5):325−330.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467−476.
- Pratt S, Kenney L, Scott SD, Wu AW. How to develop a second victim support program: a toolkit for health care organizations. Jt Comm J Qual Patient Saf. 2012;38(5):235−240,193.
- forYOU Team. Caring for our own. University of Missouri Health System website. http://www.muhealth.org/about/quality-of-care/office-of-clinical-effectiveness/foryou-team/. Accessed December 18, 2016.
- Second victim trajectory. Columbia, MO: University of Missouri Health System; 2009. http://www.muhealth.org/app/files/public/1390/6StagesRecovery.pdf. Accessed December 19, 2016.
Postexposure management of infectious diseases
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
People who have been exposed to an infectious disease should be evaluated promptly and systematically, whether they are healthcare professionals at work,1 patients, or contacts of patients. The primary goals are to prevent acquisition and transmission of the infection, allay the exposed person’s anxiety, and avoid unnecessary interventions and loss of work days.1,2 Some may need postexposure prophylaxis.
ESSENTIAL ELEMENTS OF POSTEXPOSURE MANAGEMENT
Because postexposure management can be challenging, an experienced clinician or expert consultant (eg, infectious disease specialist, infection control provider, or public health officer) should be involved. Institution-specific policies and procedures for postexposure prophylaxis and testing should be followed.1,2
Postexposure management should include the following elements:
- Immediate care of the wound or other site of exposure in cases of blood-borne exposures and tetanus- and rabies-prone injuries. This includes thoroughly washing with soap and water or cleansing with an antiseptic agent, flushing affected mucous membranes with water, and debridement of devitalized tissue.1–6
- Deciding whether postexposure prophylaxis is indicated and, if so, the type, dose, route, and duration.
- Initiating prophylaxis as soon as possible.
- Determining an appropriate baseline assessment and follow-up plan for the exposed individual.
- Counseling exposed women who are pregnant or breast-feeding about the risks and benefits of postexposure prophylaxis to mother, fetus, and infant.
- Identifying required infection control precautions, including work and school restriction, for exposed and source individuals.
- Counseling and psychological support for exposed individuals, who need to know about the risks of acquiring the infection and transmitting it to others, infection control precautions, benefits, and adverse effects of postexposure prophylaxis, the importance of adhering to the regimen, and the follow-up plan. They must understand that this treatment may not completely prevent the infection, and they should seek medical attention if they develop fever or any symptoms or signs of the infection of concern.1,2
IS POSTEXPOSURE PROPHYLAXIS INDICATED?
Postexposure management begins with an assessment to determine whether the exposure is likely to result in infection; whether the exposed individual is susceptible to the infection of concern or is at greater risk of complications from it than the general population; and whether postexposure prophylaxis is needed. This involves a complete focused history, physical examination, and laboratory testing of the potentially exposed individual and of the source, if possible.1,2
Postexposure prophylaxis should begin as soon as possible to maximize its effects while awaiting the results of further diagnostic tests. However, if the exposed individual seeks care after the recommended period, prophylactic therapy can still be effective for certain infections that have a long incubation period, such as tetanus and rabies.5,6 The choice of regimen should be guided by efficacy, safety, cost, toxicity, ease of adherence, drug interactions, and antimicrobial resistance.1,2
HOW GREAT IS THE RISK OF INFECTION?
Exposed individuals are not all at the same risk of acquiring a given infection. The risk depends on:
- Type and extent of exposure (see below)
- Characteristics of the infectious agent (eg, virulence, infectious dose)
- Status of the infectious source (eg, whether the disease is in its infectious period or is being treated); effective treatment can shorten the duration of microbial shedding and subsequently reduce risk of transmission of certain infections such as tuberculosis, meningococcal infection, invasive group A streptococcal infection, and pertussis7–10
- Immune status of the exposed individual (eg, prior infection or vaccination), since people who are immune to the infection of concern usually do not need postexposure prophylaxis2
- Adherence to infection prevention and control principles; postexposure prophylaxis may not be required if the potentially exposed individual was wearing appropriate personal protective equipment such as a surgical mask, gown, and gloves and was following standard precautions.1
WHO SHOULD BE RESTRICTED FROM WORK OR SCHOOL?
Most people without symptoms who were exposed to most types of infections do not need to stay home from work or school. However, susceptible people, particularly healthcare providers exposed to measles, mumps, rubella, and varicella, should be excluded from work while they are capable of transmitting these diseases, even if they have no symptoms.11,12 Moreover, people with symptoms with infections primarily transmitted via the airborne, droplet, or contact route should be restricted from work until no longer infectious.1,2,7,9–15
Most healthcare institutions have clear protocols for managing occupational exposures to infectious diseases, in particular for blood-borne pathogens such as human immunodeficiency virus (HIV). The protocol should include appropriate evaluation and laboratory testing of the source patient and exposed healthcare provider, as well as procedures for counseling the exposed provider, identifying and procuring an initial prophylactic regimen for timely administration, a mechanism for formal expert consultation (eg, with an in-house infectious diseases consultant), and a plan for outpatient follow-up.
The next section reviews postexposure management of common infections categorized by mode of transmission, including the risk of transmission, initial and follow-up evaluation, and considerations for postexposure prophylaxis.
BLOOD-BORNE INFECTIONS
Blood-borne pathogens can be transmitted by accidental needlesticks or cuts or by exposure of the eyes, mucous membranes, or nonintact skin to blood, tissue, or other potentially infectious body fluids—cerebrospinal, pericardial, pleural, peritoneal, synovial, and amniotic fluid, semen, and vaginal secretions. (Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are considered noninfectious for blood-borne pathogens unless they contain blood.16)
Healthcare professionals are commonly exposed to blood-borne pathogens as a result of needlestick injuries, and these exposures tend to be underreported.17
When someone has been exposed to blood or other infectious body fluids, the source individual and the exposed individual should be assessed for risk factors for hepatitis B virus, hepatitis C virus, HIV, and other blood-borne pathogens.3,4,16,18 If the disease status for these viruses is unknown, the source and exposed individual should be tested in accordance with institutional policies regarding consent to testing. Testing of needles or sharp instruments implicated in an exposure is not recommended.3,4,16,18
Determining the need for prophylaxis after exposure to an unknown source such as a disposed needle can be challenging. Assessment should be made on a case-by-case basis, depending on the known prevalence of the infection of concern in the local community. The risk of transmission in most source-unknown exposures is negligible.3,4,18 However, hepatitis B vaccine and hepatitis B immunoglobulin should be used liberally as postexposure prophylaxis for previously unvaccinated healthcare providers exposed to an unknown source.3,4,16,18
Hepatitis B
Hepatitis B virus (Table 1) is the most infectious of the common blood-borne viruses. The risk of transmission after percutaneous exposure to hepatitis B-infected blood ranges from 1% to 30% based on hepatitis Be antigen status and viral load (based on hepatitis B viral DNA).1,2,4,16
Hepatitis B vaccine or immunoglobulin, or both, are recommended for postexposure prophylaxis in pregnant women, based on evidence that perinatal transmission was reduced by 70% to 90% when these were given within 12 to 24 hours of exposure.4,16,19
Hepatitis C
The risk of infection after percutaneous exposure to hepatitis C virus-infected blood is estimated to be 1.8% per exposure.16 The risk is lower with exposure of a mucous membrane or nonintact skin to blood, fluids, or tissues from hepatitis C-infected patients.16,18
Since there is no effective postexposure prophylactic regimen, the goal of postexposure assessment of hepatitis C is early identification of infection (by monitoring the patient to see if he or she seroconverts) and, if infection is present, referral to an experienced clinician for further evaluation (Table 1). However, data supporting the utility of direct-acting anti-hepatitis C antiviral drugs as postexposure prophylaxis after occupational exposure to hepatitis C are lacking.
