User login
Acute kidney injury after hip or knee replacement: Can we lower the risk?
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
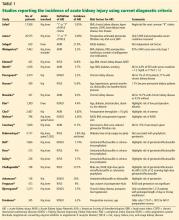
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
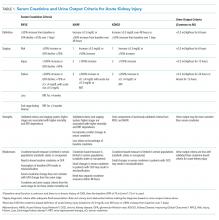
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
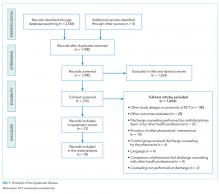
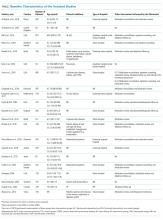
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
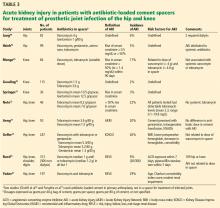
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
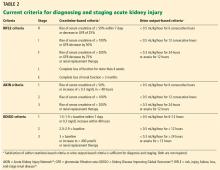
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
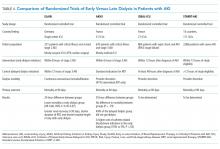
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
KEY POINTS
- Using current diagnostic criteria, the incidence of acute kidney injury complicating primary total joint arthroplasty may be nearly 10%, and 25% after placement of an antibiotic-loaded cement spacer to treat infection.
- In primary total joint arthroplasty, significant risk factors include older age, higher body mass index, chronic kidney disease, comorbidity, anemia, perioperative transfusion, aminoglycoside prophylaxis and treatment, preoperative heart murmur, and renin-angiotensin-aldosterone system blockade.
- Acute kidney injury may arise from infection, systemic administration of nephrotoxic antibiotics, and elution of antibiotics from antibiotic-loaded cement.
- No randomized controlled trial aimed at reducing acute kidney injury in these settings has been published; however, suggestions for practice modification are made based on the available data.
Unusual effects of common antibiotics
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
KEY POINTS
- Piperacillin-induced encephalopathy and seizure can occur on a continuum, with progressive dysarthria, tremor, and confusion culminating in tonic-clonic seizures.
- Monocycline-induced lupus can present as myalgia, arthralgia, serositis, constitutional symptoms, and a positive antinuclear antibody titer. The effect is not dose-dependent.
- Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been reported with quinolones and sulfonamides.
- QT-interval prolongation is a class effect of quinolones and is more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, or bradycardia, or in women.
Updates in Management and Timing of Dialysis in Acute Kidney Injury
Acute kidney injury (AKI) is a common complication in hospitalized patients, affecting one in five inpatients1,2 and more than half of patients in intensive care units (ICU).3 The incidence of AKI appears to be increasing over time.4 Potential contributing factors include an aging population, rising prevalence of comorbid conditions such as heart failure and chronic kidney disease (CKD), using nephrotoxic agents, and increasing complexity of surgical procedures.5,6 AKI during a hospital stay is associated with a two to 10-fold increased risk of inhospital mortality,1,2,7-10 longer hospital length of stay,7,10 higher risk for hospital readmissions,11 and higher healthcare costs.7 Patients who survive an episode of AKI have a higher risk for CKD and dialysis-dependence,9 even after an episode of reversible AKI.12 Despite its clinical importance, several areas of controversy remain regarding the management of AKI and, in particular, the optimal timing of renal replacement therapy (RRT) in patients with AKI. The purpose of this manuscript is to review the approaches to diagnosis and management of AKI in hospitalized patients. We also review recent evidence regarding the timing of dialysis in patients with AKI. This journal recently reviewed the differential diagnosis and diagnostic evaluation of AKI, which is not covered here.13
DEFINITION OF ACUTE KIDNEY INJURY
AKI refers to an acute change in kidney function characterized by an increase in serum creatinine and/or a reduction in urine output. It is a clinical syndrome caused by a broad range of etiologies and may be related to primary kidney pathology and/or systemic illness. Until 2004, there was no standard definition for AKI and over 30 different definitions were found in the literature, which resulted in wide variation in the reported incidence and outcomes of AKI and made it challenging to apply an evidence-based approach to patient care. In 2004, the Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE)14 criteria for AKI were proposed, which were modified to the Acute Kidney Injury Network (AKIN)15 criteria in 2007 (Table 1). Multiple studies show that the RIFLE and AKIN criteria for AKI are associated with higher mortality1,2,8,10 and increased risk for requiring RRT.1,10
International clinical practice guidelines for AKI were released by Kidney Disease: Improving Global Outcomes (KDIGO) in 2012, which included a standardized definition of AKI that was adapted from the previously validated RIFLE and AKIN definitions.16 Patients are considered to have AKI when the serum creatinine rises by as little as 0.3 mg/dL. It is notable that when the baseline serum creatinine is high, there is more inherent variability in the serum creatinine measurement; thus, patients with CKD have a higher risk of being misclassified as having AKI.17 Although the KDIGO definition for AKI is commonly used in research settings, components of this definition have not been well validated, and it is not widely used in clinical practice. Other renal professional societies still recommend an individualized approach to the diagnosis of AKI, taking into account other factors such as trajectories in kidney function, fluid balance, electrolyte abnormalities, comorbid conditions, and clinical context.18,19 While we endorse the KDIGO approach to the categorization of AKI severity, in practice, a more patient-centered approach is generally required to guide the optimal approach to determining the etiology of AKI and guiding management.
GENERAL MANAGEMENT OF ACUTE KIDNEY INJURY
All patients with AKI should have close monitoring of their serum creatinine and urine output. Noninvasive diagnostic studies (urine microscopy, postvoid residual, and renal ultrasound) should be considered based on the clinical scenario. General management strategies include treatment of the reversible causes of AKI and optimization of volume status, hemodynamics, and nutritional status (Table 2).
Reversible Causes of Acute Kidney Injury
The first step in the treatment of AKI is to identify and treat readily reversible causes of AKI such as volume depletion, hypotension, infection, and urinary obstruction. Nephrotoxins should be avoided and all medications should be reviewed and adjusted for kidney function, particularly those that may affect mental status. Avoid opiates with noxious or active metabolites, including meperidine and morphine. Instead, hydromorphone, fentanyl, and methadone are preferred in patients with AKI. Other commonly used medications that require dose adjustment include gabapentin, baclofen, metoclopramide, H2 antagonists, many commonly prescribed antibiotics (penicillins, most cephalosporins, carbapenems, quinolones, and sulfa drugs), many hypoglycemic agents, and insulin. For patients on RRT, dosing is dependent on dialysis modality. Consultation with a hospital pharmacist is recommended when RRT modalities are initiated or changed.
Intravenous Fluids
Patients with AKI should have their volume status assessed and receive adequate resuscitation with intravenous fluids to promote renal perfusion. However, the optimal type and volume of fluid to give in AKI remains controversial. Colloid-containing solutions are theoretically confined to the intravascular space and should pose a lower risk for pulmonary edema compared with crystalloids. However, these solutions are costly, are not associated with any meaningful benefit,20-22 and may even be associated with potential harm.22-27
The most commonly used colloid worldwide is hydroxyethyl starch (HES). Its potential adverse effects include anaphylactoid reactions, coagulopathy, and AKI. HES is cleared by the kidneys and can cause osmotic nephrosis, a form of AKI characterized by vacuole formation and proximal renal tubular damage.28 Randomized controlled trials have shown an increased risk of AKI, RRT use, and mortality in critically ill patients who were resuscitated with HES.22,26,27 HES is not currently recommended in patients who are critically ill or have impaired kidney function and sepsis guidelines advise against its use.29
In the United States, albumin is the most common colloid-containing solution used for intravascular volume resuscitation. Albumin has been shown to be safe for volume resuscitation in critically ill patients,20 but there is no proven advantage to using albumin over saline with respect to mortality, length of hospital stay, duration of mechanical ventilation, duration of RRT, or number of organ systems failure.20,21 Furthermore, albumin may be harmful in certain patient populations. In patients with traumatic brain injury, albumin resuscitation is associated with higher mean intracranial pressures23 and long-term mortality.24 In a retrospective study of patients undergoing cardiac surgery, albumin administration was associated with more than twice the risk of AKI compared with crystalloids.25 In contrast, in patients with cirrhosis, intravenous albumin lowers the rate of AKI when administered in the setting of a large volume paracentesis30 or spontaneous bacterial peritonitis.31 Outside of these narrow settings, current evidence does not support the use of intravenous albumin to prevent AKI and we would not endorse the use of intravenous albumin as a part of the treatment paradigm for established AKI.