Human immunodeficiency virus
The estimated risk of HIV transmission from a known infected source after percutaneous exposure is 0.3%, and after mucosal exposures it is 0.09%.20
If postexposure prophylaxis is indicated, it should be a three-drug regimen (Table 1).3,18 The recommended antiretroviral therapies have been proven effective in clinical trials of HIV treatment, not for postexposure prophylaxis per se, but they are recommended because they are effective, safe, tolerable, and associated with high adherence rates.3,16,18,21 If the source individual is known to have HIV infection, information about his or her stage of infection, CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic viral resistance testing will guide the choice of postexposure prophylactic regimen.3,18
The clinician should give the exposed patient a starter pack of 5 to 7 days of medication, give the first dose then and there, and arrange follow-up with an experienced clinician within a few days of the exposure to determine whether a complete 30-day course is needed.3,16,18
SEXUALLY TRANSMITTED INFECTIONS
In the case of sexually transmitted infections, “exposure” means unprotected sexual contact with someone who has a sexually transmitted infection.22 People with sexually transmitted infections often have no symptoms but can still transmit the infection. Thus, people at risk should be identified and screened for all suspected sexually transmitted infections.23–25
Patients with sexually transmitted infections should be instructed to refer their sex partners for evaluation and treatment to prevent further transmission and reinfection. Assessment of exposed partners includes a medical history, physical examination, microbiologic testing for all potential sexually transmitted infections, and eligibility for hepatitis A virus, hepatitis B virus, and human papillomavirus vaccines.22 Ideally, exposed partners should be reassessed within 1 to 2 weeks to follow up testing results and to monitor for side effects of and adherence to postexposure prophylaxis, if applicable.
Public health departments should be notified of sexually transmitted infections such as gonorrhea, chlamydia, chancroid, and syphilis.22
Expedited partner therapy, in which index patients deliver the medication or a prescription for it directly to their partners, is an alternative for partner management where legally allowed by state and local health departments (see www.cdc.gov/std/ept/legal/).22
Recommended postexposure prophylactic regimens for sexually transmitted infections (Table 2) are based on their efficacy in the treatment of these infections.22,26–28 The regimen for HIV prophylaxis is the same as in Table 1.3,18,26
Chlamydia
Chlamydia is the most commonly reported communicable disease in the United States. The risk of transmission after sexual intercourse with a person who has an active infection is approximately 65% and increases with the number of exposures.22,29
Gonorrhea
Infection with Neisseria gonorrhoeae is the second most commonly reported communicable disease in the United States. The transmission rate of gonorrhea after sex with someone who has it ranges from 50% to 93%.22 When prescribing postexposure prophylaxis for gonorrhea, it is essential to consider the risk of antimicrobial resistance and local susceptibility data.22
Human immunodeficiency virus
Risk of HIV transmission through sexual contact varies depending on the nature of the exposure, ranging from 0.05% to 0.5%.30
Syphilis
The risk of transmission of syphilis in its early stages (primary and secondary) after sexual exposure is approximately 30%. Transmission requires open lesions such as chancres in primary syphilis and mucocutaneous lesions (mucous patches, condyloma lata) in secondary syphilis.22
After sexual assault
In cases of sexual assault, the risk of sexually transmitted infections may be increased due to trauma and bleeding. Testing for all sexually transmitted infections, including HIV, should be considered on a case-by-case basis.22
Survivors of sexual assault have been shown to be poorly compliant with follow-up visits, and thus provision of postexposure prophylaxis at the time of initial assessment is preferable to deferred treatment.22 The recommended regimen should cover chlamydia, gonorrhea, and trichomoniasis (a single dose of intramuscular ceftriaxone 250 mg, oral azithromycin 1 g, and either oral metronidazole 2 g or tinidazole 2 g), in addition to HIV if the victim presents within 72 hours of exposure (Table 2).22,26
Hepatitis B virus vaccine, not immunoglobulin, should be given if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. Both hepatitis B vaccine and immunoglobulin should be given to unvaccinated survivors if the assailant is known to be hepatitis B surface antigen-positive.22
Human papillomavirus vaccination is recommended for female survivors ages 9 to 26 and male survivors ages 9 to 21.
Emergency contraception should be given if there is a risk of pregnancy.22,26
In many jurisdictions, sexual assault centers provide trained examiners through Sexual Assault Nurse Examiners to perform evidence collection and to provide initial contact with the aftercare resources of the center.
Advice on medical management of sexual assault can be obtained by calling National PEPline (888–448–4911).
INFECTIONS TRANSMITTED BY THE AIRBORNE ROUTE
Airborne transmission of infections occurs by inhalation of droplet nuclei (diameter ≤ 5 μm) generated by coughing and sneezing. Certain procedures (eg, administration of nebulized medication, sputum induction, bronchoscopy) also generate droplets and aerosols, which can transmit organisms.1
Measles
Measles (Table 3) is highly contagious; up to 90% of susceptible individuals develop measles after exposure. The virus is transmitted by direct contact with infectious droplets and by the airborne route. It remains infectious in the air and on surfaces for up to 2 hours; therefore, any type of exposure, even transient, is an indication for postexposure prophylaxis in susceptible individuals.11
Both the measles, mumps, rubella (MMR) vaccine and immune globulin may prevent or modify disease severity in susceptible exposed individuals if given within 3 days of exposure (for the vaccine) or within 6 days of exposure (for immune globulin).31,32
Tuberculosis
Mycobacterium tuberculosis is transmitted from patients with pulmonary or laryngeal tuberculosis, particularly if patients cough and are sputum-positive for acid-fast bacilli. Patients with extrapulmonary tuberculosis or latent tuberculosis infection are not infectious.1,7
Postexposure management of tuberculosis occurs through contact investigation of a newly diagnosed index case of tuberculosis disease. Contacts are categorized as household contacts, close nonhousehold contacts (those having regular, extensive contact with the index case), casual contacts, and transient community contacts. The highest priority for contact investigations should be household contacts, close nonhousehold or casual contacts at high risk of progressing to tuberculosis disease (eg, those with HIV, those on dialysis, or transplant recipients), and unprotected healthcare providers exposed during aerosol-generating procedures.7,33
Postexposure management includes screening exposed individuals for tuberculosis symptoms and performing tuberculin skin testing or interferon-gamma release assay (blood testing) for those who had previously negative results (Table 3). Chest radiography is recommended for exposed immunocompromised individuals, due to high risk of tuberculosis disease and low sensitivity of skin or blood testing, and for those with a documented history of tuberculosis or previous positive skin or blood test.7,33,34
A positive tuberculin skin test for persons with recent contact with tuberculosis is defined as a wheal 5 mm or larger on baseline or follow-up screening. Prior bacillus Calmette-Guérin vaccination status should not be used in the interpretation of tuberculin skin testing in the setting of contact investigation.7,33
All exposed asymptomatic people with a positive result on testing should be treated for latent tuberculosis infection, since treatment reduces the risk of progression to tuberculosis disease by 60% to 90% .7,33,35–37
Varicella and disseminated herpes zoster
Varicella zoster virus is transmitted by direct contact with vesicular fluid of skin lesions and inhalation of aerosols from vesicular fluid or respiratory tract secretions. Varicella (chickenpox) is highly contagious, with a secondary attack rate in susceptible household contacts of 85%.12 Herpes zoster is less contagious than varicella.38
Postexposure prophylaxis against varicella is recommended for susceptible individuals who had household exposure, had face-to-face contact with an infectious patient while indoors, or shared the same hospital room with the patient.12
Postexposure prophylactic options for varicella and herpes zoster include varicella vaccine (not zoster vaccine) and varicella zoster immune globulin (Table 3).12,38–40
Varicella vaccine is approximately 90% effective if given within 3 days of exposure, and 70% effective if given within 5 days.12,39
Antiviral agents should be given if the exposed individual develops manifestations of varicella or herpes zoster.12,38
INFECTIONS TRANSMITTED BY THE DROPLET ROUTE
Droplet transmission occurs when respiratory droplets carrying infectious agents travel directly across short distances (3–6 feet) from the respiratory tract of the infected to mucosal surfaces of the susceptible exposed individual. Droplets are generated during coughing, sneezing, talking, and aerosol-generating procedures. Indirect contact with droplets can also transmit infection.1
Group A streptococcal infection
Although group A streptococcal infection (Table 4) may spread to close contacts of the index case and in closed populations (eg, military recruit camps, schools, institutions), secondary cases of invasive group A streptococcal infection rarely occur in family and institutional contacts.9,41,42
Postexposure prophylaxis for contacts of people with invasive group A streptococcal infection is debated, because it is unknown if antibiotic therapy will decrease the risk of acquiring the infection. It is generally agreed that it should not be routinely given to all contacts. The decision should be based on the clinician’s assessment of each individual’s risk and guidance from the local institution. If indicated, postexposure prophylaxis should be given to household and close contacts, particularly in high-risk groups (eg, Native Americans and those with risk factors such as old age, HIV infection, diabetes mellitus, heart disease, chickenpox, cancer, systemic corticosteroid therapy, other immunosuppressive medications, intravenous drug use, recent surgery or childbirth).9,41,42
Influenza
Influenza (Table 4) causes a significant burden in healthcare settings, given its prevalence and potential to cause outbreaks of severe respiratory illness in hospitalized patients and residents of long-term-care facilities.13,43
Neuraminidase inhibitors are effective as prophylaxis after unprotected exposure to influenza, particularly in outbreak situations. However, their use is not widely recommended, since overuse could lead to antiviral resistance. In selected cases, postexposure prophylaxis may be indicated for close contacts who are at high risk of complications of influenza (eg, age 65 or older, in third trimester of pregnancy or 2 weeks postpartum, morbid obesity, chronic comorbid conditions such as a cardiopulmonary and renal disorder, immunocompromising condition) or who are in close contact with persons at high risk of influenza-related complications.13,44,45
Meningococcal disease
N meningitidis is transmitted from individuals with meningococcal disease or from asymptomatic carriers.8
Postexposure prophylaxis is effective in eradicating N meningiditis and is recommended for all close contacts of patients with invasive meningococcal disease (Table 4).46 Close contacts include household contacts, childcare and preschool contacts, contacts exposed in dormitories or military training centers, those who had direct contact with the index case’s respiratory secretions (eg, intimate kissing, mouth-to-mouth resuscitation, unprotected contact during endotracheal intubation or endotracheal tube management), and passengers seated directly next to an index case on airplane flights of longer than 8 hours.