Many renal and critical care guidelines recommend initial fluid resuscitation with isotonic crystalloids except in specific circumstances (ie, hemorrhagic shock), with consideration of albumin in select cases (ie, severe sepsis or cirrhosis).16,18,19,29 That stated, the optimal type of crystalloid solution that should be used in resuscitation remains unclear. Because of its low cost, normal (0.9%) saline is the most commonly used solution, but it can result in hyperchloremic metabolic acidosis, which can cause renal vasoconstriction and may be associated with mortality in critically ill patients.32 A prospective study found that administration of chloride-liberal fluids (including normal saline) to critically ill patients was associated with nearly twice the risk of AKI and RRT use compared with chloride-restrictive fluids,33 but a subsequent trial found no difference in AKI or mortality among patients receiving saline versus a balanced crystalloid (Plasma-Lyte 148).34 A recent pair of large, randomized control trials compared outcomes in patients at a single center who were resuscitated with normal saline versus balanced crystalloid solutions (Lactated Ringer’s or Plasma-Lyte A).35,36 In critically ill patients, the use of balanced crystalloid solutions was associated with a lower risk of the composite outcome of mortality, new RRT, or persistent kidney impairment, but there were no differences in any of the individual components of the composite outcome.35 In noncritically ill patients, there were no differences in the number of hospital-free days based on the type of crystalloid solution used.36 In the absence of compelling evidence for using balanced crystalloid solutions, we continue to use normal saline for initial fluid resuscitation, but to avoid severe hyperchloremia and acidosis, we will consider switching to a balanced solution (Lactated Ringer’s, Plasma-Lyte, or Normosol) for large volume resuscitation (>2 L), particularly in critically ill patients.
Diuretics
As above, volume status is a key component in the management of patients with AKI. In patients with AKI and hypervolemia, loop diuretics are often given prior to the initiation of RRT. Loop diuretics act on the sodium-potassium-chloride cotransporters in the thick ascending limb of the loop of Henle to increase urinary losses of these ions and urine volume. Loop diuretics are dose-dependent, and often, higher doses are needed (eg, furosemide 100 mg intravenous dose) in patients with AKI, since the diuretic effect depends on the proximal tubular secretion of the drug into the urine. The role of diuretics in AKI is controversial and some observational data suggest an increased mortality risk with diuretic use in patients with AKI.37 In critically ill patients with acute lung injury, diuretic use improved survival, which was attributed to better control of volume overload.38 But, a meta-analysis of 11 randomized controlled trials failed to demonstrate that diuretics directly improved survival or recovery of AKI.39 Moreover, randomized controlled trials found that diuretics given to a patient with AKI requiring RRT did not improve recovery of kidney function.40,41 The KDIGO guidelines recommend that diuretics should not be routinely used for AKI except in the management of volume overload.16
Nutritional Targets in Acute Kidney Injury
Critically ill patients have high protein catabolic rates, which put them at increased risk for malnutrition, which in turn is associated with mortality. Patients who receive continuous RRT (CRRT) may lose 5-10 g of protein and 10-15 g of amino acids daily, and these patients may have protein requirements that are twice the usual recommended daily protein intake.16 But excess protein administration can result in high urea generation and azotemia unrelated to the patient’s kidney function. Blood urea nitrogen may also be disproportionately elevated in conditions where tubular reabsorption of urea is increased, such as in volume depletion, diuretic use, corticosteroid use, and gastrointestinal bleeding. Interpretation of blood urea nitrogen results must be made in the appropriate clinical context, with recognition that azotemia alone may not be a good surrogate marker of the patient’s underlying kidney function. We recommend dietary consultation in critically ill patients with AKI to ensure that adequate, but not excessive, protein is administered.
RENAL REPLACEMENT THERAPY IN ACUTE KIDNEY INJURY
In patients with AKI, RRT is initiated for control of volume overload, electrolyte abnormalities, acidemia, or uremic symptoms or complications that are refractory to medical management (Table 3). In a nonoliguric patient, fluid and electrolyte abnormalities can oftentimes be managed medically. Patients with oligoanuria (generally defined as urine output less than 400 mL/day or <20 mL/hour), however, require nephrology evaluation for consideration of RRT. Early nephrology consultation (within 48 hours of AKI diagnosis) may be associated with lower dialysis dependence and mortality in critically ill patients with AKI.42 The decision to initiate dialysis is individualized based on the patient’s comorbid conditions, urine output, and trajectory of kidney function.
Timing of Renal Replacement Therapy
The optimal timing of dialysis initiation in patients with AKI is not known. Theoretically, earlier initiation of dialysis could allow for better volume and electrolyte control and prevent the development of more serious complications of kidney failure such as uremic seizures, encephalopathy, and pericarditis. However, RRT is associated with its own risks and earlier initiation may expose the patient to unnecessary procedures and complications that might delay renal recovery. A meta-analysis of predominantly observational data found that earlier initiation of RRT in AKI was associated with lower 28-day mortality, greater renal recovery, decreased duration of RRT, and decreased ICU length of stay.43 Subsequently, two prospective trials reported conflicting results regarding associations between dialysis timing and outcomes in patients with severe AKI (Table 4).44,45
The Early vs Late Initiation of Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (ELAIN) was a prospective, single-center randomized trial in Germany of 231 critically ill, predominantly surgical ICU patients (about half postcardiac surgery) with at least KDIGO stage 2 AKI.44 Patients were randomized to early (within eight hours of developing KDIGO stage 2 AKI) or delayed (within 12 hours of developing KDIGO stage 3 AKI) RRT initiation; patients in the early RRT group initiated dialysis on average 20 hours earlier than the patients in the late group. All patients were treated with continuous venovenous hemodiafiltration. Early RRT initiation was associated with a 34% lower risk of mortality at 90 days, shorter hospital length of stay, and shorter RRT duration compared with delayed RRT initiation. There was no difference between groups in dialysis dependence at 90 days, but there was a lower risk of dialysis dependence at one year.46The Artificial Kidney Initiation in Kidney Injury Study (AKIKI)45 was a prospective, multicenter randomized trial in France that compared early versus delayed strategies of RRT initiation in 620 critically ill, mostly medical ICU patients with severe AKI (KDIGO stage 3). The median time between randomization and RRT initiation was two hours for the early and 57 hours for the delayed strategy groups. There were no differences between groups in length of hospital or ICU stay, vasopressor use, dialysis dependence, or 60-day survival. The early strategy group had a higher incidence of catheter-related bloodstream infections (10% vs 5%) and hypophosphatemia (22% vs 15%) compared with that of the delayed strategy group. Patients in the delayed strategy group regained normal urine output sooner than in the early strategy group. Approximately half of the patients in the delayed strategy group avoided RRT altogether. The authors of AKIKI concluded that there was no benefit to the early strategy of RRT in critically ill patients with severe AKI, and a delayed strategy of RRT initiation may avoid unnecessary RRT and reduce catheter-related infectious complications.
How can we interpret these discrepant results? Although ELAIN found a benefit to earlier RRT initiation in AKI, it has limited generalizability to medical ICU patients, who have higher mortality and whose outcomes might be less affected by dialysis timing. Patients in ELAIN had a high prevalence of congestive heart failure and CKD; it is possible that select patient populations may derive greater benefit from earlier RRT initiation. Although both ELAIN and AKIKI used the standardized criteria for RRT initiation, neither study could incorporate important clinical factors such as trajectory of kidney function, comorbid conditions, or symptoms, which play a significant role in the decision-making process in real-world clinical practice. Additional large-scale, multicenter trials are needed to guide the timing of RRT in critically ill patients with AKI. The Initiation of Dialysis Early Versus Delayed in the ICU (IDEAL-ICU)47 and Standard versus Accelerated Initiation of RRT in Acute Kidney Injury (STARRT-AKI)48 studies are currently underway and hope to provide clearer guidance regarding the optimal timing of RRT initiation in AKI (Table 4). Until further evidence is available, experts recommend taking into consideration the trajectory of kidney disease, concurrent organ dysfunction, and expected need for fluid and solute control when making decisions regarding RRT initiation in AKI.16
DIALYSIS MODALITIES IN ACUTE KIDNEY INJURY
When RRT is required in patients with AKI, the dialysis modality is often determined by local availability. CRRT and sustained low-efficiency dialysis (SLED) are thought to be better tolerated than intermittent hemodialysis in hemodynamically unstable patients, although a randomized controlled trial could not demonstrate a survival difference between these modalities.49 In general, in settings where CRRT or SLED is available, these modalities are favored for patients with hemodynamic instability, but practice patterns vary widely.
CONCLUSION
Among hospitalized patients, AKI is common and associated with a higher risk of mortality. Although serum creatinine and urine output criteria are used to define AKI, other clinical factors (comorbid conditions, volume status, and trajectory of kidney function decline) can inform the assessment and management of patients with AKI. General strategies for AKI management include treatment of reversible conditions, optimization of volume status, hemodynamics, and nutritional status. The optimal timing of RRT in critically ill patients with AKI is not known, with unclear mortality benefit of earlier dialysis initiation. Two large-scale randomized controlled trials regarding early versus delayed dialysis timing in AKI are currently underway and will hopefully provide clarity in the near future.
Disclosures
Dr. Yu and Dr. Kamal have nothing to disclose. Dr. Chertow is an advisor to DURECT Corporation.
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. PubMed
2. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913-1917. PubMed
3. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411-1423. PubMed
4. Wald R, McArthur E, Adhikari NKJ, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870-877. PubMed
5. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
6. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28. PubMed
7. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
8. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538-546. PubMed
9. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
10. Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292-1298. PubMed
11. Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis. 2015;65(2):275-282. PubMed
12. Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477-485. PubMed
13. Cooper CM, Fenves AZ. Before you call renal: acute kidney injury for hospitalists. J Hosp Med. 2015;10(6):403-408. PubMed
14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Workgroup A. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2): R31. PubMed
16. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
17. Lin J, Fernandez H, Shashaty MG, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10(10):1723-1731. PubMed
18. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672. PubMed
19. James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):673-685. PubMed
20. Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256. PubMed
21. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412-1421. PubMed
22. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901-1911. PubMed
23. Cooper DJ, Myburgh J, Heritier S, et al. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013;30(7):512-518. PubMed
24. Myburgh J, Cooper J, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884. PubMed
25. Frenette AJ, Bouchard J, Bernier P, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care. 2014;18(6):602. PubMed
26. Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethyl starch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357(9260):911-916. PubMed
27. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124-134. PubMed
28. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51(3):491-503. PubMed
29. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. PubMed
30. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. PubMed
31. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. PubMed
32. Boniatti MM, Cardoso PRC, Castilho RK, Vieira SRR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26(2):175-179. PubMed
33. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama-J Am Med Assoc. 2012;308(15):1566-1572. PubMed
34. Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. Jama-J Am Med Assoc. 2015;314(16):1701-1710. PubMed
35. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. PubMed
36. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. PubMed
37. Mehta RL, Pascual MT, Soroko S, Chertow GM, Group PS. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547-2553. PubMed
38. Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966-973. PubMed
39. Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283-293. PubMed
40. Cantarovich F, Rangoonwala B, Lorenz H, Verho M, Esnault VL, High-Dose Flurosemide in Acute Renal Failure Study Group. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402-409. PubMed
41. van der Voort PH, Boerma EC, Koopmans M, et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med. 2009;37(2):533-538. PubMed
42. Costa e Silva VT, Liano F, Muriel A, Diez R, de Castro I, Yu L. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLoS One. 2013;8(8):e70482. PubMed
43. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. PubMed
44. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190-2199. PubMed
45. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122-133. PubMed
46. Meersch M, Kullmar M, Schmidt C, et al. Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol. 2018;29(3):1011-1019. PubMed
47. Barbar SD, Binquet C, Monchi M, Bruyere R, Quenot JP. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15:270. PubMed
48. Smith OM, Wald R, Adhikari NK, et al. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. PubMed
49. Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379-385. PubMed
Acute kidney injury (AKI) is a common complication in hospitalized patients, affecting one in five inpatients1,2 and more than half of patients in intensive care units (ICU).3 The incidence of AKI appears to be increasing over time.4 Potential contributing factors include an aging population, rising prevalence of comorbid conditions such as heart failure and chronic kidney disease (CKD), using nephrotoxic agents, and increasing complexity of surgical procedures.5,6 AKI during a hospital stay is associated with a two to 10-fold increased risk of inhospital mortality,1,2,7-10 longer hospital length of stay,7,10 higher risk for hospital readmissions,11 and higher healthcare costs.7 Patients who survive an episode of AKI have a higher risk for CKD and dialysis-dependence,9 even after an episode of reversible AKI.12 Despite its clinical importance, several areas of controversy remain regarding the management of AKI and, in particular, the optimal timing of renal replacement therapy (RRT) in patients with AKI. The purpose of this manuscript is to review the approaches to diagnosis and management of AKI in hospitalized patients. We also review recent evidence regarding the timing of dialysis in patients with AKI. This journal recently reviewed the differential diagnosis and diagnostic evaluation of AKI, which is not covered here.13
DEFINITION OF ACUTE KIDNEY INJURY
AKI refers to an acute change in kidney function characterized by an increase in serum creatinine and/or a reduction in urine output. It is a clinical syndrome caused by a broad range of etiologies and may be related to primary kidney pathology and/or systemic illness. Until 2004, there was no standard definition for AKI and over 30 different definitions were found in the literature, which resulted in wide variation in the reported incidence and outcomes of AKI and made it challenging to apply an evidence-based approach to patient care. In 2004, the Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE)14 criteria for AKI were proposed, which were modified to the Acute Kidney Injury Network (AKIN)15 criteria in 2007 (Table 1). Multiple studies show that the RIFLE and AKIN criteria for AKI are associated with higher mortality1,2,8,10 and increased risk for requiring RRT.1,10
International clinical practice guidelines for AKI were released by Kidney Disease: Improving Global Outcomes (KDIGO) in 2012, which included a standardized definition of AKI that was adapted from the previously validated RIFLE and AKIN definitions.16 Patients are considered to have AKI when the serum creatinine rises by as little as 0.3 mg/dL. It is notable that when the baseline serum creatinine is high, there is more inherent variability in the serum creatinine measurement; thus, patients with CKD have a higher risk of being misclassified as having AKI.17 Although the KDIGO definition for AKI is commonly used in research settings, components of this definition have not been well validated, and it is not widely used in clinical practice. Other renal professional societies still recommend an individualized approach to the diagnosis of AKI, taking into account other factors such as trajectories in kidney function, fluid balance, electrolyte abnormalities, comorbid conditions, and clinical context.18,19 While we endorse the KDIGO approach to the categorization of AKI severity, in practice, a more patient-centered approach is generally required to guide the optimal approach to determining the etiology of AKI and guiding management.