Postexposure prophylaxis is not indicated for those who had brief contact, those who had contact that did not involve exposure to oral or respiratory secretions, or for close contacts of patients with N meningitidis isolated in nonsterile sites only (eg, oropharynyx, trachea, conjunctiva).8,46
Pertussis
Pertussis is highly contagious, with a secondary attack rate of approximately 80% in susceptible individuals. Approximately one-third of susceptible household contacts develop pertussis after exposure.10
Postexposure prophylaxis for pertussis should be given to all household and close contacts (Table 4).10,47
Rubella
Transmission occurs through droplets or direct contact with nasopharyngeal secretions of an infectious case. Neither MMR vaccine nor immunoglobulin has been shown to prevent rubella in exposed contacts, and they are not recommended.11
INFECTIONS TRANSMITTED BY DIRECT CONTACT
Direct contact transmission includes infectious agents transmitted from an infected or colonized individual to another, whereas indirect contact transmission involves a contaminated intermediate object or person (eg, hands of healthcare providers, electronic thermometers, surgical instruments).1
There are no available postexposure prophylactic regimens for the organisms most commonly transmitted by this route (eg, methicillin-resistant Staphylococcus aureus, Clostridium difficile), but transmission can be prevented with adherence to standard precautions, including hand hygiene.1
Hepatitis A
Person-to-person transmission of hepatitis A virus occurs via the fecal-oral route. Common-source outbreaks and sporadic cases can occur from exposure to food or water contaminated with feces.1,15
Postexposure prophylaxis is indicated only for nonimmune close contacts (eg, household and sexual contacts) (Table 5). Without this treatment, secondary attack rates of 15% to 30% have been reported among households.15,48 Both hepatitis A vaccine and immune globulin are effective in preventing and ameliorating symptomatic hepatitis A infection. Advantages of vaccination include induction of longer-lasting immunity (at least 2 years), greater ease of administration, and lower cost than immune globulin.15,48
Scabies
Scabies is an infestation of the skin by the mite Sarcoptes scabiei var hominis. Person-to-person transmission typically occurs through direct, prolonged skin-to-skin contact with an infested person (eg, household and sexual contacts). However, crusted scabies can be transmitted after brief skin-to-skin contact or by exposure to bedding, clothing, or furniture used by the infested person.
All potentially infested persons should be treated concomitantly (Table 5).14,49
INFECTIONS TRANSMITTED BY MAMMAL BITES AND INJURIES
Bites and injury wounds account for approximately 1% of all visits to emergency departments.50 Human bites are associated with a risk of infection by blood-borne pathogens, herpes simplex infection, and bacterial infections (eg, skin and soft-tissue infections, bacteremia). Animal bites are associated with a risk of bacterial infections, rabies, tetanus, hepatitis B virus, and monkeypox.50
Rabies
Human rabies (Table 5) is almost always fatal. Essential factors in determining the need for postexposure prophylaxis include knowledge of the epidemiology of animal rabies in the area where the contact occurred and the species of animal involved, availability of the animal for observation or rabies testing, health status of the biting animal, and vaccination history of both the animal and exposed individual.6 Clinicians should seek assistance from public health officials for evaluating exposures and determining the need for postexposure prophylaxis in situations that are not routine.51
High-risk wild animals associated with rabies in North America include bats, raccoons, skunks, foxes, coyotes, bobcats, and woodchucks. Bats are the most common source of human rabies infections in the United States, and transmission can occur from minor, sometimes unnoticed, bites. The types of exposures that require postexposure prophylaxis include bites, abrasions, scratches, and contamination of mucous membranes or open wound with saliva or neural tissue of a suspected rabid animal.
Human-to-human transmission of rabies can rarely occur through exposure of mucous membrane or nonintact skin to an infectious material (saliva, tears, neural tissue), in addition to organ transplantation.6
Animal capture and testing is a strategy for excluding rabies risk and reducing the need for postexposure prophylaxis. A dog, cat, or ferret that bites a person should be confined and observed for 10 days without administering postexposure prophylaxis for rabies, unless the bite or exposure is on the face or neck, in which case this treatment should be given immediately.6 If the observed biting animal lives and remains healthy, postexposure prophylaxis is not recommended. However, if signs suggestive of rabies develop, postexposure prophylaxis should be given and the animal should be euthanized, with testing of brain tissue for rabies virus. Postexposure prophylaxis should be discontinued if rabies testing is negative.
The combination of rabies vaccine and human rabies immunoglobulin is nearly 100% effective in preventing rabies if administered in a timely and accurate fashion after exposure (Table 5).6
Tetanus
Tetanus transmission can occur through injuries ranging from small cuts to severe trauma and through contact with contaminated objects (eg, bites, nails, needles, splinters, neonates whose umbilical cord is cut with contaminated surgical instruments, and during circumcision or piercing with contaminated instruments).5
Tetanus is almost completely preventable with vaccination, and timely administration of postexposure prophylaxis (tetanus toxoid-containing vaccine, tetanus immune globulin) decreases disease severity (Table 5).2,5,52
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(suppl 2): S65–S164.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–45.
- Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34: 875–892.
- Schille S, Murphy TV, Sawyer M, et al; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1–19.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:13–15.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57:1–28.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R, Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb Mortal Wkly Rep 2005; 54:1–141.
- Cohn AC, MacNeil JR, Clark TA, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–28.
- Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–959.
- Tiwari T, Murphy TV, Moran J; National Immunization Program, Centers for Disease Control and Prevention (CDC). Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep 2005; 54:1–16.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62:1–34.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1–40.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Centers for Disease Control and Prevention (CDC). Scabies. www.cdc.gov/parasites/scabies/. Accessed November 4, 2016.
- Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2006; 55:1–23.
- US Public Health Service. Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001; 50:1–52.
- Treakle AM, Schultz M, Giannakos GP, Joyce PC, Gordin FM. Evaluating a decade of exposures to blood and body fluids in an inner-city teaching hospital. Infect Control Hosp Epidemiol 2011; 32:903–907.
- New York State Department of Health AIDS Institute. Update: HIV prophylaxis following non-occupational exposure. www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure/. Accessed November 4, 2016.
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983; 2:1099–1102.
- Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 2006; 20:805–812.
- McAllister J, Read P, McNulty A, Tong WW, Ingersoll A, Carr A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: safety, tolerability and adherence. HIV Med 2014; 15:13–22.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- US Preventive Services Task Force (USPSTF). Final recommendation statement: chlamydia and gonorrhea: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Human immunodeficiency virus (HIV) infection: screening. www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed November 4, 2016.
- US Preventive Services Task Force (USPSTF). Screening for syphilis. www.uspreventiveservicestaskforce.org/uspstf/uspssyph.htm#update. Accessed November 4, 2016.
- Smith DK, Grohskopf LA, Black RJ, et al; US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep 2005; 54:1–20.
- Lin JS, Donegan SP, Heeren TC, et al. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 1998; 178:1707–1712.
- Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis 2002; 29:38–43.
- Gülmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011; (5):CD000220.
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218.