GENERAL MANAGEMENT OF ACUTE KIDNEY INJURY
All patients with AKI should have close monitoring of their serum creatinine and urine output. Noninvasive diagnostic studies (urine microscopy, postvoid residual, and renal ultrasound) should be considered based on the clinical scenario. General management strategies include treatment of the reversible causes of AKI and optimization of volume status, hemodynamics, and nutritional status (Table 2).
Reversible Causes of Acute Kidney Injury
The first step in the treatment of AKI is to identify and treat readily reversible causes of AKI such as volume depletion, hypotension, infection, and urinary obstruction. Nephrotoxins should be avoided and all medications should be reviewed and adjusted for kidney function, particularly those that may affect mental status. Avoid opiates with noxious or active metabolites, including meperidine and morphine. Instead, hydromorphone, fentanyl, and methadone are preferred in patients with AKI. Other commonly used medications that require dose adjustment include gabapentin, baclofen, metoclopramide, H2 antagonists, many commonly prescribed antibiotics (penicillins, most cephalosporins, carbapenems, quinolones, and sulfa drugs), many hypoglycemic agents, and insulin. For patients on RRT, dosing is dependent on dialysis modality. Consultation with a hospital pharmacist is recommended when RRT modalities are initiated or changed.
Intravenous Fluids
Patients with AKI should have their volume status assessed and receive adequate resuscitation with intravenous fluids to promote renal perfusion. However, the optimal type and volume of fluid to give in AKI remains controversial. Colloid-containing solutions are theoretically confined to the intravascular space and should pose a lower risk for pulmonary edema compared with crystalloids. However, these solutions are costly, are not associated with any meaningful benefit,20-22 and may even be associated with potential harm.22-27
The most commonly used colloid worldwide is hydroxyethyl starch (HES). Its potential adverse effects include anaphylactoid reactions, coagulopathy, and AKI. HES is cleared by the kidneys and can cause osmotic nephrosis, a form of AKI characterized by vacuole formation and proximal renal tubular damage.28 Randomized controlled trials have shown an increased risk of AKI, RRT use, and mortality in critically ill patients who were resuscitated with HES.22,26,27 HES is not currently recommended in patients who are critically ill or have impaired kidney function and sepsis guidelines advise against its use.29
In the United States, albumin is the most common colloid-containing solution used for intravascular volume resuscitation. Albumin has been shown to be safe for volume resuscitation in critically ill patients,20 but there is no proven advantage to using albumin over saline with respect to mortality, length of hospital stay, duration of mechanical ventilation, duration of RRT, or number of organ systems failure.20,21 Furthermore, albumin may be harmful in certain patient populations. In patients with traumatic brain injury, albumin resuscitation is associated with higher mean intracranial pressures23 and long-term mortality.24 In a retrospective study of patients undergoing cardiac surgery, albumin administration was associated with more than twice the risk of AKI compared with crystalloids.25 In contrast, in patients with cirrhosis, intravenous albumin lowers the rate of AKI when administered in the setting of a large volume paracentesis30 or spontaneous bacterial peritonitis.31 Outside of these narrow settings, current evidence does not support the use of intravenous albumin to prevent AKI and we would not endorse the use of intravenous albumin as a part of the treatment paradigm for established AKI.
Many renal and critical care guidelines recommend initial fluid resuscitation with isotonic crystalloids except in specific circumstances (ie, hemorrhagic shock), with consideration of albumin in select cases (ie, severe sepsis or cirrhosis).16,18,19,29 That stated, the optimal type of crystalloid solution that should be used in resuscitation remains unclear. Because of its low cost, normal (0.9%) saline is the most commonly used solution, but it can result in hyperchloremic metabolic acidosis, which can cause renal vasoconstriction and may be associated with mortality in critically ill patients.32 A prospective study found that administration of chloride-liberal fluids (including normal saline) to critically ill patients was associated with nearly twice the risk of AKI and RRT use compared with chloride-restrictive fluids,33 but a subsequent trial found no difference in AKI or mortality among patients receiving saline versus a balanced crystalloid (Plasma-Lyte 148).34 A recent pair of large, randomized control trials compared outcomes in patients at a single center who were resuscitated with normal saline versus balanced crystalloid solutions (Lactated Ringer’s or Plasma-Lyte A).35,36 In critically ill patients, the use of balanced crystalloid solutions was associated with a lower risk of the composite outcome of mortality, new RRT, or persistent kidney impairment, but there were no differences in any of the individual components of the composite outcome.35 In noncritically ill patients, there were no differences in the number of hospital-free days based on the type of crystalloid solution used.36 In the absence of compelling evidence for using balanced crystalloid solutions, we continue to use normal saline for initial fluid resuscitation, but to avoid severe hyperchloremia and acidosis, we will consider switching to a balanced solution (Lactated Ringer’s, Plasma-Lyte, or Normosol) for large volume resuscitation (>2 L), particularly in critically ill patients.
Diuretics
As above, volume status is a key component in the management of patients with AKI. In patients with AKI and hypervolemia, loop diuretics are often given prior to the initiation of RRT. Loop diuretics act on the sodium-potassium-chloride cotransporters in the thick ascending limb of the loop of Henle to increase urinary losses of these ions and urine volume. Loop diuretics are dose-dependent, and often, higher doses are needed (eg, furosemide 100 mg intravenous dose) in patients with AKI, since the diuretic effect depends on the proximal tubular secretion of the drug into the urine. The role of diuretics in AKI is controversial and some observational data suggest an increased mortality risk with diuretic use in patients with AKI.37 In critically ill patients with acute lung injury, diuretic use improved survival, which was attributed to better control of volume overload.38 But, a meta-analysis of 11 randomized controlled trials failed to demonstrate that diuretics directly improved survival or recovery of AKI.39 Moreover, randomized controlled trials found that diuretics given to a patient with AKI requiring RRT did not improve recovery of kidney function.40,41 The KDIGO guidelines recommend that diuretics should not be routinely used for AKI except in the management of volume overload.16
Nutritional Targets in Acute Kidney Injury
Critically ill patients have high protein catabolic rates, which put them at increased risk for malnutrition, which in turn is associated with mortality. Patients who receive continuous RRT (CRRT) may lose 5-10 g of protein and 10-15 g of amino acids daily, and these patients may have protein requirements that are twice the usual recommended daily protein intake.16 But excess protein administration can result in high urea generation and azotemia unrelated to the patient’s kidney function. Blood urea nitrogen may also be disproportionately elevated in conditions where tubular reabsorption of urea is increased, such as in volume depletion, diuretic use, corticosteroid use, and gastrointestinal bleeding. Interpretation of blood urea nitrogen results must be made in the appropriate clinical context, with recognition that azotemia alone may not be a good surrogate marker of the patient’s underlying kidney function. We recommend dietary consultation in critically ill patients with AKI to ensure that adequate, but not excessive, protein is administered.
RENAL REPLACEMENT THERAPY IN ACUTE KIDNEY INJURY
In patients with AKI, RRT is initiated for control of volume overload, electrolyte abnormalities, acidemia, or uremic symptoms or complications that are refractory to medical management (Table 3). In a nonoliguric patient, fluid and electrolyte abnormalities can oftentimes be managed medically. Patients with oligoanuria (generally defined as urine output less than 400 mL/day or <20 mL/hour), however, require nephrology evaluation for consideration of RRT. Early nephrology consultation (within 48 hours of AKI diagnosis) may be associated with lower dialysis dependence and mortality in critically ill patients with AKI.42 The decision to initiate dialysis is individualized based on the patient’s comorbid conditions, urine output, and trajectory of kidney function.
Timing of Renal Replacement Therapy
The optimal timing of dialysis initiation in patients with AKI is not known. Theoretically, earlier initiation of dialysis could allow for better volume and electrolyte control and prevent the development of more serious complications of kidney failure such as uremic seizures, encephalopathy, and pericarditis. However, RRT is associated with its own risks and earlier initiation may expose the patient to unnecessary procedures and complications that might delay renal recovery. A meta-analysis of predominantly observational data found that earlier initiation of RRT in AKI was associated with lower 28-day mortality, greater renal recovery, decreased duration of RRT, and decreased ICU length of stay.43 Subsequently, two prospective trials reported conflicting results regarding associations between dialysis timing and outcomes in patients with severe AKI (Table 4).44,45
The Early vs Late Initiation of Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (ELAIN) was a prospective, single-center randomized trial in Germany of 231 critically ill, predominantly surgical ICU patients (about half postcardiac surgery) with at least KDIGO stage 2 AKI.44 Patients were randomized to early (within eight hours of developing KDIGO stage 2 AKI) or delayed (within 12 hours of developing KDIGO stage 3 AKI) RRT initiation; patients in the early RRT group initiated dialysis on average 20 hours earlier than the patients in the late group. All patients were treated with continuous venovenous hemodiafiltration. Early RRT initiation was associated with a 34% lower risk of mortality at 90 days, shorter hospital length of stay, and shorter RRT duration compared with delayed RRT initiation. There was no difference between groups in dialysis dependence at 90 days, but there was a lower risk of dialysis dependence at one year.46The Artificial Kidney Initiation in Kidney Injury Study (AKIKI)45 was a prospective, multicenter randomized trial in France that compared early versus delayed strategies of RRT initiation in 620 critically ill, mostly medical ICU patients with severe AKI (KDIGO stage 3). The median time between randomization and RRT initiation was two hours for the early and 57 hours for the delayed strategy groups. There were no differences between groups in length of hospital or ICU stay, vasopressor use, dialysis dependence, or 60-day survival. The early strategy group had a higher incidence of catheter-related bloodstream infections (10% vs 5%) and hypophosphatemia (22% vs 15%) compared with that of the delayed strategy group. Patients in the delayed strategy group regained normal urine output sooner than in the early strategy group. Approximately half of the patients in the delayed strategy group avoided RRT altogether. The authors of AKIKI concluded that there was no benefit to the early strategy of RRT in critically ill patients with severe AKI, and a delayed strategy of RRT initiation may avoid unnecessary RRT and reduce catheter-related infectious complications.
How can we interpret these discrepant results? Although ELAIN found a benefit to earlier RRT initiation in AKI, it has limited generalizability to medical ICU patients, who have higher mortality and whose outcomes might be less affected by dialysis timing. Patients in ELAIN had a high prevalence of congestive heart failure and CKD; it is possible that select patient populations may derive greater benefit from earlier RRT initiation. Although both ELAIN and AKIKI used the standardized criteria for RRT initiation, neither study could incorporate important clinical factors such as trajectory of kidney function, comorbid conditions, or symptoms, which play a significant role in the decision-making process in real-world clinical practice. Additional large-scale, multicenter trials are needed to guide the timing of RRT in critically ill patients with AKI. The Initiation of Dialysis Early Versus Delayed in the ICU (IDEAL-ICU)47 and Standard versus Accelerated Initiation of RRT in Acute Kidney Injury (STARRT-AKI)48 studies are currently underway and hope to provide clearer guidance regarding the optimal timing of RRT initiation in AKI (Table 4). Until further evidence is available, experts recommend taking into consideration the trajectory of kidney disease, concurrent organ dysfunction, and expected need for fluid and solute control when making decisions regarding RRT initiation in AKI.16
DIALYSIS MODALITIES IN ACUTE KIDNEY INJURY
When RRT is required in patients with AKI, the dialysis modality is often determined by local availability. CRRT and sustained low-efficiency dialysis (SLED) are thought to be better tolerated than intermittent hemodialysis in hemodynamically unstable patients, although a randomized controlled trial could not demonstrate a survival difference between these modalities.49 In general, in settings where CRRT or SLED is available, these modalities are favored for patients with hemodynamic instability, but practice patterns vary widely.
CONCLUSION
Among hospitalized patients, AKI is common and associated with a higher risk of mortality. Although serum creatinine and urine output criteria are used to define AKI, other clinical factors (comorbid conditions, volume status, and trajectory of kidney function decline) can inform the assessment and management of patients with AKI. General strategies for AKI management include treatment of reversible conditions, optimization of volume status, hemodynamics, and nutritional status. The optimal timing of RRT in critically ill patients with AKI is not known, with unclear mortality benefit of earlier dialysis initiation. Two large-scale randomized controlled trials regarding early versus delayed dialysis timing in AKI are currently underway and will hopefully provide clarity in the near future.
Disclosures
Dr. Yu and Dr. Kamal have nothing to disclose. Dr. Chertow is an advisor to DURECT Corporation.
Acute kidney injury (AKI) is a common complication in hospitalized patients, affecting one in five inpatients1,2 and more than half of patients in intensive care units (ICU).3 The incidence of AKI appears to be increasing over time.4 Potential contributing factors include an aging population, rising prevalence of comorbid conditions such as heart failure and chronic kidney disease (CKD), using nephrotoxic agents, and increasing complexity of surgical procedures.5,6 AKI during a hospital stay is associated with a two to 10-fold increased risk of inhospital mortality,1,2,7-10 longer hospital length of stay,7,10 higher risk for hospital readmissions,11 and higher healthcare costs.7 Patients who survive an episode of AKI have a higher risk for CKD and dialysis-dependence,9 even after an episode of reversible AKI.12 Despite its clinical importance, several areas of controversy remain regarding the management of AKI and, in particular, the optimal timing of renal replacement therapy (RRT) in patients with AKI. The purpose of this manuscript is to review the approaches to diagnosis and management of AKI in hospitalized patients. We also review recent evidence regarding the timing of dialysis in patients with AKI. This journal recently reviewed the differential diagnosis and diagnostic evaluation of AKI, which is not covered here.13
DEFINITION OF ACUTE KIDNEY INJURY
AKI refers to an acute change in kidney function characterized by an increase in serum creatinine and/or a reduction in urine output. It is a clinical syndrome caused by a broad range of etiologies and may be related to primary kidney pathology and/or systemic illness. Until 2004, there was no standard definition for AKI and over 30 different definitions were found in the literature, which resulted in wide variation in the reported incidence and outcomes of AKI and made it challenging to apply an evidence-based approach to patient care. In 2004, the Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE)14 criteria for AKI were proposed, which were modified to the Acute Kidney Injury Network (AKIN)15 criteria in 2007 (Table 1). Multiple studies show that the RIFLE and AKIN criteria for AKI are associated with higher mortality1,2,8,10 and increased risk for requiring RRT.1,10
International clinical practice guidelines for AKI were released by Kidney Disease: Improving Global Outcomes (KDIGO) in 2012, which included a standardized definition of AKI that was adapted from the previously validated RIFLE and AKIN definitions.16 Patients are considered to have AKI when the serum creatinine rises by as little as 0.3 mg/dL. It is notable that when the baseline serum creatinine is high, there is more inherent variability in the serum creatinine measurement; thus, patients with CKD have a higher risk of being misclassified as having AKI.17 Although the KDIGO definition for AKI is commonly used in research settings, components of this definition have not been well validated, and it is not widely used in clinical practice. Other renal professional societies still recommend an individualized approach to the diagnosis of AKI, taking into account other factors such as trajectories in kidney function, fluid balance, electrolyte abnormalities, comorbid conditions, and clinical context.18,19 While we endorse the KDIGO approach to the categorization of AKI severity, in practice, a more patient-centered approach is generally required to guide the optimal approach to determining the etiology of AKI and guiding management.
GENERAL MANAGEMENT OF ACUTE KIDNEY INJURY
All patients with AKI should have close monitoring of their serum creatinine and urine output. Noninvasive diagnostic studies (urine microscopy, postvoid residual, and renal ultrasound) should be considered based on the clinical scenario. General management strategies include treatment of the reversible causes of AKI and optimization of volume status, hemodynamics, and nutritional status (Table 2).