- Rice P, Young Y, Cohen B, Ramsay M. MMR immunization after contact with measles virus. Lancet 2004; 363:569–570.
- Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunization for preventing measles. Cochrane Database Syst Rev 2014; 4:CD010056.
- National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54:1–47.
- Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1–25.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Morb Mortal Wkly Rep 2000; 49:1–51.
- Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med 2014; 161:419–428.
- Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–1653.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2008; 57:1–30.
- Macartney K, Heywood A, McIntyre P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev 2014; 6:CD001833.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62: 574–576.
- Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep 2006; 32(suppl 2):1–26.
- Steer JA, Lamagni T, Healy B, et al. Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect 2012; 64:1–18.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697.
- Fiore AE, Fry A, Shay D, et al; Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–24.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965.
- Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev 2013; 10:CD004785.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007: CD004404.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080–1084.
- FitzGerald D, Grainger RJ, Reid A. Interventions for preventing the spread of infestation in close contacts of people with scabies. Cochrane Database Syst Rev 2014; 2:CD009943.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–e52.
- Rupprecht CE, Briggs D, Brown CM, et al; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies—recommendations of the Advisory Committee on Immunization Practice. MMWR Recomm Rep 2010; 59:1–9.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012; 61:468–470.
KEY POINTS
- Whether to give prophylactic therapy depends on the transmissibility of the infection, the susceptibility of the exposed individual, and the risk of infection-related complications.
- Postexposure prophylactic therapy should begin as soon as possible, while awaiting results of further diagnostic tests, to maximize the chances of preventing or ameliorating the infection.
- Keeping up-to-date with current institutional policies and national guidelines is essential. Sources include US Public Health Service guidelines and reports from the US Centers for Disease Control and Prevention, as well as consultation with an expert healthcare provider (eg, infectious diseases physician, infection control provider, public health officer).
Parsimonious blood use and lower transfusion triggers: What is the evidence?
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
For decades, physicians believed in the benefit of prompt transfusion of blood to keep the hemoglobin level at arbitrary, optimum levels, ie, close to normal values, especially in the critically ill, the elderly, and those with coronary syndromes, stroke, or renal failure.
However, the evidence supporting arbitrary hemoglobin values as an indication for transfusion was weak or nonexistent. Also, blood transfusion can have complications and adverse effects, and blood is costly and scarce. These considerations prompted research into when blood transfusion should be considered, and recommendations that it should be used more sparingly than in the past.
This review offers a perspective on the evidence supporting restrictive blood use. First, we focus on hemodilution studies that demonstrated that humans can tolerate anemia. Then, we look at studies that compared a restrictive transfusion strategy with a liberal one in patients with critical illness and active bleeding. We conclude with current recommendations for blood transfusion.
EVIDENCE FROM HEMODILUTION STUDIES
Hemoglobin is essential for tissue oxygenation, but the serum hemoglobin concentration is just one of several factors involved.1–5 In anemia, the body can adapt not only by increasing production of red blood cells, but also by:
- Increasing cardiac output
- Increasing synthesis of 2,3-diphosphoglycerate (2,3-DPG), with a consequent shift in the oxyhemoglobin dissociation curve to the right, allowing enhanced release of oxygen at the tissue level
- Moving more carbon dioxide into the blood (the Bohr effect), which decreases pH and also shifts the dissociation curve to the right.
Just 20 years ago, physicians were using arbitrary cutoffs such as hemoglobin 10 g/dL or hematocrit 30% as indications for blood transfusion, without reasonable evidence to support these values. Not until acute normovolemic hemodilution studies were performed were we able to progressively appraise how well patients could tolerate lower levels of hemoglobin without significant adverse outcomes.
Acute normovolemic hemodilution involves withdrawing blood and replacing it with crystalloid or colloid solution to maintain the volume.6
Initial studies were done in animals and focused on the safety of acute anemia regarding splanchnic perfusion. Subsequently, studies proved that healthy, elderly, and stable cardiac patients can tolerate acute anemia with normal cardiovascular response. The targets in these studies were modest at first, but researchers aimed progressively for more aggressive hemodilution with lower hemoglobin targets and demonstrated that the body can tolerate and adapt to more severe anemia.6–8
Studies in healthy patients
Weiskopf et al9 assessed the effect of severe anemia in 32 conscious healthy patients (11 presurgical patients and 21 volunteers not undergoing surgery) by performing acute normovolemic hemodilution with 5% human albumin, autologous plasma, or both, with a target hemoglobin level of 5 g/dL. The process was done gradually, obtaining aliquots of blood of 500 to 900 mL. Cardiac index increased, along with a mild increase in oxygen consumption with no increase in plasma lactate levels, suggesting that in conscious healthy patients, tissue oxygenation remains adequate even in severe anemia.
Leung et al10 addressed the electrocardiographic changes that occur with severe anemia (hemoglobin 5 g/dL) in 55 healthy volunteers. Three developed transient, reversible ST-segment depression, which was associated with a higher heart rate than in the volunteers with no electrocardiographic changes; however, the changes were reversible and asymptomatic, and thus were considered physiologic and benign.
Hemodilution in healthy elderly patients
Spahn et al11 performed 6 and 12 mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch in 20 patients older than 65 years (mean age 76, range 65–88) without underlying coronary disease.
The patients’ mean hemoglobin level decreased from 11.6 g/dL to 8.8 g/dL. Their cardiac index and oxygen extraction values increased adequately, with stable oxygen consumption during hemodilution. There were no electrocardiographic signs of ischemia.
Hemodilution in coronary artery disease
Spahn et al12 performed hemodilution studies in 60 patients (ages 35–81) with coronary artery disease managed chronically with beta-blockers who were scheduled for coronary artery bypass graft surgery. Hemodilution was performed with 6- and 12-mL/kg isovolemic exchange of blood for 6% hydroxyethyl starch maintaining normovolemia and stable filling pressures. Hemoglobin levels decreased from 12.6 g/dL to 9.9 g/dL. The hemodilution process was done before the revascularization. The authors monitored hemodynamic variables, ST-segment deviation, and oxygen consumption before and after each hemodilution.
There was a compensatory increase in cardiac index and oxygen extraction with consequent stable oxygen consumption. These changes were independent of patient age or left ventricular function. In addition, there were no electrocardiographic signs of ischemia.
Licker et al13 studied the hemodynamic effect of preoperative hemodilution in 50 patients with coronary artery disease undergoing coronary artery bypass graft surgery, performing transesophageal echocardiography before and after hemodilution. The patients underwent isovolemic exchange with iso-oncotic starch to target a hematocrit of 28%.
Acute normovolemic hemodilution triggered an increase in cardiac stroke volume, which had a direct correlation with an increase in the central venous pressure and the left ventricular end-diastolic area. No signs of ischemia were seen in these patients on electrocardiography or echocardiography (eg, left ventricular wall-motion abnormalities).
Hemodilution in mitral regurgitation
Spahn et al14 performed acute isovolemic hemodilution with 6% hydroxyethyl starch in 20 patients with mitral regurgitation. The cardiac filling pressures were stable before and after hemodilution; the mean hemoglobin value decreased from 13 to 10.3 g/dL. The cardiac index and oxygen extraction increased proportionally, with stable oxygen consumption; these findings were the same regardless of whether the patient was in normal sinus rhythm or atrial fibrillation.
Effect of hemodilution on cognition
Weiskopf et al15 assessed the effect of anemia on executive and memory function by inducing progressive acute isovolemic anemia in 90 healthy volunteers (age 29 ± 5), reducing their hemoglobin values to 7, 6, and 5 g/dL and performing repetitive neuropsychological and memory testing before and after the hemodilution, as well as after autologous blood transfusion to return their hemoglobin level to 7 g/dL.
There were no changes in reaction time or error rate at a hemoglobin concentration of 7 g/dL compared with the performance at a baseline hemoglobin concentration of 14 g/dL. The volunteers got slower on a mathematics test at hemoglobin levels of 6 g/dL and 5 g/dL, but their error rate did not increase. Immediate and delayed memory were significantly impaired at hemoglobin of 5 g/dL but not at 6 g/dL. All tests normalized with blood transfusion once the hemoglobin level reached 7 g/dL.15
Weiskopf et al16 subsequently investigated whether giving supplemental oxygen to raise the arterial partial pressure of oxygen (Pao2) to 350 mm Hg or greater would overcome the neurocognitive effects of severe acute anemia. They followed a protocol similar to the one in the earlier study15 and induced anemia in 31 healthy volunteers, age 28 ± 4 years, with a mean baseline hemoglobin concentration of 12.7 g/dL.