Reversible Causes of Acute Kidney Injury
The first step in the treatment of AKI is to identify and treat readily reversible causes of AKI such as volume depletion, hypotension, infection, and urinary obstruction. Nephrotoxins should be avoided and all medications should be reviewed and adjusted for kidney function, particularly those that may affect mental status. Avoid opiates with noxious or active metabolites, including meperidine and morphine. Instead, hydromorphone, fentanyl, and methadone are preferred in patients with AKI. Other commonly used medications that require dose adjustment include gabapentin, baclofen, metoclopramide, H2 antagonists, many commonly prescribed antibiotics (penicillins, most cephalosporins, carbapenems, quinolones, and sulfa drugs), many hypoglycemic agents, and insulin. For patients on RRT, dosing is dependent on dialysis modality. Consultation with a hospital pharmacist is recommended when RRT modalities are initiated or changed.
Intravenous Fluids
Patients with AKI should have their volume status assessed and receive adequate resuscitation with intravenous fluids to promote renal perfusion. However, the optimal type and volume of fluid to give in AKI remains controversial. Colloid-containing solutions are theoretically confined to the intravascular space and should pose a lower risk for pulmonary edema compared with crystalloids. However, these solutions are costly, are not associated with any meaningful benefit,20-22 and may even be associated with potential harm.22-27
The most commonly used colloid worldwide is hydroxyethyl starch (HES). Its potential adverse effects include anaphylactoid reactions, coagulopathy, and AKI. HES is cleared by the kidneys and can cause osmotic nephrosis, a form of AKI characterized by vacuole formation and proximal renal tubular damage.28 Randomized controlled trials have shown an increased risk of AKI, RRT use, and mortality in critically ill patients who were resuscitated with HES.22,26,27 HES is not currently recommended in patients who are critically ill or have impaired kidney function and sepsis guidelines advise against its use.29
In the United States, albumin is the most common colloid-containing solution used for intravascular volume resuscitation. Albumin has been shown to be safe for volume resuscitation in critically ill patients,20 but there is no proven advantage to using albumin over saline with respect to mortality, length of hospital stay, duration of mechanical ventilation, duration of RRT, or number of organ systems failure.20,21 Furthermore, albumin may be harmful in certain patient populations. In patients with traumatic brain injury, albumin resuscitation is associated with higher mean intracranial pressures23 and long-term mortality.24 In a retrospective study of patients undergoing cardiac surgery, albumin administration was associated with more than twice the risk of AKI compared with crystalloids.25 In contrast, in patients with cirrhosis, intravenous albumin lowers the rate of AKI when administered in the setting of a large volume paracentesis30 or spontaneous bacterial peritonitis.31 Outside of these narrow settings, current evidence does not support the use of intravenous albumin to prevent AKI and we would not endorse the use of intravenous albumin as a part of the treatment paradigm for established AKI.
Many renal and critical care guidelines recommend initial fluid resuscitation with isotonic crystalloids except in specific circumstances (ie, hemorrhagic shock), with consideration of albumin in select cases (ie, severe sepsis or cirrhosis).16,18,19,29 That stated, the optimal type of crystalloid solution that should be used in resuscitation remains unclear. Because of its low cost, normal (0.9%) saline is the most commonly used solution, but it can result in hyperchloremic metabolic acidosis, which can cause renal vasoconstriction and may be associated with mortality in critically ill patients.32 A prospective study found that administration of chloride-liberal fluids (including normal saline) to critically ill patients was associated with nearly twice the risk of AKI and RRT use compared with chloride-restrictive fluids,33 but a subsequent trial found no difference in AKI or mortality among patients receiving saline versus a balanced crystalloid (Plasma-Lyte 148).34 A recent pair of large, randomized control trials compared outcomes in patients at a single center who were resuscitated with normal saline versus balanced crystalloid solutions (Lactated Ringer’s or Plasma-Lyte A).35,36 In critically ill patients, the use of balanced crystalloid solutions was associated with a lower risk of the composite outcome of mortality, new RRT, or persistent kidney impairment, but there were no differences in any of the individual components of the composite outcome.35 In noncritically ill patients, there were no differences in the number of hospital-free days based on the type of crystalloid solution used.36 In the absence of compelling evidence for using balanced crystalloid solutions, we continue to use normal saline for initial fluid resuscitation, but to avoid severe hyperchloremia and acidosis, we will consider switching to a balanced solution (Lactated Ringer’s, Plasma-Lyte, or Normosol) for large volume resuscitation (>2 L), particularly in critically ill patients.
Diuretics
As above, volume status is a key component in the management of patients with AKI. In patients with AKI and hypervolemia, loop diuretics are often given prior to the initiation of RRT. Loop diuretics act on the sodium-potassium-chloride cotransporters in the thick ascending limb of the loop of Henle to increase urinary losses of these ions and urine volume. Loop diuretics are dose-dependent, and often, higher doses are needed (eg, furosemide 100 mg intravenous dose) in patients with AKI, since the diuretic effect depends on the proximal tubular secretion of the drug into the urine. The role of diuretics in AKI is controversial and some observational data suggest an increased mortality risk with diuretic use in patients with AKI.37 In critically ill patients with acute lung injury, diuretic use improved survival, which was attributed to better control of volume overload.38 But, a meta-analysis of 11 randomized controlled trials failed to demonstrate that diuretics directly improved survival or recovery of AKI.39 Moreover, randomized controlled trials found that diuretics given to a patient with AKI requiring RRT did not improve recovery of kidney function.40,41 The KDIGO guidelines recommend that diuretics should not be routinely used for AKI except in the management of volume overload.16
Nutritional Targets in Acute Kidney Injury
Critically ill patients have high protein catabolic rates, which put them at increased risk for malnutrition, which in turn is associated with mortality. Patients who receive continuous RRT (CRRT) may lose 5-10 g of protein and 10-15 g of amino acids daily, and these patients may have protein requirements that are twice the usual recommended daily protein intake.16 But excess protein administration can result in high urea generation and azotemia unrelated to the patient’s kidney function. Blood urea nitrogen may also be disproportionately elevated in conditions where tubular reabsorption of urea is increased, such as in volume depletion, diuretic use, corticosteroid use, and gastrointestinal bleeding. Interpretation of blood urea nitrogen results must be made in the appropriate clinical context, with recognition that azotemia alone may not be a good surrogate marker of the patient’s underlying kidney function. We recommend dietary consultation in critically ill patients with AKI to ensure that adequate, but not excessive, protein is administered.
RENAL REPLACEMENT THERAPY IN ACUTE KIDNEY INJURY
In patients with AKI, RRT is initiated for control of volume overload, electrolyte abnormalities, acidemia, or uremic symptoms or complications that are refractory to medical management (Table 3). In a nonoliguric patient, fluid and electrolyte abnormalities can oftentimes be managed medically. Patients with oligoanuria (generally defined as urine output less than 400 mL/day or <20 mL/hour), however, require nephrology evaluation for consideration of RRT. Early nephrology consultation (within 48 hours of AKI diagnosis) may be associated with lower dialysis dependence and mortality in critically ill patients with AKI.42 The decision to initiate dialysis is individualized based on the patient’s comorbid conditions, urine output, and trajectory of kidney function.
Timing of Renal Replacement Therapy
The optimal timing of dialysis initiation in patients with AKI is not known. Theoretically, earlier initiation of dialysis could allow for better volume and electrolyte control and prevent the development of more serious complications of kidney failure such as uremic seizures, encephalopathy, and pericarditis. However, RRT is associated with its own risks and earlier initiation may expose the patient to unnecessary procedures and complications that might delay renal recovery. A meta-analysis of predominantly observational data found that earlier initiation of RRT in AKI was associated with lower 28-day mortality, greater renal recovery, decreased duration of RRT, and decreased ICU length of stay.43 Subsequently, two prospective trials reported conflicting results regarding associations between dialysis timing and outcomes in patients with severe AKI (Table 4).44,45
The Early vs Late Initiation of Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (ELAIN) was a prospective, single-center randomized trial in Germany of 231 critically ill, predominantly surgical ICU patients (about half postcardiac surgery) with at least KDIGO stage 2 AKI.44 Patients were randomized to early (within eight hours of developing KDIGO stage 2 AKI) or delayed (within 12 hours of developing KDIGO stage 3 AKI) RRT initiation; patients in the early RRT group initiated dialysis on average 20 hours earlier than the patients in the late group. All patients were treated with continuous venovenous hemodiafiltration. Early RRT initiation was associated with a 34% lower risk of mortality at 90 days, shorter hospital length of stay, and shorter RRT duration compared with delayed RRT initiation. There was no difference between groups in dialysis dependence at 90 days, but there was a lower risk of dialysis dependence at one year.46The Artificial Kidney Initiation in Kidney Injury Study (AKIKI)45 was a prospective, multicenter randomized trial in France that compared early versus delayed strategies of RRT initiation in 620 critically ill, mostly medical ICU patients with severe AKI (KDIGO stage 3). The median time between randomization and RRT initiation was two hours for the early and 57 hours for the delayed strategy groups. There were no differences between groups in length of hospital or ICU stay, vasopressor use, dialysis dependence, or 60-day survival. The early strategy group had a higher incidence of catheter-related bloodstream infections (10% vs 5%) and hypophosphatemia (22% vs 15%) compared with that of the delayed strategy group. Patients in the delayed strategy group regained normal urine output sooner than in the early strategy group. Approximately half of the patients in the delayed strategy group avoided RRT altogether. The authors of AKIKI concluded that there was no benefit to the early strategy of RRT in critically ill patients with severe AKI, and a delayed strategy of RRT initiation may avoid unnecessary RRT and reduce catheter-related infectious complications.
How can we interpret these discrepant results? Although ELAIN found a benefit to earlier RRT initiation in AKI, it has limited generalizability to medical ICU patients, who have higher mortality and whose outcomes might be less affected by dialysis timing. Patients in ELAIN had a high prevalence of congestive heart failure and CKD; it is possible that select patient populations may derive greater benefit from earlier RRT initiation. Although both ELAIN and AKIKI used the standardized criteria for RRT initiation, neither study could incorporate important clinical factors such as trajectory of kidney function, comorbid conditions, or symptoms, which play a significant role in the decision-making process in real-world clinical practice. Additional large-scale, multicenter trials are needed to guide the timing of RRT in critically ill patients with AKI. The Initiation of Dialysis Early Versus Delayed in the ICU (IDEAL-ICU)47 and Standard versus Accelerated Initiation of RRT in Acute Kidney Injury (STARRT-AKI)48 studies are currently underway and hope to provide clearer guidance regarding the optimal timing of RRT initiation in AKI (Table 4). Until further evidence is available, experts recommend taking into consideration the trajectory of kidney disease, concurrent organ dysfunction, and expected need for fluid and solute control when making decisions regarding RRT initiation in AKI.16
DIALYSIS MODALITIES IN ACUTE KIDNEY INJURY
When RRT is required in patients with AKI, the dialysis modality is often determined by local availability. CRRT and sustained low-efficiency dialysis (SLED) are thought to be better tolerated than intermittent hemodialysis in hemodynamically unstable patients, although a randomized controlled trial could not demonstrate a survival difference between these modalities.49 In general, in settings where CRRT or SLED is available, these modalities are favored for patients with hemodynamic instability, but practice patterns vary widely.
CONCLUSION
Among hospitalized patients, AKI is common and associated with a higher risk of mortality. Although serum creatinine and urine output criteria are used to define AKI, other clinical factors (comorbid conditions, volume status, and trajectory of kidney function decline) can inform the assessment and management of patients with AKI. General strategies for AKI management include treatment of reversible conditions, optimization of volume status, hemodynamics, and nutritional status. The optimal timing of RRT in critically ill patients with AKI is not known, with unclear mortality benefit of earlier dialysis initiation. Two large-scale randomized controlled trials regarding early versus delayed dialysis timing in AKI are currently underway and will hopefully provide clarity in the near future.
Disclosures
Dr. Yu and Dr. Kamal have nothing to disclose. Dr. Chertow is an advisor to DURECT Corporation.
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. PubMed
2. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913-1917. PubMed
3. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411-1423. PubMed
4. Wald R, McArthur E, Adhikari NKJ, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870-877. PubMed
5. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
6. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28. PubMed
7. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
8. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538-546. PubMed
9. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
10. Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292-1298. PubMed
11. Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis. 2015;65(2):275-282. PubMed
12. Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477-485. PubMed
13. Cooper CM, Fenves AZ. Before you call renal: acute kidney injury for hospitalists. J Hosp Med. 2015;10(6):403-408. PubMed
14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Workgroup A. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2): R31. PubMed
16. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
17. Lin J, Fernandez H, Shashaty MG, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10(10):1723-1731. PubMed
18. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672. PubMed
19. James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):673-685. PubMed
20. Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256. PubMed
21. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412-1421. PubMed
22. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901-1911. PubMed
23. Cooper DJ, Myburgh J, Heritier S, et al. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013;30(7):512-518. PubMed
24. Myburgh J, Cooper J, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884. PubMed
25. Frenette AJ, Bouchard J, Bernier P, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care. 2014;18(6):602. PubMed
26. Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethyl starch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357(9260):911-916. PubMed
27. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124-134. PubMed
28. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51(3):491-503. PubMed
29. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. PubMed
30. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. PubMed
31. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. PubMed
32. Boniatti MM, Cardoso PRC, Castilho RK, Vieira SRR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26(2):175-179. PubMed
33. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama-J Am Med Assoc. 2012;308(15):1566-1572. PubMed
34. Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. Jama-J Am Med Assoc. 2015;314(16):1701-1710. PubMed
35. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. PubMed
36. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. PubMed
37. Mehta RL, Pascual MT, Soroko S, Chertow GM, Group PS. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547-2553. PubMed
38. Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966-973. PubMed
39. Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283-293. PubMed
40. Cantarovich F, Rangoonwala B, Lorenz H, Verho M, Esnault VL, High-Dose Flurosemide in Acute Renal Failure Study Group. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402-409. PubMed
41. van der Voort PH, Boerma EC, Koopmans M, et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med. 2009;37(2):533-538. PubMed
42. Costa e Silva VT, Liano F, Muriel A, Diez R, de Castro I, Yu L. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLoS One. 2013;8(8):e70482. PubMed
43. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. PubMed
44. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190-2199. PubMed
45. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122-133. PubMed
46. Meersch M, Kullmar M, Schmidt C, et al. Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol. 2018;29(3):1011-1019. PubMed
47. Barbar SD, Binquet C, Monchi M, Bruyere R, Quenot JP. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15:270. PubMed
48. Smith OM, Wald R, Adhikari NK, et al. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. PubMed
49. Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379-385. PubMed
1. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. PubMed
2. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913-1917. PubMed
3. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411-1423. PubMed
4. Wald R, McArthur E, Adhikari NKJ, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870-877. PubMed
5. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
6. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28. PubMed
7. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
8. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538-546. PubMed
9. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
10. Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292-1298. PubMed
11. Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis. 2015;65(2):275-282. PubMed
12. Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477-485. PubMed