When the volunteers reached a hemoglobin concentration of 5.7 ± 0.3 g/dL, they were significantly slower on the mathematics test, and their delayed memory was significantly impaired. Then, in a double-blind fashion, they were given either room air or oxygen. Oxygen increased the Pao2 to 406 mm Hg and normalized neurocognitive performance.
Hemodilution studies in surgical patients
A 2015 meta-analysis17 of 63 studies involving 3,819 surgical patients compared the risk of perioperative allogeneic blood transfusion as well as the overall volume of transfused blood in patients undergoing preoperative acute normovolemic hemodilution vs a control group. Though the overall data showed that the patients who underwent acute normovolemic hemodilution needed fewer transfusions and less blood (relative risk [RR] 0.74, 95% confidence interval [CI] 0.63–0.88, P = .0006), the authors noted significant heterogeneity and publication bias.
However, the hemodilution studies paved the way for justifying a more conservative and restrictive transfusion strategy, with a hemoglobin cutoff value of 7 g/dL, and in acute anemia, using oxygen to overcome acute neurocognitive effects while searching for and correcting the cause of the anemia.
STUDIES OF RESTRICTIVE VS LIBERAL TRANSFUSION STRATEGIES
Studies in critical care and high-risk patients
Hébert et al18 randomized 418 critical care patients to a restrictive transfusion approach (in which they were given red blood cells if their hemoglobin concentration dropped below 7.0 g/dL) and 420 patients to a liberal strategy (given red blood cells if their hemoglobin concentration dropped below 10.0 g/dL). Mortality rates (restrictive vs liberal strategy) were as follows:
- Overall at 30 days 18.7% vs 23.3%, P = .11
- In the subgroup with less-severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20), 8.7% vs 16.1%, P = .03
- In the subgroup under age 55, 5.7% vs 13%, P = .02
- In the subgroup with clinically significant cardiac disease, 20.5% vs 22.9%, P = .69
- In the hospital, 22.2% vs 28.1%; P = .05.
This study demonstrated that parsimonious blood use did not worsen clinical outcomes in critical care patients.
Carson et al19 evaluated 2,016 patients age 50 and older who had a history of or risk factors for cardiovascular disease and a baseline hemoglobin level below 10 g/dL who underwent surgery for hip fracture. Patients were randomized to two transfusion strategies based on threshold hemoglobin level: restrictive (< 8 g/dL) or liberal (< 10 g/dL). The primary outcome was death or inability to walk without assistance at 60-day follow-up. The median number of units of blood used was 2 in the liberal group and 0 in the restrictive group.
There was no significant difference in the rates of the primary outcome (odds ratio [OR] 1.01, 95% CI 0.84–1.22), infection, venous thromboembolism, or reoperation. This study demonstrated that a liberal transfusion strategy offered no benefit over a restrictive one.
Rao et al20 analyzed the impact of blood transfusion in 24,112 patients with acute coronary syndromes enrolled in three large trials. Ten percent of the patients received at least 1 blood transfusion during their hospitalization, and they were older and had more complex comorbidity.
At 30 days, the group that had received blood had higher rates of death (adjusted hazard ratio [HR] 3.94, 95% CI 3.26–4.75) and the combined outcome of death or myocardial infarction (HR 2.92, 95% CI 2.55–3.35). Transfusion in patients whose nadir hematocrit was higher than 25% was associated with worse outcomes.
This study suggests being cautious about routinely transfusing blood in stable patients with ischemic heart disease solely on the basis of arbitrary hematocrit levels.
Carson et al,21 however, in a later trial, found a trend toward worse outcomes with a restrictive strategy than with a liberal one. Here, 110 patients with acute coronary syndrome or stable angina undergoing cardiac catheterization were randomized to a target hemoglobin level of either at least 8 mg/dL or at least 10 g/dL. The primary outcome (a composite of death, myocardial infarction, or unscheduled revascularization 30 days after randomization) occurred in 14 patients (25.5%) in the restrictive group and 6 patients (10.9%) in the liberal group (P = .054), and 7 (13.0%) vs 1 (1.8%) of the patients died (P = .032).
Murphy et al22 similarly found trends toward worse outcomes with a restrictive strategy in cardiac patients. The investigators randomized 2,007 elective cardiac surgery patients with a postoperative hemoglobin level lower than 9 g/dL to a hemoglobin transfusion threshold of either 7.5 or 9 g/dL. Outcomes (restrictive vs liberal strategies):
- Transfusion rates 53.4% vs 92.2%
- Rates of the primary outcome (a serious infection [sepsis or wound infection] or ischemic event [stroke, myocardial infarction, mesenteric ischemia, or acute kidney injury] within 3 months):
35.1% vs 33.0%, OR 1.11, 95% CI 0.91–1.34, P = .30) - Mortality rates 4.2% vs 2.6%, HR 1.64, 95% CI 1.00–2.67, P = .045
- Total costs did not differ significantly between the groups.
These studies21,22 suggest the need for more definitive trials in patients with active coronary disease and in cardiac surgery patients.
Holst et al23 randomized 998 intensive care patients in septic shock to hemoglobin thresholds for transfusion of 7 vs 9 g/dL. Mortality rates at 90 days (the primary outcome) were 43.0% vs 45.0%, RR 0.94, 95% CI 0.78–1.09, P = .44.
This study suggests that even in septic shock, a liberal transfusion strategy has no advantage over a parsimonious one.
Active bleeding, especially active gastrointestinal bleeding, poses a significant stress that may trigger empirical transfusion even without evidence of the real hemoglobin level.
Villanueva et al24 randomized 921 patients with severe acute upper-gastrointestinal bleeding to two groups, with hemoglobin transfusion triggers of 7 vs 9 g/dL. The findings were impressive:
- Freedom from transfusion 51% vs 14% (P < .001)
- Survival rates at 6 weeks 95% vs 91% (HR 0.55, 95% CI 0.33–0.92, P = .02)
- Rebleeding 10% vs 16% (P = .01).
Patients with peptic ulcer disease as well as those with cirrhosis stage Child-Pugh class A or B had higher survival rates with a restrictive transfusion strategy.
The RELIEVE trial25 compared the effect of a restrictive transfusion strategy in elderly patients on mechanical ventilation in 6 intensive care units in the United Kingdom. Transfusion triggers were hemoglobin 7 vs 9 g/dL, and the mortality rate at 180 days was 55% vs 37%, RR 0.68, 95% CI 0.44–1.05, P = .073.
Meta-analyses and observational studies
Rohde et al26 performed a systematic review and meta-analysis of 17 trials with 7,456 patients, which revealed that a restrictive strategy is associated with a lower risk of nosocomial infection, including pneumonia, wound infection, and sepsis.
The pooled risk of all serious infections was 10.6% in the restrictive group and 12.7% in the liberal group. Even after adjusting for the use of leukocyte reduction, the risk of infection was lower in the restrictive strategy group (RR 0.83, 95% CI 0.69–0.99). With a hemoglobin threshold of less than 7.0 g/dL, the risk of serious infection was 14% lower. Although this was not statistically significant overall (RR 0.86, 95% CI 0.72–1.02), the difference was statistically significant in the subgroup undergoing orthopedic surgery (RR 0.72, 95% CI 0.53–0.97) and the subgroup presenting with sepsis (RR 0.51, 95% CI 0.28–0.95).
Salpeter et al27 performed a meta-analysis and systematic review of three randomized trials (N = 2,364) comparing a restrictive hemoglobin transfusion trigger (hemoglobin < 7 g/dL) vs a more liberal trigger. The groups with restrictive transfusion triggers had lower rates of:
- In-hospital mortality (RR 0.74, 95% CI 0.60–0.92)
- Total mortality (RR 0.80, 95% CI 0.65–0.98)
- Rebleeding (RR 0.64, 95% CI 0.45–0.90)
- Acute coronary syndrome (RR 0.44, 95% CI 0.22–0.89)
- Pulmonary edema (RR 0.48, 95% CI 0.33–0.72)
- Bacterial infections (RR 0.86, 95% CI 0.73–1.00).
Wang et al28 performed a meta-analysis of 4 randomized controlled trials in patients with upper-gastrointestinal bleeding comparing restrictive (hemoglobin < 7 g/dL) vs liberal transfusion strategies. The primary outcomes were death and rebleeding. The restrictive strategy was associated with:
- A lower mortality rate (OR 0.52, 95% CI 0.31–0.87, P = .01)
- A lower rebleeding rate (OR 0.26, 95% CI 0.03–2.10, P = .21)
- Shorter hospitalizations (P = .009)
- Less blood transfused (P = .0005).
Vincent et al,29 in a prospective observational study of 3,534 patients in intensive care units in 146 facilities in Western Europe, found a correlation between transfusion and mortality. Transfusion was done most often in elderly patients and those with a longer stay in the intensive care unit. The 28-day mortality rate was 22.7% in patients who received a transfusion and 17.1% in those who did not (P = .02). The more units of blood the patients received, the more likely they were to die, and receiving more than 4 units was associated with worse outcomes (P = .01).
Dunne et al30 performed a study of 6,301 noncardiac surgical patients in the Veterans Affairs Maryland Healthcare System from the National Veterans Administration Surgical Quality Improvement Program from 1995 to 2000. Multiple logistic regression analysis revealed that the composite of low hematocrit before and after surgery and high transfusion rates (> 4 units per hospitalization) were associated with higher rates of death (P < .01) and postoperative pneumonia (P ≤ .05) and longer hospitalizations (P < .05). The risk of pneumonia increased proportionally with the decrease in hematocrit.
These findings support pharmacologic optimization of anemia with hematinic supplementation before surgery to decrease the risk of needing a transfusion, often with parenteral iron. The fact that the patient’s hemoglobin can be optimized preoperatively by nontransfusional means may decrease the likelihood of blood transfusion, as the hemoglobin will potentially remain above the transfusion threshold. For example, if a patient has a preoperative hemoglobin level of 10 g/dL, and it is optimized up to 12, then if postoperatively the hemoglobin level drops 3 g/dL instead of reaching the threshold of 7 g/dL, the nadir will be just 9 g/dL, far above that transfusion threshold.
Brunskill et al,31 in a Cochrane review of 6 trials with 2,722 patients undergoing surgery for hip fracture, found no difference in rates of mortality, functional recovery or postoperative morbidity with a restrictive transfusion strategy (hemoglobin target > 8 g/dL vs a liberal one (> 10 g/dL). However, the quality of evidence was rated as low. The authors concluded that there is no justification for liberal red blood cell transfusion thresholds (10 g/dL), and a more restrictive transfusion threshold is preferable.
Weinberg et al32 found that, in trauma patients, receiving more than 6 units of blood was associated with poor prognosis, and outcomes were worse when the blood was older than 2 weeks. However, the effect of blood age is not significant when using smaller transfusion volumes (1 to 2 units of red blood cells).
Studies in sickle cell disease
Sickle cell disease patients have high levels of hemoglobin S, which causes erythrocyte sickling and increases blood viscosity. Transfusion with normal erythrocytes increases the amount of hemoglobin A (the normal variant).33,34
In trials in surgical patients,35,36 conservative strategies for preoperative blood transfusion aiming at a hemoglobin level of 10 g/dL were as effective in preventing postoperative complications as decreasing the hemoglobin S levels to 30% by aggressive exchange transfusion.35
In nonsurgical patients, blood transfusion should be based on formal risk-benefit assessments. Therefore, the expert panel report on sickle cell management advises against blood transfusion in sickle cell patients with uncomplicated vaso-occlusive crises, priapism, asymptomatic anemia, or acute kidney injury in the absence of multisystem organ failure.34
Is hemoglobin the most relevant marker?
Most studies that compared restrictive and liberal transfusion strategies focused on using a lower hemoglobin threshold as the transfusion trigger, not on using fewer units of blood. Is the amount of blood transfused more important than the hemoglobin threshold? Perhaps a study focused both on a restrictive vs liberal strategy and also on the minimum amount of blood that each patient may benefit from would help to answer this question.
We should beware of routinely using the hemoglobin concentration as a threshold for transfusion and a surrogate marker of transfusion benefit because changes in hemoglobin concentration may not reflect changes in absolute red cell mass.37 Changes in plasma volume (an increase or decrease) affect the hematocrit concentration without necessarily affecting the total red cell mass. Unfortunately, red cell mass is very difficult to measure; hence, the hemoglobin and hematocrit values are used instead. Studies addressing changes in red cell mass may be needed, perhaps even to validate using the hemoglobin concentration as the sole indicator for transfusion.
Is fresh blood better than old blood?
Using blood that is more than 14 days old may be associated with poor outcomes, for several possible reasons. Red blood cells age rapidly in refrigeration, and usually just 75% may remain viable 24 hours after phlebotomy. Adenosine triphosphate and 2,3-DPG levels steadily decrease, with a consequent decrease in capacity for appropriate tissue oxygen delivery. In addition, loss of membrane phospholipids causes progressive rigidity of the red cell membrane with consequent formation of echynocytes after 14 to 21 days.38,39
The use of blood more than 14 days old in cardiac surgery patients has been associated with worse outcomes, including higher rates of death, prolonged intubation, acute renal failure, and sepsis.40 Similar poor outcomes have been seen in trauma patients.32
Lacroix et al,41 in a multicenter, randomized trial in critically ill adults, compared the outcomes of transfusion of fresh packed red cells (stored < 8 days) or old blood (stored for a mean of 22 days). The primary outcome was the mortality rate at 90 days: 37.0% in the fresh-blood group vs 35.3% in the old-blood group (HR 1.1, 95% CI 0.9–1.2, P = .38).
The authors concluded that using fresh blood compared with old blood was not associated with a lower 90-day mortality rate in critically ill adults.
RISKS ASSOCIATED WITH TRANSFUSION
Infections
The risk of infection from blood transfusion is small. Human immunodeficiency virus (HIV) is transmitted in 1 in 1.5 million transfused blood components, and hepatitis C virus in 1 in 1.1 million; these odds are similar to those of having a fatal airplane accident (1 in 1.7 million per flight). Hepatitis B virus infection is more common, the reported incidence being 1 in 357,000.42
Noninfectious complications
Transfusion-associated circulatory overload occurs in 4% to 6% of patients who receive a transfusion. Therefore, circulatory overload is a greater danger from transfusion than infection is.42
Febrile nonhemolytic transfusion reactions occur in 1.1% of patients with prestorage leukoreduction.
Transfusion-associated acute lung injury occurs in 0.8 per 10,000 blood components transfused.
Errors associated with blood transfusion include, in decreasing order of frequency, transfusion of the wrong blood component, handling and storage errors, inappropriate administration of anti-D immunoglobulin, and avoidable, delayed, or insufficient transfusions.43
Surgery and condition-specific complications of red blood cell transfusion
Cardiovascular surgery. Transfusion is associated with a higher risk of postoperative stroke, respiratory failure, acute respiratory distress syndrome, prolonged intubation time, reintubation, in-hospital death, sepsis, and longer postoperative length of stay.44
Malignancy. The use of blood in this setting has been found to be an independent predictor of recurrence, decreased survival, and increased risk of lymphoplasmacytic and marginal-zone lymphomas.44–47
Vascular, orthopedic, and other surgeries. Transfusion is associated with a higher risk of death, thromboembolic events, acute kidney injury, death, composite morbidity, reoperation, sepsis, and pulmonary complications.44
ST-segment elevation myocardial infarction, sepsis, and intensive care unit admissions. Transfusion is associated with an increased risk of rebleeding, death, and secondary infections.44
COST OF RED BLOOD CELL TRANSFUSION
Up to 85 million units of red blood cells are transfused per year worldwide, 15 million of them in the United States.42 At our hospital in 2013, 1 unit of leukocyte-reduced red blood cells cost $957.27, which included the costs of acquisition, processing, banking, patient testing, administration, and monitoring.
The Premier Healthcare Alliance48 analyzed data from 7.4 million discharges from 464 hospitals between April 2011 and March 2012. Blood use varied significantly among hospitals, and the hospitals in the lowest quartile of blood use had better patient outcomes. If all the hospitals used as little blood as those in the lowest quartile and had outcomes as good, blood product use would be reduced by 802,716 units, with savings of up to $165 million annually.
In addition to the economic cost of blood transfusion, the clinician must be aware of the cost in terms of comorbidities caused by unnecessary blood transfusion.49,50
RECOMMENDATIONS FROM THE AABB
In view of all the current compelling evidence, a restrictive approach to transfusion is the single best strategy to minimize adverse outcomes.51 Below, we outline the current recommendations from the AABB (formerly the American Association of Blood Banks),42 which are similar to the national clinical guideline on blood transfusion in the United Kingdom,52 and have recently been updated, confirming the initial recommendations.53
In critical care patients, transfusion should be considered if the hemoglobin concentration is 7 g/dL or less.