13. Cooper CM, Fenves AZ. Before you call renal: acute kidney injury for hospitalists. J Hosp Med. 2015;10(6):403-408. PubMed
14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Workgroup A. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2): R31. PubMed
16. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
17. Lin J, Fernandez H, Shashaty MG, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10(10):1723-1731. PubMed
18. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672. PubMed
19. James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):673-685. PubMed
20. Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256. PubMed
21. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412-1421. PubMed
22. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901-1911. PubMed
23. Cooper DJ, Myburgh J, Heritier S, et al. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013;30(7):512-518. PubMed
24. Myburgh J, Cooper J, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884. PubMed
25. Frenette AJ, Bouchard J, Bernier P, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care. 2014;18(6):602. PubMed
26. Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethyl starch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357(9260):911-916. PubMed
27. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124-134. PubMed
28. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51(3):491-503. PubMed
29. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. PubMed
30. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. PubMed
31. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. PubMed
32. Boniatti MM, Cardoso PRC, Castilho RK, Vieira SRR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26(2):175-179. PubMed
33. Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama-J Am Med Assoc. 2012;308(15):1566-1572. PubMed
34. Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. Jama-J Am Med Assoc. 2015;314(16):1701-1710. PubMed
35. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. PubMed
36. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. PubMed
37. Mehta RL, Pascual MT, Soroko S, Chertow GM, Group PS. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547-2553. PubMed
38. Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966-973. PubMed
39. Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283-293. PubMed
40. Cantarovich F, Rangoonwala B, Lorenz H, Verho M, Esnault VL, High-Dose Flurosemide in Acute Renal Failure Study Group. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402-409. PubMed
41. van der Voort PH, Boerma EC, Koopmans M, et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med. 2009;37(2):533-538. PubMed
42. Costa e Silva VT, Liano F, Muriel A, Diez R, de Castro I, Yu L. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLoS One. 2013;8(8):e70482. PubMed
43. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. PubMed
44. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190-2199. PubMed
45. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122-133. PubMed
46. Meersch M, Kullmar M, Schmidt C, et al. Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol. 2018;29(3):1011-1019. PubMed
47. Barbar SD, Binquet C, Monchi M, Bruyere R, Quenot JP. Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15:270. PubMed
48. Smith OM, Wald R, Adhikari NK, et al. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. PubMed
49. Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379-385. PubMed
© 2019 Society of Hospital Medicine
Postpartum psychosis: Protecting mother and infant
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
Organizing the P in a SOAP note
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
The Subjective, Objective, Assessment, Plan (SOAP) format of the progress note is widely recognized by clinicians in many specialties, including p
The Plan section should be organized in a way that is systematic and relevant across many psychiatric settings, including outpatient, inpatient, emergency room, jail, pediatric, geriatric, addiction, and consultation-liaison. To best accomplish this, I have designed a format for this section that consists of 6 categories:
1. Safety: Which safety issues need to be addressed?
Examples: If your patient is an inpatient, what precautions are required? If outpatient, Tarasoff? Involuntary hold? Police presence? Child or Adult Protective Services? Access to a firearm?
2. Collateral: Would it be helpful to obtain collateral information from any source?
Examples: Family? Friend? Caregiver? Teacher? Primary care clinician? Therapist? Past medical or psychiatric records?
3. Medical: Are there any medical tests or resources to consider?
Continue to: Examples...
Examples: Laboratory studies or imaging? Consult with a specialist from another field? Nursing orders?
4. Nonpharmacologic: What interventions or assessments would be helpful?
Examples: Psychotherapy? Cognitive testing? Social work? Case manager? Housing assistance? Job coach?
5. Pharmacologic: What interventions or assessments would be helpful? (I placed this category fifth to slow myself down and consider other strategies before quickly jumping to prescribe a medication.)
Examples: Medication? Long-acting injectable? Check pill count? Prescription drug monitoring program?
Continue to: 6. Disposition/follow-up...
6. Disposition/follow-up: What is the disposition/follow-up plan?
Examples: If outpatient, what is the time frame? If inpatient or an emergency room, when should the patient be discharged?
Using these 6 categories in the P section of my SOAP notes has helped me stay organized and think holistically about each patient I assess and treat. I hope other clinicians find this format helpful.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
1. Pearce PF, Ferguson LA, George GS, et al. The essential SOAP note in an EHR age. Nurse Pract. 2016;41(2):29-36.
2. Foreman T, Dickstein LJ, Garakani A, et al (eds). A resident’s guide to surviving psychiatric training, 3rd ed. Washington, DC: American Psychiatric Association; 2015.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed August 20, 2018.
COMBS: Feeling positive about negative symptoms of schizophrenia
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
Negative symptoms of schizophrenia—such as social withdrawal, avolition, avoidance, lack of spontaneity, anhedonia, poverty of speech, and blunted affect—often persist after successful treatment of positive symptoms, such as hallucinations and delusions.1 Negative symptoms can be debilitating and are associated with poor social and occupational outcomes, as well as cognitive dysfunction. Currently, treatments for negative symptoms are not nearly as effective as treatments for positive symptoms. The mnemonic COMBS can be used to easily recall 3 treatment modalities often used to address negative symptoms.
COgnitive-behavioral therapy
Cognitive-behavioral therapy (CBT) and other psychosocial therapies derived from it, such as social skills training, recovery-oriented cognitive therapy, motivation and enhancement therapy, and cognitive-behavioral social skills training (CBSST), have shown to be effective for treating negative symptoms.2 In a study of 149 patients with schizophrenia, CBSST reduced symptoms of avolition and apathy and improved functioning outcomes.2
Medications
Antipsychotics. Although second-generation antipsychotics (SGAs) were initially promising, accumulating clinical experience and research have shown that these agents have limited efficacy for treating negative symptoms.1 Unlike first-generation antipsychotics, SGAs do not cause affective blunting, and are effective at treating depressive symptoms; however, depressive symptoms can sometimes be difficult to distinguish from negative symptoms. Improvement of depressive symptoms observed with SGA treatment could be mistakenly interpreted as alleviation of negative symptoms; however, clinical trials that focused specifically on treating negative symptoms have found no specific efficacy of SGAs.1
Antidepressants. Although clinical trials and meta-analyses have had mixed results,1 antidepressants appear to be safe add-on treatments with small efficacy for negative symptoms.
Anticonvulsants have long been used as augmentation to antipsychotics for patients with treatment-resistant schizophrenia; however, there is no evidence that these medications can improve negative symptoms.1
Stimulants. There is no strong evidence that stimulants could be an efficacious treatment for negative symptoms.1
Other pharmacologic agents,1 such as acetylcholine-related medications, oxytocin, and medications with a mechanism of action that is related to an inflammatory response and immunologic pathways (ie, minocycline), are being evaluated for treating negative symptoms. Research into the efficacy of glutamate-related agents also appears to be continuing.1
Continue to: Brain Stimulation therapies
Brain Stimulation therapies
Transcranial magnetic stimulation (TMS), transdirect current stimulation (tDCS), vagus nerve stimulation, and deep brain stimulation have been evaluated for treating negative symptoms. A recent meta-analysis of randomized controlled trials comparing the effects of brain stimulation with sham interventions in patients with schizophrenia found that TMS and tDCS that targeted the left dorsolateral prefrontal cortex effectively reduced the severity of negative symptoms.3
The Table1-3 summarizes available treatments for negative symptoms of schizophrenia and their efficacies. Although research investigating the improvement of negative symptoms is currently insufficient, CBT-related therapies and antidepressants appear to be helpful. For more information, see “Treating negative symptoms of schizophrenia” (C
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
1. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry. 2016;3:133-150.
2. Granholm E, Holden J, Worley M. Improvement in negative symptoms and functioning in cognitive-behavioral social skills training for schizophrenia: mediation by defeatist performance attitudes and asocial beliefs. Schizophr Bull. 2018;44(3):653-661.
3. Kennedy NI, Lee WH. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77.
Impact of Pharmacist-led Discharge Counseling on Hospital Readmission and Emergency Department Visits: A Systematic Review and Meta-analysis
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
© 2020 Society of Hospital Medicine
Hepatitis vaccination update
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.
The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3
As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site
Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.
The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3
As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site
Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.
The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3
As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site
Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.