In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered if the hemoglobin concentration is 8 g/dL or less or if the patient has signs or symptoms of anemia such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
In hemodynamically stable patients with acute coronary syndrome, there is not enough evidence to allow a formal recommendation for or against a liberal or restrictive transfusion threshold.
Consider both the hemoglobin concentration and the symptoms when deciding whether to give a transfusion. This recommendation is shared by a National Institutes of Health consensus conference,54 which indicates that multiple factors related to the patient’s clinical status and oxygen delivery should be considered before deciding to transfuse red blood cells.
The Society of Hospital Medicine55 and the American Society of Hematology56 concur with a parsimonious approach to blood use in their Choosing Wisely campaigns. The American Society of Hematology recommends that if transfusion of red blood cells is necessary, the minimum number of units should be given that relieve the symptoms of anemia or achieve a safe hemoglobin range (7–8 g/dL in stable noncardiac inpatients).57
New electronic tools can monitor the ordering and use of blood products in real time and can identify the hemoglobin level used as the trigger for transfusion. They also provide data on blood use by physician, hospital, and department. These tools can reveal current practice at a glance and allow sharing of best practices among peers and institutions.52
CONSIDER TRANSFUSION FOR HEMOGLOBIN BELOW 7 G/DL
The routine use of blood has come under scrutiny, given its association with increased healthcare costs and morbidity. The accepted practice in stable medical patients is a restrictive threshold approach for blood transfusion, which is to consider (not necessarily give) a single unit of packed red blood cells for a hemoglobin less than 7 g/dL.
However, studies in acute coronary syndrome patients and postoperative cardiac surgery patients have not shown the restrictive threshold to be superior to a liberal threshold in terms of outcomes and costs. This variability suggests the need for further studies to determine the best course of action in different patient subpopulations (eg, surgical, oncologic, trauma, critical illness).
Also, a limitation of most of the clinical studies was that only the hemoglobin concentration was used as a marker of anemia, with no strict assessment of changes in red cell mass with transfusion.
Despite the variability in certain populations, the overall weight of current evidence favors a restrictive approach to blood transfusion (hemoglobin < 7 g/dL), although perhaps in patients who have active coronary disease or are undergoing cardiac surgery, a more lenient threshold (< 8 g/dL) for transfusion should be considered.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
- Shander A, Gross I, Hill S, Javidroozi M, Sledge S; College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus 2013; 11:193–202.
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 2007; 21:163–171.
- Tánczos K, Molnár Z. The oxygen supply-demand balance: a monitoring challenge. Best Pract Res Clin Anaesthesiol 2013; 27:201–207.
- Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin 2004; 20:187–212.
- Spinelli E, Bartlett RH. Anemia and transfusion in critical care: physiology and management. J Intensive Care Med 2016; 31:295–306.
- Jamnicki M, Kocian R, Van Der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth 2003; 17:747–754.
- Monk TG. Acute normovolemic hemodilution. Anesthesiol Clin North America 2005; 23:271–281.
- Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion 2004; 44(suppl 2):26S–34S.
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–221.
- Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology 2000; 93:1004–1010.
- Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg 1996; 82:681–686.
- Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. Anesth Analg 1996; 82:687–694.
- Licker M, Ellenberger C, Sierra J, Christenson J, Diaper J, Morel D. Cardiovascular response to acute normovolemic hemodilution in patients with coronary artery diseases: assessment with transesophageal echocardiography. Crit Care Med 2005; 33:591–597.
- Spahn DR, Seifert B, Pasch T, Schmid ER. Haemodilution tolerance in patients with mitral regurgitation. Anaesthesia 1998; 53:20–24.
- Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 2000; 92:1646–1652.
- Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 2002; 96:871–877.
- Zhou X, Zhang C, Wang Y, Yu L, Yan M. Preoperative acute normovolemic hemodilution for minimizing allogeneic blood transfusion: a meta-analysis. Anesth Analg 2015; 121:1443–1455.
- Hébert P, Wells G, Blajchman M, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999: 340:409–417.
- Carson JL, Terrin ML, Noveck H, et al; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453–2462.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165:964.e1–971.e1.
- Murphy GJ, Pike K, Rogers CA, et al; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015; 372:997–1008.
- Holst LB, Haase N, Wetterslev J, et al; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371:1381–1391.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21.
- Walsh TS, Boyd JA, Watson D, et al; RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013; 41:2354–2363.
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care–associated infection after red blood cell transfusion. JAMA 2014; 311:1317–1326.
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3.
- Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol 2013; 19:6919–6927.
- Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288:1499–1507.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002; 102:237–244.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015; 4:CD009699.
- Weinberg JA, McGwin G Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma 2008; 65:279–284.
- Steinberg M. Management of sickle cell disease. N Engl J Med 1999; 340:1021–1030.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease. JAMA 2014; 312:1033–1048.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995; 333:206–213.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013; 381:930–938.
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013; 381:1845–1854.
- Holme S. Current issues related to the quality of stored RBCs. Transfus Apher Sci 2005; 33:55–61.
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999; 39:277–281.
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358:1229–1239.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418.
- Carson JL, Grossman BJ, Kleinman S, et al; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58.
- Bolton-Maggs P, Watt A, Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf. Accessed November 30, 2016.
- Shander A, Javidroozi M, Ozawa S, Hare GMT. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011; 107(suppl 1):i41–i59.
- Reeh M, Ghadban T, Dedow J, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg 2016 Oct 11. [Epub ahead of print]
- Elmi M, Mahar A, Kagedan D, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016; 59:322–329.
- Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection. Ann Surg 2016; Sep 14. [Epub ahead of print] PubMed PMID: 27631770.
- Premiere Analysis. Standardization of blood utilization practices could provide opportunity for improved outcomes, reduced costs. A Premiere Healthcare Alliance Analysis. 2012.
- Simeone F, Franchi F, Cevenini G, et al. A simple clinical model for planning transfusion quantities in heart surgery. BMC Med Inform Decis Mak 2011; 11:44.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354.
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK). www.ncbi.nlm.nih.gov/books/NBK11822/.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016 Oct 12. doi: 10.1001/jama.2016.9185. [Epub ahead of print]
- Consensus conference. Perioperative red blood cell transfusion. JAMA 1988; 260:2700–2703.
- Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med 2013; 8:486–492.
- Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Blood 2013; 122:3879–3883.
- Haemonetics IMPACT Online. The Blood Management Company. www.haemonetics.com/Products/Services/Consulting Services/IMPACT Online.aspx. Accessed November 30, 2016.
KEY POINTS
- In critical care patients, transfusion should be considered when the hemoglobin concentration reaches 7 g/dL or less.
- In postoperative patients and hospitalized patients with preexisting cardiovascular disease, transfusion should be considered at a hemoglobin concentration of 8 g/dL or less or for symptoms such as chest pain, orthostatic hypotension, or tachycardia unresponsive to fluid resuscitation, or heart failure.
- Consider both the hemoglobin concentration and the symptoms when deciding whether to give a patient a transfusion.
Benign prostatic hyperplasia: Evaluation and medical management in primary care
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
KEY POINTS
- Watchful waiting is appropriate for patients with mild to moderate symptoms that cause minimal bother.
- Patients with severe or bothersome symptoms should be offered pharmacotherapy, not only to improve symptoms but also to reduce the risk of disease progression.
- Several effective, minimally invasive surgical options are available for patients whose symptoms do not respond to medical therapy. These patients and those with abnormal findings on diagnostic evaluation warrant referral to a urologist for further evaluation.
Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
KEY POINTS
- For most patients in this category, the benefits of anticoagulation outweigh the risks.
- Although they are not perfect, scoring systems have been developed to predict the risk of stroke without anticoagulation and the risk of bleeding with anticoagulation.
- The decision-making process is complex and should be shared with the patient and the patient’s family and caregivers.
REIGNITE the desire: Tackle burnout in psychiatry
Burnout among psychiatric clinicians can lead to reduced job satisfaction, poorer quality of patient care, and depression.1 Signs of burnout include a feeling of cynicism (eg, negative attitudes toward patients), overwhelming exhaustion (eg, feeling depleted), and a sense of ineffectiveness (eg, reduced productivity).1 Workplace variables and other factors that could perpetuate burnout among psychiatrists include, but are not limited to:
- too much work
- chronic staff shortages
- working with difficult patients
- inability to meet self-imposed demands
- a lack of meaningful relationships with colleagues and supervisors.1,2
The mnemonic REIGNITE provides strategies to reduce the risk of burnout.1,3
Recognize your limits. Although saying “no” may be difficult for mental health clinicians, saying “yes” too often can be detrimental. Techniques for setting limits without alienating colleagues include:
- declining tasks (“I appreciate you thinking of me to do that, but I can’t complete it right now”)
- delaying an answer (“Let me ponder what you are asking”)
- delegating tasks (“I could really use your help”)
- avoid taking on too much (“I thought that I could do that extra task, but I realize that taking on the additional assignment isn’t going to work out”).
Expand your portfolio. Developing a diverse work portfolio (eg, teaching part-time) could diminish stagnation. Adding regenerative activities (eg, outdoor activities) could be restorative.
Itemize your priorities. Ask yourself what is important to you. Is it work? If so, can work be modified so it continues to be rewarding without resulting in burnout? If it isn’t work, then what is? Money? Family? Evaluating what is important and pursuing those priorities could increase overall life satisfaction.
Go after your passions. What do you like to do aside from work? Do you paint or play a musical instrument? Pursuing hobbies and interests can revitalize your spirit.
Now. We as a profession are notorious for saying to ourselves, “I will get to it (being happy) someday.” We delay happiness until we catch up with work, save enough money, and so on. This approach is unrealistic. It is better to live in the present because there are a finite number of days to seize the day. Focus your energy in the moment.
Interact. Isolating oneself will lead to burnout. If you are in solo practice, connect with other providers or get involved in community activities. If you work with other providers, interact with them in a meaningful manner (eg, don’t complain but rather air your concerns, accept honest feedback, be open to suggestions, and seek assistance; it is acceptable to admit that you can’t do everything).
Take time off and take care of yourself. Although that seems intuitive, psychiatrists, as a group, don’t do a good job of it. Waiting until you are burned out to take a vacation is counterproductive because you will be too drained to enjoy it. Taking care of your physical and mental health is equally important.
Enjoyment in and at work. We make a difference in our patients’ lives throughthe emotional connections we develop with them. By viewing what we do as fulfilling a higher calling, we can learn to enjoy what we do rather than feeling burdened by it. Advocating for better recognition—whether financial, institutional, or social—can create opportunities for personal satisfaction.
1. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15(2):103-111.
2. Bressi C, Porcellana M, Gambini O, et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985-988.
3. Bohnert P, O’Connell A. How to avoid burnout
Burnout among psychiatric clinicians can lead to reduced job satisfaction, poorer quality of patient care, and depression.1 Signs of burnout include a feeling of cynicism (eg, negative attitudes toward patients), overwhelming exhaustion (eg, feeling depleted), and a sense of ineffectiveness (eg, reduced productivity).1 Workplace variables and other factors that could perpetuate burnout among psychiatrists include, but are not limited to:
- too much work
- chronic staff shortages
- working with difficult patients
- inability to meet self-imposed demands
- a lack of meaningful relationships with colleagues and supervisors.1,2
The mnemonic REIGNITE provides strategies to reduce the risk of burnout.1,3
Recognize your limits. Although saying “no” may be difficult for mental health clinicians, saying “yes” too often can be detrimental. Techniques for setting limits without alienating colleagues include:
- declining tasks (“I appreciate you thinking of me to do that, but I can’t complete it right now”)
- delaying an answer (“Let me ponder what you are asking”)
- delegating tasks (“I could really use your help”)
- avoid taking on too much (“I thought that I could do that extra task, but I realize that taking on the additional assignment isn’t going to work out”).
Expand your portfolio. Developing a diverse work portfolio (eg, teaching part-time) could diminish stagnation. Adding regenerative activities (eg, outdoor activities) could be restorative.
Itemize your priorities. Ask yourself what is important to you. Is it work? If so, can work be modified so it continues to be rewarding without resulting in burnout? If it isn’t work, then what is? Money? Family? Evaluating what is important and pursuing those priorities could increase overall life satisfaction.
Go after your passions. What do you like to do aside from work? Do you paint or play a musical instrument? Pursuing hobbies and interests can revitalize your spirit.
Now. We as a profession are notorious for saying to ourselves, “I will get to it (being happy) someday.” We delay happiness until we catch up with work, save enough money, and so on. This approach is unrealistic. It is better to live in the present because there are a finite number of days to seize the day. Focus your energy in the moment.
Interact. Isolating oneself will lead to burnout. If you are in solo practice, connect with other providers or get involved in community activities. If you work with other providers, interact with them in a meaningful manner (eg, don’t complain but rather air your concerns, accept honest feedback, be open to suggestions, and seek assistance; it is acceptable to admit that you can’t do everything).
Take time off and take care of yourself. Although that seems intuitive, psychiatrists, as a group, don’t do a good job of it. Waiting until you are burned out to take a vacation is counterproductive because you will be too drained to enjoy it. Taking care of your physical and mental health is equally important.
Enjoyment in and at work. We make a difference in our patients’ lives throughthe emotional connections we develop with them. By viewing what we do as fulfilling a higher calling, we can learn to enjoy what we do rather than feeling burdened by it. Advocating for better recognition—whether financial, institutional, or social—can create opportunities for personal satisfaction.
Burnout among psychiatric clinicians can lead to reduced job satisfaction, poorer quality of patient care, and depression.1 Signs of burnout include a feeling of cynicism (eg, negative attitudes toward patients), overwhelming exhaustion (eg, feeling depleted), and a sense of ineffectiveness (eg, reduced productivity).1 Workplace variables and other factors that could perpetuate burnout among psychiatrists include, but are not limited to:
- too much work
- chronic staff shortages
- working with difficult patients
- inability to meet self-imposed demands
- a lack of meaningful relationships with colleagues and supervisors.1,2
The mnemonic REIGNITE provides strategies to reduce the risk of burnout.1,3
Recognize your limits. Although saying “no” may be difficult for mental health clinicians, saying “yes” too often can be detrimental. Techniques for setting limits without alienating colleagues include:
- declining tasks (“I appreciate you thinking of me to do that, but I can’t complete it right now”)
- delaying an answer (“Let me ponder what you are asking”)
- delegating tasks (“I could really use your help”)
- avoid taking on too much (“I thought that I could do that extra task, but I realize that taking on the additional assignment isn’t going to work out”).
Expand your portfolio. Developing a diverse work portfolio (eg, teaching part-time) could diminish stagnation. Adding regenerative activities (eg, outdoor activities) could be restorative.
Itemize your priorities. Ask yourself what is important to you. Is it work? If so, can work be modified so it continues to be rewarding without resulting in burnout? If it isn’t work, then what is? Money? Family? Evaluating what is important and pursuing those priorities could increase overall life satisfaction.
Go after your passions. What do you like to do aside from work? Do you paint or play a musical instrument? Pursuing hobbies and interests can revitalize your spirit.
Now. We as a profession are notorious for saying to ourselves, “I will get to it (being happy) someday.” We delay happiness until we catch up with work, save enough money, and so on. This approach is unrealistic. It is better to live in the present because there are a finite number of days to seize the day. Focus your energy in the moment.
Interact. Isolating oneself will lead to burnout. If you are in solo practice, connect with other providers or get involved in community activities. If you work with other providers, interact with them in a meaningful manner (eg, don’t complain but rather air your concerns, accept honest feedback, be open to suggestions, and seek assistance; it is acceptable to admit that you can’t do everything).
Take time off and take care of yourself. Although that seems intuitive, psychiatrists, as a group, don’t do a good job of it. Waiting until you are burned out to take a vacation is counterproductive because you will be too drained to enjoy it. Taking care of your physical and mental health is equally important.
Enjoyment in and at work. We make a difference in our patients’ lives throughthe emotional connections we develop with them. By viewing what we do as fulfilling a higher calling, we can learn to enjoy what we do rather than feeling burdened by it. Advocating for better recognition—whether financial, institutional, or social—can create opportunities for personal satisfaction.
1. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15(2):103-111.
2. Bressi C, Porcellana M, Gambini O, et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985-988.
3. Bohnert P, O’Connell A. How to avoid burnout
1. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15(2):103-111.
2. Bressi C, Porcellana M, Gambini O, et al. Burnout among psychiatrists in Milan: a multicenter survey. Psychiatr Serv. 2009;60(7):985-988.
3. Bohnert P, O’Connell A. How to avoid burnout